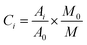Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
GC-MS combined with multivariate analysis for the determination of the geographical origin of Elsholtzia rugulosa Hemsl. in Yunnan province
Chaopei Zhengac,
Sifeng Yangbc,
Dequan Huangbc,
Deshou Maoc,
Jianhua Chenc,
Chengming Zhangc,
Weisong Kongc,
Xin Liuc,
Yong Xuc,
Yiqin Wuc,
Zhengfeng Lic,
Jin wang *c and
Yanqing Ye*a
*c and
Yanqing Ye*a
aCollege of Chemical and Environment, Yunnan Minzu University, Kunming 650500, China. E-mail: yey-qing@163.com
bCollege of Chinese National Medicine, Yunnan Minzu University, Kunming 650500, China
cResearch and Development Center, China Tobacco Yunnan Industrial Co., Ltd, Kunming, 650231, China. E-mail: wangjin@iccas.ac.cn
First published on 2nd August 2022
Abstract
Elsholtzia rugulosa Hemsl., a Chinese herbal medicine, may have the potential to treat COVID-19. The geographical origin has a significant influence on the quality and application of E. rugulosa. In this paper, gas chromatography-mass spectrometry (GC-MS) combined with principal component analysis (PCA) and hierarchical cluster analysis (HCA) and other multivariate statistical analyses were performed for the identification of E. rugulosa. origins. The results showed that the volatile components of E. rugulosa. from different origins were significantly different. PCA and HCA can clearly distinguish the E. rugulosa of Lijiang and Fumin, and Dali and Yongsheng can be distinguished but with a certain overlap. The correlation of different components of was investigated by Pearson correlation. The results showed that E. rugulosa. characteristic component Elsholtzia ketone is regulated by terpenoid metabolism. The discriminant functions of different origins are constructed by Fisher stepwise discrimination, and its initial verification accuracy and leave-one-out cross-validation accuracy were 100% and 87.5%, respectively.
1. Introduction
At present, the Corona Virus Disease 2019 (COVID-19) is a global pandemic, and finding a suitable special medicine is an urgent problem to be solved. Elsholtzia rugulosa Hemsl. has been shown to have good antiviral, anti-influenza, and anti-inflammatory effects and may have the potential to treat and alleviate COVID-19.1–6,9,16 E. rugulosa is a medicine and food homologous plant with a special fragrance.1,2 It is known as a local herbal tea, medicinal herb, and honey plant, mainly distributed in Southwest China, especially in Yunnan Province.3–7 In this region, the local ethnic minorities use it to treat colds, fever, flu, and diarrhea.4–10 In addition to the above-mentioned medicinal functions, recent studies have shown that many compounds isolated from E. rugulosa have anti-cancer and even relieve Alzheimer's symptoms.4–18 Through phytochemical methods, a variety of terpenoids, polyphenols, flavonoids, and other non-volatile components were identified, such as anti-inflammatory triterpene ursolic acid,16,18 antioxidant polyphenol rosmarinic acid,26 anti-influenza and anti-Alzheimer's flavonoid Luteolin,11,12 etc. In the essential oil of E. rugulosa, β-dehydroelsholtzia ketone and Elsholtzia ketone were identified. In addition, various volatile medicinal components such as linalool,27–29 piperitone,30,31 isospathulenol,31,32 β-bourbonene,34 caryophyllene,35 α-humulene,32–36 spathulenol, artemisyl ketone,34–36 etc. have been identified from the 21 species of Elsholtzia. As a herbal medicine, the safety, authenticity, and efficacy of E. rugulosa are all affected by the geographical origin. Therefore, it is necessary to trace the origin.Origin traceability is an essential guarantee for the authenticity of the product, and the rights and interests of consumers. There are many research reports on the origin traceability of agricultural products such as wine, cheese, beef, rice, and wheat.19–22 These techniques of origin traceability include isotope analysis, multielement analysis, infrared spectroscopy, LC-MS, DNA method, etc. Gas chromatography-mass spectrometry (GC-MS) has good reproducibility, high sensitivity, and excellent separation and identification of volatile components.23 The GC-MS determination of volatile components can be used to make a preliminary evaluation of the medicinal efficacy of herbal medicines and further combined with chemometric methods can realize the traceability of its origin.
The primary aim of this research was to trace the origin of E. rugulosa from different origins. For the first time, GC-MS analysis technology, combined with multivariate statistical methods such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and correlation analysis were used to analyze the volatilization of E. rugulosa from different origins. The E. rugulosa geographical origin discriminant functions were constructed by linear discriminant analysis. They were verified by initial validation and leave-one-out cross-validation (LOOCV).
2. Materials and methods
2.1 Reagents and chemicals
The HPLC-grade solvents methanol, chloroform, dichloromethane, ethyl acetate, and n-hexane were purchased from Beijing Chemical Reagent Company. Deuterated toluene (d8, >97%) as an internal standard was obtained from Sigma-Aldrich. Ultrapure water was prepared by a Milli-Q Water System (Millipore, Billerica, USA).2.2 Sample collection and preparation
The 16 E. rugulosa flower samples were collected from 4 different county of yunnan province in 2018–2019, including 4 from Fuming county (25° 22′N, 102° 34′E, 1685 m a.s.l), 4 from Dali county-level city (25° 42′N, 99° 93′E, 1976 m a.s.l), 4 from Yongsheng county (26° 32′N, 100° 66′E, 2140 m a.s.l), and 4 from Lijiang county (26° 86N, 100° 25′E, 2418 m a.s.l), with the geographic data from https://www.geodata.cn/. The locations of 4 sampling areas are marked on the Yunnan map shown in Fig. 1.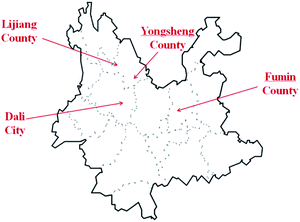 | ||
| Fig. 1 Geographical origins of the E. rugulosa samples from Yunnan Province: Lijiang (LJ), Yongsheng (YS), Fumin (FM), and Dali (DL). | ||
Samples were collected in the fall. All samples were dried in the sun and ground to obtain the powder.
2.3 GC-MS analysis
0.1 g ground sample was weighed in 10 mL centrifuge tube and 1.5 mL of 1 μg mL−1 deuterated toluene ethyl acetate solution was added. Then, the mixture was ultrasonically treated for 10 min, centrifuged at 4000 rpm for 8 min, filtered, 500 μL supernatant was transferred to a chromatography injection vial for the GC-MS test.Gas chromatography (TRACE 1310, Thermo Scientific, USA) coupled with mass spectrometry (ISQ 7000, Thermo Scientific, USA) was employed to evaluate the volatile components in the sample. The column used was a DB-35 GC column (30 m × 0.25 mm × 0.25 μm) (Agilent, USA). The inlet temperature was 280 °C, and the helium gas flow rate through the column was 1 mL min−1. The injection volume was 1 mL, and the split ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The initial oven temperature was 80 °C, heated to 275 °C at a rate of 60 °C min−1, then raised to 295 °C at a rate of 1 °C min−1, and held for 1 min. The transfer line and the ion source temperatures were 300 and 280 °C, respectively. The ionization mode was the electron impact at 70 eV. The solvent delay was 2 min.
1. The initial oven temperature was 80 °C, heated to 275 °C at a rate of 60 °C min−1, then raised to 295 °C at a rate of 1 °C min−1, and held for 1 min. The transfer line and the ion source temperatures were 300 and 280 °C, respectively. The ionization mode was the electron impact at 70 eV. The solvent delay was 2 min.
2.4 Data pre-treatment and statistical analysis
The data pre-treatment is preprocessed as follows:31,35,36Among them: Ci represents the content of the measured odor components; Ai is the peak area of each compound; A0 represents the peak area of the internal standard substance; M0 represents the mass of the internal standard substance; M is the mass of the sample taken. The data is then used for statistical analysis.
Analysis of variance (ANOVA) was carried out for each element using SPSS 22.0 software (IBM, US). The significance level was P < 0.05. And the SPSS 22.0 is also used to correlation analysis, Fisher Discriminant analysis, initial validation, LOOCV. PCA multivariate analysis was performed by SIMCA-P 11 (Sartorius Stedim, Germany). Hierarchical cluster analysis (HCA), Heatmap and other plots were used OriginPro 2017 (OriginLab, US).
3. Results and discussion
3.1 GC/MS analysis
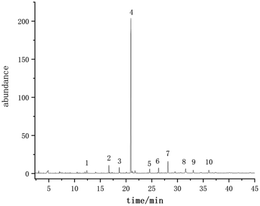
The volatile compounds of E. rugulosa flowers from 4 different regions were evaluated by GC-MS. The total ion chromatogram is shown in Fig. 2. Volatile compounds were identified by the comparison of the mass spectra with the commercial mass spectra libraries (NIST27 and WILEY7). Nearly 80 volatile components were separated and determined, and the data were analyzed by ANOVA, and the results are shown in Table 1. Among them, Elsholtzia ketone, linalool, artemisyl ketone, humulenol, isospathulenol, andrographolide, and many chemical components isolated from E. rugulosa have bio-medical activities such as anti-cancer, anti-inflammatory, anti-oxidation, anti-malarial, and anti-influenza virus.5–18 These compounds are also the material basis of the medicinal effect of E. rugulosa. It can be seen from Table 1 that under the significance level P < 0.05, the differential volatile components of E. rugulosa in the four origins reach more than 50, indicating that the origin has a great influence on the volatile components of E. rugulosa. In addition to E. rugulosa. characteristic components Elsholtzia ketone and β-Dehydroelsholtzia ketone, a variety of biologically active or medicinal components are also identified, such as artemisyl ketone, linalool, piperitone, isospathulenol, β-bourbonene, dimethyl-heptadienone, caryophyllene, α-humulene, spathulenol, (1R,7S,E)-7-isopropyl-4,10-dimethylenecyclodec-5-enol, andrographolide, thunbergol, squalene, β-sitosterol, and so on.| RT (min) | Compounds | Lijiang (μg g−1) | Dali (μg g−1) | Yongsheng (μg g−1) | Fumin (μg g−1) | F | P-Value |
|---|---|---|---|---|---|---|---|
| a The data are expressed as the mean ± the standard deviation.b "—" Not detected. | |||||||
| 3.23 | 2,4-Dimethyl-1-heptene | 0.56 ± 0.13 | 0.25 ± 0.25 | 0.21 ± 0.06 | 0.38 ± 0.25 | 2.49 | 0.11 |
| 3.63 | 3-Methyl-butanoic acid | 2.34 ± 0.23 | 0.3 ± 0.3 | 0.28 ± 0.03 | 5.23 ± 0.35 | 175.99 | 0.00 |
| 4.96 | 3-Methyl-2-butenoic acid | 11.18 ± 2.28 | 13.32 ± 2.92 | 6.17 ± 5.06 | 7.03 ± 0.91 | 1.77 | 0.21 |
| 5.43 | Furan | 1 ± 0.07 | 1.03 ± 0.16 | 0.42 ± 0.43 | 0.86 ± 0.11 | 1.74 | 0.21 |
| 6.09 | 3-Methyl-2(5H)-furanone | 3.49 ± 0.37 | 3.97 ± 1.05 | 1.8 ± 1.56 | 1.85 ± 0.13 | 5.93 | 0.01 |
| 7.14 | 2-Methylpropyl 3-methylbutanoate | — | — | — | 1.85 ± 0.06 | 2478.50 | 0.00 |
| 8.8 | trans-Linalool oxide (furanoid) | 1.65 ± 0.12 | 2.35 ± 0.56 | 1.01 ± 0.96 | 2.13 ± 0.08 | 3.22 | 0.06 |
| 8.8 | (2R,5S)-linalool oxide(5) acetate | 1.87 ± 0.12 | 1.74 ± 1.15 | 1 ± 0.67 | 2.67 ± 0.12 | 1.95 | 0.17 |
| 9.65 | l-Linalool | 0.81 ± 0.07 | 1.92 ± 0.54 | 0.84 ± 0.79 | 0.78 ± 0.02 | 15.70 | 0.00 |
| 10.81 | 2-Methyl-2-propenoic acid | 2.56 ± 0.34 | 5.24 ± 0.97 | 2.18 ± 2.18 | 2.24 ± 0.21 | 18.09 | 0.00 |
| 11.55 | Linalool oxide (pyranoid) | 1.1 ± 0.11 | 2.41 ± 0.34 | 0.95 ± 1.03 | 1.07 ± 0.05 | 17.89 | 0.00 |
| 12.08 | Benzoic acid, 2-hydroxy-, methyl ester | 1.22 ± 0.11 | 0.99 ± 0.19 | 0.43 ± 0.4 | 0.92 ± 0.26 | 1.55 | 0.25 |
| 12.23 | 2-Cyano-2-methyl-1-cyclobutanol | 0.93 ± 0.1 | 1.82 ± 0.42 | 0.78 ± 0.75 | — | 29.29 | 0.00 |
| 12.24 | Artemisyl ketone | 3.32 ± 1.93 | 6.57 ± 0.83 | 3.11 ± 2.49 | 2.34 ± 0.22 | 13.25 | 0.00 |
| 12.39 | Elsholtzia ketone | 7.92 ± 1.04 | 3.12 ± 0.72 | 1.63 ± 1.06 | 42.08 ± 2.33 | 564.41 | 0.00 |
| 13.84 | Piperitone | 4.74 ± 0.25 | 12.6 ± 2.44 | 5.1 ± 5.38 | 4.33 ± 0.19 | 26.57 | 0.00 |
| 15.35 | β-Dehydroelsholtzia ketone | 101.9 ± 5.79 | 107.73 ± 18.27 | 43.93 ± 45.4 | 50.38 ± 2.92 | 13.90 | 0.00 |
| 15.91 | 4-Acetyl-1-carbamoyl-5-methylpyrazole | 18.16 ± 0.99 | 21.76 ± 2.95 | 8.57 ± 9.36 | 7.91 ± 0.6 | 28.80 | 0.00 |
| 17.67 | β-Bourbonene | 0.72 ± 0.42 | 1.15 ± 0.3 | 0.62 ± 0.38 | 0.73 ± 0.07 | 0.82 | 0.51 |
| 17.69 | Dimethyl – heptadienone | 3.61 ± 0.27 | 6.8 ± 0.91 | 2.66 ± 2.94 | 1.75 ± 0.17 | 36.18 | 0.00 |
| 18.47 | Methyl 5-hydroxyiminopentanoate | 1.1 ± 0.08 | — | 0.03 ± 0.04 | 2.93 ± 0.26 | 313.16 | 0.00 |
| 18.61 | Caryophyllene | 15.09 ± 0.95 | 14.86 ± 2.49 | 6.1 ± 6.23 | 25.05 ± 2.08 | 16.68 | 0.00 |
| 18.88 | Hepta-2,4-dienoic acid, methyl ester | 1.03 ± 0.08 | 1.55 ± 0.25 | 0.63 ± 0.66 | — | 52.42 | 0.00 |
| 19.56 | α-Humulene | 3.11 ± 0.21 | 2.79 ± 0.42 | 1.14 ± 1.17 | 4 ± 0.38 | 6.73 | 0.01 |
| 20.63 | Bicyclogermacrene | — | 0.28 ± 0.49 | 0.26 ± 0.2 | 0.85 ± 0.1 | 2.71 | 0.09 |
| 21.84 | 1-(2-Methoxyethenyl)cyclopropane | 58.78 ± 2.18 | 86.98 ± 10.21 | 33.12 ± 38.22 | 28.58 ± 2.63 | 51.04 | 0.00 |
| 22.62 | (+) Spathulenol | 3.31 ± 0.21 | 6.49 ± 0.76 | 2.49 ± 2.84 | 2.87 ± 0.35 | 43.35 | 0.00 |
| 22.71 | (−)-Caryophyllene oxide | 4.76 ± 0.2 | 7.33 ± 0.88 | 2.8 ± 3.21 | 3.19 ± 0.33 | 43.20 | 0.00 |
| 23.19 | 2-[2-H3]Methylpyrazine | 2.35 ± 0.2 | 3.69 ± 0.5 | 1.46 ± 1.58 | 0.9 ± 0.13 | 60.23 | 0.00 |
| 23.38 | (−)-Humulene epoxide II | 1.04 ± 0.05 | 1.4 ± 0.19 | 0.55 ± 0.61 | 2.07 ± 0.19 | 17.98 | 0.00 |
| 23.96 | Humulenol-II | 2.41 ± 1.43 | 3.72 ± 0.68 | 1.94 ± 1.29 | 0.81 ± 0.5 | 3.17 | 0.06 |
| 24.03 | 11,11-Dimethyl-4,8-dimethylenebicyclo[7.2.0]undecan-3-ol | 1.33 ± 0.09 | 2.47 ± 0.28 | 0.95 ± 1.08 | 0.67 ± 0.47 | 17.32 | 0.00 |
| 24.48 | Isoaromadendrene epoxide | 4.43 ± 0.27 | 6.64 ± 0.84 | 2.58 ± 2.88 | 2.13 ± 0.23 | 45.55 | 0.00 |
| 24.82 | (1R,7S,E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol | 5.37 ± 0.26 | 8.52 ± 0.95 | 3.24 ± 3.74 | 2.1 ± 0.23 | 77.36 | 0.00 |
| 24.95 | 4-Isopropenyl-4,7-dimethyl-1-oxaspiro[2.5]octane | 8.81 ± 0.42 | 8.02 ± 0.91 | 3.12 ± 3.47 | 5.7 ± 0.52 | 13.99 | 0.00 |
| 25.14 | 6-Heptyl-2,3,4,5-tetrahydropyridine | 1.21 ± 0.1 | 2.2 ± 0.41 | 0.9 ± 0.93 | — | 48.81 | 0.00 |
| 25.88 | 1,1,4,7-Tetramethyldecahydro-1H-cyclopropa[e]azulene-4,7-diol | 1.71 ± 0.11 | 3.46 ± 0.44 | 1.34 ± 1.51 | 1.59 ± 0.17 | 37.71 | 0.00 |
| 26.21 | Nerolidol-epoxyacetate | 7.67 ± 0.13 | 5.28 ± 0.64 | 2.02 ± 2.32 | 4.7 ± 2.14 | 3.65 | 0.04 |
| 26.26 | Isospathulenol | 0.25 ± 0.25 | 0.88 ± 0.22 | 0.45 ± 0.3 | 1.7 ± 2.62 | 0.61 | 0.62 |
| 26.47 | Cholestan-3-ol, 2-methylene-, (3β,5α)- | 1.98 ± 0.13 | 1.64 ± 0.21 | 0.66 ± 0.69 | 1.07 ± 0.15 | 14.24 | 0.00 |
| 26.79 | (1R,2S,4S,5R,7R)-5-Isopropyl-1-methyl-3,8-dioxatricyclo[5.1.0.02,4]octane | 1.31 ± 0.14 | 1.07 ± 0.19 | 0.47 ± 0.43 | — | 32.68 | 0.00 |
| 26.99 | ((4-Methoxyphenyl)dimethylacetyl)pyrrolidine | — | 1.27 ± 0.52 | 0.6 ± 0.52 | — | 21.51 | 0.00 |
| 27.1 | (−)-Isolongifolol, methyl ether | 2.61 ± 0.3 | 2.47 ± 0.08 | 0.95 ± 1.08 | 1.17 ± 0.14 | 24.26 | 0.00 |
| 27.66 | cis-Z-α-bisabolene epoxide | 1.59 ± 0.14 | 1.85 ± 0.36 | 0.78 ± 0.76 | 1.09 ± 0.14 | 6.20 | 0.01 |
| 27.84 | Diepicedrene-1-oxide | 12.37 ± 0.68 | 11.98 ± 1.29 | 4.65 ± 5.19 | 9.95 ± 0.91 | 4.48 | 0.02 |
| 28.44 | α-Kessyl acetate | 1.2 ± 0.13 | 0.8 ± 0.15 | 0.36 ± 0.31 | 0.75 ± 0.44 | 2.16 | 0.15 |
| 28.63 | 6,10,14-Trimethyl-2-Pentadecanone | 2.84 ± 0.14 | 1.94 ± 0.3 | 0.79 ± 0.81 | 1.13 ± 0.34 | 23.32 | 0.00 |
| 29.18 | 4,4,8-Trimethyltricyclo[6.3.1.0(1,5)]dodecane-2,9-diol | 9.68 ± 0.56 | 10.73 ± 1.3 | 4.2 ± 4.63 | 7.21 ± 0.69 | 11.97 | 0.00 |
| 30.85 | 3-Methyl-2-butenoic acid, cyclobutyl ester | 0.4 ± 0.23 | 1.16 ± 0.28 | 0.56 ± 0.43 | — | 22.58 | 0.00 |
| 31.11 | Hexadecanoic acid | 17.75 ± 1.33 | 13.37 ± 1.87 | 5.52 ± 5.55 | 6.04 ± 0.93 | 28.71 | 0.00 |
| 32.93 | Oxalic acid, cyclohexyl decyl ester | 1.69 ± 0.6 | 1.19 ± 0.05 | 0.61 ± 0.47 | — | 16.62 | 0.00 |
| 33.31 | Carbonic acid, eicosyl vinyl ester | — | 0.13 ± 0.22 | 0.12 ± 0.09 | 0.76 ± 0.46 | 4.03 | 0.03 |
| 33.46 | N-Methyl-3-azabicyclo[3.1.0]hex-1-ylamine | — | 0.2 ± 0.34 | 0.18 ± 0.14 | — | 4.24 | 0.03 |
| 34.12 | (Z)-18-Octadec-9-enolide | 6.32 ± 0.63 | 2.97 ± 1.87 | 1.82 ± 0.96 | 1.53 ± 0.46 | 10.89 | 0.00 |
| 34.23 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | 10.37 ± 0.9 | 4.19 ± 0.87 | 1.99 ± 1.56 | 2.88 ± 1.06 | 36.47 | 0.00 |
| 34.75 | Octadecanoic acid | 2.74 ± 0.3 | 1.66 ± 0.34 | 0.77 ± 0.63 | 0.68 ± 0.08 | 24.98 | 0.00 |
| 34.87 | Tetradecanamide | 1.08 ± 0.27 | 1.19 ± 0.36 | 0.61 ± 0.41 | 2.84 ± 2.46 | 1.23 | 0.34 |
| 34.93 | Hexadecanamide | 4.07 ± 0.83 | 4.17 ± 0.99 | 2 ± 1.54 | 3.56 ± 2.19 | 0.49 | 0.70 |
| 35.53 | Andrographolide | 0.17 ± 0.29 | 1.05 ± 0.23 | 0.52 ± 0.37 | — | 22.60 | 0.00 |
| 37.81 | Ethyl linoleate | 2.2 ± 0.84 | 2.6 ± 0.96 | 1.47 ± 0.8 | 3.63 ± 1.17 | 1.24 | 0.34 |
| 37.93 | 9-Octadecenamide | 33.31 ± 7.63 | 34.99 ± 8.27 | 16.96 ± 12.75 | 47.42 ± 11.1 | 1.40 | 0.29 |
| 39.29 | Thunbergol | 1.31 ± 0.09 | 1.81 ± 0.34 | 0.75 ± 0.76 | 0.84 ± 0.11 | 16.51 | 0.00 |
| 39.71 | 2-Methylhexacosane | 3.89 ± 0.49 | 1.13 ± 0.11 | 0.58 ± 0.42 | 0.98 ± 0.15 | 65.93 | 0.00 |
| 40.29 | Docosane | 1.57 ± 2.72 | 2.98 ± 1.82 | 2.51 ± 0.5 | 2.51 ± 1.48 | 0.45 | 0.72 |
| 40.3 | Triacontane | 0.67 ± 0.39 | — | 0.13 ± 0.18 | — | 8.89 | 0.00 |
| 40.31 | Heptacosane | 7.83 ± 0.74 | 5.9 ± 0.87 | 2.5 ± 2.4 | 6.5 ± 2.43 | 1.13 | 0.38 |
| 41.39 | 3-Ethyl-tetracosane | 0.79 ± 0.07 | — | 0.02 ± 0.03 | — | 368.24 | 0.00 |
| 41.65 | 2-Methyl-eicosane | 1.36 ± 0.09 | — | 0.03 ± 0.04 | — | 623.81 | 0.00 |
| 42.27 | Henicosanal | 0.35 ± 0.6 | 0.9 ± 0.55 | 0.68 ± 0.15 | — | 2.33 | 0.13 |
| 42.72 | 11-Decyl-tetracosane | 3.71 ± 0.29 | 1.46 ± 0.9 | 0.88 ± 0.48 | 1.24 ± 0.16 | 16.08 | 0.00 |
| 42.74 | 11-Decyl-docosane | — | — | — | 1.74 ± 0.27 | 7.67 | 0.00 |
| 43.28 | Celidoniol, deoxy- | 2.68 ± 0.15 | 1.54 ± 0.31 | 0.67 ± 0.62 | 2.99 ± 0.32 | 16.81 | 0.00 |
| 43.28 | Pentacosane | 7.84 ± 0.5 | 4.37 ± 0.68 | 1.85 ± 1.78 | 6.31 ± 0.89 | 14.71 | 0.00 |
| 43.55 | β-Sitosterol | 5.06 ± 1.04 | 2.75 ± 2.46 | 2.08 ± 0.75 | 0.97 ± 1.04 | 3.32 | 0.06 |
| 43.89 | Hentriacontane | 39.09 ± 19.15 | 29.63 ± 17.41 | 22.06 ± 5.4 | 40.13 ± 23.5 | 0.17 | 0.91 |
| 44.22 | 13-Docosenamide, (Z)- | 29.01 ± 1.19 | 31.4 ± 5.96 | 12.85 ± 13.26 | 33.39 ± 1.51 | 0.98 | 0.43 |
| 44.79 | Squalene | 2.71 ± 0.47 | — | 0.16 ± 0.22 | — | 100.12 | 0.00 |
| 45.78 | 2-Methyl-octacosane | 5.77 ± 0.37 | 2.97 ± 0.59 | 1.31 ± 1.18 | 4.41 ± 0.96 | 9.01 | 1.0 |
Under the high significance of difference level (F > 50, P < 0.05), the E. rugulosa pharmaceutical components with the highest relative content of the four origins were Elsholtzia ketone in Fumin, (1R,7S,E)-7-isopropyl-4,10-dimethylenecyclodec-5-enol in Dali, squalene in Lijiang, and these compounds can be labeled as origin characteristic medicinal components. However, in this condition, Yongsheng E. rugulosa has no pharmaceutical components whose content is significantly bigger than that of other origins.
3.2 PCA and HCA analysis
PCA is an unsupervised classification method, which can objectively and directly reflect the classification of samples.37 It is commonly used as a reduction analysis method in multi-statistical analysis. PCA was performed for 4 different geo-origins of E. rugulosa using the Simca, and the results are shown in Fig. 3. As shown in Fig. 3, PCA first primary component variance interpretation rate is 47.2%, and the second primary component is 21.3%. Its total variance interpretation rate was 78.5%. As seen in the PCA plots, Fumin and Lijiang can be well distinguished when clustered together. However, Dali and Yongsheng can be partially distinguished, and the E. rugulosa of the two origins are very close.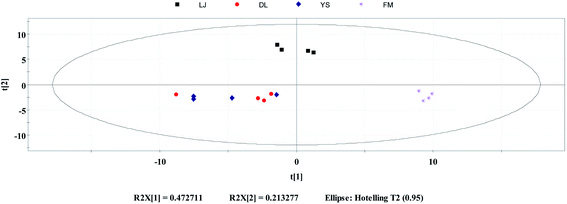 | ||
| Fig. 3 Principal component analysis: distribution of E. rugulosa samples from 4 origins in Yunnan Province. | ||
It shows that the differences in volatile components of Fumin and Lijiang E. rugulosa can be clearly distinguished by PCA, while the difference of Dali and Yongsheng components is small. It may be due to their geographical and climatic characteristics. Fumin has a typical subtropical mountain monsoon climate with the lowest altitude, while Lijiang has a low-latitude warm temperate plateau monsoon climate with the highest altitude, so these two origins of E. rugulosa have obvious characteristics, respectively.25,26 Dali and Yongsheng belong to the transition from the subtropical mountain monsoon climate to the low-latitude warm temperate mountain monsoon climate, and the altitudes are relatively close.25,26 Therefore, the E. rugulosa characteristics of the two regions are relatively similar, and the difference is not obvious.
In terms of administrative division, Lijiang county and Yongsheng county are under the jurisdiction of Lijiang Autonomous Prefecture, while Dali county is under the jurisdiction of Dali Autonomous Prefecture. At the same time, Yongsheng E. rugulosa has similar characteristics to Dali, but is different from Lijiang county, indicating that the plants' characteristics are mainly influenced by geographical and climatic rather than administrative divisions.
The chemical composition of plant, indeed, is partially related to geographical and climatic conditions that are different among different geographical areas.24,38,39 Geographical and climatic conditions affect plants directly via the regulation of their biosynthetic pathways or indirectly via their effects on vine physiology and phenology.38 Ahmed found that the seasonality, water, geography, light factors, altitude, and temperature can cause 50% variation in secondary metabolites.39 Rienth research on grapes showed that temperature, water, and solar radiation govern the synthesis and degradation of primary (sugars, amino acids, organic acids, etc.) and secondary (phenolic and volatile flavor compounds and their precursors) metabolites.38 In the future, the influence of various geographical and climatic conditions on the metabolites of E. rugulosa will be systematically studied.
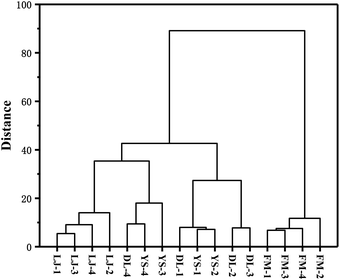
HCA is also an unsupervised objective classification method.37 It is a method of classifying samples according to the degree of similarity, which reflects the implicit similarity between samples. It combines the most similar samples together according to the degree of similarity between observations or variables and clusters the samples in a successive aggregation manner until all samples are finally clustered into one class. Generally, the smaller the critical value, the more similar the samples are.37 HCA analysis was carried out using OriginPro 2017 for E. rugulosa of 4 different origins. As shown in Fig. 3, when the critical value is between 45.2 and 89.3, Fumin E. rugulosa can be distinguished from the other three types of yebazi. When the critical value is between 14.3 and 18.4, Lijiang E. rugulosa can be distinguished. When the critical value is between 18.4 and 45.2, Dali and Yongsheng E. rugulosa can be divided into two types, but there is a certain intersection. Therefore, the two groups of Fumin and Lijiang E. rugulosa were well separated and clustered into two categories, respectively. In contrast, the Dali and Yongsheng E. rugulosa were poorly separated and clustered into three categories with a certain crossover. The results were consistent with the PCA results. In addition, it can be seen from Fig. 4 that the distance between Fumin and the other three origins is the largest, and the difference is the most obvious. The Lijiang samples are closer to the samples of Dali and Yongsheng, and the samples are more similar.
3.3 Correlation analysis
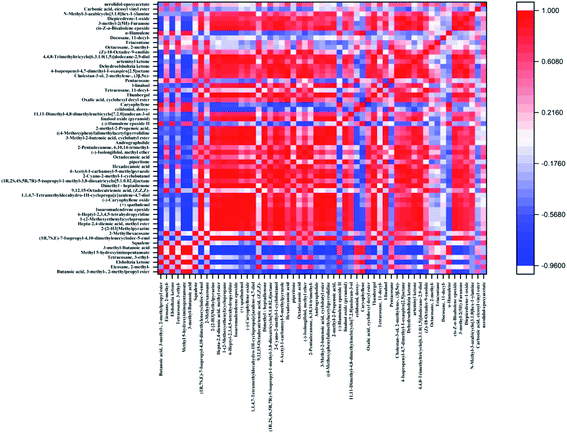
The correlation analysis of different components in E. rugulosa is of great significance for studying the correlation, metabolic pathway, and metabolic network among E. rugulosa metabolites. It can be used to understand the influence of geographical and climatic conditions on metabolites and the interaction process of different metabolites.In this paper, the Pearson correlation coefficient between the compounds with a significance level of P < 0.05 was calculated, and the results are shown in Fig. 5. The absolute value of the correlation coefficient is limited to be above 0.85, and the p < 0.05, as the significant correlation condition. Under this condition, a total of 57 compounds, composed of 1596 pairs of compounds, of which 341 compound pairs with significant correlation were found, 300 pairs were positively correlated, and 41 pairs were negatively correlated (Fig. 5). In general, the positively correlated metabolite pairs have similar chemical composition, biological function, and homogeneous characteristics. Among them, the characteristic components of E. rugulosa Elsholtzia ketone are positively correlated with 2-methylpropyl 3-methylbutanoate, methyl 5-hydroxyiminopentanoate, 3-methyl-butanoic acid, caryophyllene, and negatively correlated with (1R,7S,E)-7-isopropyl-4,10-dimethylenecyclodec-5-enol, isoaromadendrene epoxide, (−)-isolongifolol, beta-dehydroelsholtzia ketone and so on. Elsholtzia ketone and 3-methyl-butanoic acid (hemiterpene derivatives), 3-methyl-butanoic acid (hemiterpene derivatives), caryophyllene (sesquiterpene) are all terpenoid metabolites and positively affected by terpenoid biosynthesis.16 Dehydroelsholtzia ketone may be obtained by dehydrogenation of Elsholtzia ketone, so they are negatively correlated. Therefore, Elsholtzia ketone is regulated by terpenoid metabolism. The results show that different geographical conditions have a great impact on terpenoid metabolic pathways.
3.4 E. rugulosa origin traceability functions and verification
From the above analysis, it can be seen that the volatile compounds of E. rugulosa from different origins have certain characteristics. Fisher discriminant is established based on the idea of variance.40 The principle is to minimize the variance between groups and maximize the variance between groups. Fisher discrimination is the most commonly used classic discrimination method. Compared with “black box” discrimination such as neural network and fuzzy mathematics, etc., Fisher discrimination is “white box” discrimination. It can give the most influential components and their relative weights.40 Therefore, it is often used in the discriminant analysis of samples.To further trace the origin of E. rugulosa from different origins, we constructed the origin traceability functions using the Fisher discriminant analysis. The GC-MS data were imported into the SPSS software, followed by step-by-step analysis and build the Fisher discrimination functions. Final screening of nine compounds as the representative function variable components are shown in Table 2.
| Compounds | Geo-Origin | |||
|---|---|---|---|---|
| DL | FM | LJ | YS | |
| 2-Methylpropyl 3-methylbutanoate | −10110.886 | 740![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116.143 116.143 |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 993.793 993.793 |
−26440.045 |
| Artemisyl ketone | 130.395 | −8997.888 | −939.531 | 334.505 |
| Methyl 5-hydroxyiminopentanoate | 1851.893 | −139925.356 | −14677.245 | 4740.107 |
| Humulenol-II | −75.955 | 4919.775 | 441.177 | −206.877 |
| Isospathulenol | −65.962 | 4431.237 | 429.954 | −165.276 |
| 2-Pentadecanone, 6,10,14-trimethyl- | −293.419 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916.656 916.656 |
2536.984 | −828.038 |
| Carbonic acid, eicosyl vinyl ester | −125.041 | 8120.202 | 682.405 | −339.309 |
| Eicosane, 2-methyl- | −2480.404 | 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451.104 451.104 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678.870 678.870 |
−6246.729 |
| Octacosane, 2-methyl- | 121.254 | −7925.902 | −707.265 | 337.847 |
| Constants | −147.078 | −474916.482 | −5953.955 | −787.601 |
The results of the functions are shown in Table 2. The four discriminant functions formed by these 9 indicators have variances of 82.0%, 13.5%, 3.4%, and 1.1% of the total variance, respectively, and the cumulative variance explanation rate is 100%. The resulting Fisher discriminant functions were the E. rugulosa origin discriminant function of 4 origins. In practical application, it is only necessary to substitute the contents of the above 9 indicators detected in the blind sample into the above functions, and the largest function value is the origin of the sample.
The validity of the model was verified by initial validation and LOOCV, and the results are shown in Table 3. In the initial verification results, all E. rugulosa from the four origins were correctly classified, and the initial validation accuracy was 100%. The initial verification training set and the verification set are the same set, and its results have a certain bias, and the accuracy rate is relatively high. Therefore, it is necessary to verify it with LOOCV.41,42
| Method | Statistics | Geo-Origin | Predicted Geo-Origin | ||||
|---|---|---|---|---|---|---|---|
| DL | LJ | YS | FM | total | |||
| a The total initial determination accuracy is 100%.b The total LOOCV accuracy rate is 87.5%. | |||||||
| Initial verificationa | Number | DL | 4 | 0 | 0 | 0 | 4 |
| LJ | 0 | 4 | 0 | 0 | 4 | ||
| YS | 0 | 0 | 4 | 0 | 4 | ||
| FM | 0 | 0 | 0 | 0 | 4 | ||
| Accuracy% | DL | 100 | 0 | 0 | 0 | 100 | |
| LJ | 0 | 100 | 0 | 0 | 100 | ||
| YS | 0 | 0 | 100 | 0 | 100 | ||
| FM | 0 | 0 | 0 | 100 | 100 | ||
| LOOCVb | Number | DL | 3 | 0 | 1 | 0 | 4 |
| LJ | 0 | 4 | 0 | 0 | 4 | ||
| YS | 1 | 0 | 3 | 0 | 4 | ||
| FM | 0 | 0 | 0 | 4 | 4 | ||
| Accuracy% | DL | 75 | 0 | 25 | 0 | 100 | |
| LJ | 0 | 100 | 0 | 0 | 100 | ||
| YS | 25 | 0 | 75 | 0 | 100 | ||
| FM | 0 | 0 | 0 | 100 | 100 | ||
Leave-one-out cross-validation (LOOCV) is a special case of cross-validation. In each validation, nearly all the data except for a single observation are used for training, and the model is tested on that single observation. An accuracy estimate obtained using LOOCV is known to be almost unbiased. It is widely used when the available data are very rare.41,42 In the LOOCV results as shown in Table 3, the E. rugulosa of Lijiang and Fumin were all correctly classified, and the LOOCV accuracy was 100%; while the E. rugulosa of Dali, three were classified as Dali and one was classified as Yongsheng, and the LOOCV accuracy was 75%; E. rugulosa of Yongsheng, three classifications are Yongsheng, one is classified as Dali, and the LOOCV accuracy was 75%; the overall leave-one-out cross-validation accuracy rate of the model was 87.5%.
The analysis results of LOOCV were consistent with the results of PCA and HCA, which may be caused by the similarity of the volatile components of Yongsheng and Dali E. rugulosa. It indicates that the GC-MS combined with multivariate statistical analysis for origin traceability has certain limitations, namely, when the geographical climate of the origin is similar and then result in the difference of volatile herbal volatile components may not be obvious, the classification is prone to misjudgment. However, herbal medicines with insignificant differences in chemical composition from different origins also have small differences in efficacy. Therefore, this misjudgment of origin has a limited impact on the efficacy and use of Chinese herbal medicines.
To sum up, the E. rugulosa origin traceability based on Fisher's stepwise discriminant analysis, the initial verification accuracy was 100%, and LOOCV accuracy was 87.5%, which can realize the identification of most E. rugulosa origins.
4. Conclusions
In this study, volatile components were determined in 16 E. rugulosa flower samples from 4 regions including Lijiang, Dali, Yongsheng, and Fumin. The GC-MS data were analyzed using ANOVA. The results showed that more than 17 active ingredients were identified in E. rugulosa. The origin characteristic medicinal components of E. rugulosa are Elsholtzia ketone in Fumin, (1R, 7S, e)-7-isopropyl-4,10-dimethylenecyclodec-5-enol in Dali, squalene in Lijiang, respectively, while Yongsheng has no obvious high medicinal components. PCA and HCA analysis show that the E. rugulosa samples of Fumin and Lijiang can be clearly classified, Dali and Yongsheng E. rugulosa can be partially distinguished but there is a certain overlap. This is due to the large difference in geography and climate between Fumin and Lijiang, while Dali and Yongsheng belong to the middle of the geographical climate transition zone with a certain crossover. In addition, the Pearson correlation coefficient was used to study the correlation of the chemical components of the E. rugulosa components. The results showed that E. rugulosa characteristic component Elsholtzia ketone is regulated by terpenoid metabolism. The effective identification of E. rugulosa origin is achieved by Fisher step-by-step discriminant analysis, and the initial verification and LOOCV accuracy of the E. rugulosa origin traceability functions are 100% and 87.5%, respectively.The GC-MS combined with multivariate statistical analysis can not only determine the volatile components of Chinese herbal medicines, but also realize their origin traceability, which can be widely used in the efficacy evaluation and origin traceability. The E. rugulosa volatile compounds are rich in active antiviral ingredients, which may have efficacy in preventing, anti- or/and ease COVID-19, and it will be further studied in the future.
Conflicts of interest
There are no conflicts to declare.References
- G. She, Z. Guo, H. Lv and D. She, New flavonoid glycosides from Elsholtzia rugulosa Hemsl, Molecules, 2009, 14, 4190–4196 CrossRef CAS PubMed.
- R. Liu, F. Meng, L. Zhang, A. Liu, H. Qin, X. Lan, L. Li and G. Du, Luteolin isolated from the medicinal plant Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in beta-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells, Molecules, 2011, 16, 2084–2096 CrossRef CAS PubMed.
- B. Liu, A. J. Deng, J. Q. Yu, A. L. Liu, G. H. Du and H. L. Qin, Chemical constituents of the whole plant of Elsholtzia rugulosa, J. Asian Nat. Prod. Res., 2012, 14, 89–96 CrossRef CAS PubMed.
- H. B. Han, Y. Diao, H. P. Wei, S. Q. Wan and D. F. Zhang, ICP-AES Determination of Elements in Elsholtzia rugulosa Hemsl. after Microwave Assisted Digestion, Appl. Mech. Mater., 2014, 543–547, 1136–1139 Search PubMed.
- X. M. Zhang and L. M. Wolfe, Stigma receptivity over the lifetime of the hermaphroditic flower of Elsholtzia rugulosa was negatively correlated with pollen viability, Plant Signaling Behav., 2016, 11(12), e1259052 CrossRef PubMed.
- X. M. Zhang, Floral volatile sesquiterpenes of Elsholtzia rugulosa (Lamiaceae) selectively attract Asian honey bees, J. Appl. Entomol., 2018, 142, 359–362 CrossRef CAS.
- L. Zhang, A. Tao and F. Zhao, The complete chloroplast genome sequence of the medicinal plant Elsholtzia rugulosa Hemsl (Labiatae), Mitochondrial DNA, 2020, 5, 1548–1549 CrossRef.
- F. P. Zhang, Q. Y. Yang and S. B. Zhang, Dual Effect of Phenolic Nectar on Three Floral Visitors of Elsholtzia rugulosa (Lamiaceae) in SW China, PLoS One, 2016, 11, e0154381 CrossRef PubMed.
- F. P. Zhang, Q. Y. Yang, G. Wang and S. B. Zhang, Multiple functions of volatiles in flowers and leaves of Elsholtzia rugulosa (Lamiaceae) from southwestern China, Sci. Rep., 2016, 6, 2761 Search PubMed.
- L. Zhao, L. Hou, H. Sun, X. Yan, X. Sun, J. Li, Y. Bian, Y. Chu and Q. Liu, Apigenin Isolated from the Medicinal Plant Elsholtzia rugulosa Prevents β-Amyloid 25–35-Induces Toxicity in Rat Cerebral Microvascular Endothelial Cells, Molecules, 2011, 16, 4005–4019 CrossRef CAS.
- A. L. Liu, B. Liu, H. L. Qin, S. M. Lee, Y. T. Wang and G. H. Du, Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa, Planta Med., 2008, 74, 847–851 CrossRef CAS PubMed.
- A. Liu, Discovery and Characterization of Novel Influenza Neuraminidase Inhibitors from Chinese Herbs by Integrative Approaches. University of Macau Macao SAR, China, 2009 Search PubMed.
- H. Li, T. Nakashima, T. Tanaka, Y. J. Zhang, C. R. Yang and I. Kouno, Two new maltol glycosides and cyanogenic glycosides from Elsholtzia rugulosa Hemsl, J. Nat. Med., 2008, 62, 75–78 CrossRef CAS PubMed.
- G. Y. Zuo, G. C. Wang, Y. B. Zhao, G. L. Xu, X. Y. Hao, J. Han and Q. Zhao, Screening of Chinese medicinal plants for inhibition against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA), J. Ethnopharmacol., 2008, 120, 287–290 CrossRef CAS PubMed.
- V. S. Rana, L. R. Devi, M. Verdeguer and M. A. Blazquez, Elsholtzia blandaBenth: New Citral-rich Chemotypes from India, J. Herbs, Spices Med. Plants, 2012, 18, 132–139 CrossRef.
- A. L. Liu, S. M. Y. Lee, Y. T. Wang and G. H. Du, Elsholtzia: review of traditional uses, chemistry and pharmacology, J. Chin. Pharm. Sci., 2007, 16, 73–78 CAS.
- L. Yip, S. Pei, J. B. Hudson and G. Towers, Screening of medicinal plants from yunnan province in southwest china for antiviral activity, J. Ethnopharmacol., 1991, 34, 1 CrossRef CAS PubMed.
- Z. Guo, Z. Liu, X. Wang, W. Liu, R. Jiang, R. Cheng and G. She, Elsholtzia: phytochemistry and biological activities, Chem. Cent. J., 2012, 6, 14 CrossRef PubMed.
- P. L. Fernández-Cáceres, M. J. Martín and F. Pablos, et al., Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content, J. Agric. Food Chem., 2001, 49, 4775 CrossRef PubMed.
- K. Katerinopoulou, A. Kontogeorgos, C. E. Salmas, A. Patakas and A. Ladavos, Geographical Origin Authentication of Agri-Food Products: Alpha Review, Foods, 2020, 9, 489 CrossRef PubMed.
- K. Schlesier, C. Fauhl-Hassek, M. Forina, V. Cotea, E. Kocsi, R. Schoula, F. van Jaarsveld and R. Wittkowski, Characterisation and determination of the geographical origin of wines. Part I: overview, Eur. Food Res. Technol., 2009, 230, 1–13 CrossRef CAS.
- B. Krajnc, L. Bontempo, J. Luis Araus, M. Giovanetti, C. Alegria, M. Lauteri, A. Augusti, N. Atti, S. Smeti, F. Taous, N. E. Amenzou, M. Podgornik, F. Camin, P. Reis, C. Máguas, M. Bučar Miklavčič and N. Ogrinc, Selective Methods to Investigate Authenticity and Geographical Origin of Mediterranean Food Products, Food Rev. Int., 2020, 37, 656–682 CrossRef.
- S. Fang, J. Ning, W. J. Huang, G. Zhang, W. W. Deng and Z. Zhang, Identification of geographical origin of Keemun black tea based on its volatile composition coupled with multivariate statistical analyses, J. Sci. Food Agric., 2019, 99, 4344–4352 CrossRef CAS PubMed.
- J. Yang, W. Zhang and W. Feng, et al., Geographical distribution of testate amoebae in Tibet and northwestern Yunnan and their relationships with climate, Hydrobiologia, 2006, 559, 297–304 CrossRef.
- X. Duan, Y. Tao and C. Duan, A fine mesh climate division and the selection of representative climate stations in Yunan Province, Trans Atmos Sci, 2011, 34, 336–342 Search PubMed.
- L. Liu, Q. Gao, Z. Zhang and X. Zhang, Elsholtzia rugulosa: phytochemical profile and antioxidant, anti-alzheimer's disease, antidiabetic, antibacterial, cytotoxic and hepatoprotective activities, Plant Foods Hum. Nutr., 2022, 77, 62–67 CrossRef CAS PubMed.
- H. B. Hu and X. D. Zheng, Extraction, separation and antimicrobial activity of volatile oil from Elsholtzia rugulosa, Chin. J. Hosp. Pharm., 2006, 26, 14–16 CAS.
- S. S. Kim, H. Oh, J. S. Baik, T. H. Oh, P. Y. Yun, C. S. Kim, N. H. Lee and C. G. Hyun, Chemical composition and biological activities of Elsholtzia splendens essential oil, J. Appl. Biol. Chem., 2008, 51, 69–72 CrossRef CAS.
- H. B. Hu, H. Cao, Y. F. Jian and X. D. Zheng, Extraction and antibacterial activity of active constituents of Elsholtzia ciliata Hyland, Pratac. Sci., 2007, 24, 36–39 CAS.
- Z. W. Li and T. H. Zhou, Studies on the components of essential oils of Elsholtzia splendens Nakai ex F. Maekawa (I) and Origanum vulgare L (II), Acta Pharmacol. Sin., 1983, 18, 363–368 CAS.
- S. C. Hyang and C. M. Kyung, Aroma-active compounds of Elsholtzia splendens using AEDA and HS-SPME-GC-O dilution analysis, Flavour Fragrance J., 2008, 23, 58–64 CrossRef.
- L. P. Sun, J. Wang, S. H. Kang, S. Z. Zheng and X. W. Sheng, Studies on the chemical constituents of volatile oils from Elsholtzia densa Benth var calycocarpa, J. Northwest Norm. Univ., Nat. Sci., 2000, 36, 48–49 CAS.
- H. Q. Du, X. Zhao and H. J. Fang, Components of essential oils of Elsholtzia stauntonii Benth, Chin. J. Pharm. Anal., 1989, 9, 18–21 CAS.
- F. J. He, X. F. Shi, H. G. Li, Y. J. Tian, J. P. Yang, W. Su, J. K. Chen and Y. J. Zhao, Chemical components of essential oils of Elsholtzia patrini Garcke, Chin. J. Pharm. Anal., 1995, 15, 20–22 CAS.
- G. Liu, H. Wang, B. H. Zhou and J. C. Song, GC-MS analysis of essential oil from Elsholtzia ciliata, Chin. J. Exp. Tradit. Med. Formulae, 2006, 12, 18–21 CAS.
- L. Pudziuvelyte, M. Stankevicius, A. Maruska, V. Petrikaite, O. Ragazinskiene and G. Draksiene, et al. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods, Ind. Crops Prod., 2017, 107, 90–96 CrossRef CAS.
- H. He, C. Tian, G. Jin and K. Han, Principal component analysis and fisher discriminant analysis of environmental and ecological quality, and the impacts of coal mining in an environmentally sensitive area, Environ. Monit. Assess., 2020, 192, 207–215 CrossRef PubMed.
- M. Rienth, N. Vigneron, P. Darriet, C. Sweetman and S. D. Castellarin, Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario-a review, Front. Plant Sci., 2021, 12, 643258 CrossRef PubMed.
- S. Ahmed, T. Griffin, D. Kraner, K. Schaffner, D. Sharma and A. Leitch, et al., Environmental factors variably impact tea secondary metabolites in the context of climate change: a systematic review, Front. Plant Sci., 2019, 10, 939 CrossRef PubMed.
- D. M. Witten and R. Tibshirani, Penalized classification using fisher’s linear discriminant, J. Roy. Stat. Soc. B Stat. Methodol., 2011, 73, 753–772 CrossRef PubMed.
- B. Gandek, J. Ware, N. K. Aaronson, G. Apolone and M. Sullivan, Cross-validation of item selection and scoring for the sf-12 health survey in nine countries: results from the iqola project. international quality of life assessment, J. Clin. Epidemiol., 1998, 51, 1171–1178 CrossRef CAS PubMed.
- P. Refaeilzadeh, L. Tang, and H. Liu Cross-Validation, Encyclopedia of database systems, 2009, vol. 5, pp. 532–538 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |