Open Access Article
Open Access ArticleNaphthalene diimide-based random terpolymer acceptors for constructing all-polymer solar cells with enhanced fill factors†
Baitian He *a,
Longfei Liu
*a,
Longfei Liu b,
Yan Liua,
Guiting Chen
b,
Yan Liua,
Guiting Chen *a,
Manjun Xiao*b and
Chuanbo Daia
*a,
Manjun Xiao*b and
Chuanbo Daia
aSchool of Chemistry and Environment, Jiaying University, Meizhou 514015, P. R. China. E-mail: baitian-he@foxmail.com; 576146400@qq.com
bCollege of Chemistry, Key Lab of Environment-Friendly Chemistry and Application, (Ministry of Education), Xiangtan University, Xiangtan 411105, P. R. China. E-mail: xmj0704@163.com
First published on 16th June 2022
Abstract
All-polymer solar cells (all-PSCs) with mechanical and thermal stability have potential for applications in flexible devices. Polymer acceptors based on naphthalene diimide (NDI) have been widely studied because of their strong electron affinity, high electron mobility, and high mechanical reliability. However, controlling the film morphology of the polymer–polymer blends of NDI-based all-PSCs is difficult. Consequently, all-PSCs based on NDI building blocks exhibit a low fill factor (FF) and a lower power-conversion efficiency (PCE) than state-of-the-art polymer solar cells. In this work, we added a small amount of dicyanodistyrylbenzene (DCB) unit to the NDI-based polymer acceptor N2200 through random copolymerization and synthesized a series of NDI-based terpolymer acceptors PNDIx, where x is the molar concentration of DCB units relative to NDI units. PNDI5 and PNDI10, corresponding to 5% and 10% molar concentrations of DCB, respectively, showed lower crystallization and good miscibility with PBDB-T, a widely used electron-donating copolymer, than the terpolymer based on DCB-free N2200. Moreover, compared to the PBDB-T:N2200 device, the PNDI5-based device exhibited a much higher PCE (8.01%), and an enhanced FF of 0.75 in all-PSCs. These results indicate that ternary random copolymerization is a convenient and effective strategy for optimizing the film morphology of NDI-based polymers, and that the resulting terpolymer acceptor is a promising n-type acceptor for constructing high-performance all-PSCs.
Introduction
All-polymer solar cells (all-PSCs), composed of a polymeric electron donor and a polymeric electron acceptor, display morphological stability and flexibility.1–4 Thus, they have gained increasing attention and were being considered for commercial applications.5–7 However, the entanglement of polymer chains in polymer–polymer blends disfavors the formation of a phase-separated morphology that can facilitate charge separation and transportation.8–12 Consequently, the overall photovoltaic performances of current all-PSCs are less than those of devices based on small-molecule acceptors. Much effort has been devoted to developing highly efficient polymer acceptors, which are mainly constructed from several electron deficient fragments, such as naphthalene diimide (NDI),13–16 perylene diimide (PDI),17,18 B ← N bridged bipyridine (BNBP),19,20 a fused-ring electron acceptor (FREA),21–23 bithiophene imide (BTI).6,24 Recently, a series of new polymer acceptors with high light-absorption capacity had been constructed from Y6, a small-molecule building block. These acceptors can increase the power conversion efficiency (PCE) of all-PSCs to 15%.25–29NDI-based acceptors, especially poly[[N,N′-bis(2-octyldodecyl)-naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl-alt]-5,5′-(2,2′-bithiophene)] (N2200), were candidate polymer acceptors for manufacturing commercial all-PSCs, owing to their ease of synthesis, strong electron affinities, high electron mobilities, and high mechanical reliabilities.30 However, N2200 exhibited strong crystalline and poor miscibility with polymer donors, resulting in large crystalline domains between polymer donor and polymer acceptor, which leads to suboptimal morphology and retard the performance of all-PSCs.31,32 Consequently, only several collocations could offer a PCE over 8%. Pre-aggregating donor and acceptor polymers in 2-methyl tetrahydrofuran, processing active layers with an environmentally friendly solvent, and preparing active layers via slot-die coating could help mitigate the problems associated with N2200.12,13,33 Moreover, several research groups have modified the molecular architecture of N2200 to control the crystallinity of NDI-based polymer acceptors. These researchers incorporated another donor or acceptor unit to construct N2200 derivatives with a D1–A–D2–A or D–A1–D–A2 structure. For example, Jenekhe et al.,14 Wang et al.,34 Huang et al.,35 Chen et al.,36 and Kim et al.37 had optimized the molecular structures of NDI-based polymers through a terpolymerization strategy. Therefore, random copolymerization is a potential technique for fine-tuning the crystallinity of NDI-based polymer acceptors to effectively control film morphology.
In this study, we incorporated a small amount of dicyanodistyrylbenzene (DCB) unit into N2200 through random polymerization to control the molecular topology of N2200 and generate a series of new terpolymer acceptors. The synthesized terpolymer acceptors are denoted as PNDI5 and PNDI10, as they contained 5% and 10% molar concentrations of DCB, respectively (Scheme 1). Previous research had reported that introducing cyanovinylene into photoactive materials can control polymer crystallization and promote exciton dissociation and electron transport.38,39 We found that the terpolymer acceptor crystallization temperature (Tc) gradually decreased with increasing DCB concentration, as this led to a decrease in the rigidity of the molecular backbone and in intermolecular stacking.20 Compared with PNDI10-based devices, devices based on the PNDI5 and a PBDB-T (poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b]dithiophene))-alt-(5,5-(1,3-di-2-thienyl-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c]dithiophene-4,8-dione))]) donor exhibited more favorable film morphologies, more balanced hole/electron mobilities, and lower bimolecular recombination, leading to enhanced PCE of up to 8.01% with a higher Jsc of 12.9 mA cm−2 and fill factor (FF) of 0.75. These results demonstrate that random copolymerization, which changes the molecular structures of NDI-based polymer acceptors, is an effective strategy for manufacturing all-PSCs with favorable morphologies and enhanced efficiencies.
 | ||
| Scheme 1 (a) Molecular structures of polymer donor and terpolymer acceptors; (b) energy levels of PBDB-T, PNDI5, PNDI10 and N2200. | ||
Results and discussion
A series of NDI-based terpolymer acceptors was synthesized via Stille polymerization of bistannylated bithiophene (Th2-SnMe3), dibromo-NDI (NDI-Br2), and a (2E, 2′E)-3,3′-(2,5-bis(2-ethylhexyloxy)-1,4-phenylene)bis(2-(5-(trimethylstannyl)thiophen-2-yl)acrylonitrile) (DCB-SnMe3) as the starting materials. The synthetic route and detailed procedures for the preparation of the copolymers are described in the ESI (Fig. S1†). The synthesized terpolymer acceptors (PNDI5 and PNDI10) were dissolved in common organic solvents, such as toluene, chloroform, and chlorobenzene, or transformed into uniform films by spin-casting. The average molecular weights of the copolymers were determined via high-temperature gel permeation chromatography in 1,2,4-trichlorobenzene, with respect to a polystyrene standard (Table 1). The number-average molecular weights of N2200, PNDI5, and PNDI10 were 75.7, 68.5, and 60.6 kDa, with polydispersity indices of 1.83, 1.85, and 1.93, respectively.| Copolymers | Mna (kDa) | PDIa | λmaxsol (nm) | λmaxfilm (nm) | HOMOb (eV) | LUMOb (eV) | Egcv (eV) | Tm (°C) | TC (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a Determined by gel permeation chromatography (1,2,4-trichlorobenzene) against PS standards.b Measured by cyclic voltammetry. | |||||||||
| N2200 | 75.7 | 1.83 | 706 | 718 | −5.90 | −3.84 | 2.06 | 304 | 326 |
| PNDI5 | 68.5 | 1.85 | 675 | 696 | −5.92 | −3.85 | 2.07 | 292 | 313 |
| PNDI10 | 60.6 | 1.93 | 645 | 688 | −5.93 | −3.87 | 2.06 | 284 | 305 |
The thermal properties of the polymer acceptors were evaluated via differential scanning calorimetry (Fig. 1). The melting temperatures (Tm) and crystallization temperatures (Tc) of the PNDI5 (321 °C and 298 °C) and PNDI10 (316 °C and 286 °C) copolymers were less than those of N2200 (328 °C and 307 °C, respectively). The reduction in Tm and Tc is attributable to the reduced intermolecular stacking of the copolymers owing to the increased backbone disorder.32 Thermogravimetric analysis (TGA) (Fig. S6, ESI†) shows that the onset decomposition temperatures of the copolymers with 5% weight loss (Td) were 380.6 °C, 454.8 °C, 435.4 °C and 420.7 °C for PBDB-T, N2200, PNDI5 and PNDI10, respectively, indicating good thermal stability of the copolymers.
 | ||
| Fig. 1 Differential scanning calorimetric characteristics of N2200, PNDI5, and PNDI10. The measurement was performed at a heating/cooling rate of 10 °C min−1 under nitrogen. | ||
Optical and electrochemical properties
The normalized ultraviolet-visible absorption spectra of the polymer acceptors (N2200, PNDI5, PNDI10), both in chloroform solution and as thin films, are shown in Fig. 2, and the detailed parameters are listed in Table 1. The spectra of all of the polymer acceptors in solution showed two absorption bands characteristic to donor–acceptor conjugated polymers: a short-wavelength absorption band at approximately 370 nm, attributed to the π–π* transition of the main chain, and a low-energy band (500–800 nm), attributed to the intra-molecular charge-transfer effect. The low-energy band of the terpolymer acceptor exhibited a hypsochromic shift, due to the backbone planarity of the polymer being lower than that of N2200.40 The spectra of the thin-film polymer acceptors showed red-shifted absorption peaks, which indicated the effective stacking of polymer chains in the solid state. Moreover, the PNDI5 and PNDI10 polymer acceptors exhibited complementary absorption with the PBDB-T donor. | ||
| Fig. 2 Normalized UV-vis absorption spectra of copolymers in chloroform solutions (a) and as thin films (b). | ||
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of polymer acceptors were measured by cyclic voltammetry (CV) (Fig. S7, ESI†), and the detailed parameters are listed in Table 1. The synthesized terpolymer acceptors exhibited identical HOMO energy levels (−5.9 eV). Owing to the effect of the DCB unit, the LUMO of the acceptor polymers were slightly less than that of the reference polymer (N2200), which may be related to electron withdrawal from the polymer backbone.22
Photovoltaic properties
Bulk heterojunction all-PSCs were fabricated to evaluate the photovoltaic properties of the synthesized terpolymer acceptors, which had the conventional configuration of ITO/PEDOT:PSS/PBDB-T:acceptor/PFN-Br/Al. A thin layer (∼5 nm) of water-/alcohol-soluble poly[(9,9-bis(3-((N,N-dimethyl)-N-ethylammonium)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctylfluorene)]dibromide (PFN-Br) was used as the cathode interlayer because it can facilitate electron collection through the formation of an interfacial dipole.41 To obtain the optimal photovoltaic performances, the device based on PBDB-T:PNDI5 was initially chose to optimize processing condition from the donor-to-acceptor weight ratios, thermal annealing temperatures, volume of additive, and thickness of the active layer. The data are detailed in ESI (Table S1–S4†). The current density–voltage (J–V) curves and external quantum efficiency (EQE) spectra of the optimized devices are shown in Fig. 3, and the relevant photovoltaic parameters are summarized in Table 2. | ||
Fig. 3 (a) J–V characteristics and (b) EQE spectra of devices with photoactive layer of PBDB-T:acceptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, wt 0.5, wt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt). wt). | ||
| Active layera | Voc (V) | Jsc (mA cm−2) | Jsc, EQEc (mA cm−2) | FF (%) | PCEb (max) (%) |
|---|---|---|---|---|---|
a D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = 1 A = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5; all blend films were processed by chlorobenzene with 0.5 vol% DIO.b Best PCE values are obtained from 12 separate devices.c Obtained from the integration of EQE spectra. 0.5; all blend films were processed by chlorobenzene with 0.5 vol% DIO.b Best PCE values are obtained from 12 separate devices.c Obtained from the integration of EQE spectra. |
|||||
| PBDB-T:N2200 | 0.87 ± 0.01 | 11.00 ± 0.13 | 10.78 | 67.22 ± 0.53 | 6.45 ± 0.10 (6.55) |
| PBDB-T: PNDI5 | 0.87 ± 0.01 | 12.32 ± 0.01 | 11.80 | 74.94 ± 0.02 | 8.01 ± 0.01 (8.02) |
| PBDB-T: PNDI10 | 0.86 ± 0.01 | 10.93 ± 0.19 | 10.65 | 72.00 ± 0.49 | 6.70 ± 0.12 (6.82) |
All of the devices showed the similar open-circuit voltage (Voc). The device based on PBDB-T-N2200 (control device) showed a PCE of 6.60% (Jsc = 11.00 mA cm−2, FF = 67.22%), consistent with previous results reported for a similar active layer.42 The PNDI5-based device considerably outperformed the control device by achieving a PCE of 8.01% (Jsc = 12.32 mA cm−2; FF = 74.94%). The higher FF of the PNDI5-based device was due to its balanced hole/electron mobilities and more homogeneous morphology than the control device. Moreover, compared with the PNDI5-based device, the PNDI10-based device, with a higher DCB loading, exhibited a slightly lower PCE (6.81%; Jsc = 10.93 mA cm−2; FF = 72.00%), because of its disordered molecular packing and unfavorable blend morphology.36
The EQE spectral of the devices are shown in Fig. 3b. All blend films showed a broad photocurrent response from 300 to 850 nm, consistent with their absorption spectra. This indicates that both the polymer acceptor and PBDB-T polymer donor in the blend films exhibited a photocurrent. Devices based on PBDB-T-PNDI5 showed better spectral responses at 350–750 nm than the N2200-based device, which is consistent with the higher Jsc of the former devices. The enhanced Jsc values of the devices based on a random polymer acceptor correlated well with the changes in their EQE spectral responses.
Charge generation, transport properties and recombination
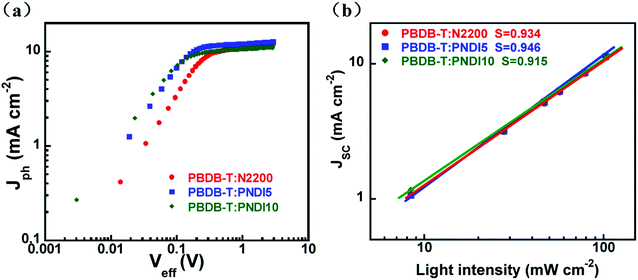
To reveal the exciton dissociation and charge collection processes of the all-PSCs based on a PBDB-T:acceptor, we investigated the dependence of the photocurrent density (Jph, Jph = JL − JD, where JL and JD are the light and dark current densities, respectively) on the effective voltage (Veff, Veff = V0 − Vbias, where V0 is the voltage when Jph is 0 and Vbias is the applied bias voltage.) at an illumination of 100 mW cm−2,43,44 and the relevant characteristics are shown in Fig. 4a. We presume that at a high Veff (>2 V), all photogenerated excitons were dissociated into free charges and collected by electrodes, and the Jph reaches saturation (Jsat). Thus, the maximum exciton generation rates (Gmax = Jsat/qL), where Jsat is the saturated photocurrent density, q is the electronic charge, and L is the thickness of the active layer of devices, are summarized in Table S5.† The PNDI5-based devices presented slightly higher Gmax values (7.93 × 1027) than the N2200-based devices (7.43 × 1027), which indicates that the PNDI5-based device generated excitons faster than N2200 counterparts, consistent with the Jsc values. In addition, the charge-dissociation probability P(E, T) was determined by normalizing Jph with Jsat. Under short-circuit conditions, the P(E, T) values the N2200- and PNDI5-based devices were 91.60% and 94.49%, respectively. These results indicate that the PNDI5-based device exhibited better exciton dissociation rates and more efficient charge collection than the N2200-based device.In addition, the charge transport properties of blended films were evaluated via the space–charge-limited current method. The corresponding J–V characteristics are shown in Fig. S3 (ESI†), and the carrier mobility is summarized in Table 3. The hole/electron mobility balances (μh/μe), which depended on the DCB molar concentration, exhibited nonmonotonic behaviors. A balanced carrier mobility is a crucial factor affecting the FF of all-PSCs. Among all of the blends, the PNDI5-based blend film showed the most balanced μh/μe value (3.5), while the N2200- and PNDI10-based blends showed unbalanced μh/μe values (38 and 5.8, respectively). These results suggest that the incorporation of a small amount of DCB into the N2200 main chains resulted in a balanced mobility of charge carriers in blend films, which is beneficial for charge extraction and collection. Thus, the PNDI5-based devices achieved the best FF values in all-PSCs. Moreover, a photoluminescence quenching experiment quenching experiments were performed to investigate exciton diffusion and dissociation in blend films (Fig. S9, ESI†). The quenching of the photoluminescence intensity of the PNDI5 blend films was more pronounced than the blend films of N2200 or PNDI10, indicating more efficient exciton dissociation and charge generation in the blend film with PBDB-T:PNDI5. This result is consistent with the significantly enhanced PCE of the PNDI5-based devices.
| PBDB-T:Acceptor | μh (cm2 V−1 s−1) | μe (cm2 V−1 s−1) | μh/μe |
|---|---|---|---|
| N2200 | 1.52 × 10−3 | 4.01 × 10−5 | 38 |
| PNDI5 | 1.00 × 10−3 | 2.85 × 10−4 | 3.5 |
| PNDI10 | 1.80 × 10−3 | 3.08 × 10−4 | 5.8 |
We further investigated the charge recombination behavior of all-PSCs by measuring the dependence of their Jsc on illumination intensity. The correlation between Jsc and illumination intensity (P) is quantitatively as Jsc ∝ (Plight)S, where Plight is the light intensity and S is the exponential factor.45 When the bimolecular recombination of the charge carriers is weak, Jsc shows a linear dependence on Plight with the S value almost equal to 1. The S values from the fitted line for the N2200-, PNDI5-, and PNDI10-based all-PSCs were 0.934, 0.946, 0.915, respectively (Fig. 4b), indicating that there was very weak bimolecular recombination in the device based on PBDB-T-PNDI5. The weak bimolecular recombination is associated with balanced hole and electron transport.
Film morphology
The morphological properties of optimized blend films were investigated via atomic force microscopy (Fig. 5a–c) and transmission electron microscopy (Fig. 5d–f). The PBDB-T:N2200 blend film exhibited granular aggregates across its surface and a root-mean-square (RMS) roughness of 2.33 nm. The PBDB-T:PNDI5 blend film formed smoother percolated fibrous structures with a slightly lower RMS roughness of 2.10 nm, suggesting that the DCB concentration of the polymer acceptor can be controlled to optimize the film morphology for charge transport (i.e., enhanced hole/electron mobility) in the bulk-heterojunction films. Moreover, the transmission electron microscopy images revealed that the blend film based on PBDB-T:PNDI5 showed blurred whiskers, which are beneficial for efficient charge transport in blend films and thus contributed to the excellent FF and higher Jsc of the all-PSCs devices.21 | ||
| Fig. 5 AFM height images (5 × 5 μm2) and TEM images for all-PSCs based on PBDB-T:N2200 (a and d), PBDB-T:PNDI5 (b and e), PBDB-T: PNDI10 (c and f) under optimized conditions. | ||
Conclusion
We developed two terpolymer acceptors, PNDI5 and PNDI10, by introducing different numbers of DCB units into bithiophene and 2,6-dibromonaphthalene-1,4,5,8-tetracarboxylic-N,N′-bis(2-octyldodecyl)diimide. The crystallinity and nanostructures of the resulting donor–acceptor blends could be fine-tuned by adjusting the DCB concentration in the acceptor. All-PSCs based on PBDB-T:PNDI5 blend exhibited a high PCE of 8.01%, which was considerably greater than that of the device based on PBDB-T-N2200 (6.60%). The enhanced photovoltaic performances of the PBDB-T:PNDI5-based devices was due to their balanced bulk charge mobility, reduced recombination, and optimized microstructure morphology. These results indicate that the construction of terpolymer acceptors based on N2200 is a promising approach for manufacturing all-PSCs.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011141), the characteristic Innovation Project for Higher Education Institution of Guangdong Province (No. 2019KTSCX170).References
- C. Lee, S. Lee, G.-U. Kim, W. Lee and B. J. Kim, Chem. Rev., 2019, 119, 8028–8086 CrossRef CAS PubMed.
- Z. Genene, W. Mammo, E. Wang and M. R. Andersson, Adv. Mater., 2019, 29, 1807275 CrossRef PubMed.
- P. Cheng and Y. Yang, Acc. Chem. Res., 2020, 53, 1218–1228 CrossRef CAS PubMed.
- J. Qin, L. Lan, S. Chen, F. Huang, H. Shi, W. Chen, H. Xia, K. Sun and C. Yang, Adv. Funct. Mater., 2020, 30, 2002529 CrossRef CAS.
- C. Zhao, J. Wang, J. Jiao, L. Huang and J. Tang, J. Mater. Chem. C, 2020, 8, 28–43 RSC.
- J. Du, K. Hu, L. Meng, I. Angunawela, J. Zhang, S. Qin, A. L.- Pelaez, C. Zhu, Z. Zhang, H. Ade and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 15181–15185 CrossRef CAS PubMed.
- H. Sun, H. Yu, Y. Shi, J. Yu, Z. Peng, X. Zhang, B. Liu, J. Wang, R. Singh, J. Lee, Y. Li, Z. Wei, Q. Liao, Z. Kan, L. Ye, H. Yan, F. Gao and X. Guo, Adv. Mater., 2020, 32, 2002183 CrossRef PubMed.
- Y. Zhang, Y. Xu, M. J. Ford, F. Li, J. Sun, X. Ling, Y. Wang, J. Gu, J. Yuan and W. Ma, Adv. Energy Mater., 2018, 8, 1800029 CrossRef.
- N. J. Zhou, H. Lin, S. J. Lou, X. G. Yu, P. J. Guo, E. F. Manley, S. Loser, P. Hartnett, H. Huang, M. R. Wasielewski, L. X. Chen, R. P. H. Chang, A. Facchetti and T. J. Marks, Adv. Energy Mater., 2014, 4, 1300785 CrossRef.
- R. H. Lee, L. C. Yang, J. Y. Wua and R. J. Jeng, RSC Adv., 2017, 7, 1016–1025 RSC.
- W. J. Chen, Y. C. Cheng, D. W. Kuo, C. T. Chen, B. T. Liu, R. J. Jeng and R. H. Lee, RSC Adv., 2018, 8, 31478–31489 RSC.
- D. Mori, H. Benten, I. Okada, H. Ohkita and S. Ito, Adv. Energy Mater., 2014, 4, 1301006 CrossRef.
- Z. Li, L. Ying, P. Zhu, W. Zhong, N. Li, F. Liu, F. Huang and Y. Cao, Energy Environ. Sci., 2019, 12, 157–163 RSC.
- B. Fan, W. Zhong, L. Ying, D. Zhang, M. Li, Y. Lin, R. Xia, F. Liu, H.-L. Yip, N. Li, Y. Ma, C. J. Brabec, F. Huang and Y. Cao, Nat. Commun., 2019, 10, 4100 CrossRef PubMed.
- B. Xie, K. Zhang, Z. Hu, H. Fang, B. Lin, Q. Yin, B. He, S. Dong, L. Ying, W. Ma, F. Huang, H. Yan and Y. Cao, Sol. RRL, 2019, 4, 1900385 CrossRef.
- N. B. Kolhe, D. K. Tran, H. Lee, D. Kuzuhara, N. Yoshimoto, T. Koganezawa and S. A. Jenekhe, ACS Energy Lett., 2019, 4, 1162–1170 CrossRef CAS.
- Y. Guo, Y. Li, O. Awartani, H. Han, J. Zhao, H. Ade, H. Yan and D. Zhao, Adv. Mater., 2017, 29, 1700309 CrossRef PubMed.
- S. Li, H. Zhang, W. Zhao, L. Ye, H. Yao, B. Yang, S. Zhang and J. Hou, Adv. Energy Mater., 2016, 6, 1501991 CrossRef.
- X. Long, Z. Ding, C. Dou, J. Zhang, J. Liu and L. Wang, Adv. Mater., 2016, 28, 6504–6508 CrossRef CAS PubMed.
- R. Zhao, N. Wang, Y. Yu and J. Liu, Chem. Mater., 2020, 32, 1308–1314 CrossRef CAS.
- Z.-G. Zhang, Y. Yang, J. Yao, L. Xue, S. Chen, X. Li, W. Morrison, C. Yang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 13503–13507 CrossRef CAS PubMed.
- H. Yao, F. Bai, H. Hu, L. Arunagiri, J. Zhang, Y. Chen, H. Yu, S. Chen, T. Liu, J. Y. L. Lai, Y. Zou, H. Ade and H. Yan, ACS Energy Lett., 2019, 4, 417–422 CrossRef CAS.
- J. Wu, Y. Meng, X. Guo, L. Zhu, F. Liu and M. Zhang, J. Mater. Chem. A, 2019, 7, 16190–16196 RSC.
- H. Sun, Y. Tang, C. W. Koh, S. Ling, R. Wang, K. Yang, J. Yu, Y. Shi, Y. Wang, H. Y. Woo and X. Guo, Adv. Mater., 2019, 31, 1807220 CrossRef PubMed.
- T. Jia, J. Zhang, W. Zhong, Y. Liang, K. Zhang, S. Dong, L. Ying, F. Liu, X. Wang, F. Huang and Y. Cao, Nano Energy, 2020, 72, 104718 CrossRef CAS.
- W. Wang, Q. Wu, R. Sun, J. Guo, Y. Wu, M. Shi, W. Yang, H. Li and J. Min, Joule, 2020, 4, 1070–1086 CrossRef CAS.
- Z. Luo, T. Liu, R. Ma, Y. Xiao, L. Zhan, G. Zhang, H. Sun, F. Ni, G. Chai, J. Wang, C. Zhong, Y. Zou, X. Guo, X. Lu, H. Chen, H. Yan and C. Yang, Adv. Mater., 2020, 30, 2005942 CrossRef PubMed.
- A. Tang, J. Li, B. Zhang, J. Peng and E. Zhou, ACS Macro Lett., 2020, 9, 706–712 CrossRef CAS PubMed.
- Q. Fan, Q. An, Y. Lin, Y. Xia, Q. Li, M. Zhang, W. Su, W. Peng, C. Zhang, F. Liu, L. Hou, W. Zhu, D. Yu, M. Xiao, E. Moons, F. Zhang, T. D. Anthopoulos, O. Inganäs and E. Wang, Energy Environ. Sci., 2020, 13, 5017–5027 RSC.
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dotz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679 CrossRef CAS PubMed.
- C. Mu, P. Liu, W. Ma, K. Jiang, J. Zhao, K. Zhang, Z. Chen, Z. Wei, Y. Yi, J. Wang, S. Yang, F. Huang, A. Facchetti, H. Ade and H. Yan, Adv. Mater., 2014, 26, 7224 CrossRef CAS PubMed.
- J. W. Jung, J. W. Jo, C. C. Chueh, F. Liu, W. H. Jo, T. P. Russell and A. K. Jen, Adv. Mater., 2015, 27, 3310 CrossRef CAS PubMed.
- L. Zhu, W. Zhong, C. Qiu, B. Lyu, Z. Zhou, M. Zhang, J. Song, J. Xu, J. Wang, J. Ali, W. Feng, Z. Shi, X. Gu, L. Ying, Y. Zhang and F. Liu, Adv. Mater., 2019, 31, 1902899 CrossRef CAS PubMed.
- Z. Li, X. Xu, W. Zhang, X. Meng, W. Ma, A. Yartsev, O. Inganas, M. R. Andersson, R. A. Janssen and E. Wang, J. Am. Chem. Soc., 2016, 138, 10935–10944 CrossRef CAS PubMed.
- X. Liu, C. Zhang, C. Duan, M. Li, Z. Hu, J. Wang, F. Liu, N. Li, C. J. Brabec, R. A. J. Janssen, G. C. Bazan, F. Huang and Y. Cao, J. Am. Chem. Soc., 2018, 140, 8934–8943 CrossRef CAS PubMed.
- D. Chen, J. Yao, L. Chen, J. Yin, R. Lv, B. Huang, S. Liu, Z.-G. Zhang, C. Yang, Y. Chen and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 1–6 CrossRef.
- J.-W. Lee, M. Sung, D. Kim, S. Lee, H. You, F. S. Kim, Y.-H. Kim, B. J. Kim and S.-K. Kwon, Chem. Mater., 2020, 32, 2572–2582 CrossRef CAS.
- B. He, Q. Yin, X. Yang, L. Liu, X.-F. Jiang, J. Zhang, F. Huang and Y. Cao, J. Mater. Chem. C, 2017, 5, 8774–8781 RSC.
- B. He, Q. Yin, J. Zhang, T. Jia, X. Yang, X.-F. Jiang, F. Huang and Y. Cao, Chin. J. Chem., 2018, 36, 406 CrossRef CAS.
- R. Steyrleuthner, R. Di Pietro, B. A. Collins, F. Polzer, S. Himmelberger, M. Schubert, Z. Chen, S. Zhang, A. Salleo, H. Ade, A. Facchetti and D. Neher, J. Am. Chem. Soc., 2014, 136, 4245–4256 CrossRef CAS PubMed.
- Z. Hu, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2017, 60, 571–582 CrossRef CAS.
- Y. Zhang, Y. Xu, M. J. Ford, F. Li, J. Sun, X. Ling, Y. Wang, J. Gu, J. Yuan and W. Ma, Adv. Energy Mater., 2018, 8, 1800029 CrossRef.
- B. Fan, L. Ying, Z. Wang, B. He, X.-F. Jiang, F. Huang and Y. Cao, Energy Environ. Sci., 2017, 10, 1243–1251 RSC.
- J.-L. Wu, F.-C. Chen, Y.-S. Hsiao, F.-C. Chien, P. Chen, C.-H. Kuo, M. H. Huang and C.-S. Hsu, ACS Nano, 2011, 5, 959–967 CrossRef CAS PubMed.
- M. M. Mandoc, F. B. Kooistra, J. C. Hummelen, B. de Boer and P. W. M. Blom, Appl. Phys. Lett., 2007, 91, 263505 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: additional figures as mentioned in the text, including synthesis of monomer and polymer, and device fabrication and characterizations. See https://doi.org/10.1039/d2ra03062d |
| This journal is © The Royal Society of Chemistry 2022 |