Open Access Article
Open Access ArticlePrecise immunological evaluation rationalizes the design of a self-adjuvanting vaccine composed of glycan antigen, TLR1/2 ligand, and T-helper cell epitope†
Tsung-Che Chang‡
a,
Yoshiyuki Manabe‡ *ab,
Keita Itoa,
Ryuku Yamamotoa,
Kazuya Kabayamaab,
Shino Ohshimac,
Yoshie Kametani*c,
Yukari Fujimoto
*ab,
Keita Itoa,
Ryuku Yamamotoa,
Kazuya Kabayamaab,
Shino Ohshimac,
Yoshie Kametani*c,
Yukari Fujimoto d,
Chun-Cheng Lin
d,
Chun-Cheng Lin e and
Koichi Fukase*ab
e and
Koichi Fukase*ab
aDepartment of Chemistry, Graduate School of Science, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan. E-mail: Koichi@chem.sci.osaka-u.ac.jp
bForefront Research Center, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan
cFaculty of Medicine, School of Medicine, Tokai University, 143 Shimokasuya, Isehara-shi, Kanagawa 259-1193, Japan
dDepartment of Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama, Kanagawa 223-8522, Japan
eDepartment of Chemistry, National Tsing Hua University, 101 Sec. 2, Kuang Fu Rd., Hsinchu 30013, Taiwan
First published on 29th June 2022
Abstract
Sialyl-Tn (STn), overexpressed on various tumors, has been investigated for its application in anti-cancer vaccine therapy. However, Theratope, an STn-based vaccine, failed in the phase III clinical trial due to poor immunogenicity and epitope suppression by the foreign carrier protein. We therefore developed a self-adjuvanting STn based-vaccine, a conjugate of clustered STn (triSTn) antigen, TLR1/2 ligand (Pam3CSK4), and T-helper (Th) cell epitope, and found that this three-component self-adjuvanting vaccine effectively resulted in the production of anti-triSTn IgG antibodies. We herein analyzed immune responses induced by this self-adjuvanting vaccine in detail. We newly synthesized two-component vaccines, i.e., Pam3CSK4- or Th epitope-conjugated triSTn, as references to evaluate the immune-stimulating functions of Pam3CSK4 and Th epitope. Immunological evaluation of the synthesized vaccine candidates revealed that Pam3CSK4 was essential for antibody production, indicating that the uptake of triSTn antigen by antigen-presenting cells (APCs) was promoted by the recognition of Pam3CSK4 by TLR1/2. The function of the Th epitope was also confirmed. Th cell activation was important for boosting antibody production and IgG subclass switching. Furthermore, flow cytometric analyses of immune cells, including T cells, B cells, dendritic cells, and other monocytes, were first employed in the evaluation of self-adjuvanting vaccines and revealed that the three-component vaccine was able to induce antigen-specific immune responses for efficient antibody production without excessive inflammatory responses. Importantly, the co-administration of Freund's adjuvants was suggested to cause excessive myeloid cell accumulation and decreased plasma cell differentiation. These results demonstrate that vaccines can be designed to achieve the desired immune responses via the bottom-up construction of each immune element.
Introduction
Tumor-associated carbohydrate antigens (TACAs),1 which are overexpressed on tumor cells and rarely expressed on normal cells, can be used for cancer vaccine therapy.2 In fact, many vaccines have entered clinical trials, including phase III trials. However, no TACA-based cancer vaccine has been approved for clinical use yet. Since TACAs employed in clinical trials are small T cell-independent antigens, they exhibit poor immunogenicity and cannot induce immunoglobulin (Ig) class switching. Therefore, they have been conjugated with immunogenic carrier proteins, such as keyhole limpet hemocyanin (KLH), tetanus toxoid (TT), or diphtheria toxin (CRM197).3 Although these carrier proteins possess T cell epitopes and the conjugates can elicit a strong immune response, they can sometimes induce suppression of the target epitope-specific reaction, reducing their efficacy.4,5It has been necessary to control adverse reactions while ensuring efficacy for current vaccine development. Self-adjuvanting vaccines, the conjugates of antigens and immunoenhancers, are expected to induce antigen-specific immune responses without excessive inflammation.6–8 In addition, they have well-defined structures and hence facilitate quality control of vaccine products.
In the self-adjuvanting vaccine strategy, T-helper (Th) cell epitopes and innate immune ligands are usually conjugated as immunoenhancers. Conjugation with Th epitopes can induce T cell-dependent immune responses, such as antigen-specific B cell activation and IgG class switching. Kunz et al. introduced various Th epitopes or multiple Th epitopes in MUC1 glycopeptide-based vaccines and evaluated their effects.9,10
Innate immune ligands, especially Toll-like receptor (TLR) agonists, are promising adjuvants and have been applied to practical use.11–20 Since TLRs are expressed on antigen presenting cells (APCs) such as macrophages, B cells, and dendritic cells, the conjugation of antigens with TLR ligands is expected to promote antigen uptake by APCs and also to upregulate adoptive immune responses through the induction of costimulatory molecules such as CD80 (B7-1) and CD86 (B7-2) responsible for T cell activation. The TLR family is divided into largely two groups, which contain extracellular and intracellular receptors.13,21–23 Especially, TLR-1, TLR-2 and TLR-6, which are of the former TLRs, tend to induce humoral immune responses, whereas intracellular TLRs enhance Th1 and cellular immune responses. Although cancer immunity has focused on cellular immunity, humoral immunity might exert strong effects to overcome the cancer if functional anti-cancer IgGs are extensively produced.
Extracellular TLR ligands, such as TLR2 ligands and TLR4 ligands have been mainly utilized to develop self-adjuvanting vaccines. Trumenba, a recombinant lipoprotein with a TLR2-stimulating unit, is an FDA-approved self-adjuvanting vaccine that is used against Neisseria meningitidis group B disease.24 Actually, TLR2 agonists, including Pam3CSK4, have often been used in many self-adjuvanting vaccines considering their immunostimulation efficacy and ease of synthesis.25–33 Boons et al. reported that a tumor-associated MUC1 glycopeptide conjugated with TLR1/2 ligand (Pam3CSK4) and a Th epitope induced high IgG titers against the MUC1 antigen.25,26 We have also previously reported Pam3CSK4-conjugated self-adjuvanting vaccines.30–32 TLR4 ligands have also been used for self-adjuvanting vaccines. Guo et al. reported that self-adjuvanting vaccines containing the mild TLR4 agonist monophosphoryl lipid A elicit TACA-specific IgG antibodies without Th epitope conjugation.34–37 Codée et al. also developed a self-adjuvanting vaccine conjugated with CRX-527, a lipid A analogue.38 The lipidic property of TLR2 and TLR4 ligands might also contribute to the adjuvant effects via the formation of aggregates, which enables multivalent interactions between the conjugated antigens and B cell receptors (BCRs) to promote BCR clustering. In fact, Kiessling et al. have revealed that polymeric multivalent ligands induce BCR clustering to activate B cell responses.39 The importance of the lipid moiety for the self-adjuvating property was also suggested based on the adjuvant effects in several TACA-based vaccines.33,40–42 Intracellular TLR ligands and other innate immune ligands have been also employed in self-adjuvanting vaccines. A TLR9 agonist (CpG-ODNs) was less effective for antibody production compared to Pam3CSK4.26 A self-adjuvanting vaccine using a CD1d ligand (α-galactosylceramide),43–47 a zwitterionic polysaccharide,48–50 and a TLR7 ligand51 have also been investigated.
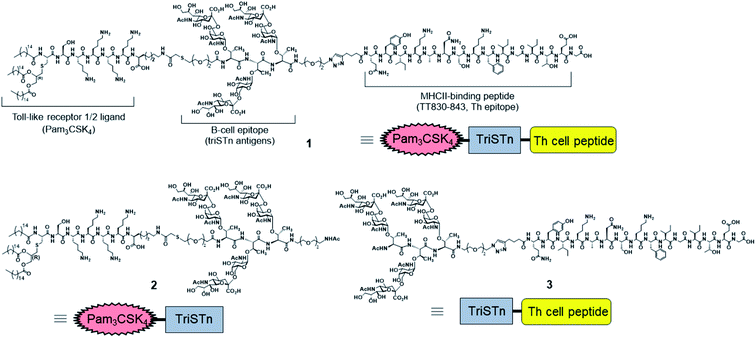
Despite many reports of excellent self-adjuvanting vaccines, most previous studies have focused on the antibody titer and level of secreted cytokines to evaluate the efficacy of self-adjuvanting vaccines, especially TACA-based vaccines. Exceptionally, BenMohamed et al. reported the efficacy of synthetic conjugated vaccines composed of the clustered tetravalent Tn carbohydrate antigen with Th (CD4+) and Tc (CD8+) epitopes as an antitumor vaccine in a mouse tumor model, though they did not analyze the innate immune responses.52 To understand the high efficacy of self-adjuvanting vaccines with low inflammatory effects, the cellular responses should be investigated. However, the role and effect of each component in self-adjuvanting vaccines have not been investigated well at the cellular level. Hence, in this study, we evaluated the immunological responses of the three-component self-adjuvanting vaccine 1,30 composed of trimeric Sialyl-Tn cluster (TACA), Pam3CSK4, and a Th cell epitope, in comparison to those with Pam3CSK4- or Th epitope-conjugated triSTn (vaccine 2 and 3, respectively; Fig. 1). We herein analyzed the production of antibodies, as well as the activation of various immune cells, including T cells, B cells, and dendritic cells, by flow cytometry with staining for each cell marker. These analyses provided clear evidence that the covalently linked three-component vaccine can efficiently induce specific immune responses without causing unnecessary inflammation. A combination of the TLR2 ligand and Th epitope was found to be essential for the activation of B cells and ACPs, as well as T cells. Namely, we herein demonstrated the immunological rationality of the design of three-component vaccines, offering essential information for their clinical development.
In general, glycoconjugate vaccines require the co-administration of adjuvants such as alum for clinical use and Freund's adjuvants for experimental use.31,53 Freund's adjuvants are often used in mouse experiments, but are not approved for use in humans and are not recommended for preclinical studies. Until now, conflicting results have been reported on the effects of co-administrating additional adjuvants in the self-adjuvanting system, and no detailed immunological analyses have been performed. This study clearly revealed that the co-administration of Freund's adjuvants induced nonspecific immune responses to interfere with the antigen-selective immune responses, proposing guidelines that additional adjuvants should not be added to the self-adjuvanting vaccines.
Sialyl-Tn antigen [STn, NeuAcα(2,6)GalNAcα-O-Ser/Thr] is abundantly expressed on many epithelium-derived tumors (e.g., breast, pancreas, prostate, lung, colorectal, gastric, and ovarian)54,55 and correlates with invasion and aggressive potential. Therefore, cancer vaccines targeting STn-related mucins have been developed and investigated over the past several decades.56–60 Theratope (STn-KLH conjugate) was developed as a therapeutic vaccine for the treatment of metastatic breast cancer.61 However, it failed in the phase III clinical trial because statistical significance was not observed in the extension of overall survival and disease progression time.62,63 Post hoc analysis revealed that overall survival was significantly improved in patients exhibiting a high antibody titer against ovine submaxillary mucin (OSM), whereas the antibody titer against STn did not correlate with the overall survival. Since OSM multivalently expresses STn, the clustered STn seemed to be an appropriate antigen for vaccines against breast cancer. Moreover, the clustered STn structures are expressed on the mucins of most adenocarcinomas with high specificity.64–66 Therefore, anti-STn vaccines that can effectively induce immune responses against clustered STn are expected to effectively target cancer cells. The three-component self-adjuvanting vaccine 1, developed by our group, effectively produced IgG antibodies, which selectively recognized clustered STn (triSTn) over STn.30 Thus, we herein analyzed immunological responses induced by 1 in detail to obtain the guidelines for efficient TACA-based cancer vaccine development.
Results and discussion
The vaccine candidates in this study and their chemical structures are presented in Table 1 and Fig. 1, respectively. Two-component conjugated vaccines, Pam3CSK4-triSTn 2 and triSTn-Th epitope 3, were newly synthesized to investigate the function of the TLR2 ligand and Th immunomodulator (Schemes S1–S3 and Fig. S1–S6†). The preparation of three-component vaccine 1, composed of triSTn antigen, Pam3CSK4, and Th epitope, has been previously reported.30 Compound 1 was used for immunization with or without Freund's adjuvants (V1′ and V1, respectively), whereas compounds 2 and 3 were used for vaccination without Freund's adjuvants (V2 and V3).| Vaccine candidate | Immunization compounds |
|---|---|
| V1 | Pam3CSK4-triSTn-Th epitope 1 |
| V1′ | Pam3CSK4-triSTn-Th epitope 1 + Freund's adjuvant |
| V2 | Pam3CSK4-triSTn 2 |
| V3 | triSTn-Th epitope 3 |
Immunization was performed according to the standard protocol. Compounds 1–3 in phosphate-buffered saline (PBS) were intraperitoneally administrated to 8 week-old wild-type BALB/c mice on day 1. The immunization schedule included the administration of three booster doses for each mouse on days 14, 28, and 42 via injection of the same vaccine materials. Regarding V1′, The first immunization was performed with complete Freund's adjuvant, whereas the booster doses were with incomplete Freund's adjuvant. Blood was collected from each mouse before immunization on day 0 (blank controls) and 1 week after each immunization (days 8, 21, 35, and 49). The blood samples were used to prepare plasma via the standard method. TriSTn-, STn-, and linker (composed of PEG linker and tri Thr without STn glycan)-immobilized bovine serum albumin (BSA-TriSTn, BSA-STn, and BSA-linker, respectively)30 were used to evaluate the antibody titers against each epitope by enzyme-linked immunosorbent assay (ELISA).
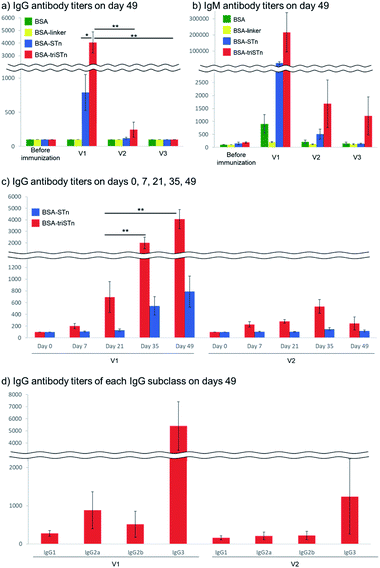
An estimation of antibody production after four doses clearly revealed the usefulness of the three-component vaccine 1 (Fig. 2a and b). In our previous report,30 1 induced robust immune responses, whereas the mixture of the respective components (STn antigen, Pam3CSK4, and Th epitope) did not induce significant antibody production (Fig. S7†), indicating the importance of covalent linkage for antigen-specific immune responses. Furthermore, the titer with V1 was slightly higher than that for V1′ (Fig. S7†), suggesting that additional adjuvant administration did not improve antibody production. Moreover, the present study clearly demonstrates that robust immune responses to the three-component vaccine 1 require the conjugation of both Pam3CSK4 and Th epitope; the antibody titers for the two-component vaccine 2, Pam3CSK4-triSTn conjugate, were much lower than those induced by the three-component vaccine 1, whereas vaccine 3, Th epitope-triSTn conjugate, did not induce IgG production. V2 was able to induce a small amount of antibodies, whereas V3 was not, indicating that Pam3CSK4 was essential for antibody production. Probably, promotion of antigen uptake through the recognition by TLR is required for the initiation of immune responses.67 It should also be mentioned that V1 produced antibodies that selectively recognize tri-STn over STn, as expected.
Next, we investigated the booster effect of vaccine candidates V1 and V2 (Fig. 2c). V1 resulted in a steady increase in IgG titers from day 0 to day 49, showing a significant booster effect as observed in the previous report of V1′ (Fig. S8†). Conversely, V2 did not show any booster effect; IgG titers induced by V2 did not change from day 7 to 49. These results indicated that the Th epitope is necessary for Th cell activation to induce robust immune responses. In addition, memory B cells with highly specific IgG expression might not be induced without Th epitope stimulation, and repetitive naïve B cell activation might result in the poor production of highly specific antibodies. This tendency was also observed for IgM antibody production (Fig. S9†), demonstrating that the Th epitope plays an important role in boosting IgM antibody production, as well as in IgM to IgG class switching.9,10
The Th-epitope also influenced the produced IgG subclasses (Fig. 2d and S10†). The IgG3 isotype of anti-triSTn antibodies was observed as a major subclass with V1, V1′, and V2 vaccinations. IgG2a and IgG2b antibodies were significantly induced by V1 and V1′. Mouse IgG3 can be produced against thymus-independent antigens such as bacterial carbohydrates without T cell assistance.68,69 Thus, IgG2a and IgG2b antibody production with V1 and V1′ suggested that the three-component vaccine 1 induced adaptive T cell-mediated immunity and IgG subclass switching.
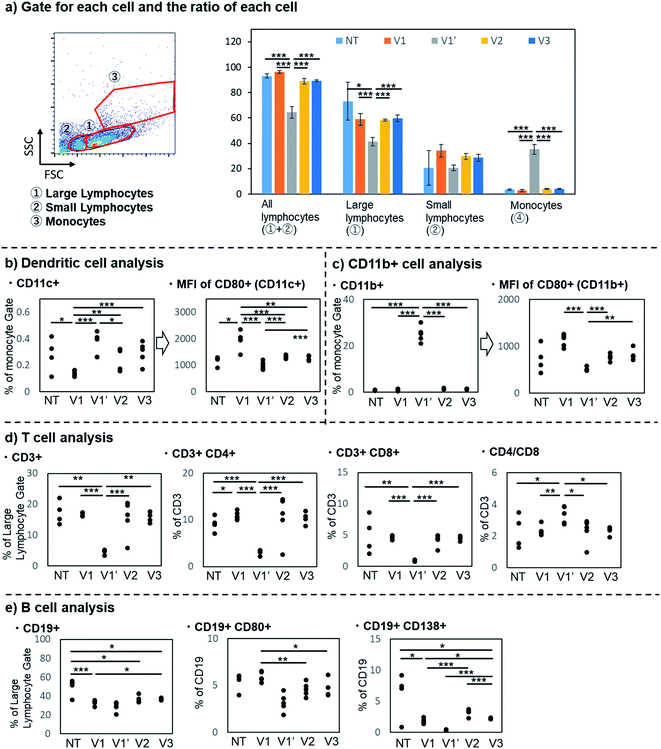
To evaluate the detailed immune responses induced by each vaccine candidate, immune cells from the spleen of each mouse collected on day 49 were analyzed by flow cytometry (Fig. 3). In addition, immune responses of non-vaccine treated mice (NT) were also analyzed as a control. Size-fractionation and staining with antibodies against the corresponding immune cell markers provided information on the activation and proportion of respective cells.
The number of spleen cells was first counted for each mouse. The spleen cells were divided into large lymphocytes, small lymphocytes, and monocytes based on forward scatter (FSC) and side scatter (SSC) (Fig. 3a). The co-administration of Freund's adjuvants increased the total number of immune cells (Fig. S28†), especially the number of monocytes, resulting in a highly skewed monocyte/lymphocyte ratio (average monocyte ratio, NT: 3.5%, V1: 2.8%, V1′: 35.2%, V2: 4.1%, V3: 4.0%, cf. usual: less than 10%).
The increased expression of CD80, an activation marker of APCs, including dendritic cells and macrophages, with the three-component vaccine 1 was confirmed (Fig. 3b and c). V1 resulted in a higher mean fluorescence intensity (MFI) for CD80 than the other candidates. It is well-known that TLR2 ligands upregulate the expression of CD80. The present result indicates that the co-activation of TLR2 and MHC class II efficiently promotes CD80 expression, which is responsible for the subsequent T cell activation.
Although the number of dendritic cells and other monocytes (involving macrophages), stained with antibodies against CD11c and CD11b, respectively, were increased with the co-administration of Freund's adjuvants (V1′), CD80 expression decreased on these cells. Namely, the co-administration of Freund's adjuvants increased the number of myeloid-gated cells, but the co-receptor (CD80) expression on these cells was suppressed in the spleen. These results indicated that Freund's adjuvants might induce monocyte accumulation in the spleen and consequently suppress their activation. The development of suppressive cells, such as myeloid-derived suppressor cells (MDSCs), might be enhanced by the excess stimulation by Freund's adjuvants.70
T cell analysis revealed that V1 activated T cells without affecting the Th/Tc balance. Spleen cells of immunized mice were stained with antibodies against a T cell marker (CD3) and differentiated cell markers [CD4: helper T (Th) cell marker, CD8: cytotoxic T (Tc) cell marker] (Fig. 3d). For all candidates, the Th(CD4+)/Tc(CD8+) ratio was almost the same as that for nonimmunized BALB/c mice except with V1′; the CD4+/CD8+ ratios for NT, V1, V1′, V2, and V3 were 1.8, 2.3, 3.3, 2.5, and 2.3, respectively, whereas that of nonimmunized BALC/c mice was reported to be approximately 2.3.71,72 The IgG titer was significantly higher with V1 than with V2 (Fig. 2a), clearly indicating that the conjugated Th epitope was functional. However, the total Th cell number and ratio were not remarkably increased by the conjugation with Th epitope (V1 and V3), suggesting only the selective activation of specific Th cells by V1 and V3. Considering that Tc responses are also important for anti-tumor immunity, the induction of Th responses without affecting the Th/Tc balance is desirable. In contrast, the co-administration of Freund's adjuvants (V1′) decreased the proportion of activated T cells (both Th and Tc), indicating that the co-administration of additional adjuvants like Freund's adjuvant should be avoided for self-adjuvanting vaccines.
B cell analysis also suggested that V1 can effectively stimulate the immune system to induce antibody production (Fig. 3e). The three conjugated vaccines 1, 2, and 3 did not significantly affect the number of B cells, which were stained with the antibody against a B cell marker (CD19). Meanwhile, V1, the three-component vaccine 1 administered without Freund's adjuvants, resulted in the highest ratio of B cells expressing an activated B cell marker (CD80). V1 exhibited higher differentiating activity than V2, whereas both V1 and V2 may directly stimulate B cells via TLR2,73 suggesting that B cells need to be activated by an interaction between B cells and T cells via presentation of the Th epitope by MHC class II to the T cell receptor, as well as the recognition of co-stimulatory molecules such as CD80 on B cells by CD28 on T cells.
In contrast, V1′ showed a significantly lower ratio of CD80+ B cells, suggesting that the excessive immune responses induced by Freund's adjuvants might suppress B cell activation. In addition, the proportion and number of plasma/plasmablasts, which are involved in antibody production, stained with an antibody against CD138, were decreased with the co-administration of Freund's adjuvants (average plasma/plasmablast ratio, NT: 6.1, V1: 1.8, V1′: 0.3, V2: 3.2, and V3: 2.2). These results also suggest that the co-administration of additional Freund's adjuvants with self-adjuvanting vaccines is not desirable.
Li et al. also reported that the co-administration of Freund's adjuvant with self-adjuvanting vaccines reduced antibody production, though they did not analyze the cellular responses.28,74 In contrast, Toth et al. reported that coadministration of Freund's adjuvant enhanced the antibody production by self-adjuvanting vaccines.41 However, since they used a weak TLR2 ligand, it is likely that additional stimulation with Freund's adjuvant resulted in antibody production. Our results indicate that the co-administration of additional adjuvants to self-adjuvanting vaccines should be avoided because it elicits non-specific inflammatory responses.
Conclusions
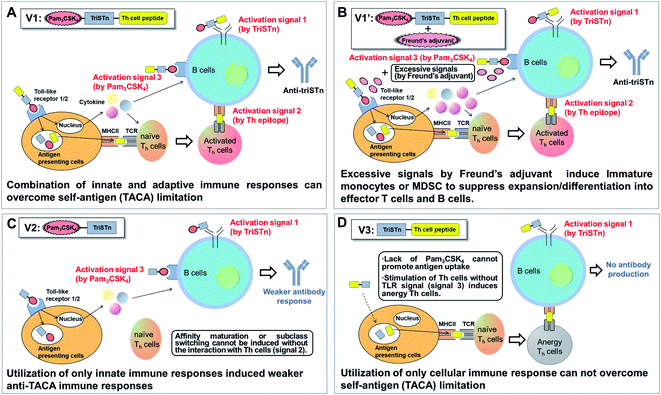
In conclusion, our analyses revealed that the three-component vaccine 1 induced robust immunity toward triSTn antigen as designed. Since vaccine 1 induced innate and adaptive immunity to effectively activate T cells, B cells, dendritic cells, and macrophages, V1 overcame the self-antigen (TACA) limitation (Fig. 4A). B cells are synergistically stimulated via direct interactions with V1 (activation signal 1), the interaction with T cells activated by dendritic cells (activation signal 2), and cytokines induced by a TLR agonist (activation signal 3) to efficiently achieve affinity maturation and class switching of anti-triSTn antibodies.75 Conjugation with not only TLR ligand but also Th epitope has already been reported to be required to overcome the poor immunogenicity of T cell-independent TACA.33,41,42 Our analysis revealed that Th epitope and TLR ligand conjugation are essential for the activation of B cells and APCs, as well as T cells, demonstrating the immunological rationality of the design of three-component vaccines. Of course, the importance of covalent linkage between the triStn antigen, Pam3CSK4, and Th epitope should be emphasized to obtain antigen-specific immune responses.Importantly, the co-administration of Freund's adjuvants with vaccine 1 was suggested to cause splenomegaly, containing increased number of myeloid cells, which might include immature monocytes or MDSCs, to suppress expansion/differentiation into effector T cells and B cells (Fig. 4B).76,77 This study demonstrated that the co-administration of an additional adjuvant induces nonspecific immune responses to interfere with the antigen-selective immune responses.
In contrast, the Pam3CSK4-triSTn conjugate V2 produced a weaker anti-triSTn humoral immune response than V1 (Fig. 4C). Although V2 can stimulate immune cells through TLR1/2 to produce anti-triSTn antibodies (activation signal 1 and 3), it cannot induce T cell–B cell interactions via the TCR, MHC, and antigen peptides due to the absence of a Th epitope and cannot promote the affinity maturation and class switching.
In spite of the trimeric presentation of STn antigen and conjugation with Th epitope, V3 did not trigger the production of any anti-triSTn IgG antibodies partly because of the small molecular weight of the glycopeptide (Fig. 4D). Lipophilic Pam3CSK4-conjugated vaccines tend to form supramolecular aggregates, which function as carriers.31,32 Kunz and Li et al. previously reported that self-adjuvanting vaccines composed of MUC1 glycopeptides and a T-cell epitope with Freund's adjuvants effectively induced antibodies against MUC1 glycopeptides, probably the molecular sizes of the vaccines were large enough for the antibody production.9 However, addition of Freund's adjuvant to V3 may not improve its immunogenicity because of its low molecular weight. Promotion of antigen uptake to APCs through the recognition by TLR might be essential step to initiate immune responses. The interaction between T cells and APCs without TLR stimulation might also induce the anergy of T cells due to the insufficient expression of MHC II and B7.78 Hence, the intrinsic limitation of TACAs, self-antigens, probably cannot be overcome only by the involvement of a Th epitope.
Overall, our results indicate that the activation of both innate and adaptive immune responses is crucial in the design of fully synthetic self-adjuvanting TACA-based anti-cancer vaccines. Our results demonstrate that vaccines can be designed to achieve the desired immune responses through bottom-up construction of the necessary immune elements.
Data availability
We have provided all the necessary data in the ESI.†Author contributions
T-C. C. synthesized all compounds employed in this study. T-C. C., K. I. and R. Y. performed ELISA assay. S. O. and Y. K. performed in vivo vaccination and flowcytometry analysis. Y. M., K. K., Y. K., Y. F., C–C. L., and K. F. designed the experiments. T-C. C., Y. M., Y. K., and K. F. mainly wrote the manuscript. All authors reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported in part by JSPS KAKENHI grant number 20H05675, JSPS KAKENHI grant number 20H00404, JSPS KAKENHI grant number 20K05727, JSPS KAKENHI grant number 20H04709, JSPS KAKENHI grant number 21H05074, and AMED grant number 20lm0203007j0004, AMED grant number 21ek0109444h0002.Notes and references
- S. S. Pinho and C. A. Reis, Nat. Rev. Cancer, 2015, 15, 540–555 CrossRef CAS PubMed.
- R. M. Wilson and S. J. Danishefsky, J. Am. Chem. Soc., 2013, 135, 14462–14472 CrossRef CAS PubMed.
- M. E. Pichichero, Hum. Vaccines Immunother., 2013, 9, 2505–2523 CrossRef CAS PubMed.
- M. P. Schutze, C. Leclerc, M. Jolivet, F. Audibert and L. Chedid, J. Immunol., 1985, 135, 2319–2322 CAS.
- H. Findlow and R. Borrow, Hum. vaccines immunother., 2016, 12, 226–230 CrossRef CAS PubMed.
- N. Gaidzik, U. Westerlind and H. Kunz, Chem. Soc. Rev., 2013, 42, 4421–4442 RSC.
- D. M. McDonald, S. N. Byrne and R. J. Payne, Front. Chem., 2015, 3, 60 Search PubMed.
- Q. Li and Z. Guo, Molecules, 2018, 23, 1583 CrossRef PubMed.
- H. Cai, M.-S. Chen, Z.-Y. Sun, Y.-F. Zhao, H. Kunz and Y.-M. Li, Angew. Chem., Int. Ed., 2013, 52, 6106–6110 CrossRef CAS PubMed.
- B. Palitzsch, S. Hartmann, N. Stergiou, M. Glaffig, E. Schmitt and H. Kunz, Angew. Chem., Int. Ed., 2014, 53, 14245–14249 CrossRef CAS PubMed.
- A. Bendelac and R. Medzhitov, J. Exp. Med., 2002, 195, F19–F23 CrossRef CAS PubMed.
- M. A. Anwar, M. Shah, J. Kim and S. Choi, Med. Res. Rev., 2019, 39, 1053–1090 CrossRef CAS PubMed.
- B. Pulendran, P. S. Arunachalam and D. T. O'Hagan, Nat. Rev. Drug Discovery, 2021, 20, 454–475 CrossRef CAS PubMed.
- C. W. Cluff, Adv. Exp. Med. Biol., 2010, 667, 111–123 CrossRef PubMed.
- V. Mata-Haro, C. Cekic, M. Martin, P. M. Chilton, C. R. Casella and T. C. Mitchell, Science, 2007, 316, 1628–1632 CrossRef CAS PubMed.
- C. R. Casella and T. C. Mitchell, Cell. Mol. Life Sci., 2008, 65, 3231 CrossRef CAS PubMed.
- A. M. Krieg, A.-K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky and D. M. Klinman, Nature, 1995, 374, 546–549 CrossRef CAS PubMed.
- H. Hemmi, O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda and S. Akira, Nature, 2000, 408, 740–745 CrossRef CAS PubMed.
- A. M. Krieg, Annu. Rev. Immunol., 2002, 20, 709–760 CrossRef CAS PubMed.
- A. M. Krieg, Nat. Rev. Drug Discovery, 2006, 5, 471–484 CrossRef CAS PubMed.
- D. N. Toussi and P. Massari, Vaccines, 2014, 2, 323–353 CrossRef CAS PubMed.
- S. Mukherjee, S. Karmakar and S. P. S. Babu, Braz. J. Infect. Dis., 2016, 20, 193–204 CrossRef PubMed.
- M. Luchner, S. Reinke and A. Milicic, Pharmaceutics, 2021, 13, 142 CrossRef CAS PubMed.
- A. Gandhi, P. Balmer and L. J. York, Postgrad. Med., 2016, 128, 548–556 CrossRef PubMed.
- S. Ingale, M. A. Wolfert, J. Gaekwad, T. Buskas and G. J. Boons, Nat. Chem. Biol., 2007, 3, 663–667 CrossRef CAS PubMed.
- A.-B. M. Abdel-Aal, V. Lakshminarayanan, P. Thompson, N. Supekar, J. M. Bradley, M. A. Wolfert, P. A. Cohen, S. J. Gendler and G.-J. Boons, ChemBioChem, 2014, 15, 1508–1513 CrossRef CAS PubMed.
- A. Kaiser, N. Gaidzik, T. Becker, C. Menge, K. Groh, H. Cai, Y.-M. Li, B. Gerlitzki, E. Schmitt and H. Kunz, Angew. Chem., Int. Ed., 2010, 49, 3688–3692 CrossRef CAS PubMed.
- H. Cai, Z.-Y. Sun, Z.-H. Huang, L. Shi, Y.-F. Zhao, H. Kunz and Y.-M. Li, Chem.–Eur. J., 2013, 19, 1962–1970 CrossRef CAS PubMed.
- P. Thompson, V. Lakshminarayanan, N. T. Supekar, J. M. Bradley, P. A. Cohen, M. A. Wolfert, S. J. Gendler and G.-J. Boons, Chem. Commun., 2015, 51, 10214–10217 RSC.
- T.-C. Chang, Y. Manabe, Y. Fujimoto, S. Ohshima, Y. Kametani, K. Kabayama, Y. Nimura, C.-C. Lin and K. Fukase, Angew. Chem., Int. Ed., 2018, 57, 8219–8224 CrossRef CAS PubMed.
- Q. Feng, Y. Manabe, K. Kabayama, T. Aiga, A. Miyamoto, S. Ohshima, Y. Kametani and K. Fukase, Chem.–Asian J., 2019, 14, 4268–4273 CrossRef CAS PubMed.
- T. Aiga, Y. Manabe, K. Ito, T.-C. Chang, K. Kabayama, S. Ohshima, Y. Kametani, A. Miura, H. Furukawa, H. Inaba, K. Matsuura and K. Fukase, Angew. Chem., Int. Ed., 2020, 59, 17705–17711 CrossRef CAS PubMed.
- V. Lakshminarayanan, P. Thompson, M. A. Wolfert, T. Buskas, J. M. Bradley, L. B. Pathangey, C. S. Madsen, P. A. Cohen, S. J. Gendler and G.-J. Boons, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 261–266 CrossRef CAS PubMed.
- Q. Wang, Z. Zhou, S. Tang and Z. Guo, ACS Chem. Biol., 2012, 7, 235–240 CrossRef CAS PubMed.
- Z. Zhou, M. Mondal, G. Liao and Z. Guo, Org. Biomol. Chem., 2014, 12, 3238–3245 RSC.
- G. Liao, Z. Zhou, S. Suryawanshi, M. A. Mondal and Z. Guo, ACS Cent. Sci., 2016, 2, 210–218 CrossRef CAS PubMed.
- L. Wang, S. Feng, S. Wang, H. Li, Z. Guo and G. Gu, J. Org. Chem., 2017, 82, 12085–12096 CrossRef CAS PubMed.
- N. R. M. Reintjens, E. Tondini, A. R. de Jong, N. J. Meeuwenoord, F. Chiodo, E. Peterse, H. S. Overkleeft, D. V. Filippov, G. A. van der Marel, F. Ossendorp and J. D. C. Codée, J. Med. Chem., 2020, 63, 11691–11706 CrossRef CAS PubMed.
- E. B. Puffer, J. K. Pontrello, J. J. Hollenbeck, J. A. Kink and L. L. Kiessling, ACS Chem. Biol., 2007, 2, 252–262 CrossRef CAS PubMed.
- C. Pifferi, A. Ruiz-de-Angulo, D. Goyard, C. Tiertant, N. Sacristán, D. Barriales, N. Berthet, J. Anguita, O. Renaudet and A. Fernández-Tejada, Chem. Sci., 2020, 11, 4488–4498 RSC.
- A.-B. M. Abdel-Aal, D. El-Naggar, M. Zaman, M. Batzloff and I. Toth, J. Med. Chem., 2012, 55, 6968–6974 CrossRef CAS PubMed.
- B. L. Wilkinson, S. Day, L. R. Malins, V. Apostolopoulos and R. J. Payne, Angew. Chem., Int. Ed., 2011, 50, 1635–1639 CrossRef CAS PubMed.
- M. Cavallari, P. Stallforth, A. Kalinichenko, D. C. K. Rathwell, T. M. A. Gronewold, A. Adibekian, L. Mori, R. Landmann, P. H. Seeberger and G. De Libero, Nat. Chem. Biol., 2014, 10, 950–956 CrossRef CAS PubMed.
- R. J. Anderson, B. J. Compton, C.-w. Tang, A. Authier-Hall, C. M. Hayman, G. W. Swinerd, R. Kowalczyk, P. Harris, M. A. Brimble, D. S. Larsen, O. Gasser, R. Weinkove, I. F. Hermans and G. F. Painter, Chem. Sci., 2015, 6, 5120–5127 RSC.
- B. J. Compton, C.-w. Tang, K. A. Johnston, T. L. Osmond, C. M. Hayman, D. S. Larsen, I. F. Hermans and G. F. Painter, Org. Lett., 2015, 17, 5954–5957 CrossRef CAS PubMed.
- X.-G. Yin, X.-Z. Chen, W.-M. Sun, X.-S. Geng, X.-K. Zhang, J. Wang, P.-P. Ji, Z.-Y. Zhou, D. J. Baek, G.-F. Yang, Z. Liu and J. Guo, Org. Lett., 2017, 19, 456–459 CrossRef CAS PubMed.
- P. G. Chen, H. G. Hu, Z. Y. Sun, Q. Q. Li, B. D. Zhang, J. J. Wu, W. H. Li, Y. F. Zhao, Y. X. Chen and Y. M. Li, Mol. Pharm., 2020, 17, 417–425 CAS.
- R. A. De Silva, Q. Wang, T. Chidley, D. K. Appulage and P. R. Andreana, J. Am. Chem. Soc., 2009, 131, 9622–9623 CrossRef CAS PubMed.
- F. Berti and R. Adamo, ACS Chem. Biol., 2013, 8, 1653–1663 CrossRef CAS PubMed.
- M. Shi, K. A. Kleski, K. R. Trabbic, J.-P. Bourgault and P. R. Andreana, J. Am. Chem. Soc., 2016, 138, 14264–14272 CrossRef CAS PubMed.
- G. P. P. Gential, T. P. Hogervorst, E. Tondini, M. J. van de Graaff, H. S. Overkleeft, J. D. C. Codée, G. A. van der Marel, F. Ossendorp and D. V. Filippov, Bioorg. Med. Chem. Lett., 2019, 29, 1340–1344 CrossRef CAS PubMed.
- I. Bettahi, G. Dasgupta, O. Renaudet, A. A. Chentoufi, X. Zhang, D. Carpenter, S. Yoon, P. Dumy and L. BenMohamed, Cancer Immunol. Immunother., 2008, 58, 187 CrossRef PubMed.
- A. S. McKee and P. Marrack, Curr. Opin. Immunol., 2017, 47, 44–51 CrossRef CAS PubMed.
- Y. Cao, P. Stosiek, G. F. Springer and U. Karsten, Histochem. Cell Biol., 1996, 106, 197 CrossRef CAS PubMed.
- C. Bernardo, C. Costa, T. Amaro, M. Goncalves, P. Lopes, R. Freitas, F. Gartner, F. Amado, J. A. Ferreira and L. Santos, Anticancer Res., 2014, 34, 735–744 CAS.
- G. D. MacLean, M. A. Reddish, R. R. Koganty and B. M. Longenecker, J. Immunother. Emphasis Tumor Immunol., 1996, 19, 59–68 CrossRef CAS PubMed.
- R. Kircheis, P. Vondru, A. Nechansky, R. Ohler, H. Loibner, G. Himmler and G. C. Mudde, Bioconjugate Chem., 2005, 16, 1519–1528 CrossRef CAS PubMed.
- J. Wu and Z. Guo, Bioconjugate Chem., 2006, 17, 1537–1544 CrossRef CAS PubMed.
- S. Julien, G. Picco, R. Sewell, A. S. Vercoutter-Edouart, M. Tarp, D. Miles, H. Clausen, J. Taylor-Papadimitriou and J. M. Burchell, Br. J. Cancer, 2009, 100, 1746–1754 CrossRef CAS PubMed.
- F. Yang, X.-J. Zheng, C.-X. Huo, Y. Wang, Y. Zhang and X.-S. Ye, ACS Chem. Biol., 2011, 6, 252–259 CrossRef CAS PubMed.
- L. A. Holmberg and B. M. Sandmaier, Expert Rev. Vaccines, 2004, 3, 655–663 CrossRef CAS PubMed.
- D. Miles, H. Roché, M. Martin, T. J. Perren, D. A. Cameron, J. Glaspy, D. Dodwell, J. Parker, J. Mayordomo, A. Tres, J. L. Murray, N. K. Ibrahim and t. T. S. Group, Oncologist, 2011, 16, 1092–1100 CrossRef CAS PubMed.
- N. K. Ibrahim, J. L. Murray, D. Zhou, E. A. Mittendorf, D. Sample, M. Tautchin and D. Miles, J. Cancer, 2013, 4, 577–584 CrossRef PubMed.
- C. K. Ching, S. W. Holmes, G. K. Holmes and R. G. Long, Gut, 1993, 34, 1722–1725 CrossRef CAS PubMed.
- S. Zhang, L. A. Walberg, S. Ogata, S. H. Itzkowitz, R. R. Koganty, M. Reddish, S. S. Gandhi, B. M. Longenecker, K. O. Lloyd and P. O. Livingston, Cancer Res., 1995, 55, 3364–3368 CAS.
- M. A. Reddish, L. Jackson, R. Rao Koganty, D. Qiu, W. Hong and B. Michael Longenecker, Glycoconjugate J., 1997, 14, 549–560 CrossRef CAS PubMed.
- Y. Arai, K. Yokoyama, Y. Kawahara, Q. Feng, I. Ohta, A. Shimoyama, S. Inuki, K. Fukase, K. Kabayama and Y. Fujimoto, Org. Biomol. Chem., 2018, 16, 3824–3830 RSC.
- R. M. Perlmutter, D. Hansburg, D. E. Briles, R. A. Nicolotti and J. M. Davie, J. Immunol., 1978, 121, 566–572 CAS.
- G. P. Der Balian, J. Slack, B. L. Clevinger, H. Bazin and J. M. Davie, J. Exp. Med., 1980, 152, 209–218 CrossRef CAS PubMed.
- E. Ribechini, I. Eckert, A. Beilhack, N. Du Plessis, G. Walzl, U. Schleicher, U. Ritter and M. B. Lutz, JCI Insight, 2020, 4, e128664 CrossRef PubMed.
- J. Sprent and M. Schaefer, Immunol. Today, 1989, 10, 17–23 CrossRef CAS PubMed.
- L. M. Pinchuk and N. M. Filipov, Immun. Ageing, 2008, 5, 1 CrossRef PubMed.
- E. P. Browne, Immunology, 2012, 136, 370–379 CrossRef CAS PubMed.
- Z.-H. Huang, L. Shi, J.-W. Ma, Z.-Y. Sun, H. Cai, Y.-X. Chen, Y.-F. Zhao and Y.-M. Li, J. Am. Chem. Soc., 2012, 134, 8730–8733 CrossRef CAS PubMed.
- Primer to the Immune Response, (Second Edn), ed. T. W. Mak, M. E. Saunders and B. D. Jett, Academic Cell, Boston, 2014, pp. 111–142 Search PubMed.
- J. L. Rastad and W. R. Green, Immunohorizons, 2018, 2, 87–106 CrossRef CAS PubMed.
- F. Veglia, M. Perego and D. Gabrilovich, Nat. Immunol., 2018, 19, 108–119 CrossRef CAS PubMed.
- R. H. Schwartz, Annu. Rev. Immunol., 2003, 21, 305–334 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03286d |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2022 |