Open Access Article
Open Access ArticleTwo-photon absorption of 28-hetero-2,7-naphthiporphyrins: expanded carbaporphyrinoid macrocycles†
Emma Robbins ab,
Radosław Deska
ab,
Radosław Deska a,
Katarzyna Ślusarek
a,
Katarzyna Ślusarek c,
Marta Dudek
c,
Marta Dudek a,
Marek Samoć
a,
Marek Samoć a,
Lechosław Latos-Grażyński
a,
Lechosław Latos-Grażyński c,
Bartosz Szyszko
c,
Bartosz Szyszko *c and
Katarzyna Matczyszyn
*c and
Katarzyna Matczyszyn *a
*a
aAdvanced Materials Engineering and Modelling Group, Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeże Wyspianskiego 27, 50-370 Wrocław, Poland. E-mail: katarzyna.matczyszyn@pwr.edu.pl
bLaboratoire PEIRENE, Université de Limoges, 123 Avenue Albert Thomas, 87060 Limoges, France
cDepartment of Chemistry, University of Wrocław, 14 F. Joliot-Curie St., 50-383 Wrocław, Poland. E-mail: bartosz.szyszko@chem.uni.wroc.pl
First published on 6th July 2022
Abstract
The one- and two-photon absorption (1PA and 2PA) properties of three expanded aceneporphyrinoids, 28-thia-, 28-selena- and 28-tellura-2,7-naphthiporphyrin, have been studied. The open-aperture Z-scan technique was used to determine two-photon absorption cross-sections in the near infrared range using an amplified femtosecond laser system. The maximum values of the cross sections were found to be 99, 200 and 650 GM at 900 nm and 1, 13 and 31 GM at 1400 nm for the three investigated compounds, respectively. These results demonstrate enhanced 2PA properties compared with well-known porphyrin photosensitizers, such as Foscan®, showing the potential of porphyrin core modification for optimizing infrared nonlinear absorbers.
Introduction
Carbaporphyrinoids are porphyrin analogues characterized by a presence of carbon atom(s) within the coordination cavity of the macrocycle.1 This profound structural alteration of archetypical porphyrin framework results in systems providing an unusual coordination environment composed of nitrogen donors and carbon atoms capable of metal or metalloid binding.2In this respect, the carbaporphyrinoids embedding polycyclic aromatic hydrocarbon (PAH) subunits, namely aceneporphyrinoids, were found to be particularly intriguing, as it was demonstrated that the PAH motif is a crucial factor dictating the overall properties of the hybrid molecule.3–5 The extent to which the electronic properties are altered depends on the nature of the PAH moiety present and whether it takes part in the π-delocalization with the rest of the macrocycle. In recent years aceneporphyrinoids have received a lot of attention due to their atypical π-conjugation,6,7 their aromaticity varying from nonaromatic to antiaromatic,8,9 and their ability to form stable organometallic complexes with metals of uncommon oxidation states.6,10–12 The incorporation of a particular PAH into the macrocycle can be accomplished in a way that the cavity size exceeds 16 atoms, typical for a porphyrin/porphyrinoid of regular size.1,13,14 The resultant macrocycles are considered expanded carbaporphyrinoids.15
One selected type of a PAH embedded porphyrinoid is that of naphthiporphyrin. Up to date a series of its constitutional isomers have been synthesized, including 1,3-,16,17 1,4-,16,18 1,5-,19 and 1,7-naphthiporphyrinoids.4 These macrocycles contribute porphyrin-like donor atoms within the precisely designed cavity and are becoming more and more desirable for applications within organoelement chemistry and organometallic chemistry.2,20 The naphthiporphyrins have also been demonstrated to display exceptional conformational flexibility.21–23
The molecular properties of carbaporphyrinoids can be further altered by the incorporation of heteroatoms24–26 such as O, S, Se, or Te within the macrocyclic cavity. In most cases, the modification significantly affects the electronic properties of the molecule, which in turn changes the spectral, structural, and coordination properties.3,5,27
In a previous study, a new constitutional isomer of naphthiporphyrin was designed and synthesized, namely 28-hetero-2,7-naphthiporphyrin.28 The compound was proven to act as a macrocyclic ligand, as demonstrated by the formation of organophosphorus(V) complexes.
It has been well established that two-photon absorption (2PA) properties are affected by both structural and electronic properties of molecules, such as the extent of the π-conjugation length,29–34 and the planarity and aromaticity of the molecule.35–41 However, the photophysical properties for heteroporphyrins, and particularly carbaporphyrinoids, have not been extensively studied despite their potential use in applications such as photodynamic therapy,42 sensor development,43 and as catalysts44 and anion-binding agents.45 Herein, we provide details on the 2PA properties, characterized using the Z-scan technique,46,47 of 28-hetero-2,7-naphthiporphyrins differing in the presence of sulfur (1-S), selenium (1-Se) and tellurium (1-Te) atoms within the macrocyclic cavity (Fig. 1). To the best of our knowledge, these are some of the very few carbaporphyrinoids for which the nonlinear optical properties were measured using this technique.48 Several examples of previous works describing the 2PA properties of atypical porphyrinoids include N-confused hexaphyrins,49 expanded porphyrins such as cyclo[n]pyrrole, amethyrin and rubyrin,39 and biradicaloid meso-substituted [26]hexaphyrin.50
Results and discussion
One-photon absorption
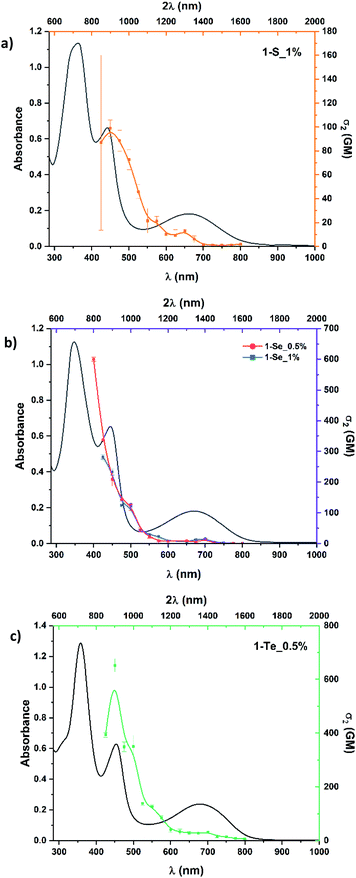
The three investigated compounds 1-S, 1-Se and 1-Te were dissolved in ultrapure dichloromethane to ensure no chemical changes of the molecules took place during the measurements. When dissolved, they formed dark green solutions. The UV-Vis spectra contained an intense Soret-like band located in the 347–358 nm range, as well as a second relatively intense band in the 443–452 nm range (see Fig. 2). | ||
| Fig. 2 UV-Vis absorption spectra of 1-S (concentration = 1% w/w), 1-Se (concentration = 1% w/w) and 1-Te (concentration = 0.5% w/w) measured in DCM. | ||
They also display a broad absorption band extending through the 500–850 nm range, with maxima at 661 nm for 1-S, 670 nm for 1-Se (for both 0.5 and 1%) and 679 nm for 1-Te. These bands and spectral patterns are characteristics of nonaromatic carbaporphyrinoids.36 As the heterocyclic ring D is varied in the macrocycles from thiophene to selenophene to tellurophene (Fig. 1), the absorption bands display a modest bathochromic (red) shift. This observed red-shift is a common effect and has been well-documented for heteroporphyrins51 and heterocarbaporphyrinoids,19,28 likely due to the increasing metallic nature and electron delocalization of group 16 elements (the chalcogens).52 Due to the differing sizes of the heteroatoms in the 28th position, the conformation of the macrocycle is affected, and may also have some effect on the photophysical properties, due to changes in aromaticity and π-electron delocalization.53
Two-photon absorption
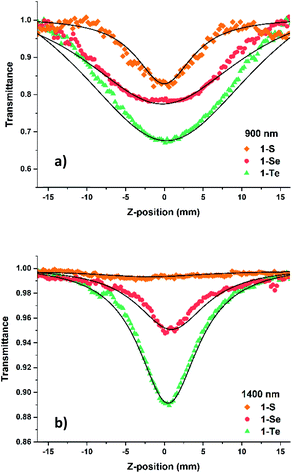
Changing the heterocyclic ring D from thiophene to selenophene to tellurophene, leads to changes in the electron delocalizability and charge transfer of the molecules, due to the differences of the three heteroatoms with tellurium being the heaviest and less electronegative than selenium and sulfur.54 The presence of these heavy atoms not only red-shifts the 1PA band, but has also been known to enhance the intersystem crossing (ISC) of a molecule.55–57 Since 2PA, a third-order non-linear optical phenomenon, is known to be influenced by charge transfer efficiency58,59 which is related to electron donor properties, we have compared the 2PA cross-section (σ2) values of 1-S, 1-Se and 1-Te by using the femtosecond open-aperture Z-scan technique.60–62 In order to determine the maximum σ2 values for these compounds, we have measured the two-photon absorption at excitation wavelengths in the ranges corresponding to the doubled wavelength region of the 1PA bands, where the one-photon absorption contribution to the open aperture signal (which may be due to the excited state absorption process and/or absorption saturation) could be manageable, between 850 and 1600 nm for 1-S_1, 1-Se_1, and 1-Te_0.5, and between 800 and 1600 nm for 1-Se_0.5. The 2PA cross-sections (σ2), which are a measure of the probability of simultaneous absorption of two photons by a molecule were computed and the values are presented in Fig. 3 alongside the one-photon spectra plotted against the doubled wavelength. This way of presenting the 2PA data rests on the assumption that the same excited states can be reachable by both 1PA and 2PA, which is a normal occurrence for non-centrosymmetric molecules.63 One should note, however, that the relative 2PA strengths of various absorption bands do not have to follow the patterns seen in 1PA spectra.The examples of open aperture Z-scan curves are displayed below for two wavelengths, 900 and 1400 nm, which are at approximately twice the wavelengths of 1PA peaks (Fig. 4 and Table 1). It is evident that the 1-S and 1-Se display weaker nonlinear absorption compared to that of 1-Te, with the nonlinear absorption being almost indiscernible for 1-S at 1400 nm. As expected, the two differently concentrated solutions of 1-Se complex (0.5% and 1%) exhibit the nonlinear absorption that roughly scales with the concentration, thus, as seen in Table 1, the cross sections calculated from those data are in agreement.
| Sample | 2PA λ (nm) | σ2 (GM) | σ2/MW (GM g−1) |
|---|---|---|---|
| 1-S_1 | 900 | 99 ± 6.8 | 0.143 |
| 1400 | 1.4 ± 0.8 | 0.002 | |
| 1-Se_0.5 | 900 | 209 ± 22 | 0.272 |
| 1400 | 13.2 ± 3 | 0.017 | |
| 1-Se_1 | 900 | 233 ± 10 | 0.303 |
| 1400 | 15.7 ± 1.5 | 0.020 | |
| 1-Te_0.5 | 900 | 652 ± 25 | 0.826 |
| 1400 | 31.5 ± 3 | 0.040 |
Examination of the data indicates that all compounds show a relatively weak TPA maximum at around 1400 nm with the value of σ2 being about twice higher for 1-Te compared to 1-Se, and about 6 times higher when compared to 1-S. One does expect that shorter wavelength maxima may appear at around 700 and 900 nm. This, however, cannot be determined rigorously because of the overlap of those ranges with the tail of the 1PA absorption. The presence of the band at around 900 nm can be invoked as the existence of a knee-like feature in all two-photon spectra.64 The increase of σ2 to 600 GM at 800 nm for 1-Se is likely to arise from the presence of excited state absorption. The 1PA contribution is probably still too high at 800 nm, leading to the observed increase in calculated 2PA cross-section value due to excited state absorption or absorption saturation. The cross sections determined here exceed 650 GM for 1-Te. Still larger cross-sections may be present at shorter wavelengths, but the evidence for that is hard to obtain because of the influence of 1PA.65
From the results presented above, we can further support the fact that changing from sulfur to selenium, and to the heavier tellurium atom, yields larger 2PA cross-sections. There is increased electron density at the center of these heavy atoms,66–69 which has been interpreted to be due to the increased electron donation down the chalcogen family because of the decreased electronegativity of each element. This may lead to improved charge transfer70 and thus, lead to increased 2PA cross-sections. Further studies of photophysical properties of each compound would be helpful to confirm this mechanism. Such studies may shed more light on the role of electron donation and the heavy atom effect, which also promotes intersystem crossing due to atoms with large atomic numbers being able to induce strong spin–orbit coupling (SOC).56,71,72 The heavy atom effect has been shown to cause changes to photophysical properties,73–76 observed here by the increasing 2PA cross-section as the heavy atom becomes heavier. Both effects being of great interest for novel materials for related photochemical and photophysical applications, such as photodynamic therapy,77–79 optical limiting,80–82 optical data storage,83,84 2PA imaging,85,86 microfabrication,87,88 and more.
While the main interest in the present study has been in nonlinear absorption properties of the investigated molecules, the closed-aperture Z-scan measurements on their solutions in principle allow for the determination of both the nonlinear absorptive and refractive properties. These are most often presented as the real (Re (γ)) and imaginary (Im (γ)) parts of the cubic hyperpolarizability (γ) of the studied compounds.89–91
Fig. 5 shows the spectra of the complex hyperpolarizability, γ (−ω; ω, −ω, ω) that were derived from the Z-scan measurements. For all three compounds the absolute values of the real and imaginary parts of γ are of the order of 10−33 esu. While the imaginary part is related to the two-photon cross section and its dispersion follows that of the spectral dependence of σ2, on the other hand, the real part appears to be negative in almost the whole spectral region investigated which is typical for compounds with strong two-photon absorption. One may note that, thanks to relatively high concentrations of the solutions, the solute contribution to the total refractive nonlinearity of the solution was relatively high leading to acceptably low errors in the determination of Re (γ). As the chalcogen changes from S to Se to Te, we see an increase in the hyperpolarizability (γ) value. Previous studies suggest that the heavier heteroatom is responsible for decreases in the transition energy between the ground and excited state involved in the transition that contributes to γ.92,93 Thus, this change in transition energy may also lead to the enhanced 2PA cross-sections observed as we move down the chalcogen group.
 | ||
| Fig. 5 Spectra of the real and imaginary parts of the hyperpolarizability γ, derived from the Z-scan measurements for samples 1-S_1 (a), 1-Se_0.5 and 1-Se_1 (b) and 1-Te_0.5 (c). | ||
The measured 2PA cross-sections, for 1-Te in particular, show a marked improvement upon traditional macrocyclic molecules. The distinct advantage of these compounds is their ability to absorb at much longer wavelengths (1400 nm) compared to commonly used porphyrins, Foscan® (652 nm) and meso-tetra-4-hydroxyphenylporphyrin (648 nm),94 for the treatment of head and neck cancers, demonstrating their potential for future applications.
Experimental
Solvents were obtained commercially and used without purification.Synthesis
1-S, 1-Se, and 1-Te were synthesized as described earlier.28UV-Vis
All UV-Vis spectra were recorded on a JASCO V-730 spectrophotometer.Z-scan
The two-photon absorption properties have been determined using the Z-scan technique.95 The experiments were performed over a wide range of wavelengths (800–1600 nm), achieved by employing an Astrella Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sapphire amplifier (output wavelength 800 nm) pumping a TOPAS Prime optical parametric amplifier equipped with a NirUVis frequency mixer, which delivered wavelength tunable pulses with a duration of about 50 fs and the repetition rate of 1 kHz. The output beam was selected with a polarization separator and attenuated using neutral density filters. DCM solutions of 1-S_1, 1-Se_0.5, 1-Se_1 and 1-Te_0.5 were placed in stoppered 1 mm glass cuvettes. Two different concentrations of solutions were studied for 1-Se: 0.5% (1-Se_0.5) and 1% (1-Se_1) w/w while the concentration of 1-S was 1% w/w, and 1-Te was 0.5% w/w. In a Z-scan experiment the sample is moved along the axis of the incident beam (z-direction), prior to and past the focal point, and the so-called open and closed aperture signals are recorded: corresponding to the overall transmittance of the sample and that of the central part of the beam as selected by an aperture in the far field, respectively. These signals, as well as the signal corresponding to the incident beam, were viewed on an oscilloscope and digitized and transferred to a computer via a National Instruments BNC-2110 Terminal Block and National Instruments PCI-6143 Multifunction I/O Device. The obtained results for the three compounds were calibrated by using Z-scan measurements performed on a fused silica plate, for which the nonlinear refractive index was assumed employing literature data and compared with measurements of an identical glass cuvette filled solely with the solvent.96–98 The obtained data were analyzed using a custom fitting program that utilised equations derived by Sheik-Bahae, et al.60
Sapphire amplifier (output wavelength 800 nm) pumping a TOPAS Prime optical parametric amplifier equipped with a NirUVis frequency mixer, which delivered wavelength tunable pulses with a duration of about 50 fs and the repetition rate of 1 kHz. The output beam was selected with a polarization separator and attenuated using neutral density filters. DCM solutions of 1-S_1, 1-Se_0.5, 1-Se_1 and 1-Te_0.5 were placed in stoppered 1 mm glass cuvettes. Two different concentrations of solutions were studied for 1-Se: 0.5% (1-Se_0.5) and 1% (1-Se_1) w/w while the concentration of 1-S was 1% w/w, and 1-Te was 0.5% w/w. In a Z-scan experiment the sample is moved along the axis of the incident beam (z-direction), prior to and past the focal point, and the so-called open and closed aperture signals are recorded: corresponding to the overall transmittance of the sample and that of the central part of the beam as selected by an aperture in the far field, respectively. These signals, as well as the signal corresponding to the incident beam, were viewed on an oscilloscope and digitized and transferred to a computer via a National Instruments BNC-2110 Terminal Block and National Instruments PCI-6143 Multifunction I/O Device. The obtained results for the three compounds were calibrated by using Z-scan measurements performed on a fused silica plate, for which the nonlinear refractive index was assumed employing literature data and compared with measurements of an identical glass cuvette filled solely with the solvent.96–98 The obtained data were analyzed using a custom fitting program that utilised equations derived by Sheik-Bahae, et al.60
Conclusions and outlook
The investigation of one- and two-photon absorption properties of expanded heteroporphyrins 1-S, 1-Se and 1-Te demonstrates an improvement upon well-known porphyrin photosensitizers used e.g., in photodynamic therapy. While the measured 2PA cross-sections may appear moderate in comparison to much larger molecules investigated in this context, it is important that reasonable values can be obtained even at the relatively long wavelength of 1400 nm and order of magnitude higher values are seen at 900 nm for 1-Se and 1-Te, exceeding values for well-known PDT photosensitizers such as Foscan®, which has σ2 = 28 ± 8 GM, in DMSO at 775 nm.99Further investigations would be required to determine whether the modification of the porphyrinoid core can be utilized to build more extended porphyrin-like systems with sizeable enhanced 2PA properties.
Author contributions
Conceptualization: B. S. and K. M.; formal analysis: E.R.; funding acquisition: M. S., B. S. and K. M.; investigation: E. R. and R. D.; supervision: K. M.; validation: K. S., M. S., L. L. G. and B. S.; visualization: E. R., R. D., K. S., M. S., L. L. G., B. S. and K. M.; writing – original draft: E. R.; writing – review & editing: E. R., K. S., M. S., L. L. G., B. S., and K. M.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant (agreement no. 764837). The work at the University of Wrocław was funded by National Science Centre of Poland (2017/26/D/ST5/00184). MS and RD acknowledge the NCN Harmonia grant 2016/22/M/ST4/00275. KM and MD thanks National Science Centre of Poland for the grant no. 2019/35/B/ST4/03280.References
- T. D. Lash, Chem. Rev., 2017, 117, 2313–2446 CrossRef CAS PubMed.
- B. Szyszko and L. Latos-Grażyński, Chem. Soc. Rev., 2015, 44, 3588–3616 RSC.
- K. Laxman, A. Kumar and M. Ravikanth, Asian J. Org. Chem., 2020, 9, 162–180 CrossRef CAS.
- J.-H. Hong, A. S. Aslam, M. Ishida, S. Mori, H. Furuta and D.-G. Cho, J. Am. Chem. Soc., 2016, 138, 4992–4995 CrossRef CAS PubMed.
- A. Sinha and M. Ravikanth, J. Org. Chem., 2021, 86, 6100–6110 CrossRef CAS PubMed.
- B. Szyszko, L. Latos-Grażyński and L. Szterenberg, Chem. Commun., 2012, 48, 5004–5006 RSC.
- B. Szyszko, P. Rymut, M. Matviyishyn, A. Białońska and L. Latos-Grażyński, Angew. Chem., Int. Ed., 2020, 59, 20137–20146 CrossRef CAS PubMed.
- M.-C. Yoon, S. Cho, M. Suzuki, A. Osuka and D. Kim, J. Am. Chem. Soc., 2009, 131, 7360–7367 CrossRef CAS PubMed.
- T. Tanaka and A. Osuka, Chem. Rev., 2017, 117, 2584–2640 CrossRef CAS PubMed.
- B. Szyszko, A. Białońska, L. Szterenberg and L. Latos-Grażyński, Angew. Chem., 2015, 127, 5014–5018 CrossRef.
- T. D. Lash, D. A. Colby and L. F. Szczepura, Inorganic chemistry, 2004, 43, 5258–5267 CrossRef CAS PubMed.
- K. Kupietz, M. J. Białek, K. Hassa, A. Białońska and L. Latos-Grażyński, Inorg. Chem., 2019, 58, 12446–12456 CrossRef CAS PubMed.
- J. L. Sessler and D. Seidel, Angew. Chem., Int. Ed., 2003, 42, 5134–5175 CrossRef CAS PubMed.
- M. Bröring, H. J. Dietrich, J. Dörr, G. Hohlneicher, J. Lex, N. Jux, C. Pütz, M. Roeb, H. Schmickler and E. Vogel, Angew. Chem., Int. Ed., 2000, 39, 1105–1108 CrossRef.
- B. Szyszko and L. Latos-Grażyński, Angew. Chem., Int. Ed., 2020, 59, 16874–16901 CrossRef CAS PubMed.
- T. D. Lash, A. M. Young, J. M. Rasmussen and G. M. Ferrence, J. Org. Chem., 2011, 76, 5636–5651 CrossRef CAS PubMed.
- T. D. Lash, S. T. Chaney and D. T. Richter, J. Org. Chem., 1998, 63, 9076–9088 CrossRef CAS.
- B. Szyszko and L. Latos-Grażyński, Organometallics, 2011, 30, 4354–4363 CrossRef CAS.
- B. Szyszko, E. Pacholska-Dudziak and L. Latos-Grażyński, J. Org. Chem., 2013, 78, 5090–5095 CrossRef CAS PubMed.
- T. D. Lash, Chem.–Asian J., 2014, 9, 682–705 CrossRef CAS PubMed.
- R. Misra and T. K. Chandrashekar, Acc. Chem. Res., 2008, 41, 265–279 CrossRef CAS PubMed.
- N. Shivran, S. C. Gadekar and V. G. Anand, Chem.–Asian J., 2017, 12, 6–20 CrossRef CAS PubMed.
- T. Koide, K. Youfu, S. Saito and A. Osuka, Chem. Commun., 2009, 6047–6049 RSC.
- T. D. Lash, Eur. J. Org. Chem., 2007, 2007, 5461–5481 CrossRef.
- B. Szyszko, M. Małecki, A. Berlicka, M. J. Białek, A. Białońska, K. Kupietz, E. Pacholska-Dudziak and L. Latos-Grażyński, Chem.–Eur. J., 2016, 22, 7602–7608 CrossRef CAS PubMed.
- S. Pushpan, M. Kumar and T. Chandrashekar, J. Chem. Soc., Perkin Trans., 1999, 2, 961–968 Search PubMed.
- R. L. Hill, M. Gouterman and A. Ulman, Inorg. Chem., 1982, 21, 1450–1455 CrossRef CAS.
- B. Szyszko, M. Matviyishyn, S. Hirka, E. Pacholska-Dudziak, A. Białońska and L. Latos-Grażyński, Org. Lett., 2019, 21, 7009–7014 CrossRef CAS PubMed.
- K. Kurotobi, K. S. Kim, S. B. Noh, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2006, 45, 3944–3947 CrossRef CAS PubMed.
- Y. Inokuma, N. Ono, H. Uno, D. Y. Kim, S. B. Noh, D. Kim and A. Osuka, Chem. Commun., 2005, 3782–3784 RSC.
- Y. Nakamura, S. Y. Jang, T. Tanaka, N. Aratani, J. M. Lim, K. S. Kim, D. Kim and A. Osuka, Chem.–Eur. J., 2008, 14, 8279–8289 CrossRef CAS PubMed.
- T. K. Ahn, K. S. Kim, D. Y. Kim, S. B. Noh, N. Aratani, C. Ikeda, A. Osuka and D. Kim, J. Am. Chem. Soc., 2006, 128, 1700–1704 CrossRef CAS PubMed.
- H. Rath, J. Sankar, V. PrabhuRaja, T. K. Chandrashekar, A. Nag and D. Goswami, J. Am. Chem. Soc., 2005, 127, 11608–11609 CrossRef CAS PubMed.
- R. Misra, R. Kumar, T. K. Chandrashekar, A. Nag and D. Goswami, Org. Lett., 2006, 8, 629–631 CrossRef CAS PubMed.
- I. Hisaki, S. Hiroto, K. S. Kim, S. B. Noh, D. Kim, H. Shinokubo and A. Osuka, Angew. Chem., 2007, 119, 5217–5220 CrossRef.
- J.-Y. Shin, K. S. Kim, M.-C. Yoon, J. M. Lim, Z. S. Yoon, A. Osuka and D. Kim, Chem. Soc. Rev., 2010, 39, 2751–2767 RSC.
- S. Gokulnath and T. K. Chandrashekar, J. Chem. Sci., 2008, 120, 137–142 CrossRef CAS.
- T. K. Ahn, J. H. Kwon, D. Y. Kim, D. W. Cho, D. H. Jeong, S. K. Kim, M. Suzuki, S. Shimizu, A. Osuka and D. Kim, J. Am. Chem. Soc., 2005, 127, 12856–12861 CrossRef CAS PubMed.
- Z. S. Yoon, J. H. Kwon, M.-C. Yoon, M. K. Koh, S. B. Noh, J. L. Sessler, J. T. Lee, D. Seidel, A. Aguilar and S. Shimizu, J. Am. Chem. Soc., 2006, 128, 14128–14134 CrossRef CAS PubMed.
- M.-C. Yoon, R. Misra, Z. S. Yoon, K. S. Kim, J. M. Lim, T. K. Chandrashekar and D. Kim, J. Phys. Chem. B, 2008, 112, 6900–6905 CrossRef CAS PubMed.
- J.-Y. Shin, J. M. Lim, Z. S. Yoon, K. S. Kim, M.-C. Yoon, S. Hiroto, H. Shinokubo, S. Shimizu, A. Osuka and D. Kim, J. Phys. Chem. B, 2009, 113, 5794–5802 CrossRef CAS PubMed.
- B. Habermeyer and R. Guilard, Photochem. Photobiol. Sci., 2018, 17, 1675–1690 CrossRef CAS PubMed.
- R. Paolesse, S. Nardis, D. Monti, M. Stefanelli and C. Di Natale, Chem. Rev., 2017, 117, 2517–2583 CrossRef CAS PubMed.
- N. Mihara, Y. Yamada, H. Takaya, Y. Kitagawa, K. Igawa, K. Tomooka, H. Fujii and K. Tanaka, Chem.–Eur. J., 2019, 25, 3369–3375 CrossRef CAS PubMed.
- A. Srinivasan, M. R. Kumar, R. Pandian, S. Mahajan, K. S. Pushpan, B. Sridevi, S. J. Narayanan and T. Chandrashekar, J. Porphyrins Phthalocyanines, 1998, 2, 305–314 CrossRef CAS.
- S. Drouet, A. Merhi, D. Yao, M. P. Cifuentes, M. G. Humphrey, M. Wielgus, J. Olesiak-Banska, K. Matczyszyn, M. Samoc and F. Paul, Tetrahedron, 2012, 68, 10351–10359 CrossRef CAS.
- L. Mazur, R. Kolkowski, K. Matczyszyn, F. Mathevet, P. Rannou, A.-J. Attias and M. Samoc, Opt. Mater., 2012, 34, 1682–1685 CrossRef CAS.
- X.-J. Liu, J.-K. Feng, A.-M. Ren and X. Zhou, Chem. Phys. Lett., 2003, 373, 197–206 CrossRef CAS.
- J. H. Kwon, T. K. Ahn, M.-C. Yoon, D. Y. Kim, M. K. Koh, D. Kim, H. Furuta, M. Suzuki and A. Osuka, J. Phys. Chem. B, 2006, 110, 11683–11690 CrossRef CAS PubMed.
- T. Koide, K. Furukawa, H. Shinokubo, J.-Y. Shin, K. S. Kim, D. Kim and A. Osuka, J. Am. Chem. Soc., 2010, 132, 7246–7247 CrossRef CAS PubMed.
- L. Latos-Grażyński, E. Pacholska, P. J. Chmielewski, M. M. Olmstead and A. L. Balch, Inorg. Chem., 1996, 35, 566–573 CrossRef.
- K. T. Mahmudov, M. N. Kopylovich, M. F. C. G. da Silva and A. J. Pombeiro, Dalton Trans., 2017, 46, 10121–10138 RSC.
- A. Kumar, K. Laxman and M. Ravikanth, Org. Lett., 2019, 21, 8726–8730 CrossRef CAS PubMed.
- S. Wang, J. Shang, C. Yan, W. Wang, C. Yuan, H.-L. Zhang and X. Shao, Org. Chem. Front., 2019, 6, 263–272 RSC.
- J. Pirillo, B. C. De Simone and N. Russo, Theor. Chem. Acc., 2016, 135, 1–5 Search PubMed.
- J. C. Koziar and D. O. Cowan, Acc. Chem. Res., 1978, 11, 334–341 CrossRef CAS.
- K. M. Farrell, M. M. Brister, M. Pittelkow, T. I. Sølling and C. E. Crespo-Hernández, J. Am. Chem. Soc., 2018, 140, 11214–11218 CrossRef CAS PubMed.
- B. Küçüköz, G. Sevinç, E. Yildiz, A. Karatay, F. Zhong, H. Yılmaz, Y. Tutel, M. Hayvalı, J. Zhao and H. Yaglioglu, Phys. Chem. Chem. Phys., 2016, 18, 13546–13553 RSC.
- S. Tekin, B. Küçüköz, H. Yılmaz, G. Sevinç, M. Hayvalı, H. G. Yaglioglu and A. Elmali, J. Photochem. Photobiol., A, 2013, 256, 23–28 CrossRef CAS.
- M. Sheik-Bahae, A. A. Said, T.-H. Wei, Y.-Y. Wu, D. J. Hagan, M. Soileau and E. W. Van Stryland, Sensitive measurement of optical nonlinearities using a single beam, San Diego, CA, 1989 Search PubMed.
- L. M. Mazur, T. Roland, S. Leroy-Lhez, V. Sol, M. Samoc, I. D. W. Samuel and K. Matczyszyn, J. Phys. Chem. B, 2019, 123, 4271–4277 CrossRef CAS PubMed.
- E. W. Van Stryland and M. Sheik-Bahae, in Characterization Techniques and Tabulations for Organic Nonlinear Materials, Marcel Dekker, Inc, 1st edn, 1998, pp. 671–708 Search PubMed.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed. Engl., 2009, 48, 3244–3266 CrossRef CAS PubMed.
- S. J. Zelewski, K. C. Nawrot, A. Zak, M. Gladysiewicz, M. Nyk and R. Kudrawiec, J. Phys. Chem. Lett., 2019, 10, 3459–3464 CrossRef CAS PubMed.
- M. Drobizhev, N. Makarov, T. Hughes and A. Rebane, J. Phys. Chem. B, 2007, 111, 14051–14054 CrossRef CAS PubMed.
- F. A. Devillanova and W.-W. Du Mont, Handbook of chalcogen chemistry: new perspectives in sulfur, selenium and tellurium, Royal Society of Chemistry, 2013 Search PubMed.
- W. Levason, S. D. Orchard and G. Reid, Organometallics, 1999, 18, 1275–1280 CrossRef CAS.
- A. J. Barton, W. Levason and G. Reid, J. Organomet. Chem., 1999, 579, 235–242 CrossRef CAS.
- A. J. Genge, S. Orchard and S. A. Pope, J. Chem. Soc., Dalton Trans., 1999,(14), 2343–2352 Search PubMed.
- H. Yılmaz, B. Küçüköz, G. Sevinç, S. Tekin, H. G. Yaglioglu, M. Hayvalı and A. Elmali, Dyes Pigm., 2013, 99, 979–985 CrossRef.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- A. Rodriguez-Serrano, V. Rai-Constapel, M. C. Daza, M. Doerr and C. M. Marian, Phys. Chem. Chem. Phys., 2015, 17, 11350–11358 RSC.
- A. Karatay, M. C. Miser, X. Cui, B. Küçüköz, H. Yılmaz, G. Sevinç, E. Akhüseyin, X. Wu, M. Hayvali and H. G. Yaglioglu, Dyes Pigm., 2015, 122, 286–294 CrossRef CAS.
- B. Sauerwein and G. B. Schuster, J. Phys. Chem., 1991, 95, 1903–1906 CrossRef CAS.
- D. G. Hilmey, M. Abe, M. I. Nelen, C. E. Stilts, G. A. Baker, S. N. Baker, F. V. Bright, S. R. Davies, S. O. Gollnick and A. R. Oseroff, J. Med. Chem., 2002, 45, 449–461 CrossRef CAS PubMed.
- C.-L. Sun, Q. Liao, T. Li, J. Li, J.-Q. Jiang, Z.-Z. Xu, X.-D. Wang, R. Shen, D.-C. Bai and Q. Wang, Chem. Sci., 2015, 6, 761–769 RSC.
- J. Bhawalkar, N. Kumar, C.-F. Zhao and P. Prasad, Journal of Lasers in Medical Sciences, 1997, 15, 201–204 CAS.
- S. Kim, T. Y. Ohulchanskyy, H. E. Pudavar, R. K. Pandey and P. N. Prasad, J. Am. Chem. Soc., 2007, 129, 2669–2675 CrossRef CAS PubMed.
- Q. Zheng, A. Bonoiu, T. Y. Ohulchanskyy, G. S. He and P. N. Prasad, Mol. Pharm., 2008, 5, 389–398 CrossRef CAS PubMed.
- J.-C. Liu, X.-Z. Li and Y. Zhang, Two-photon absorption induced optical power limiting behaviour of strong femtosecond hyper-Gaussian pulses, Beijing, China, 2016 Search PubMed.
- M. G. Silly, L. Porrès, O. Mongin, P.-A. Chollet and M. Blanchard-Desce, Chem. Phys. Lett., 2003, 379, 74–80 CrossRef CAS.
- Q. Zheng, S. K. Gupta, G. S. He, L. S. Tan and P. N. Prasad, Adv. Funct. Mater., 2008, 18, 2770–2779 CrossRef CAS.
- B. H. Cumpston, S. P. Ananthavel, S. Barlow, D. L. Dyer, J. E. Ehrlich, L. L. Erskine, A. A. Heikal, S. M. Kuebler, I. Y. S. Lee, D. McCord-Maughon, J. Qin, H. Röckel, M. Rumi, X.-L. Wu, S. R. Marder and J. W. Perry, Nature, 1999, 398, 51–54 CrossRef CAS.
- Q. Zhang, S. Yue, H. Sun, X. Wang, X. Hao and S. An, J. Mater. Chem. C, 2017, 5, 3838–3847 RSC.
- Z. F. Chang, L. M. Jing, B. Chen, M. Zhang, X. Cai, J. J. Liu, Y. C. Ye, X. Lou, Z. Zhao, B. Liu, J. L. Wang and B. Z. Tang, Chem. Sci., 2016, 7, 4527–4536 RSC.
- Q. Zhang, X. Tian, H. Zhou, J. Wu and Y. Tian, Materials, 2017, 10, 223 CrossRef PubMed.
- F. Niesler and M. Hermatschweiler, Opt. Photonik, 2016, 11, 54–57 CrossRef CAS.
- E. Scarpa, E. D. Lemma, R. Fiammengo, M. P. Cipolla, F. Pisanello, F. Rizzi and M. De Vittorio, Sens. Actuators, B, 2019, 279, 418–426 CrossRef CAS.
- Z. Pokladek, N. Ripoche, M. Betou, Y. Trolez, O. Mongin, J. Olesiak-Banska, K. Matczyszyn, M. Samoc, M. G. Humphrey and M. Blanchard-Desce, Chem.–Eur. J., 2016, 22, 10155–10167 CrossRef CAS PubMed.
- K. Matczyszyn, J. Olesiak-Banska, K. Nakatani, P. Yu, N. A. Murugan, R. Zalesny, A. Roztoczynska, J. Bednarska, W. Bartkowiak and J. Kongsted, J. Phys. Chem. B, 2015, 119, 1515–1522 CrossRef CAS PubMed.
- L. Chen, R. Hu, J. Xu, S. Wang, X. Li, S. Li and G. Yang, Spectrochim. Acta, Part A, 2013, 105, 577–581 CrossRef CAS PubMed.
- K. Kamada, T. Sugino, M. Ueda, K. Tawa, Y. Shimizu and K. Ohta, Chem. Phys. Lett., 1999, 302, 615–620 CrossRef CAS.
- K. Kamada, M. Ueda, T. Sakaguchi, K. Ohta and T. Fukumi, J. Opt. Soc. Am., 1998, 15, 838–845 CrossRef CAS.
- L. Ma, J. Moan and K. Berg, Int. J. Cancer, 1994, 57, 883–888 CrossRef CAS PubMed.
- M. Dudek, N. Tarnowicz-Staniak, M. Deiana, Z. Pokładek, M. Samoć and K. Matczyszyn, RSC Adv., 2020, 10, 40489–40507 RSC.
- J. Wang, M. Sheik-Bahae, A. A. Said, D. J. Hagan and E. W. Van Stryland, J. Opt. Soc. Am. B, 1994, 11(6), 1009–1017 CrossRef CAS.
- R. DeSalvo, M. Sheik-Bahae, A. Said, D. J. Hagan and E. W. Van Stryland, Opt. Lett., 1993, 18, 194–196 CrossRef CAS PubMed.
- M. Sheik-Bahae, J. Wang, R. DeSalvo, D. Hagan and E. Van Stryland, Opt. Lett., 1992, 17, 258–260 CrossRef CAS PubMed.
- B. Hamed, T. von Haimberger, V. Kozich, A. Wiehe and K. Heyne, J. Photochem. Photobiol., A, 2014, 295, 53–56 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Re and Im values of 1-S, 1-Se and 1-Te are available. See https://doi.org/10.1039/d2ra03167a |
| This journal is © The Royal Society of Chemistry 2022 |