 Open Access Article
Open Access ArticleA PtPdCoCuNi high-entropy alloy nanocatalyst for the hydrogenation of nitrobenzene†
Fagui Lua,
Kuan Lub,
Gui Zhaoa,
Song Zhouc,
Bowen Hea,
Yixiao Zhanga,
Jian Xuc,
Yongwang Lic,
Xi Liu *ad and
Liwei Chen*ad
*ad and
Liwei Chen*ad
aSchool of Chemistry and Chemical Engineering, In-situ Center for Physical Sciences, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: liuxi@sjtu.edu.cn; lwchen2018@sjtu.edu.cn
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
cSynCat@Beijing Synfuels China Technology Co. Ltd, Beijing 101407, China
dShanghai Jiao Tong University, China Shanghai Electrochemical Energy Device Research Center (SEED), Shanghai 200240, China
First published on 7th July 2022
Abstract
High-entropy alloys (HEAs) with multiple elements in near-equiatomic proportions hold great promise in heterogeneous catalysis because of their exceptional physicochemical properties governed by synergy. Herein, we prepared PtPdCoCuNi HEA nanoparticles via a one-step colloid-based route and tested their catalytic performance for nitrobenzene hydrogenation to aniline. The SiO2 supported PtPdCoCuNi displays 93.9% yield of aniline at 80 °C, which is 2.11 times that of PtPd/SiO2. Even at room temperature, a 47.4% yield of aniline is attained with the PtPdCoCuNi/SiO2 catalyst. DRIFTS experiments indicate formation of isolated Pt and Pd sites after alloying the transition metals and evidence a stronger interaction between the HEA catalyst and nitrobenzene. Both XPS data and DFT calculations disclose charge transfer to Pt and Pd species, which eventually enhance the interaction between nitrobenzene and the isolated metal sites and the hydrogenation activity as well. The experimental and theoretical results shed light on mechanistic understanding of the unique catalytic performance of the HEA nanocatalyst and pave a new avenue to realize the high catalytic performance of nitrobenzene hydrogenation over well-isolated noble metal sites with specific geometries.
Introduction
Aromatic amines are valuable intermediates for the synthesis of polyurethanes, dyes, rubber additives, pharmaceuticals, explosives, and fine chemicals.1 The production of aromatic amines from catalytic hydrogenation of nitroaromatics is an important industrial reaction.2 Tremendous efforts have been made to develop efficient catalysts to achieve highly efficient production of aromatic amines under mild conditions. Pt- and Pd-based catalysts have been extensively investigated for the selective hydrogenation of nitroarenes using molecular hydrogen as an environmentally benign and cheap reducing agent.1 Improving the activity of catalysts as well as reducing the cost of noble metal-based catalysts are of considerable importance for industrial applications.In contrast to conventional alloy materials based on single principal elements, high-entropy alloys (HEAs) have recently received considerable attention in various fields because of their unique physical and chemical properties.3 In principle, HEAs are produced by alloying five or more metal elements in nearly equal proportions (recently extended to 5 to 35 at%) into solid solutions with a high mixing entropy rather than an intermetallic phase.4 The resulting synergistic effect endows HEAs with high mechanical strength,5 good thermal stability,6 and catalytic activity.7 In particularly, HEAs displayed appraisable catalytic performance in electrocatalytic water splitting,3,8,9 ammonia oxidation and decomposition,10,11 and CO2 reduction.12 Mechanistic studies suggest that the ligand effect and distortion effect of HEAs, which arise from tuning the composition and geometric properties of HEAs to a large degree, could significantly modify the electronic properties of active sites and consequently improve their catalytic performance.11 The inherent synergy effect of HEAs provides possibility to boost the catalytic activity by combining suitable metals.
However, the preparation of uniform HEA nanoparticles is challenging because the difference in reduction potentials and rates of different metal precursors can easily lead to phase separation. The conventional impregnation chemical strategy, which was used to produce supported HEA nanoparticles,12 has obvious disadvantages in terms of size and composition of the nanoparticles. Although HEA nanoparticles can be produced by carbothermal shock method,10,11 this method does not allow scaled-up production due to high requirements for the instrument and thermal stability of supports. Subsequently, fast moving bed pyrolysis,3 metal–organic framework pyrolysis,9 and synergistic confinement strategy13 were explored as alternative synthetic approaches. Unfortunately, the experimental conditions crucially limit their practical applications.
Nowadays, the colloid solvothermal method has been widely used in the synthesis of bimetallic or trimetallic alloy nanoparticles.14,15 This one-pot colloid-based approach can integrate distinct metal elements into nanocrystals to form well-defined structure, by which allows the preparation of nanoparticles with uniform chemical compositions and homogenous size distributions.16,17 Therefore, a general colloidal approach to synthesis of HEA nanoparticles should be developed to provide new opportunities in catalyst design and beyond.8
In this paper, small PtPdCoCuNi HEA nanoparticles were successfully prepared by a one-pot solvothermal synthesis method and deposited on SiO2 to prepare a PtPdCoCuNi/SiO2 catalyst with uniform dispersion of HEA nanoparticles. The catalytic performance of the PtPdCoCuNi/SiO2 catalyst for nitrobenzene hydrogenation was tested and compared to counterparts with fewer metal components. The PtPdCoCuNi/SiO2 catalyst exhibits high activity even at room temperature. The catalyst was characterized by a series of techniques, including HRSTEM, XRD, XPS, and DRIFTS, to obtain mechanistic understanding of the structure–activity relationship of the HEA catalyst. Both experimental and theoretical results evidence the formation of isolated Pt and Pd active sites in HEA and the resulting charge-transfer from the cheap transition metals to the noble metals, which suggests that the modulated electronic structure could promote adsorption of nitrobenzene on Pt/Pd active sites and eventually enhance nitrobenzene hydrogenation activity.
Results and discussion
The morphology of the as-prepared PtPdCoCuNi nanoparticles was characterized by transmission electron microscopy (TEM). As shown in Fig. 1A, the nanoparticles are uniform in terms of morphology and size. An average diameter of the PtPdCoCuNi nanoparticles is around 7.1 ± 3.2 nm with a narrow size distribution. A selected area electron diffraction (SAED) pattern of the PtPdCoCuNi nanoparticles with clear diffraction polycrystalline rings (Fig. 1C) is indexed to face-centered cubic (fcc) structure. As seen in a high-resolution TEM (HRTEM) image (Fig. 1B), the measured lattice-fringe distances of the PtPdCoCuNi nanoparticles are 0.22 and 0.19 nm, corresponding to the (111) and (002) facets of fcc, respectively. After the deposition of PtPdCoCuNi nanoparticles on SiO2 and following reduction at 160 °C under H2 atmosphere, the morphology and size of supported PtPdCoCuNi nanoparticles keep unchanged, which are uniformly dispersed on SiO2 without obvious aggregation (Fig. 1D and 2A). A HRTEM image (Fig. 1E) of the post-treated nanoparticles validates negligible differences in the crystal structure. An X-ray diffraction (XRD) pattern of the PdPdCoCuNi/SiO2 catalyst is shown in Fig. 1F, in which diffraction peaks located at 40.9°, 47.5°, and 69.5° should be assigned to the (111), (002), and (022) facets, respectively (fcc Pd, JCPDF: 96-101-1111). Rietveld refinement analysis of the diffraction result indicates the formation of fcc structure with a lattice parameter of a = 3.838 Å. This value is between 3.908 Å (for fcc Pd, JCPDF: 96-101-1111) and 3.524 Å (for fcc Ni, JCPDF: 96-151-2527), suggesting presence of large local structural distortion. When the period 4 elements were alloyed into PdPt bimetallic matrix in sequence to form tri-, quad-, and quin-metallic alloy (see Fig. S3 and S4†), a gradual decrease in the value was observed, indicating apparent lattice shrinks during the progressive replacement of Pt or Pd atoms with heteroatoms. | ||
| Fig. 2 (A) STEM-ADF image of PtPdCoCuNi/SiO2 catalyst; (B) STEM-ADF image and corresponding EDS elemental mapping of PtPdCoCuNi/SiO2 catalyst. | ||
An atomically resolved scanning transmission electron microscopy annular dark field (STEM-ADF) image of a HEA nanoparticle (Fig. 2B) discloses its poly crystalline nature. The corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping displays that each metal element (Pt, Pd, Co, Cu, and Ni) is evenly distributed throughout the entire nanoparticle, confirming the successful synthesis of the HEA alloy. Inductively coupled plasma atomic emission spectroscopy (ICP–AES) data (Fig. S5†) show that the mole ratios of these five metals (Pt, Pd, Co, Cu, and Ni) range from 14% to 24%, which is consistent with the definition of HEA. We also calculated the thermodynamic properties of the synthetic PtPdCoCuNi nanoparticles based on the equations proposed by Xie et al., who supposed that the formation of HEA requires an atomic size difference δ ≤ 6.6% and an enthalpy of mixing ΔHmix between −11.6 and 3.2 kJ mol−1.11 In the present study, it is calculated that δ = 5.3%, ΔHmix = −1.35 kJ mol−1, ΔSmix = 13.26 J mol−1 K−1, and ΔGmix = −7.62 kJ mol−1 for the PtPdCoCuNi nanoparticles, in good agreement with the reference.
X-ray photoelectron spectroscopy (XPS) was performed to explore the valence states of Pt, Pd, Co, Cu, and Ni in the PtPdCoCuNi/SiO2 catalyst and its counterparts with fewer components. For the quin-metallic sample, the Pt 4f7/2 and Pt 4f5/2 peaks of the quin-metallic sample (Fig. S6A†) are centered at 70.2 and 73.6 eV respectively, which should be attributed to metallic Pt.18 The Pd 3d5/2 and Pd 3d3/2 doublet peaks (Fig. S6B†) are located at 334.5 and 339.8 eV, respectively, associated with metallic Pd.19 The binding energy of Co 2p3/2 (Fig. S6C†) is about 778.5 eV, which is consistent with metallic Co.20 The Cu 2p3/2 and Cu 2p1/2 (Fig. S6D†) with binding energies of 931.7 and 951.5 eV are attributed to metallic state of Cu.21 Furthermore, the Auger Cu LMM spectrum (Fig. S6E†) at 918.9 eV confirms the metallic state of Cu species.14 The binding energy of Ni 2p3/2 at approximately 853.0 eV (Fig. S6F†) is attributed to metallic Ni.22 The XPS analysis confirms that all the constituent elements of Pt, Pd, Co, Cu, and Ni in the HEA nanoparticles are mainly in metallic states. However, both peaks of Pt 4f (Fig. 3A) and Pd 3d (Fig. 3B) gradually shift towards lower binding energies associated with incrementally alloying of Co/Cu/Ni into PtPd matrix one-by-one, indicating the increasing charge transfer to Pt and Pd. Similar charge transfer from Co to noble metal Pd has previously been observed in CoPd alloy, disclosing that introducing cheap transition metal can alter the electronic state of Pd species, and subsequently affect their catalytic performance.23 Clearly, the characterization data validate the obvious structural and electronic alternations in the HEA nanoparticles, which should significantly influence their physicochemical properties, like catalytic reactivity.
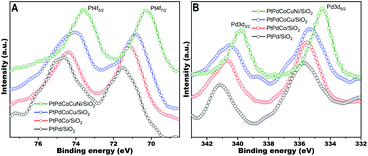 | ||
| Fig. 3 Pt 4f and Pd 3d XPS spectra of PtPd/SiO2, PtPdCo/SiO2, PtPdCoCu/SiO2, and PtPdCoCuNi/SiO2 catalysts. | ||
In this study, hydrogenation of nitrobenzene to aniline was chosen as a model reaction to demonstrate the catalytic advantage of PtPdCoCuNi/SiO2 over the bi-, tri-, quad-metallic alloy catalysts. Fig. 4A shows yields of aniline over these PtPd-based samples and their monometallic counterparts. Apparently, the hydrogenation reaction hardly occurs over the unary cheap transition metal Co/SiO2, Cu/SiO2, or Ni/SiO2 catalyst, as yields of aniline are less than 2.2% (Fig. 4A). The unary noble metal Pt/SiO2 and Pd/SiO2 catalysts exhibit relatively high activity, which is slightly lower than that of the binary PtPd/SiO2 catalyst. These results indicate that Pt and Pd are the main active species in the hydrogenation of nitrobenzene. After alloying Co, Cu, and Ni in sequence, the yield of aniline increases from 44.4% (PtPd/SiO2) to 55.6% (PtPdCo/SiO2), and 73.7% (PtPdCoCuNi/SiO2), and finally reaches 93.9% for PtPdCoCuNi/SiO2 with a mass productivity of 15.0 mmolaniline mgPt+Pd−1 h−1, which is 1.46, 2.27, and 3.41 times to those of PtPdCoCu/SiO2 (10.3 mmolaniline mgPt+Pd−1 h−1), PtPdCo/SiO2 (6.6 mmolaniline mgPt+Pd−1 h−1), and PtPd/SiO2 (4.4 mmolaniline mgPt+Pd−1 h−1), respectively. All these catalysts gave almost 100% selectivity to the target product aniline. The turnover frequency (TOF) of PtPdCoCuNi/SiO2 catalyst was higher than the values achieved by some most active Pt or Pd-based catalysts (Table S1†). In addition, the PtPdCoCuNi/SiO2 catalyst also exhibited high activity for hydrogenation of other substituted nitroarenes to the corresponding aromatic amines (Fig. S8†). The catalytic performance definitely confirms that the continuously alloying inactive transition metals greatly manipulate their catalytic performance. The excellent performance of PtPdCoCuNi/SiO2 toward nitrobenzene reduction could be ascribed to the synergistic effect obtained from the combination of elements comprising the HEAs.8,12
The effects of reaction temperature and time on hydrogenation of nitrobenzene to aniline over PtPdCoCuNi/SiO2 were also investigated in the present work. As shown in Fig. 4B, when the reaction temperature decreases from 80 to 50 °C, the two-hour yield of aniline decreases from 93.9% to 53.5%. But even at room temperature, the catalyst still displays marked activity toward nitrobenzene hydrogenation with a two-hour yield of 25.1%. Extending the reaction time favors the productivity of aniline and a 47.3% aniline yield is achieved at room temperature after 5 hours reaction without byproducts. After 5 hours' reaction, a complete conversion is obtained at 80 °C and a yield of 91.7% is obtained at 50 °C, respectively. The mass productivity of PtPdCoCuNi/SiO2 reaches 5.9 and 3.0 mmolaniline mgPt+Pd−1 h−1 at 50 and 25 °C, respectively. The supported PtPdCoCuNi catalysts with different metal loading (0.1 wt% to 4.0 wt%) for the hydrogenation of nitrobenzene were also tested, showing a typical volcano-shaped activity with an optimal loading amount of 2.0 wt% (Fig. S11†). Moreover, the influence of content of the cheap transition metals in nanoparticles on overall catalytic performance was examined, indicating that the catalytic performance dropped rapidly when the concentration of CoCuNi increased (Fig. S12†). The PtPdCoCuNi/SiO2 catalyst displays considerable reusability as the activity of PtPdCoCuNi/SiO2 decreases marginally after five cycles (Fig. 4C). The morphology of used PtPdCoCuNi/SiO2 catalyst is well maintained, and no obvious aggregation of nanoparticles appears after the reaction (Fig. S13A†). In addition, EDS elemental mapping evidences the structural stability of HEA nanoparticles (Fig. S13B†). No metal component (Pt, Pd, Co, Cu, and Ni) was detected in the reaction solution from the ICP-AES results. The slight decrease of catalytic activity may be due to the blockage of pores (Fig. S2†). These results validate that the PtPdCoCuNi/SiO2 HEA catalyst has good reusability and stability in hydrogenation of nitrobenzene. Linear relationships between −ln(1 − yield) and reaction time are obtained over PtPdCoCuNi/SiO2 and PtPd/SiO2 catalysts, which are fitted well with the first-order reaction kinetics (Fig. S14 and S15†), suggesting that the nitrobenzene hydrogenation follows a similar reaction pathway over the two catalysts.24 According to the Arrhenius plot, the apparent activation energy (Ea) is determined to be 28.9 kJ mol−1 over PtPdCoCuNi/SiO2 catalyst for the hydrogenation of nitrobenzene to aniline (Fig. 4D), which is slightly lower than the apparent activation energy of PtPd/SiO2 catalyst (37.4 kJ mol−1). The obtained activation energies is quite similar to the value previously reported in the literature,25 suggesting the catalytic hydrogenation reaction over the HEA catalyst follows the similar reaction pathway.25,26
Conclusions
Considering the similar size and morphology between PtPd and PtPdCoCuNi nanoparticles, the higher activity of PtPdCoCuNi/SiO2 catalyst should be largely attributed to promoted chemisorption over active sites.To further explore the structure–activity relationship of these PtPd-based samples for the hydrogenation reaction, diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) of CO absorption was performed (Fig. 5A). In principle, frequencies of CO adsorbed on metal sites are determined by adsorption types, including low-coordinated sites or even single atoms responsible for linear-type CO adsorption (above 2000 cm−1) and close-packed surface for bridging-type adsorption (below 1980 cm−1).27 With alloying more transition metals into the PtPd matrix, intensity of the linear CO adsorption peak and a proportion of peak area of the linear adsorption to that of total CO adsorption (Al/(Al + Ab)) increase progressively. This result indicates that Pt or Pd assembly is separated incrementally by the incorporation of cheap transition metals and consequently turned into isolated Pt or Pd sites, resulting in decrease in bridging CO adsorption as well as increase in linear CO adsorption.28 The isolated Pt or Pd sites can serve as active sites for selective hydrogenation of unsaturated aromatic nitrogen compounds.29 Moreover, the linear CO adsorption band is redshifted with increasing metal composition, indicating modulated interaction between adsorbed CO and Pt or Pd active sites. Moreover, the increased electron density of Pt/Pd evidenced by the XPS results can enhance the electron feedback from the d-orbital of metallic Pt/Pd to the antibonding orbital (π*) of CO and consequently weaken the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O bond, leading to a redshift of the linear CO adsorption stretching band.27 The components of Co/Cu/Ni serve a dual role in isolating Pt/Pd and increasing electron density of Pt/Pd. The aniline yield and mass productivity are found to be almost linearly correlated proportionally with the ratio of linear CO adsorption (Al/(Al + Ab)) for the PtPd-based catalysts (Fig. 5B), indicating that the modulated structural and electronic properties of Pt and Pd should be the main reason for the enhanced catalytic performance. In order to examine the correlation, DRIFTS spectra of nitrobenzene were also studied to disclose the interaction between nitrobenzene and active sites and thus to better understand the origin of the excellent catalytic performance of PtPdCoCuNi/SiO2 catalyst. As shown in Fig. 5C, two characteristic absorbance peaks located at about 1526 and 1344 cm−1 are observed, which are assigned to the asymmetric and symmetric stretching vibrations of nitro group, respectively.1 Similar to the CO-DRIFTS profiles, the promoted interaction between Pt/Pd active sites and adsorbed nitrobenzene was evidenced by the down-shifting of the corresponding vibrations, since the redshift of the characteristic nitro peaks should be attributed to electron transfer from metallic Pt/Pd to nitro group.30 Furthermore, nitrobenzene-TPD experiments were conducted to investigate the enhanced chemisorption of nitrobenzene on PtPd-based catalysts (Fig. 5D). The peak area and desorption temperature along with the increase in metal composition, confirming that there is the maximum chemisorption of nitrobenzene on the PtPdCoCuNi/SiO2 catalyst over other PtPd-based catalysts. Both nitrobenzene-DRIFTS and TPD results are consistent with the CO-DRIFTS and XPS data, indicating that the isolated electron-rich Pt and Pd sites are preferable for adsorption and activation of nitrobenzene for the subsequent hydrogenation reaction. Clearly, the addition of Co/Cu/Ni increases the dispersion of Pt/Pd, generating isolated Pt and Pd sites on PtPd-based catalysts. On the other hand, the incorporation of Co/Cu/Ni also increases the electron density of Pt/Pd, thus enhancing interaction between nitrobenzene and the isolated metal sites.
O bond, leading to a redshift of the linear CO adsorption stretching band.27 The components of Co/Cu/Ni serve a dual role in isolating Pt/Pd and increasing electron density of Pt/Pd. The aniline yield and mass productivity are found to be almost linearly correlated proportionally with the ratio of linear CO adsorption (Al/(Al + Ab)) for the PtPd-based catalysts (Fig. 5B), indicating that the modulated structural and electronic properties of Pt and Pd should be the main reason for the enhanced catalytic performance. In order to examine the correlation, DRIFTS spectra of nitrobenzene were also studied to disclose the interaction between nitrobenzene and active sites and thus to better understand the origin of the excellent catalytic performance of PtPdCoCuNi/SiO2 catalyst. As shown in Fig. 5C, two characteristic absorbance peaks located at about 1526 and 1344 cm−1 are observed, which are assigned to the asymmetric and symmetric stretching vibrations of nitro group, respectively.1 Similar to the CO-DRIFTS profiles, the promoted interaction between Pt/Pd active sites and adsorbed nitrobenzene was evidenced by the down-shifting of the corresponding vibrations, since the redshift of the characteristic nitro peaks should be attributed to electron transfer from metallic Pt/Pd to nitro group.30 Furthermore, nitrobenzene-TPD experiments were conducted to investigate the enhanced chemisorption of nitrobenzene on PtPd-based catalysts (Fig. 5D). The peak area and desorption temperature along with the increase in metal composition, confirming that there is the maximum chemisorption of nitrobenzene on the PtPdCoCuNi/SiO2 catalyst over other PtPd-based catalysts. Both nitrobenzene-DRIFTS and TPD results are consistent with the CO-DRIFTS and XPS data, indicating that the isolated electron-rich Pt and Pd sites are preferable for adsorption and activation of nitrobenzene for the subsequent hydrogenation reaction. Clearly, the addition of Co/Cu/Ni increases the dispersion of Pt/Pd, generating isolated Pt and Pd sites on PtPd-based catalysts. On the other hand, the incorporation of Co/Cu/Ni also increases the electron density of Pt/Pd, thus enhancing interaction between nitrobenzene and the isolated metal sites.
DFT calculations were performed to obtain insightful understanding of the electronic properties of PtPdCoCuNi in comparison with PtPd. Differential charge distributions of the two models (Fig. S16†) confirm the relatively nucleophilic nature of Pt and Pd sites (0.11e and 0.05e, respectively) in the PtPdCoCuNi matrix compared with the bimetallic PtPd system, which is in a good agreement with XPS data. In addition, the adsorption energy of nitrobenzene on PtPd and PtPdCoCuNi was calculated to identify the interaction between these active sites and the reactant molecule. All models consisted of six layers with p(6 × 6): the top three layers were relaxed and the bottom three layers were fixed. The HEA model was simply represented by the top one-layer alloy atoms, as shown in Fig. 5E. Lower adsorption energy indicates stronger adsorption. The results show that the lowest adsorption energy of nitrobenzene on the PtPd(111) surface was −2.36 eV for the hcp site, and the most stable adsorption structure of nitrobenzene on the PtPdCoCuNi(111) surface was also on the hcp site (−3.67 eV). The adsorption energy of nitrobenzene on PtPd was rather high, accounting for the lower hydrogenation activity observed for the PtPd/SiO2 catalyst. In contrast, nitrobenzene was strongly adsorbed on the PtPdCoCuNi surface. The lower adsorption energy/stronger adsorption of nitrobenzene on the PtPdCoCuNi(111) surface indicates that nitrobenzene is easily attached to PtPdCoCuNi/SiO2 with the highest possibility for reaction, which could explain the higher hydrogenation activity of PtPdCoCuNi/SiO2, validating that the interaction between PtPdCoCuNi/SiO2 and nitrobenzene is stronger than that with PtPd/SiO2. The comprehensive experimental and theoretical results prove that the formation of the HEA nanocatalyst creates highly-isolated and nucleophilic Pt and Pd active sites, and eventually modulates the chemisorption of nitrobenzene over the active sites and thereby its catalytic performance for the selective hydrogenation.
In summary, we have successfully synthesized PtPdCoCuNi HEA nanoparticles using a simple one-step solvothermal method. The PtPdCoCuNi/SiO2 catalyst exhibits the high catalytic activity for hydrogenation of nitrobenzene to aniline. The HEA nanocatalyst displays much better catalytic performance than the monometallic catalysts and other PtPd alloy catalysts. Even at room temperature, an aniline yield of 47.3% is achieved after 5 hours of reaction. The enhanced activity of PtPdCoCuNi/SiO2 is attributed to the high dispersion of Pt and Pd in HEA and the arising synergistic electronic properties of the formed active sites. This work not only provides a viable synthetic route for the fabrication of HEA nanoparticles, but also provides detailed mechanistic understanding of this unique catalyst. We expect that this new synthetic strategy will facilitate the design and synthesis of new HEA nanoparticles for catalysis or other applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2021YFA1500300) and the National Natural Science Foundation of China (Grant No. 21991153, 21991150, and 22072090). The authors acknowledge support from Synfuels China Technology Co. Ltd (Beijing).Notes and references
- Q. Zhang, J. Bu, J. Wang, C. Sun, D. Zhao, G. Sheng, X. Xie, M. Sun and L. Yu, ACS Catal., 2020, 10, 10350–10363 CrossRef CAS.
- D. Formenti, F. Ferretti, F. K. Scharnagl and M. Beller, Chem. Rev., 2019, 119, 2611–2680 CrossRef CAS PubMed.
- S. Gao, S. Hao, Z. Huang, Y. Yuan, S. Han, L. Lei, X. Zhang, R. Shahbazian-Yassar and J. Lu, Nat. Commun., 2020, 11, 2016 CrossRef CAS PubMed.
- Y. Xin, S. Li, Y. Qian, W. Zhu, H. Yuan, P. Jiang, R. Guo and L. Wang, ACS Catal., 2020, 10, 11280–11306 CrossRef CAS.
- S. W. Wu, G. Wang, Q. Wang, Y. D. Jia, J. Yi, Q. J. Zhai, J. B. Liu, B. A. Sun, H. J. Chu, J. Shen, P. K. Liaw, C. T. Liu and T. Y. Zhang, Acta Mater., 2019, 165, 444–458 CrossRef CAS.
- Y. Yao, Z. Liu, P. Xie, Z. Huang, T. Li, D. Morris, Z. Finfrock, J. Zhou, M. Jiao, J. Gao, Y. Mao, J. Miao, Z. Peng, R. Shahbazian-Yassar, C. Wang, G. Wang and L. Hu, Sci. Adv., 2020, 6, eaaz0510 CrossRef CAS PubMed.
- K. Zhao, X. Li and D. Su, Acta Phys.-Chim. Sin., 2021, 37, 2009077 Search PubMed.
- H. Li, Y. Han, H. Zhao, W. Qi, D. Zhang, Y. Yu, W. Cai, S. Li, J. Lai, B. Huang and L. Wang, Nat. Commun., 2020, 11, 5437 CrossRef CAS PubMed.
- K. Huang, B. Zhang, J. Wu, T. Zhang, D. Peng, X. Cao, Z. Zhang, Z. Li and Y. Huang, J. Mater. Chem. A, 2020, 8, 11938–11947 RSC.
- Y. Yao, Z. Huang, P. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. Chen, A. Nie, T. Pu, M. Rehwoldt, D. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. Hu, Science, 2018, 359, 1489–1494 CrossRef CAS PubMed.
- P. Xie, Y. Yao, Z. Huang, Z. Liu, J. Zhang, T. Li, G. Wang, R. Shahbazian-Yassar, L. Hu and C. Wang, Nat. Commun., 2019, 10, 4011 CrossRef PubMed.
- K. Mori, N. Hashimoto, N. Kamiuchi, H. Yoshida, H. Kobayashi and H. Yamashita, Nat. Commun., 2021, 12, 3884 CrossRef CAS PubMed.
- H. Li, H. Zhu, Q. Shen, S. Huang, S. Lu, P. Ma, W. Dong and M. Du, Chem. Commun., 2021, 57, 2637–2640 RSC.
- F. Lu, S. Zhou, S. Li, H. Jiang, B. He, J. Qi, Y. Zhang, X. Liu, J. Xu, Y. Li, X. Liu and L. Chen, J. Phys. Chem. C, 2021, 125, 23205–23211 CrossRef CAS.
- Y. Tuo, Q. Lu, C. Chen, T. Liu, Y. Pan, Y. Zhou and J. Zhang, RSC Adv., 2021, 11, 26326–26335 RSC.
- A. Holm, E. D. Goodman, J. H. Stenlid, A. Aitbekova, R. Zelaya, B. T. Diroll, A. C. Johnston-Peck, K.-C. Kao, C. W. Frank, L. G. M. Pettersson and M. Cargnello, J. Am. Chem. Soc., 2020, 142, 14481–14494 CrossRef CAS PubMed.
- L. Lin, J. Liu, X. Liu, Z. Gao, N. Rui, S. Yao, F. Zhang, M. Wang, C. Liu, L. Han, F. Yang, S. Zhang, X. D. Wen, S. D. Senanayake, Y. Wu, X. Li, J. A. Rodriguez and D. Ma, Nat. Commun., 2021, 12, 6978 CrossRef CAS PubMed.
- W. Xu, J. Chang, Y. Cheng, H. Liu, J. Li, Y. Ai, Z. Hu, X. Zhang, Y. Wang, Q. Liang, Y. Yang and H. Sun, Nano Res., 2021, 15, 965–971 CrossRef.
- Y. Bao and L. Feng, Acta Phys.-Chim. Sin., 2021, 37, 2008031 Search PubMed.
- Q. Jiang, W. Luo, Y. Piao, H. Matsumoto, X. Liu, A. Züttel, K. Parkhomenko, C. Pham-Huu and Y. Liu, ACS Catal., 2021, 11, 8087–8096 CrossRef CAS.
- Y.-B. Chang, C. Zhang, X.-L. Lu, W. Zhang and T.-B. Lu, Nano Res., 2021, 15, 195–201 CrossRef.
- Y. H. Ahmad, A. T. Mohamed, A. Kumar and S. Y. Al-Qaradawi, RSC Adv., 2021, 11, 33734–33743 RSC.
- W. Ye, X. Shi, Y. Zhang, C. Hong, C. Wang, W. M. Budzianowski and D. Xue, ACS Appl. Mater. Interfaces, 2016, 8, 2994–3002 CrossRef CAS PubMed.
- E. S. Gutterød, S. Øien-Ødegaard, K. Bossers, A.-E. Nieuwelink, M. Manzoli, L. Braglia, A. Lazzarini, E. Borfecchia, S. Ahmadigoltapeh, B. Bouchevreau, B. T. Lønstad-Bleken, R. Henry, C. Lamberti, S. Bordiga, B. M. Weckhuysen, K. P. Lillerud and U. Olsbye, Ind. Eng. Chem. Res., 2017, 56, 13206–13218 CrossRef.
- M. Turáková, T. Salmi, K. Eränen, J. Wärnå, D. Y. Murzin and M. Králik, Appl. Catal., A, 2015, 499, 66–76 CrossRef.
- L. Zhang, J. Jiang, W. Shi, S. Xia, Z. Ni and X. Xiao, RSC Adv., 2015, 5, 34319–34326 RSC.
- W. Liu, Y. Yang, L. Chen, E. Xu, J. Xu, S. Hong, X. Zhang and M. Wei, Appl. Catal., B, 2021, 282, 119569 CrossRef CAS.
- R. Li, W. Yao, Y. Jin, W. Jia, X. Chen, J. Chen, J. Zheng, Y. Hu, D. Han and J. Zhao, Chem. Eng. J., 2018, 351, 995–1005 CrossRef CAS.
- H. Wang, Q. Luo, W. Liu, Y. Lin, Q. Guan, X. Zheng, H. Pan, J. Zhu, Z. Sun, S. Wei, J. Yang and J. Lu, Nat. Commun., 2019, 10, 4998 CrossRef PubMed.
- Y. Bonita, T. P. O'Connell, H. E. Miller and J. C. Hicks, Ind. Eng. Chem. Res., 2019, 58, 3650–3658 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03145k |
| This journal is © The Royal Society of Chemistry 2022 |



