 Open Access Article
Open Access ArticleFacile synthesis of mesoporous NixCo9−xS8 hollow spheres for high-performance supercapacitors and aqueous Ni/Co–Zn batteries†
Daojun Zhang *,
Jingchao Zhang,
Jiaqi Li,
Chengxiang Li,
Yuting Li,
Yingying Liu and
Renchun Zhang
*,
Jingchao Zhang,
Jiaqi Li,
Chengxiang Li,
Yuting Li,
Yingying Liu and
Renchun Zhang
College of Chemistry and Chemical Engineering, Anyang Normal University, Anyang, 455000, Henan, China. E-mail: zhangdj0410@126.com; zhangdj0410@sohu.com; Tel: +86 372 2900040
First published on 14th July 2022
Abstract
Porous micro/nanostructure electrode materials have always contributed to outstanding electrochemical energy storage performances. Co9S8 is an ideal model electrode material with high theoretical specific capacity due to its intrinsic two crystallographic sites of cobalt ions. In order to improve the conductivity and specific capacitance of Co9S8, nickel ions were introduced to tune the electronic structure of Co9S8. The morphology design of the mesoporous hollow sphere structure guarantees cycle stability and ion diffusion. In this work, NixCo9−xS8 mesoporous hollow spheres were synthesized via a facile partial ion-exchange of Co9S8 mesoporous hollow spheres without using a template, boosting the capacitance to 1300 F g−1 at the current density of 1 A g−1. Compared with the pure Co9S8 and Ni-Co9S8-30%, Ni-Co9S8-60% exhibited the best supercapacitor performance, which was ascribed to the maximum Ni ion doping with morphology and structure retention, enhanced conductivity and stabilization of Co3+ in the structure. Therefore, Ni/Co–Zn batteries were fabricated by using a Zn plate as the anode and Ni-Co9S8-60% as the cathode, which deliver a high energy density of 256.5 W h kg−1 at the power density of 1.69 kW kg−1. Furthermore, the Ni/Co–Zn batteries exhibit a stable cycling after 3000 repeated cycles with capacitance retention of 69% at 4 A g−1. This encouranging result might provide a new perspective to optimize Co9S8-based electrodes with superior supercapacitor and Ni/Co–Zn battery performances.
1 Introduction
In recent years, as an important transition metal sulfide, Co9S8 has been regarded as one of the most attractive electrode materials with a high theoretical specific capacity (5449 F g−1 at 0.45 V),1 low cost and non-toxic nature, however, the presence of sluggish ion transport kinetics and low conductivity limits its application in the energy storage field. Thus a lot of efforts have been devoted to improving the conductivity by conductive metals or carbonaceous supports,2–6 ion-doping,7 constructing hierarchical composites,8 graphene hybridization,9,10 carbon coating,11 and constructing hetero-phase interfaces.12–14 In addition to consideration of the conductivity, the electrochemical performance is also dependent on the accessible surface area of transition metal based micro/nanomaterials,15–17 thus a lot of effect has been devoted to synthesizing electrode materials with large surface areas and porous structures. For example, the reported morphology and synthesis strategies include ion exchange reaction,6 microwave irradiation,18 sacrificial metal salt hard templates19 and derivation from metal–organic framework precursors via a sulfuration process.20,21 Considering the complexity and multiple steps of the template transformation method, it is worth developing a simple method without a template to obtain hollow sphere structures.In general, the mesoporous hollow structure can alleviate the volumetric expansion, accommodate large internal and external surface area and facile electrolyte ions diffusion reaction.22–24 For example, Zheng reported the metallic hollow Co9S8 sphere can serve as sulfur hosts in Li–S batteries and promote interfacial redox reaction kinetics, which present excellent rate performance and high stability.25 Feng designed hierarchical Co9S8@carbon hollow microspheres via a solvothermal reaction and subsequent annealing process, which also exhibited remarkable sodium storage performance with remarkable cycling stability.26 Li reported the synthesis of hollow spherical Co9S8 using presynthetic silica hollow spheres template to construct Co–SiO2 precursor and subsequent hydrothermal sulfurizing process.27 Guo et al. using bacteria as template via hydrothermal and further calcination process to fabricate porous Co9S8/CoS/C submicronspheres.28 Thus a facile one-step method to prepare Co9S8 porous spheres especially mesoporous hollow architecture is appreciated.
Transition metal sulfides especially porous hollow structures are considered as promising electrode materials for electrochemical energy storage devices (EESDs) owing to their relatively abundant, low cost, non-toxic nature, multiple electrochemical reaction sites with high capacity.29,30 Alkaline aqueous EESDs, such as supercapacitor and Ni/Co–Zn batteries, have many merits consist of environmental friendliness and quickly charge–discharge speed attribute to using high ionic conductance of OH− anions as the charge carries, thus alkaline aqueous EESDs have great potential applications in energy storage field.31,32 Especially, Co-based sulfides were considered to be good cathodes with high theoretical capacity and operating voltage, which attracted extensive research interests.33 Extensive exploring micro/nanostructure is help to increase the capacity output and rate capability of cathode.34 The structure of Co9S8 is a typical structure with the octahedral site of Co3+ and tetrahedral site of Co2+. Therefore, doping Ni ions into Co9S8 is a promising strategy to increase the Co3+ and Ni3+ content in the octahedral sites of Co9S8 lattices, which effectively synergistic increase the capacity and cycling stability of supercapacitor and Co–Ni//Zn battery. Nevertheless, the investigation of NixCo9−xS8 as cathode material in alkaline Ni–Zn battery is rarely reported till now.
Herein, a facile cation exchange approach to synthesize mesoporous hollow structured NixCo9−xS8 using the solvothermal synthesized Co9S8 microspheres as precursors. The nickel ion doping improves the conductivity of parent Co9S8 and promotes electrochemical reaction kinetics. The Ni-Co9S8-0.6 shows a battery-type faradaic electrode, which can offer additional charge storage capacity via surface redox processes, e.g., 1300 F g−1 at 1 A g−1, compared with the pure Co9S8 electrode (430 F g−1 at 1 A g−1). Furthermore, we exhibit a novel Ni/Co–Zn battery utilizing Ni-Co9S8-0.6 mesoporous hollow microspheres as cathode material, due to the high content Co3+ ions of Ni-Co9S8-0.6, the voltage window of Ni/Co–Zn battery can achieve 1.93 V vs. Zn. These special designs are helped to improve the kinetic property and rate property of the cathode material. This new Ni/Co–Zn battery shows a high power density of 1.69 kW kg−1 at the energy density of 256.46 W h kg−1.
2 Experimental
2.1 Preparation of NixCo9−xS8 mesoporous hollow microspheres
To prepare Co9S8 microspheres, 0.1 mmol of cobalt acetate tetrahydrate was dissolved in a mixture solution of N,N-dimethylformamide (4 mL), tetramethyl-ethylenediamine (3 mL), and ethanolamine (1 mL) under strong stirring for 0.5 h. Afterwards, 0.1 mmol of thioacetamide was added and further stirred for another 0.5 h. Then the solution was transferred to a 20 mL autoclave and heated at 180 °C for 12 h. The black precipitate was collected by centrifugation and dried at 60 °C for 30 min.For the synthesis of Ni-Co9S8-30% and Ni-Co9S8-60% microspheres, the equal amounts (0.03/0.06 mmol) of nickel acetate and thiourea was added to the pre-dispersed aqueous solution (6 mL) containing the as-prepared Co9S8 microspheres, then 2 mL of ethylene glycol were added with vigorous stirring, subsequently heated to 160 °C and kept for 4 h. The final samples were washed and dried in vacuum at 60 °C for 30 min. Herein, we use Ni-Co9S8-0.3 and Ni-Co9S8-0.6 to denote the 30% and 60% Ni ions added in the reaction system.
2.2 Material characterization
The resultant phase of the mesoporous Co9S8, Ni-Co9S8-0.3 and Ni-Co9S8-0.6 hollow microspheres was determined by X-ray diffraction (XRD) on a PANalytical X’ Pert operated at 40 kV and 40 mA. The morphology and microstructure were studied by SEM and TEM technique. The composition and chemical states of the samples was further analyzed by energy-dispersive X-ray spectroscopy (EDS) as well as X-ray photoelectron spectroscopy (XPS, ESCALab, 250XI), respectively. The specific surface area of the samples was measured by N2 adsorption analyzer (Gemini VII 2390).2.3 Cathode electrode preparation and measurement
For preparation supercapacitor and alkaline Ni–Zn battery cathode, the electrode slurry was prepared by mixing 16 mg of the NixCo9−xS8 powders, 2 mg of acetylene black, and 2 mg of polyvinylidene-fluoride with 110 μL of N-methylpyrrolidone. Then, the slurry was carefully coated onto the pretreated Ni foam with the area of 1 cm × 1 cm, and dried in vacuum at 80 °C for 10 h. The specific capacitance Cs of supercapacitor was calculated from following equation:where I, Δt, m, ΔV is the current density, discharge time, the mass of active materials, and the potential window, respectively.
The alkaline Ni–Zn battery tests were recorded on an electrochemical workstation (CHI 660E) using two electrode system in which Zn plate as reference and counter electrode, and Ni-Co9S8-0.6 as working electrode, 2 M KOH and 20 mM Zn(Ac)2 were used as mixture electrolytes. AC impendence tests with a platinum coil counter electrode and a saturated calomel electrode as reference electrode. The electrochemical impendence plots were collected at open circuit potential at the frequency range of 0.01 Hz–1 MHz.
3 Results and discussion
Scheme 1 shows the one-step synthesis of unique mesoporous Co9S8 hollow microspheres via a mixed solvothermal route, then Ni-doped Co9S8 can be obtained by a cation-exchange method with introducing 0.03 or 0.06 mmol nickel acetate and thiourea in a water–ethylene glycol system. Herein, we use Ni-Co9S8-0.3 and Ni-Co9S8-0.6 to denote the 30% and 60% Ni ions added in the reaction system, and aim to investigate the role of Ni doping in promoting the supercapacitor properties of Co9S8 particles.The morphology and composition of Co9S8 mesoporous hollow spheres were characterized by SEM and EDS. Fig. S1a† and 1a show SEM images of the as-prepared Co9S8 microspheres at low and high magnification, it is clearly seen that microspheres assembled by numerous interconnected nanosheets with large inner cavity, after introducing Ni ions, the hollow sphere morphology can be well preserved with thinner size and more closely stacked nanosheets. The as-prepared Co9S8 microspheres exhibited an average diameter of ∼2.65 μm (Fig. S1b†). The cracked microspheres provide insights into the hollow interior and the assembly structure. After the Ni ions doping, the microsphere size decreases to from 2.34 to 2.29 μm (Fig. 1c, e, and S1†), which may due to the breakage of the big microspheres via the second step of Ni ions exchange. As shown in the EDS element mapping (Fig. 1d), the cobalt and sulfur elements are homogenous distributed in the Co9S8 microspheres. The intensity of Ni element mapping raised following the increase of Ni content in Co9S8 matrics (Fig. 1d and f).
 | ||
| Fig. 1 SEM images and EDS element mapping of (a and b) Co9S8, (c and d) Ni-Co9S8-0.3, (e and f) Ni-Co9S8-0.6 hollow microspheres. (The scale bar of b, d, f is 800, 500 and 600 nm). | ||
The TEM image of a typical Ni-Co9S8-0.6 hollow microsphere was shown in Fig. 2a, which shows a sharp contrast between the inner cavity and cross stacking nanosheets shell. The special microstructure not only avoid the aggregation and structural collapse, but also provide more surface and inner exposed active sites, which help to achieve desirable rate and cycling performance due to the fast ion diffusion. The lattice interplanar spacing measured to be ∼0.295 nm, which matched well with the (311) plane of cobalt pentlandite Co9S8 (Fig. 2b), indicating the structure of Co9S8 was stable in moderate Ni ions doping.
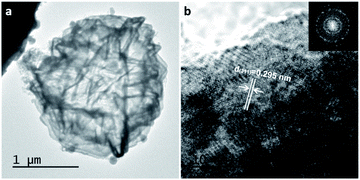 | ||
| Fig. 2 TEM images of Ni-Co9S8-0.6 hollow spheres. (a) A single Ni-Co9S8-0.6 hollow sphere, (b) HRTEM image. | ||
The phase purities of as-synthesized microspheres were further detected by X-ray power diffraction (Fig. 3a), the diffraction peaks of the three samples match well with the cubic Co9S8 phase. The XRD patterns of the three samples are similar, for example, the obtained XRD of Co9S8 mesoporous microspheres shown three obvious diffraction peaks at ∼29.8, 47.5 and 52.1°, which corresponded to the (311), (511), and (400) plane of Co9S8 (JCPDS no: 03-65-6801). Because the Co ion radius is larger than the Ni ion, the diffraction peaks of Ni ions doped microspheres especially Ni-Co9S8-0.6 present a slight moving to higher angle of 2 Theta than pure Co9S8, and the calculated d-spacing of (311) plane from Scherrer formula is 0.296 nm, which is lower than that of pure Co9S8 (0.299 nm). The XRD result is almost consistent with the HRTEM in Fig. 2b. The energy-dispersive X-ray spectroscopy (EDS) results indicated the presence of Co, S and Ni elements in the series samples. The ratio of Co/S in Co9S8 is 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, which is consistent with the stoichiometry of Co9S8. The Ni/Co/S ratio of Ni-Co9S8-0.3 and Ni-Co9S8-0.6 samples is 1.4
8, which is consistent with the stoichiometry of Co9S8. The Ni/Co/S ratio of Ni-Co9S8-0.3 and Ni-Co9S8-0.6 samples is 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6
7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 2.8
6 and 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2
6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5, respectively (Fig. S2†). Therefore, both XRD and EDS results confirmed the pure phase of the Ni doped series of NixCo9−xS8. To analyse the surface area and porous structure of the series of hollow spheres, N2 adsorption–desorption isotherms were conducted. The hysteresis loop for Co9S8 located above P/P0 = 0.8, indicating the presence of mesoporous structure (Fig. 3b). The inset is the Barrett–Joyner–Halenda (BJH) pore distribution for pure Co9S8, the mean pore size is ∼47 nm with the BET surface area of 65.92 m2 g−1. The surface area of Co9S8 (65.92 m2 g−1) is larger than Ni-Co9S8-0.3 (54.18 m2 g−1) and Ni-Co9S8-0.6 (55.73 m2 g−1) samples as shown in Fig. 3c and d. In addition, the mean pore distribution for Ni-Co9S8-0.3 and Ni-Co9S8-0.6 decreased to 29.21 and 18.08 nm after nickel ions exchange into Co9S8 structure. Thus, the porous structure facilitates electrolyte diffusion and electron transport during the redox electrochemical reaction.
6.5, respectively (Fig. S2†). Therefore, both XRD and EDS results confirmed the pure phase of the Ni doped series of NixCo9−xS8. To analyse the surface area and porous structure of the series of hollow spheres, N2 adsorption–desorption isotherms were conducted. The hysteresis loop for Co9S8 located above P/P0 = 0.8, indicating the presence of mesoporous structure (Fig. 3b). The inset is the Barrett–Joyner–Halenda (BJH) pore distribution for pure Co9S8, the mean pore size is ∼47 nm with the BET surface area of 65.92 m2 g−1. The surface area of Co9S8 (65.92 m2 g−1) is larger than Ni-Co9S8-0.3 (54.18 m2 g−1) and Ni-Co9S8-0.6 (55.73 m2 g−1) samples as shown in Fig. 3c and d. In addition, the mean pore distribution for Ni-Co9S8-0.3 and Ni-Co9S8-0.6 decreased to 29.21 and 18.08 nm after nickel ions exchange into Co9S8 structure. Thus, the porous structure facilitates electrolyte diffusion and electron transport during the redox electrochemical reaction.
Furthermore, XPS in Fig. 4 also indicated the existence of Co, S, and Ni elements in the as-obtained Ni-Co9S8-0.3 and Ni-Co9S8-0.6 microspheres and the variation of surface chemical state of Co after Ni ion doping. In the Co 2p spectra of pure Co9S8 and Ni-doped Co9S8 indicated the presence of Co2+ and Co3+ in the tetrahedral and octahedral sites. The peaks at ∼778.34 and 793.42 eV for Co9S8 were ascribed to Co3+, while those at 780.81 and 796.62 eV belonged to Co2+.35 It can be seen from Fig. 4b that the Co3+ area of Ni-Co9S8-0.6 is higher than Co9S8, the ratio of Co3+/Co2+ obtained from their respective main lines is 0.3529 for Ni-Co9S8-0.6 and 0.3248 for Co9S8. The peaks located at 712.40 and 724.30 eV, with the satellite of 718.60 and 736.41 eV, indicated the existence of Ni3+, which caused the increase of Co3+/Co2+ ratio in Ni-Co9S8-0.6 microspheres. Meanwhile, the Ni3+/Ni2+ ratio of is 1.88 for Ni-Co9S8-0.3 and 2.38 for Ni-Co9S8-0.6 (Table S1†). Thus, we conclude that the nickel ions doping supply more trivalent sites in Co9S8 structure, which is helped to regulate cationic distribution and increase the output voltage of the electrode materials. The typical binding energy peaks S 2p3/2 and S 2p1/2 were located at 161.46 and 162.68 eV, respectively, the peak located at 59.60 eV may be ascribed to a small degree of surface oxidation of the catalyst exposed to the air condition.
 | ||
| Fig. 4 (a) XPS survey of NixCo9−xS8 series hollow spheres. The high-resolution spectra of (b) Co 2p, (c) Ni 2p, and (d) S 2p. | ||
3.1 Electrochemical performance of mesoporous NixCo9−xS8 hollow microspheres
To evaluate the supercapacitance performance of the series NixCo9−xS8 samples, electrochemical tests were conducted by a standard three-electrode system in 2.0 M KOH aqueous solution, with sample coated Ni foam as work electrode, Hg/Hg2Cl2 and Pt plate as reference electrode and counter electrode. The CV curves of the Co9S8, Ni-Co9S8-0.3, and Ni-Co9S8-0.6 electrodes were plotted in Fig. 5a, S3 and S4.† Co9S8 sample present two pairs of redox peaks in the CV curve, which can be attributed to the redox reaction of Co9S8 with two Co crystallographic sites in its structure. Compared with the Co2+/Co3+ and Co3+/Co4+ redox peaks of Co9S8 electrode, the Ni doped Co9S8 exhibited an obvious increase of peak intensity and increased integration area because of the coexistence of Ni2+/Ni3+, indicated the better performance of the Ni-doped samples. Fig. 5b indicated the intercalation and surface reaction mechanism occurred in the series of NixCo9−xS8 electrodes. The corresponding diffusion contribution of Co9S8, Ni-Co9S8-0.3 and Ni-Co9S8-0.6 is 48%, 56%, and 61%, respectively, demonstrating the diffusion-controlled behavior at 5 mV s−1. Conversely, with the increase of scan rate, the capacitive contribution enhanced due to the insufficient time for intercalation reaction into the series of NixCo9−xS8 electrodes. The galvanostatic charge–discharge (GCD) plot of the three electrodes was shown in Fig. 5c and S5.† At a current density of 1 A g−1, the Ni-Co9S8-0.6 electrode exhibits the longest discharge time, furthermore, according to the galvanostatic discharge curves, the calculated capacitances of Ni-Co9S8-0.6, Ni-Co9S8-0.3, and Co9S8 electrodes are 1300, 653, and 430 F g−1, respectively. As the current density increase to 10 A g−1, the corresponding capacitance decreased to 1048, 536, and 346 F g−1 (Fig. 5d), and the corresponding capacitance retentions are 80.6%, 82.1%, and 80.5%, respectively. The above results indicated the introducing of Ni ions can effectively increase the specific capacitance and rate capability of pure Co9S8 sample. Fig. 5e showed the long-term cyclic performance of the three electrodes at 4 Ag−1. The capacity of Ni-Co9S8-0.6 electrode attenuate to 74.5% after 5000 repeated cycles. More importantly, the mesoporous hollow Ni-Co9S8-0.6 electrode exhibited a performance comparable to or higher than the recently reported sulfide-related electrodes such as rGO/Co9S8 (575.9 F g−1, 2 A g−1),36 CoNi2S4/Co9S8 composites (1183.3 F g−1, 2 A g−1),37 Ni3S4@Co9S8 tubes (1002.2 F g−1, 1 A g−1),38 CoO/Co9S8 hollow microspheres (1100 F g−1, 2 A g−1),39 GH@NC@Co9S8 composite (540 F g−1, 1 A g−1),40 Co9S8 nanowire/Ni (1191.17 F g−1, 2 mV s−1),41 0.3 cP/rGO/Co9S8 (788.9 F g−1, 1 A g−1),42 Co9S8@N-C@MoS2 (410 F g−1, 10 A g−1),43 Co3(OH)2(HPO4)2 @Co9S8/Co electrode (1279.4 F g−1, 5 mA cm−2),44 CoS@NSC-800 (289 F g−1, 1 A g−1),45 Co9S8@NiCo2S4@NF electrode (1026 F g−1, 1 A g−1),46 (Co0.94Fe0.06)9S8 hollow spheres (454 F g−1, 1 A g−1),47 Co9S8-2@CN/NF (471.1 F g−1, 0.5 A g−1),48 Co9S8–aCNT–NiCoLDH (1185.5 F g−1, 1 A g−1),49 and MnCo2S4/Co9S8/Ni composites (1058.0 F g−1, 1 A g−1).50 A more extensive comparison of super-capacitance performance of Ni-Co9S8-0.6 with other related electrodes are listed in Table S2.† Furthermore, the improved electrochemical performance of Ni-Co9S8-0.6 can be supported by the smallest electrolyte resistance (Rs) and charge transfer resistance (Rct) shown in Fig. 5f. The electrolyte resistance of Co9S8, Ni-Co9S8-0.3, and Ni-Co9S8-0.6 is 1.09, 1.29, and 0.97 Ω, respectively. Furthermore, the charge transfer resistance of Co9S8 is 3.39 Ω, which decreased to 1.39 Ω in Ni-Co9S8-0.6. The straight line with the largest slope in low frequency region also indicated the Ni-Co9S8-0.6 electrode exhibit the fast ionic diffusion efficiency and excellent reversibility (Fig. S6†).Furthermore, a typical Ni-Co9S8-0.6//Zn alkaline battery is fabricated by using Ni-Co9S8-0.6 as cathode, Zn plate as anode, and 2 M KOH+0.2 M Zn(Ac)2 as electrolytes. Fig. 6a shows the CV curves of Ni-Co9S8-0.6//Zn battery at the scan rate of 1–20 mV s−1 with the potential range of 1.2–2.0 V vs. Zn. At 1 mV s−1, the Ni-Co9S8-0.6//Zn displays a pair of redox peaks at 1.8 and 1.65 V. Even the scan rate up to 20 mV s−1, the redox peaks can be preserved, indicated the excellent reversibility of the full battery. The GCD curves obtained at different current densities were shown in Fig. 6b, the GCD curves present a charge and discharge platforms located at 1.774 and 1.675 V at 1 A g−1, with the voltage hysteresis of 0.099 V, demonstrating a less polarization and high energy conversion efficiency at the current density of 1 A g−1. Furthermore, the specific capacitance of the Ni-Co9S8-0.6//Zn battery decreased from 152 to 97 mA h g−1 with its voltage reduced from 1.675 to 1.654 V as the current density increasing from 1 to 10 A g−1. The capacitance retention of 10 A g−1 is 64%, indicating the excellent rate performance of Ni-Co9S8-0.6//Zn battery in alkaline electrolyte. Moreover, when the discharge current density came back to 1 A g−1, the battery can restore the capacitance of 140 mA h g−1 (92%), illustrating the tolerance to high speed charge–discharge reaction (Fig. 6c). The maximum charge capacitance approach to the similar aqueous Ni–Zn batteries, such as G-NCGs//Zn battery (113.8 mA h g−1, 0.5 A g−1),51 Ni3S2/Ni//Zn battery (148 mA h g−1, 0.2 A g−1),52 Zn//NiCo2O4 (112 mA h g−1, 1 A g−1),53 Zn//NiO–CNTs battery (155 mA h g−1, 1 A g−1),54 F-doped Ni–Co hydroxide (FNCH) and oxide constructed FNCH/Ni//Zn battery (88 mA h g−1, 1 A g−1) and FNCO/Ni//Zn battery (159 mA h g−1, 1 A g−1),55 ZnCo2O4−x//Zn (148.3 mA h g−1, 0.05 A g−1),56 Zn//ZnAl0.67Co1.33O4 (134 mA h g−1, 16 mA g−1),57 NiCo2O4-1//Zn (122.5 mA h g−1, 1 A g−1),58 and NiO/C–Zn (159 mA h g−1, 1 A g−1).59 Furthermore, the cyclic stability of the Ni-Co9S8-0.6//Zn battery was tested at 4 A g−1 for 3000 cycles, the capacity retention kept at 69% (Fig. 6d). The excellent rate capability of Ni-Co9S8-0.6//Zn can be supported by the small charge transfer resistance (Fig. 6e). The power density and energy density of Ni-Co9S8-0.6//Zn battery was calculated and shown in Fig. 6f, the Ni-Co9S8-0.6//Zn battery can achieve the energy density of 256.5 W h kg−1 (at 1.69 kW kg−1) and the maximum power density of 16.56 kW kg−1 (at 160.1 W h kg−1). The power/energy density of Ni-Co9S8-0.6//Zn battery are comparable with the reported Ni–Zn battery such as β-Ni(OH)2/CNFs//Zn (325 W h kg−1 at a power density of 1.23 kW kg−1),60 Co3O4@NiO NSRAs//Zn (215.51 W h kg−1 at 3.45 kW kg−1),61 Co-Ni3Se2//Zn (199.34 W h kg−1),62 P-Co3O4//Zn battery (193.7 W h kg−1 at 1.6 kW kg−1),63 N-Fe2O3−x//Zn battery (135 W h kg−1 at 0.47 kW kg−1),64 Am-NCS//Zn (254.2 Wh/kg at 3.28 kW kg−1).65 The excellent performance of Ni-Co9S8-0.6 should be ascribed to the unique mesoporous hollow spheres structure with enriched contact area and highly open structure, which facilities the mass transportation, and the Ni dopant in the Co9S8 matrix can improve the electrical conductivity.
 | ||
| Fig. 6 The electrochemical characterization of Ni-Co9S8-0.6//Zn battery (a) CV curves, (b) GCD curves, (c) rate capability, (d) the cyclic stability at 4 A g−1, (e) Nyquist plot, and (f) Ragone plot. | ||
4 Conclusions
In summary, a series of mesoporous hollow NixCo9−xS8 spheres were synthesized via a facile solvothermal method, which exhibit excellent rate capability and long-time cycling performance. Attributed to the improvement of conductivity and abundant active surface, the capacitance of Ni-doped Co9S8 increased dramatically. Remarkably, the optimized Ni-Co9S8-0.6 electrode delivery a capacity of 1300 F g−1 at 1 A g−1. Especially, a stable aqueous Ni/Co–Zn battery was constructed by Ni-Co9S8-0.6 as cathode and Zn plate as anode. The Ni-Co9S8-0.6//Zn battery delivers an energy density of 256 W h kg−1 and power density of 1.69 kW kg−1. This work provides a facile method to prepare mesoporous hollow structure and cathodes for aqueous Ni–Zn batteries.Author contributions
Daojun Zhang: methodlogy, writing and editing. Jingchao Zhang: analysis and investigation. Jiaqi Li and Yuting Li, synthesis and characterization. Chenxiang Li and Yingying Liu, software and data curation. Renchun Zhang, editing and review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (No. 21603004), the Foundation of Henan Educational Committee (22A150002), and the Science and Technology Research Project of Henan Province (222102240096).Notes and references
- S. X. Sun, J. H. Luo, Y. Qian, Y. Jin, Y. Liu, Y. G. Qiu, X. Li, C. Fang, J. T. Han and Y. H. Huang, Adv. Energy Mater., 2018, 8, 1801080 CrossRef.
- J. Pu, Z. H. Wang, K. L. Wu, N. Yu and E. H. Sheng, Phys. Chem. Chem. Phys., 2014, 16, 785 RSC.
- Y. X. Wen, Y. P. Liu, S. Dang, S. H. Tian, H. Q. Li, Z. L. Wang, D. Y. He, Z. S. Wu, G. Z. Cao and S. L. Peng, J. Power Sources, 2019, 423, 106–114 CrossRef CAS.
- Y. Q. Zhou, N. Li, L. D. Sun, X. L. Yu, C. Liu, L. Yang, S. D. Zhang and Z. Y. Wang, Nanoscale, 2019, 11, 7457–7464 RSC.
- J. Xu, Q. F. Wang, X. W. Wang, Q. Y. Xiang, B. Liang, D. Chen and G. Z. Shen, ACS Nano, 2013, 7, 5453–5462 CrossRef CAS PubMed.
- N. Zhang, W. C. Wang, C. Q. Teng, Z. X. Wu, Z. R. Ye, M. J. Zhi and Z. L. Hong, RSC Adv., 2018, 8, 27574–27579 RSC.
- E. S. Goda, A. ur Rehman, B. Pandit, A. Al-Shahat Eissa, S. E. Hong and K. R. Yoon, Chem. Eng. J., 2022, 428, 132470 CrossRef CAS.
- X. C. Hou, Y. Z. Zhang, Q. C. Dong, Y. Hong, Y. L. Liu, W. J. Wang, J. J. Shao, W. L. Si and X. C. Dong, ACS Appl. Energy Mater., 2018, 1, 3513–3520 CrossRef CAS.
- B. Q. Xie, M. Y. Yu, L. H. Lu, H. Z. Feng, Y. Yang, Y. Chen, H. D. Cui, R. B. Xiao and J. Liu, Carbon, 2019, 141, 134–142 CrossRef CAS.
- X. Z. Wang, D. C. Su, Y. H. Xiao, S. G. Xu, S. M. Fang and S. K. Cao, Electrochim. Acta, 2019, 293, 419–425 CrossRef CAS.
- L. L. Li, Y. H. Ding, H. J. Huang, D. S. Yu, S. Y. Zhang, H. Y. Chen, S. Ramakrishna and S. J. Peng, J. Colloid Interface Sci., 2019, 540, 389–397 CrossRef CAS PubMed.
- L. R. Hou, Y. Y. Shi, S. Q. Zhu, M. Rehan, G. Pang, X. G. Zhang and C. Z. Yuan, J. Mater. Chem. A, 2017, 5, 133–144 RSC.
- Q. Liu, X. D. Hong, X. Zhang, W. Wang, W. X. Guo, X. Y. Liu and M. D. Ye, Chem. Eng. J., 2019, 356, 985–993 CrossRef CAS.
- J. X. Zhang, Y. Deng, Y. Q. Wu, Z. Y. Xiao, X. B. Liu, Z. J. Li, R. R. Bu, Q. Zhang, W. Sun and L. Wang, Chem. Eng. J., 2022, 430, 132836 CrossRef CAS.
- S. Biswas, V. Sharma, T. Singh and A. Chandra, J. Mater. Chem. A, 2021, 9, 6460–6468 RSC.
- S. Biswas, A. Chowdhury and A. Chandra, Front. Mater., 2019, 6, 1–11 CrossRef.
- J. H. Ryu, B. G. Park, S. B. Kim and Y. Park, J. Appl. Electrochem., 2009, 39, 1059–1066 CrossRef CAS.
- L. Yin, L. Q. Wang, X. H. Liu, Y. S. Gai, L. H. Su, B. H. Qu and L. Y. Gong, Eur. J. Inorg. Chem., 2015, 2457–2462 CrossRef CAS.
- L. Wang, S. K. Li, F. Z. Huang, X. Y. Yu, M. J. Liu and H. Zhang, J. Power Sources, 2019, 439, 227103 CrossRef CAS.
- X. Q. Du, H. Su and X. S. Zhang, ACS Sustainable Chem. Eng., 2019, 7(19), 16917–16926 CrossRef CAS.
- L. Q. Wu, K. Y. Zhang, T. T. Wang, X. B. Xu, Y. Q. Zhao, Y. Sun, W. Zhong and Y. W. Du, ACS Appl. Nano Mater., 2018, 1, 1083–1093 CrossRef CAS.
- S. Biswas, D. Mandal, T. Singh and A. Chandra, RSC Adv., 2021, 11, 30031–30039 RSC.
- L. Zuniga, V. Agubra, D. Flores, H. Campos, J. Villareal and M. Alcoutlabi, J. Alloys Compd., 2016, 686, 733–743 CrossRef CAS.
- S. Biswas, V. Sharma, D. Mandal, A. Chowdhury, M. Chakravarty, S. Priya, C. C. Gowda, P. De, I. Singh and A. Chandra, CrystEngComm, 2020, 22, 1633–1644 RSC.
- X. F. Liu, D. Wang, X. Z. Yang, Z. Z. Zhao, H. Yang, M. Feng, W. Zhang and W. T. Zheng, ACS Appl. Energy Mater., 2019, 2, 1428–1435 CrossRef CAS.
- M. M. Yin, X. T. Feng, D. Zhao, Y. Zhao, H. S. Li, W. Zhou, H. B. Liu, X. P. Bai, H. X. Wang, C. H. Feng and Q. Z. Jiao, ACS Sustainable Chem. Eng., 2019, 7, 6122–6130 CrossRef CAS.
- H. N. Li, Y. H. Zhang, B. H. Liu, Z. H. Gao, G. F. Zhao, T. T. Liu, X. Gao, S. B. Xia and H. Guo, Sustainable Energy Fuels, 2020, 4, 2208–2219 RSC.
- X. M. Guo, W. Zhang, D. Zhang, S. L. Qian, X. Z. Tong, D. C. Zhou, J. H. Zhang and A. H. Yuan, ChemElectroChem, 2019, 6, 4571–4575 CrossRef CAS.
- M. Dai and R. Wang, Small, 2021, 17, 2006813 CrossRef CAS PubMed.
- X. Chen, Q. Liu, T. Bai, W. G. Wang, F. L. He and M. D. Ye, Chem. Eng. J., 2021, 409, 127237 CrossRef CAS.
- X. R. Hu, Y. Wang, Q. S. Wu and J. F. Li, Ionics, 2022, 28, 989–1015 CrossRef CAS.
- Y. W. Yu, X. L. Hu, S. Wang, H. D. Qiao, Z. Y. Liu, K. F. Song and X. D. Shen, Nano Res., 2022, 15, 685–693 CrossRef CAS.
- X. Y. Gao, J. M. Zhang, W. Yin and X. H. Lu, NanoSelect, 2021, 2, 1642–1660 CAS.
- H. Chen, Z. H. Shen, Z. H. Pan, Z. K. Kou, X. M. Liu, H. Zhang, Q. L. Gu, C. Guan and J. Wang, Adv. Sci., 2019, 6, 1802002 CrossRef PubMed.
- J. Pu, T. T. Wang, H. Y. Wang, Y. Tong, C. C. Lu, W. Kong and Z. H. Wang, ChemPlusChem, 2014, 79, 577–583 CrossRef CAS PubMed.
- P. Wang, C. Y. Li, W. G. Wang, J. Wang, Y. S. Zhu and Y. P. Wu, Chin. Chem. Lett., 2018, 29, 612–615 CrossRef CAS.
- F. L. Zhao, W. X. Huang, H. T. Zhang and D. M. Zhou, Appl. Surf. Sci., 2017, 426, 1206–1212 CrossRef CAS.
- H. Li, F. Yue, H. T. Xie, C. Yang, Y. Zhang, L. G. Zhang and J. D. Wang, CrystEngComm, 2018, 20, 889–895 RSC.
- Y. P. Wang, T. Zhu, Y. F. Zhang, X. Z. Kong, S. Q. Liang, G. Z. Cao and A. Q. Pan, J. Mater. Chem. A, 2017, 5, 18448–18456 RSC.
- H. T. Niu, Y. Zhang, Y. Liu, B. F. Luo, N. Xin and W. D. Shi, J. Mater. Chem. A, 2019, 7, 8503–8509 RSC.
- S. J. Patil, A. C. Lokhande, J. S. Park, J. H. Kim, Y. B. Kim, B. C. Choi, S. H. Park, S. H. Jung and D. W. Lee, J. Ind. Eng. Chem., 2018, 61, 206–215 CrossRef CAS.
- T. H. Yao, Y. L. Li, D. Q. Liu, Y. P. Gu, S. C. Qin, X. Guo, H. Guo, Y. Q. Ding, Q. M. Liu, Q. Chen, J. S. Li and D. Y. He, J. Power Sources, 2018, 379, 167–173 CrossRef CAS.
- X. C. Hou, Y. Z. Zhang, Q. C. Dong, Y. Hong, Y. L. Liu, W. J. Wang, J. J. Shao, W. L. Si and X. C. Dong, ACS Appl. Energy Mater., 2018, 1, 3513–3520 CrossRef CAS.
- Z. Q. Zhu, J. H. Lin, N. Li, R. Z. Zhang, K. F. Zhang, C. H. Zhao, G. R. Chen and C. J. Zhao, J. Phys. Chem. C, 2020, 124, 83–91 CrossRef CAS.
- Z. Xiao, G. Z. Xiao, M. H. Shi and Y. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 16436–16448 CrossRef CAS PubMed.
- Y. Y. Yang, D. L. Qian, H. Zhu, Q. Zhou, Z. Y. Zhang, Z. M. Li and Z. A. Hu, J. Alloys Compd., 2022, 898, 162850 CrossRef CAS.
- Y. N. Wang, Z. S. Meng, X. L. Gong, C. Jiang, C. X. Zhang, J. Xu, Y. X. Li, J. Y. Bao, Y. N. Cui, H. B. Wang, Y. Zeng, X. Y. Hu, S. S. Yu and H. W. Tian, Chem. Eng. J., 2022, 431, 133980 CrossRef CAS.
- Z. Wang, M. L. Wang, K. He, X. X. Hang and Y. F. Bi, Chem.–Asian J., 2021, 16, 1486–1492 CrossRef CAS PubMed.
- C. Sun, L. Sun, K. F. Fan, Y. Shi, J. L. Gu, Y. F. Lin, J. J. Hu and Y. H. Zhang, Dalton Trans., 2021, 50, 9283–9292 RSC.
- X. Wei, H. Y. Wu and L. L. Li, J. Taiwan Inst. Chem. Eng., 2020, 111, 198–204 CrossRef CAS.
- X. Y. Zhang, J. J. He, L. J. Zhou, H. Z. Zhang, Q. S. Wang, B. B. Huang, X. H. Lu, Y. X. Tong and C. S. Wang, Adv. Funct. Mater., 2021, 31, 2100443 CrossRef CAS.
- P. Hu, T. S. Wang, J. W. Zhao, C. J. Zhang, J. Ma, H. P. Du, X. G. Wang and G. L. Cui, ACS Appl. Mater. Interfaces, 2015, 7, 26396–26399 CrossRef CAS PubMed.
- J. Wang, Z. J. Jia, S. B. Li, Y. Wang, W. Guo and T. Qi, Bull. Mater. Sci., 2015, 38, 1435 CrossRef CAS.
- X. W. Wang, M. X. Li, Y. F. Wang, B. W. Chen, Y. S. Zhu and Y. P. Wu, J. Mater. Chem. A, 2015, 3, 8280–8283 RSC.
- W. Liu, Y. J. Chen, Y. Y. Wang, Q. W. Zhao, L. B. Chen, W. F. Wei and J. M. Ma, Energy Storage Mater., 2021, 37, 336–344 CrossRef.
- J. J. Huang, Y. Y. Li, R. K. Xie, J. W. Li, Z. H. Tian, G. L. Chai, Y. W. Zhang, F. L. Lai, G. J. He, C. T. Liu, T. X. Liu and D. J. L. Brett, J. Energy Chem., 2021, 58, 147–155 CrossRef.
- C. S. Pan, R. G. Nuzzo and A. A. Gewirth, Chem. Mater., 2017, 29, 9351–9359 CrossRef CAS.
- H. L. Du, J. H. Lei, K. X. Xiang, W. J. Lin, J. C. Zheng and H. Y. Liao, J. Alloys Compd., 2021, 896, 162925 CrossRef.
- J. L. Li and C. G. Chen, J. Porous Mater., 2020, 27, 1447–1454 CrossRef CAS.
- Y. Jian, D. M. Wang, M. Z. Huang, H. L. Jia, J. H. Sun, X. K. Song and M. Y. Guan, ACS Sustainable Chem. Eng., 2017, 5, 6827–6834 CrossRef CAS.
- Z. Y. Lu, X. C. Wu, X. D. Lei, Y. P. Li and X. M. Sun, Inorg. Chem. Front., 2015, 2, 184–187 RSC.
- D. A. Reddy, H. Lee, M. Gopannagari, D. P. Kumar, K. Kwon, H. D. Yoo and T. K. Kim, Int. J. Hydrogen Energy, 2020, 45, 7741–7750 CrossRef.
- F. Yang, K. Zhang, Z. Cen and K. B. Xu, J. Alloys Compd., 2021, 879, 160439 CrossRef CAS.
- C. L. Teng, C. Q. Zhang, K. S. Yin, M. X. Zhao, Y. S. Du, Q. Wu and X. H. Lu, Sci. China Mater., 2022, 65, 920–928 CrossRef CAS.
- N. Li, G. M. Qu, X. X. Zhang, S. S. Zhao, C. G. Wang, G. Zhao, P. Y. Hou and X. J. Xu, Chin. Chem. Lett., 2022, 33, 3272–3276 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: the size distribution, EDX, CV curves, and GCD curves of NixCo9−xS8 hollow microspheres. The XPS peak area ratio and comparison of the supercapacitor performance. See https://doi.org/10.1039/d2ra03022e |
| This journal is © The Royal Society of Chemistry 2022 |




