Open Access Article
Open Access ArticleBiophysical analysis of gelatin and PLGA nanoparticle interactions with complex biomimetic lung surfactant models†
W. Daear‡
 ,
K. Sule‡
,
K. Sule‡ ,
P. Lai§
,
P. Lai§
 and
E. J. Prenner
and
E. J. Prenner *
*
Department of Biological Sciences, University of Calgary, Calgary, AB T2N 1N4, Canada. E-mail: eprenner@ucalgary.ca
First published on 30th September 2022
Abstract
Biocompatible materials are increasingly used for pulmonary drug delivery, and it is essential to understand their potential impact on the respiratory system, notably their effect on lung surfactant, a monolayer of lipids and proteins, responsible for preventing alveolar collapse during breathing cycles. We have developed a complex mimic of lung surfactant composed of eight lipids mixed in ratios reported for native lung surfactant. A synthetic peptide based on surfactant protein B was added to better mimic the biological system. This model was used to evaluate the impact of biocompatible gelatin and poly(lactic-co-glycolic acid) nanoparticles. Surface pressure–area isotherms were used to assess lipid packing, film compressibility and stability, whereas the lateral organization was visualized by Brewster angle microscopy. Nanoparticles increased film fluidity and altered the monolayer collapse pressure. Bright protruding clusters formed in their presence indicate a significant impact on the lateral organization of the surfactant film. Altogether, this work indicates that biocompatible materials considered to be safe for drug delivery still need to be assessed for their potential detrimental impact before use in therapeutic applications
1. Introduction
Nanotechnology has applications in various fields of science and technology that involve medicine and biotechnology. As the name implies, materials of interest are on the nanometer scale.1,2 One of the key goals of nanotechnology applications in terms of drug delivery goes back to the German scientist Paul Ehrlich in the 1890s, who envisioned the creation of a “magic bullet”, where medicine can be targeted and delivered to a specific location of choice.3A more recent approach for this concept is the use of nanoparticles (NPs) that are designed to encapsulate, attach, or adsorb drugs and deliver them to a target location.4 Furthermore, their small size results in a large surface area to volume ratio allowing for higher drug loading per particle.5 Moreover, this large surface area offers a high capacity for chemical modification or attachment of different ligands. These surface modifications can be used to improve circulation times and aid in targeting specific cell types.
An important aspect in drug delivery NPs is the nature of the material used for the drug carrier. In order to avoid or at least reduce side effects of the carrier system, NPs formulated with biocompatible materials can be readily metabolized in the body. This also allows for controlled drug release.1
The NPs can be delivered in the human body through a variety of drug delivery routes. More recently, the pulmonary route, which is delivery through the lungs, has gained significant interest. It is non-invasive and ideally suited to directly target lung diseases. Another main reason for the scientific interest is the large surface area of the lungs of around 43–102 m,2 depending on the age and size of the individual.6–8 Furthermore, the close proximity to the vascular system allows for local and systematic drug delivery and avoids metabolic break down in the gastrointestinal tract.8 Systemic pulmonary delivery requires particles to reach the alveoli found at the terminal end of the respiratory tract.
The human lower respiratory tract is composed of the small bronchioles and the alveoli. The alveoli are the site of gas exchange between the blood and external environment where oxygen is taken up, and carbon dioxide is released. The thin alveolar barrier of around 200–500 nm9,10 is composed of three layers (Fig. S1†). The layer in contact with the blood circulation is composed of type I and type II epithelial pneumocytes. Above the epithelial cells is an aqueous layer of 50–80 nm thick containing macrophages that act as a defense mechanism by eliminating foreign substances.6,11 In humans, around 300 million alveoli comprise about 95% of the lung’s surface area. Both the large surface area and proximity and extent of vascularization are major advantages of this drug delivery route.12–14 For the NP to pass from the air into circulation it must first come into contact with the lung surfactant. This single molecular layer composed of 90% lipids and 10% protein resides on top of the aqueous layer.
The major role of this monolayer is to reduce the surface tension at the air–water interface, maintain surfactant film stability during exhalation, and aid respreading of the film during inhalation (Fig. S1†). This helps prevent lung collapse during breathing cycles.15–17 The major lipid classes found in the human lung surfactant include about 70% zwitterionic phosphatidylcholines and about 10% anionic phosphatidylglycerol of the total lipidic components.18–20 Among the phosphatidylcholines, the saturated dipalmitoylphosphatidylcholine (DPPC) makes up around 50% of the total lipid composition while the rest are the unsaturated form.18 Interestingly, the phase transition temperature of DPPC is 41 °C21 above the physiological temperature, which helps to understand its important role to ensure the stability of lung surfactant upon compression during exhalation.20 In contrast, the fast area increase upon inhalation requires fluid lipids that promote film respreading. This fluidity is provided by unsaturated lipids, such as palmitoyl-oleoylphosphatidylglycerol (POPG)22 representing a class of negatively charged lipid components. Furthermore, lung surfactant contains about 10% neutral lipids such as cholesterol.18
In addition to lipids, lung surfactant also contains about 10% surfactant proteins (SPs), which are present as SP-A, SP-B, SP-C, and SP-D.16,20 SP-B and SP-C are both hydrophobic whose functions are to aid the lipidic component of the lung surfactant in reducing the surface tension and allow respreading of the monolayer during breathing cycles.23–25 Controlled and reversible folding of the monolayer to generate multilayer stacks and reverting back to a monolayer is facilitated by the hydrophobic surfactant proteins.26 While the hydrophilic SP-A and SP-D are involved in the immune response against pathogens.27–29
In pulmonary drug delivery, it is crucial that inhaled nanoparticles have minimal impact on lung surfactant function to maintain the ability of surfactant to lower surface tension and to avoid detrimental impacts on respiratory function.
Lung surfactant contains a complex mixture of lipids with varying degrees of acyl chain length and saturation. Many groups have worked on LS model systems based on single lipids like DPPC.2,3,26,30 We have spent significant efforts to establish a complex biomimetic system31,32 and complex lung surfactant model systems have also been used in the literature.26,30,31 In an effort to even better capture the detailed lipid composition, we used custom synthesized 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC and PG, which make up 20% of the total lipid component of the lung surfactant4 but have not been characterized. The culmination of this work is presented with 7 phospholipids and cholesterol in ratios based on literature reports.33–36 The stepwise addition of specific lipids to increasingly complex models, allow to assess specific contributions to the overall observed effects.
1 PC and PG, which make up 20% of the total lipid component of the lung surfactant4 but have not been characterized. The culmination of this work is presented with 7 phospholipids and cholesterol in ratios based on literature reports.33–36 The stepwise addition of specific lipids to increasingly complex models, allow to assess specific contributions to the overall observed effects.
Another level of complexity is achieved by including surfactant proteins or protein derived peptides. SP-B is required for monolayer to multilayer transition of surfactant important for function37,38 but a synthetic SP-B construct (SP-B1–25) has been shown to perform similar functions as the full SP-B.39,40 Thus, this synthetic SP-B fragment was added to our most complex biomimetic model investigated here. To our knowledge, there is no comparable well-defined synthetic model. This system could be used as a tool for in vitro screening of different nanomaterials.
This complex lipid–peptide based LS model was used to evaluate the potential impact of two biocompatible materials on surfactant film function in terms of film stability and lateral domain organization by using monolayers at the air–water interface and Brewster angle microscopy. The first is gelatin, a natural polymer obtained from gelatin which has been investigated by our group before.32,41 The second is a synthetic polymer poly(lactic-co-glycolic) acid (PLGA), which has been used in medical devices and would healing applications.42–44
This approach aims to better understand and compare the impact of nanomaterials and their potential to impair surfactant function. As more nanomaterials are designed for drug delivery, these formulations will need to be evaluated. This in vitro method provides a tool to screen new nanomaterials before conducting in vivo studies.
2. Materials and methods
2.1. Materials
All lipids were purchased from or custom synthesized 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PC (16-1-PC) and 16
1-PC (16-1-PC) and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PG(16
1-PG(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PG) by Avanti Polar Lipids Inc. (Alabaster, AL, USA). Gelatin (type B, bloom of 225), poly(D,L-lactide-co-glycolide) (75
1-PG) by Avanti Polar Lipids Inc. (Alabaster, AL, USA). Gelatin (type B, bloom of 225), poly(D,L-lactide-co-glycolide) (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25), ethyl acetate, and glutaraldehyde (25% v/v) were purchased from Sigma-Aldrich (Oakville, ON, CA). SP-B1–25 peptide, which has a molecular weight of 2.9 kDa, was custom synthesized by AAPPTec (Kentucky, USA) to a 97.5% purity (HPLC) with the following truncated sequence:
25), ethyl acetate, and glutaraldehyde (25% v/v) were purchased from Sigma-Aldrich (Oakville, ON, CA). SP-B1–25 peptide, which has a molecular weight of 2.9 kDa, was custom synthesized by AAPPTec (Kentucky, USA) to a 97.5% purity (HPLC) with the following truncated sequence:
NH2-FPIPLPYCWLCRALIKRIQAMIPKG-COOH
These are the first 25 amino acids from the full SP-B protein sequence.40 SP-B is 79 amino acids long, but the first 25 amino acids was chosen for this work due to their role in the insertion to the lung surfactant monofilm. Acetone, methanol, hexane, and chloroform with an ACS grade were purchased from Fisher Scientific (Ontario, CA). Pluronic F-68 was a donation from collaborators at the University of Alberta (Dr Löbenberg, Faculty of Pharmacy).
2.2. Methods
For zeta potential measurements, nanoparticles were centrifuged and resuspended in a 10 mM NaCl solution at pH 7. The solution was transferred to a folded capillary cell from Malvern (Quebec, Canada) an electric field of 150 mV was applied to measure the electrophoretic mobility. Each sample was an average of at least 3 measurements.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v chloroform
4 v/v chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol to a final concentration of 1 mM as can be seen below (Table 1).
methanol to a final concentration of 1 mM as can be seen below (Table 1).
| System | Lipid composition | Molar ratio | Final concentration |
|---|---|---|---|
| PC system | DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PC 16-1PC |
5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 mM |
| PG system | DPPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPG POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SOPG SOPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PG 16-1PG |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 mM |
| 8 Lipid system | DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPG DPPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPG POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SOPG SOPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PC 16-1PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PG + cholesterol 16-1PG + cholesterol |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 +2% w/w cholesterol 2 +2% w/w cholesterol |
1 mM |
SP-B1–25 along with lipids were added at a 10% weight ratio of lipids, gelatin at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 lipid
1 lipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NP weight ratio, and PLGA at both 10
NP weight ratio, and PLGA at both 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 lipid
1 lipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NP weight ratio in 6 : 4 chloroform
NP weight ratio in 6 : 4 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol.
methanol.
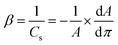 | (1) |
The reported compression modulus values describe the phases of the deposited monofilms. Values under 12 mN m−1 correspond to the gas phase, 12 mN m−1 to 100 mN m−1 reflect the liquid-expanded (LE) phase, whereas values above 100 mN m−1 correspond to the liquid condensed (LC) phase.47,48 This analysis reports on the interfacial packing elasticity of monolayers.
The lung surfactant model systems were deposited as described for the pressure–area isotherms. After a 10 min delay, compression was started until the selected surface pressure and stopped to collect images before compression was resumed. Images were obtained by using an EP3 imaging ellipsometer and corresponding EP3 software (Accurion, Goettingen, Germany). Image analysis in terms of area coverage and domain frequency was performed by using ImageJ (NIH, Bethesda, USA). Each image is representative of at least three separate trials.
3. Results and discussion
The increased interest in pulmonary drug delivery also includes the need for a better understanding of the impact of drug delivery systems on lung function. An essential element for proper lung function is the surfactant layer within the alveoli that provides the interface between the air and water layer located in top of the lung endothelial cells.While the size dependent deposition and uptake of nanoparticles is well established,49 the impact of nanomaterials on lung surfactant is less understood. We have shown previously that gelatin NPs exhibit differential interactions with zwitterionic PCs and negatively charges PGs as the latter showed lateral film reorganization by inducing clusters. Moreover, the effect was stronger for saturated DPPG over monounsaturated POPG.32
The present work expands the scope to a more complex biomimetic lung surfactant model comprising 8 lipids and a peptide from SP-B and by comparing two biocompatible materials gelatin and PLGA in the form of NPs.
3.1. Nanoparticle characterization
Gelatin NPs had a diameter of 110 nm with a polydispersity index of 0.2 and zeta potential of +17 mV at pH of 7. This indicates that the particles are mostly monodisperse in an aqueous suspension.PLGA NPs had a diameter of 141 nm with a polydispersity index of 0.13 and zeta potential of −26 mV at pH of 7. This also demonstrates a mostly monodisperse suspension.
3.2. Individual lipid systems
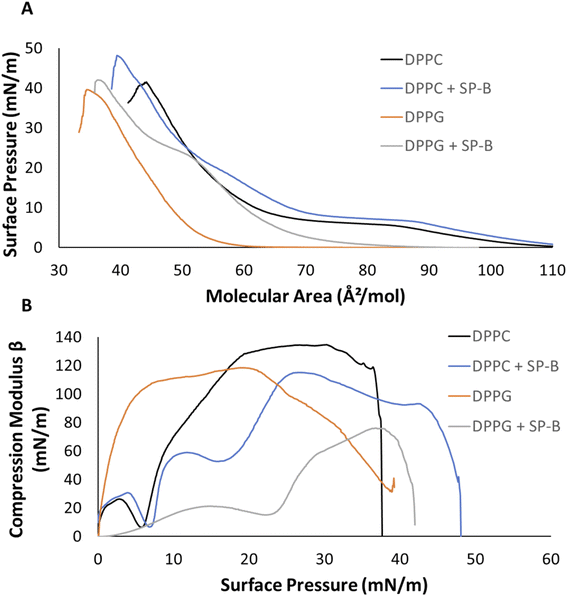
DPPC exhibits a characteristic phase coexistence region of LE and LC phases between 85 and 65 Å2 mol−1, which is shifted by the peptide to larger areas between 89 and 69 Å2 mol−1. This phase coexistence results in distinct domain formation. Upon compression, the continuous slope of DPPC is replaced by an isotherm exhibiting a shoulder at around 20 mN m−1 in the presence of SP-B1–25 (Fig. 1A, purple arrow) and another inflection at 42 mN m−1 to a gentler slope, which is indicative of a film that can withstand compression. This may be due to the formation of functional multilayers, which are important for proper surfactant function50 (Fig. 1A, purple box). SP-B1–25 peptide increased the collapse pressure by about 7 mN m−1, such film stabilization has been reported before.16,51,52
Fig. 1B shows compression modulus (Cm) data according to eqn (1) representing film elasticity. The dip in the isotherm at 6 mN m−1 corresponds to the LE-LC phase coexistence region of DPPC (Fig. 1B). Subsequently, Cm values exceeding 100 mN m−1 indicated the more rigid LC phase with a maximum of 134.3 mN m−1 (Fig. 1B). The addition of 10% by weight SP-B1–25 shifted the LE-LC phase coexistence dip to a higher surface pressure of around 8 mN m−1 (Fig. 1B). The shoulder around 20 mN m−1 in the peptide containing system is highlighted as a dip in the Cm (Fig. 1B). The maximum peak at 115 mN m−1 compared to 134.3 mN m−1 for controls represents a peptide induced increase in fluidity.
Fig. 1A presents surface pressure–area isotherms for DPPG. The negatively charged phosphatidylglycerol are the second largest lipid class found in human lung surfactant at about 10%.18,20 DPPG has the same acyl chain composition as DPPC but a smaller glycerol headgroup compared to choline. Both lipids carry a negative charge on the phosphate group, but PC is zwitterionic due to an added positive charge on the quaternary amine. DPPG films lift off at 60 Å2 mol−1 and rapidly transitions into the LC phase as seen by the sharp increase in the isotherm slope (Fig. 1A) before collapsing at a surface pressure of 40 mN m−1. SP-B1–25 caused a significant lift off shift to 89 Å2 mol−1, a distinct shoulder at 23 mN m−1 and a slight increase in the collapse pressure from 40 to 42 mN m−1 (Fig. 1A). The shoulder at 23 mN m−1 and shift to smaller molecular areas likely reflects an at least partial squeeze-out of SP-B1–25. Collapse pressures for monofilms of protein and peptides have been reported between 15 and 20 mN m−1.53,54
DPPG entered the LC phase early on during the compression isotherm showing Cm values above 100 mN m−1 (Fig. 1B) and with a maximum Cm of 118 mN m−1 at a surface pressure of 20 mN m−1. The Cm profile for SP-B1–25 displayed lower values throughout the entire compression. Once again, the shoulder at 22 mN m−1 in the pressure–area isotherm appears as a dip in the Cm graph with a reduced maximum of 76 mN m−1. This maximum is also observed at higher surface pressures closer to 38 mN m−1 whereas the maximum for DPPG controls appears at much lower pressures (Fig. 1B).
SP-B1–25 affected both DPPC and DPPG, and it is known to interact with lung surfactant through electrostatic and hydrophobic interactions, respectively.55–58 SP-B resides outside of the LC domains, preferentially interacting with the more fluid LS components59 which are primarily PG lipids. Nevertheless, the rigid sterol cholesterol is also required for proper function of LS60 and involved in SP-B function.59 A peptide induced shift to larger areas in the pressure–area isotherms has been observed before for both saturated lipids.39,61,62 Results from Fig. 1 show that SP-B1–25, increased the fluidity of both of DPPC and more so DPPG, as indicated by the lower compression moduli (Fig. 1B).
Hydrophobic SP-B and SP-C are known to facilitate adsorption and spreading of the lung surfactant during breathing cycles through the formation of surface associated LS reservoirs.63,64 Fig. S2† shows a model to better illustrate the proposed mechanism. This squeeze out effect has been seen previously as well with other systems.61,65 Lipids are squeezed out during exhalation as the alveolar surface area is reduced by forming multilayer stacks. The further reduction in surface area allows for surfactant to reach near zero surface tensions.66 SP-B is critical for this function, while SP-C is required for proper function.
3.3. Model lipid systems
Analysis of key lipid classes helps to demonstrate the impact of the peptide and illustrates the scope of information obtained from monolayer isotherms. The next step was the analysis of more complex biomimetic systems and their response to NPs made from two biodegradable materials.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acyl chained partly unsaturated lipid. The PC lipid mixture was comprised of DPPC, POPC and 16
1 acyl chained partly unsaturated lipid. The PC lipid mixture was comprised of DPPC, POPC and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PC (16-1-PC) at a molar ratio of 5.6
1-PC (16-1-PC) at a molar ratio of 5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereby both partly unsaturated lipids exist at an equivalent molar percentage33 (Fig. 2). POPC is a partly unsaturated lipid found in lung surfactant and carries both a palmitoyl and oleoyl side chains resulting in a phase transition temperature from gel to liquid-crystalline phase of −2 °C.67 The third lipid in the system, the partly unsaturated 16
1, whereby both partly unsaturated lipids exist at an equivalent molar percentage33 (Fig. 2). POPC is a partly unsaturated lipid found in lung surfactant and carries both a palmitoyl and oleoyl side chains resulting in a phase transition temperature from gel to liquid-crystalline phase of −2 °C.67 The third lipid in the system, the partly unsaturated 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC (16-1PC) carries palmitoyl and palmitoleoyl side chains. This lipid has a shorter unsaturated acyl chain and had to be custom synthesized by Avantli Polar lipids. There are limited data about its biophysical behavior but one paper using pyrene fluorescence reports a Tm of 30 °C.68 A Tm in the ∼30 °C seems too high when known melting temperatures for the 18 carbon lipids are considered. Fully saturated DSPC has a Tm of 55 °C, fully unsaturated DOPC has −17 °C and the mixed chain SOPC has been reported at 6 °C.69 DPPC has a Tm of 41 °C whereas the fully unsaturated Di-16
1 PC (16-1PC) carries palmitoyl and palmitoleoyl side chains. This lipid has a shorter unsaturated acyl chain and had to be custom synthesized by Avantli Polar lipids. There are limited data about its biophysical behavior but one paper using pyrene fluorescence reports a Tm of 30 °C.68 A Tm in the ∼30 °C seems too high when known melting temperatures for the 18 carbon lipids are considered. Fully saturated DSPC has a Tm of 55 °C, fully unsaturated DOPC has −17 °C and the mixed chain SOPC has been reported at 6 °C.69 DPPC has a Tm of 41 °C whereas the fully unsaturated Di-16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PC has −36 °C70 suggesting a Tm for the mixed chain lipid just above 0 °C.
1-PC has −36 °C70 suggesting a Tm for the mixed chain lipid just above 0 °C.
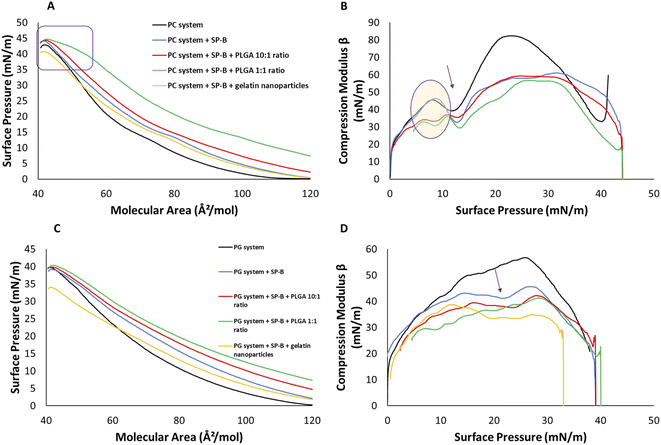
The PC system is anticipated to be more fluid and packing defects between saturated and unsaturated species are likely facilitating the insertion of SPs. The PC system enters the LE phase at a molecular area of 110 Å2 mol−1 (Fig. 2A). DPPC, the major component at 74%, is responsible for plateau region representing phase coexistence. In the presence of the unsaturated lipids it occurs later at 13 mN m−1. This PC mixture collapsed at 42 ± 0.1 mN m−1 (Fig. 2A). SP-B1–25 peptide increased the lift off area from 110 Å2 mol−1 to 120 Å2 mol−1 compared, maintained the coexistence phase region at 13 mN m−1 and induced film collapse at 44 mN m−1. The peptide induced molecular area increase is consistent with the results in Fig. 1 and literature reports.39,62,65
PLGA nanoparticles at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in the subphase caused an earlier lift off by about 8 Å2 mol−1, which indicates their interaction with the headgroups (Fig. 2A). The isotherm showed a gentle pressure increase upon compression, characteristic for the LE phase, and the collapse pressure remained at 44 mN m−1. The addition of particles to the subphase does not provide a quantitative measure of their concentration at the monofilm and considering the much higher subphase volume compared to surface area, the PLGA concentration was also increased to 1
1 ratio in the subphase caused an earlier lift off by about 8 Å2 mol−1, which indicates their interaction with the headgroups (Fig. 2A). The isotherm showed a gentle pressure increase upon compression, characteristic for the LE phase, and the collapse pressure remained at 44 mN m−1. The addition of particles to the subphase does not provide a quantitative measure of their concentration at the monofilm and considering the much higher subphase volume compared to surface area, the PLGA concentration was also increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, drastically increasing their impact. At the starting area of ∼130 mN m−1 the surface pressure was already around 5 mN m−1 (Fig. 2A). The isotherm exhibited an overall gentle LE phase slope with a minor discontinuity at 15 mN m−1 a pressure range reported for SP-B.51 The slope change at around 40 mN m−1 to a less steep slope suggested the onset of multilayer formation until the same collapse pressure of 44 mN m−1 was reached.
1, drastically increasing their impact. At the starting area of ∼130 mN m−1 the surface pressure was already around 5 mN m−1 (Fig. 2A). The isotherm exhibited an overall gentle LE phase slope with a minor discontinuity at 15 mN m−1 a pressure range reported for SP-B.51 The slope change at around 40 mN m−1 to a less steep slope suggested the onset of multilayer formation until the same collapse pressure of 44 mN m−1 was reached.
GNPs did not affect the lift off area of SP-B1–25 system at 120 Å2 mol−1 (Fig. 2A). However, upon compression, the isotherm shifted to smaller molecular areas with a reduced shoulder at 15 mN m−1 and a reduced collapse pressure of 40 mN m−1 indicating a destabilizing effect.
The Cm of the PC system displayed a dip corresponding to coexistence phase at 13 mN m−1 (Fig. 2B) and a peak modulus of around 82 mN m−1, which was much lower than DPPC alone and is due to increased fluidity of the unsaturated lipids. All other systems showed the isotherm phase coexistence shoulder as isotherm dips between 8 and 15 mN m−1 and were more fluid in nature with a maximum Cm of 56 mN m−1 compared to 82 mN m−1 (Fig. 2B). All systems were similar between 25 and 35 mN m−1 with lower Cm values for NP containing films above this range.
The PG system contains DPPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPG
POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SOPG:16
SOPG:16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16-1PG at 1
0/16-1PG at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4
4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol ratios which is derived from a mass spectroscopy study.33 POPG is also very fluid with a Tm of −2 °C.67 A Tm for SOPG has not been published but PC and PG lipids have similar transition temperatures71 and the Tm of SOPC has been reported as 6 °C.69 For the 16
2 mol ratios which is derived from a mass spectroscopy study.33 POPG is also very fluid with a Tm of −2 °C.67 A Tm for SOPG has not been published but PC and PG lipids have similar transition temperatures71 and the Tm of SOPC has been reported as 6 °C.69 For the 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1PG (16
1PG (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1PG), a similar Tm as the PC variant can be estimated, which makes the system controlled by fluid lipids.
1PG), a similar Tm as the PC variant can be estimated, which makes the system controlled by fluid lipids.
The area–pressure isotherms in Fig. 2C were more complex than our previously investigated DPPG/POPG. The PG lipid films entered the LE phase at 120 Å2 mol−1 and collapsed at a surface pressure of 40 mN m−1 (Fig. 2C). The change at 35 mN m−1 to a gentler slope could also be due to functional multilayer formation as discussed above. SP-B1–25 shifts the transition to the LE phase 12 Å2 mol−1 higher than control to 132 Å2 mol−1. The slope was gentler with a minor shoulder at 22 mN m−1 and a similar collapse pressure of 40 mN m−1. The addition of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PLGA NPs increased the molecular area, and the surface pressure to around 3 mN m−1 at the starting area of 130 Å2 mol−1. The 1
1 PLGA NPs increased the molecular area, and the surface pressure to around 3 mN m−1 at the starting area of 130 Å2 mol−1. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 addition of PLGA nanoparticles isotherm resulted in a starting pressure of 8 mN m−1. Both additions did not change the collapse pressure of 40 mN m−1 (Fig. 2C). The GNP containing system had the same lift off at 132 Å2 mol−1 as the peptide containing films. However, gelatin NPs resulted in a more gradual increase in surface pressure, a shoulder at 20 mN m−1 and earlier collapse than all other systems at 33 mN m−1 (Fig. 2C).
1 addition of PLGA nanoparticles isotherm resulted in a starting pressure of 8 mN m−1. Both additions did not change the collapse pressure of 40 mN m−1 (Fig. 2C). The GNP containing system had the same lift off at 132 Å2 mol−1 as the peptide containing films. However, gelatin NPs resulted in a more gradual increase in surface pressure, a shoulder at 20 mN m−1 and earlier collapse than all other systems at 33 mN m−1 (Fig. 2C).
The compression modulus profiles displayed a peak of 57 mN m−1 at a surface pressure of 26 mN m−1 as expected for a system enriched in unsaturated lipids (Fig. 2D). SP-B1–25 induced a dip at 22 mN m−1, reflecting the shoulder in the area–pressure isotherm, and reached a lower peak value of 46 mN m−1 indicating increased fluidity (Fig. 2D, arrow). NP additions only slightly lowered compression modulus values, especially above surface pressures of 30 mN m−1, with 35 mN m−1 for the PLGA NPs and around 28 mN m−1 for GNP which exhibited a maximum of 35 mN m−1 at lower pressures (Fig. 2D).
Like for both DPPC and DPPG lipid systems, SP-B1–25 increased the molecular area in both PC and PG lipid systems indicating peptide–lipid interactions. The peptide did not affect the stability of PC and PG systems as the collapse pressure was increased for PC by about 2 mN m−1 and maintained for the PG system.
Gelatin NPs had stronger interactions, although it must be stated that they are added to the organic phase and may be present in higher concentrations. As previously reported, the addition of GNPs decreased the collapse pressure of the single lipids (PG > PC), and similarly to PC and PG systems reported here (PG > PC, 7 mN m−1 vs. 2 mN m−1). We have previously observed preferential interaction of GNPs with PG lipids due to electrostatic interaction between positively charged GNPs and negatively charged PGs.32
The last system analyzed was the 8 lipid system which contained DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC
POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPG
DPPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPG
POPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SOPG
SOPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PC
16-1PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16-1PG at 45
16-1PG at 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4
4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol ratio and 2% by weight cholesterol based on previous reports.33–36 This is the most complex system studied which contains the major PC and PG lipids found in the human lung surfactant along with cholesterol. According to our knowledge, this system has not been studied before as a synthetic biomimetic model. Cholesterol is a comparably minor component but is essential for proper lung function like low surface tension,60 and multilayer formation.25 Its content is maintained between 5 and 10% w/w11 and higher amounts of the sterol have been linked to acute respiratory distress syndrome.72
2 mol ratio and 2% by weight cholesterol based on previous reports.33–36 This is the most complex system studied which contains the major PC and PG lipids found in the human lung surfactant along with cholesterol. According to our knowledge, this system has not been studied before as a synthetic biomimetic model. Cholesterol is a comparably minor component but is essential for proper lung function like low surface tension,60 and multilayer formation.25 Its content is maintained between 5 and 10% w/w11 and higher amounts of the sterol have been linked to acute respiratory distress syndrome.72
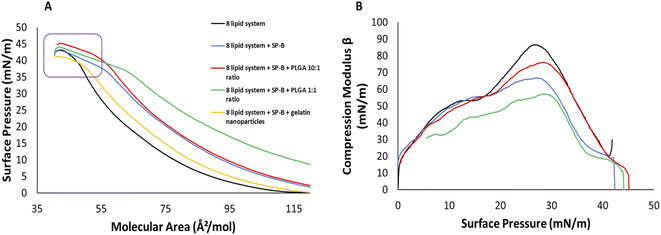
The 8 lipid system entered the LE phase at 115 Å2 mol−1, with a minor shoulder around 15 mN m−1 reflecting the pressure range of the potential partial peptide squeeze-out before collapse at a surface pressure of 42 mN m−1 (Fig. 4A). SP-B1–25 increased the lift off area to 130 Å2 mol−1, collapse occurred gradually between 40.8 and 43 mN m−1 (Fig. 3A, purple box). The 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 addition of PLGA NPs had very minor effects below 30 mN m−1 but increased the onset of the multilayer formation from 37 to 41 mN m−1. The 1
1 addition of PLGA NPs had very minor effects below 30 mN m−1 but increased the onset of the multilayer formation from 37 to 41 mN m−1. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 addition had a starting pressure of 6 mN m−1 at 135 Å2 mol−1 and larger overall areas suggest enhanced interactions in the headgroup region (Fig. 3A). A wider range of gradual collapse between 38 and 42 mN m−1 reflects a slightly reduced stability compared to the 10
1 addition had a starting pressure of 6 mN m−1 at 135 Å2 mol−1 and larger overall areas suggest enhanced interactions in the headgroup region (Fig. 3A). A wider range of gradual collapse between 38 and 42 mN m−1 reflects a slightly reduced stability compared to the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 but similar to the lipid and lipid/peptide controls. In contrast to the single lipids and the PC or PG systems, the complex surfactant model was able to withstand the presence of gelatin NPs as the area was only slightly increased with a continuous slope and only a minor reduction of collapse pressure by 2 mN m−1 to 40 mN m−1 (Fig. 3A).
1 but similar to the lipid and lipid/peptide controls. In contrast to the single lipids and the PC or PG systems, the complex surfactant model was able to withstand the presence of gelatin NPs as the area was only slightly increased with a continuous slope and only a minor reduction of collapse pressure by 2 mN m−1 to 40 mN m−1 (Fig. 3A).
The Cm data for the 8 lipid system showed a peak rigidity at 86 mN m−1 at a surface pressure of 27 mN m−1 (Fig. 3B). In the presence of both, SP-B1–25 and gelatin NPs, the monolayer elasticity was increased with a peak modulus of around 66 mN m−1. Elasticity was further increased in the presence of both SP-B1–25 and PLGA NPs at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a peak modulus of 57 mN m−1. However, PLGA NPs at a 10
1 ratio with a peak modulus of 57 mN m−1. However, PLGA NPs at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio had a much lower elasticity compared to the other NP systems with a peak modulus of 73 mN m−1 (Fig. 3B).
1 ratio had a much lower elasticity compared to the other NP systems with a peak modulus of 73 mN m−1 (Fig. 3B).
However, the major effect of SP-B1–25 on collapse can be seen in complex 8 lipid system as the observed multilayer formation allows film compression with less pressure increase compared to controls only containing lipids (Fig. 3A, purple box).9,16,51,52,73 To a lesser extend this was seen for DPPC as well, the main lipid component of lung surfactant. The squeeze out model15,66,74–76 suggests that more fluid lipids are removed from the film at lower alveolar areas and concomitant high pressures but SP-B and SP-C help in the folding and formation of surface associated reservoirs during breathing cycles, which mostly consist of unsaturated lipids (Fig. S2†). The remaining saturated lipids at the interface can achieve much lower surface tension values and thus stabilize the surfactant during exhalation potentially in the form of multilayer formation seen here.9,16,51,52,73 These data confirm the important interplay of lipids and proteins and suggest that the most complex lipid–peptide model system best reflects the currently accepted model of surfactant function.16
The key forces that determine the domain shape are line tension, favoring rounder domains79 and electrostatic dipole–dipole repulsion promoting noncircular domains.80 The single lipids systems have been widely investigated and we present changes induced by the addition of the peptide to DPPC and DPPG in the ESI.† DPPC alone displayed domain formation in the LE/LC phase coexistence region at a surface pressure of around 7 mN m−1. Depending on the experimental conditions, bean and tri-lobed shapes have been reported.79,80 In systems including 10% by weight SP-B1–25, larger domains were observed that coalesced into a homogenous film earlier than control DPPC (Fig. S4†).
Control DPPG exhibits almost fully coalesced domains at 15 mN m−1 (Fig. S5†). At surface pressures close to 30 mN m−1, bright clusters and film defects appeared that remained until monolayer collapse (Fig. S5†). These clusters signify the start of DPPG system collapse which is well documented and reported by other groups.81,82 The presence of 10% SP-B1–25 induced LE/LC phase demixing with numerous lobed domains that exhibit appreciable size variability at around 15 mN m−1, domains became smaller and more circular and film coalescence was significantly delayed (Fig. S5†). At increasing pressures, domains started to coalesce but at 30 mN m−1, the film was not entirely homogenous yet. Unlike control system, no bright clusters or film defects were seen.
The BAM results indicate that SP-B1–25 interacts with DPPC and DPPG differently. With DPPC, the peptide appeared to interact preferentially with the LC form suggested by the increased domain sizes compared to peptide free controls (Fig. S4†). However, for DPPG increased phase demixing and a delay in the formation of a homogenous LC phase was seen in DPPG + SP-B1–25 systems (Fig. S5†). A stronger interaction of positively charged SP-B with anionic lipids has been reported.83 Moreover, the preference of SP-B for the fluid LE phase has been reported as well.84
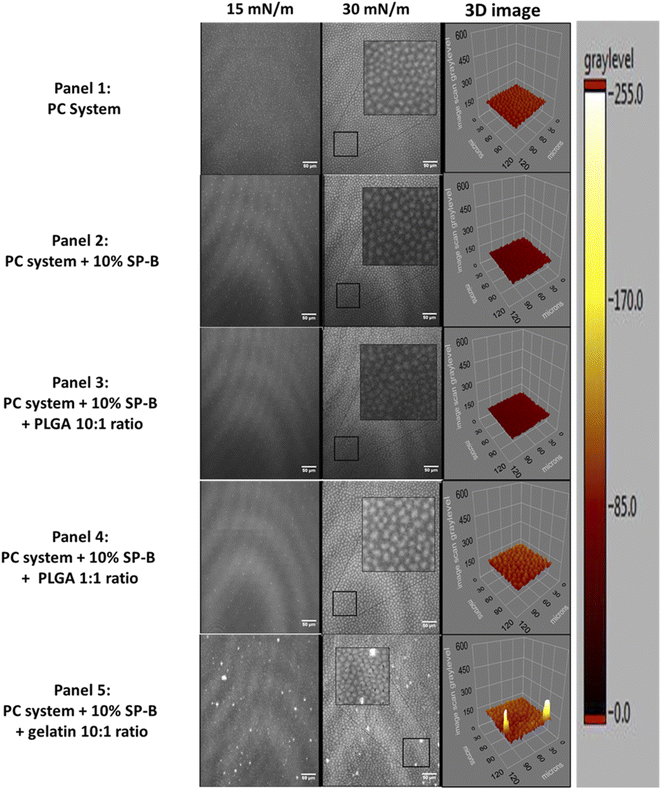
The lateral organization of the PC system is shown in (Fig. 4). The unsaturated POPC and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-PC (16-1-PC) reduced and delayed domain formation compared to saturated DPPC. Much smaller domains are observed at 15 mN m−1 (Fig. 4, panel 1) that moderately increased in size and frequency upon compression with very comparable sizes to 30 mN m−1 and no coalescence was seen until film collapse. With SP-B1–25, small domains of a similar size but significantly reduced frequency were recorded (Fig. 4, panel 2). Domains moderately increased in size at 30 mN m−1. With gelatin NPs, phase demixing can be seen at low surface pressures that persisted until monolayer collapse (Fig. 4, panel 3 inserts). Bright clusters associated with gelatin NPs as seen in both DPPC and DPPG systems were also observed throughout the compression.
1-PC (16-1-PC) reduced and delayed domain formation compared to saturated DPPC. Much smaller domains are observed at 15 mN m−1 (Fig. 4, panel 1) that moderately increased in size and frequency upon compression with very comparable sizes to 30 mN m−1 and no coalescence was seen until film collapse. With SP-B1–25, small domains of a similar size but significantly reduced frequency were recorded (Fig. 4, panel 2). Domains moderately increased in size at 30 mN m−1. With gelatin NPs, phase demixing can be seen at low surface pressures that persisted until monolayer collapse (Fig. 4, panel 3 inserts). Bright clusters associated with gelatin NPs as seen in both DPPC and DPPG systems were also observed throughout the compression.
However, domains can still be seen at around 15 mN m−1 and increased at 30 mN m−1 (Fig. 4, panel 3). The inserts in each figure are enlarged (as shown for BAM images at 30 mN m−1) to better illustrate the lateral organization. The 3D images are based on these selections and allows better visualization of topology changes such as protrusion from the monofilm.
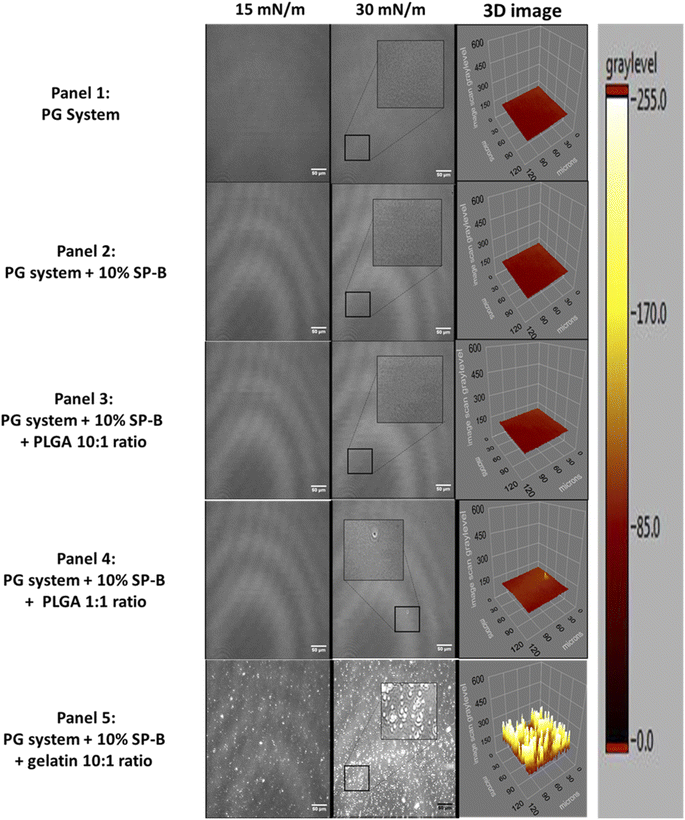
The control PG system contains about 92.5% fluid lipids and thus no domains were seen in the homogeneous LE film (Fig. 5, panel 1). Similarly, the presence of SP-B1–25 did not result in any observable changes to the lateral organization (Fig. 5, panel 2). The addition of PGLA only showed minor domain formation at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. However, the addition of GNPs induced bright clusters throughout the compression that increase in size and numbers, especially noticeable at 30 mN m−1 (Fig. 5, panel 3).
1 ratio. However, the addition of GNPs induced bright clusters throughout the compression that increase in size and numbers, especially noticeable at 30 mN m−1 (Fig. 5, panel 3).
This is in agreement with previous results which show reduction in LC domain sizes of DPPC/DPPG mixtures in the presence of SP-B.84
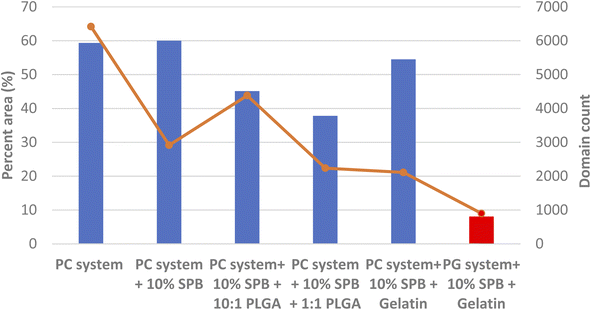
A comparison between PC and PG systems is also presented in terms of total area covered by domains in Fig. 6, which shows a progressive reduction for the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (−17%) to the 1
1 (−17%) to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (−22%). This is consistent with previous work31 that reported NP induced loss of lateral organization. These rigid domains are important as the anchor regions for hydrophobic SPs, and their disappearance could affect film folding and respreading during the breathing cycle. The more fluid PG system only showed domains with GNP, which are smaller and less frequent compared to GNP in the PC system.
1 (−22%). This is consistent with previous work31 that reported NP induced loss of lateral organization. These rigid domains are important as the anchor regions for hydrophobic SPs, and their disappearance could affect film folding and respreading during the breathing cycle. The more fluid PG system only showed domains with GNP, which are smaller and less frequent compared to GNP in the PC system.
One anomaly was PC system + 10% SPB + 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PLGA with a higher domain coverage and frequency than the higher mol ratio of PLGA (Fig. 6).
1 PLGA with a higher domain coverage and frequency than the higher mol ratio of PLGA (Fig. 6).
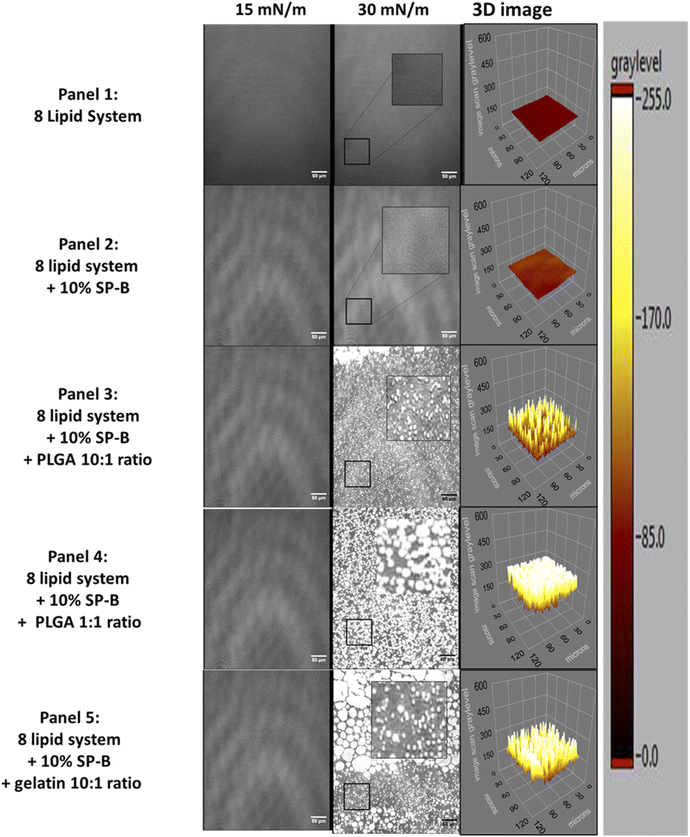
The lateral organization of the most complex biomimetic system is shown in Fig. 7. Lipid controls exhibited homogenous LE films until monolayer collapse (Fig. 7, panel 1). SP-B1–25 only induced very small intermittent domains that persisted until around 20 mN m−1 followed by homogenous film until monolayer collapse (Fig. 7, panel 2). The film organization with GNPs was similar to controls up to pressures closer to 30 mN m−1 before numerous bright clusters of varying size, including very large likely coalesced clusters are recorded, indicative of a drastically altered lateral film organization (Fig. 7, panel 3). Unlike previous reports31 these clusters only appeared after 30 mN m−1 but dominated the film structure. The more complex lipid/peptide system is more able to withstand the impact of NPs.
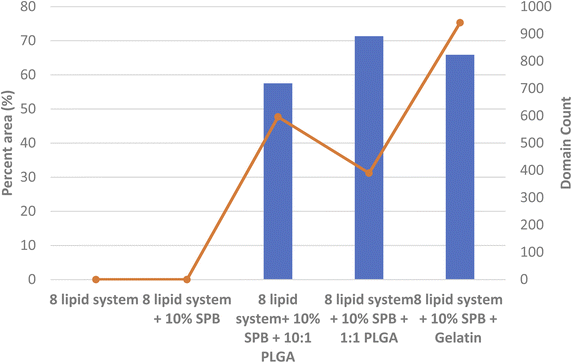
The complex mixture only shows distinct lateral domains (within the resolution of BAM imaging of 1 μm85) in the presence of nanomaterials. The different materials and concentrations have comparable effects in terms of overall domain area but GNP results in larger batches, again possible due to higher concentration within the film, nevertheless, the more heterogeneous film resulted in a higher overall domain frequency count (Fig. 8B).
Interestingly, we have observed similar 3D protrusion in biomimetic tear film models, where the polar lipid fractions is also enriched in rigid lipids such as DPPC, DPPE, ceramide and sphingomyelin. DPPC was among the lipids that showed indications for reversible multilayer formation, well below the actual film collapse.86 Indeed, analysis after cycling of these tear film models suggested that the reversible formation and reintegration of 3D protrusions could be important to prevent collapse during blinking.86
Positively charged SP-B and its 1–25 peptide has been shown to preferentially interact with anionic phospholipids.59 MD simulations highlighted the importance of PG lipids and cholesterol for SP–B interaction with lung surfactant. This was further supported by FRET studies supporting the preference for anionic lipids.87
As mentioned before, the hydrophobic SPs are important to facilitate reversible film folding during exhalation (squeeze-out), suggesting that they are, at least partially, anchored in LC lipids. DPPC is the main candidate for these interactions, and the observed significant decrease in domain size at 30 mN m−1 (Fig. S4†) suggests peptide interactions at the edge of LC domains that limit further size increases. The helical nature of these peptides56,88 could also promote the formation of helix bundles to further stabilize these structures. Similarly, smaller domains in the presence of 5% SP-B were reported for Survanta, which is a clinically derived bovine lung surfactant84 SP-B1–25 increased the line tension between the LE and LC phases of the PC system, which is energetically unfavorable, leading to smaller LC domain sizes.84
The presence of biocompatible gelatin NPs had a significant negative effect on the stability and integrity of three complex model system with the most prominent effects seen in the 8 lipid system. The bright lipid-NP clusters indicate negative effects of these biodegradable and biocompatible NPs that were thought to be safe.
4. Conclusion
A highly complex, biomimetic lung surfactant model was developed composed of the major lipids of saturated and unsaturated PCs and PGs and 2% (w/w) of the neutral lipid cholesterol. Furthermore, SP-B1–25 was added to assess its interaction with the major lipids of the lung surfactant and NPs.One of the major goals of our group is the development of a well-defined and representative model of the human lung surfactant to allow for fast initial in vitro evaluation of potential NP toxicology through the pulmonary route.
The interaction of SP-B1–25 with PC and PG lipids depended on the system. With saturated DPPC, we saw interaction with the LC phase in the form of larger LC domains. When unsaturated PC lipids were present, like in PC system, the interaction appeared to favour the LE phase. However, in both systems, we see an overall fluidization effect after a surface pressure of around 10 mN m−1 determined by compression moduli.
For negatively charged PG, interactions were mostly with the LE phase. Like for PC lipids, the peptide caused significant fluidization of PG lipids. As is expected due to positive charge at the NH2-terminal, a stronger effect in terms of significantly reduced collapse pressures was observed for PG systems.
The formation of functional multilayers as suggested here and reported in the literature is important and was shown to prevent premature buckling.89 These authors suggested that increased film stiffness could negatively affect the monolayer–multilayer conversion. The interaction of nanomaterials, which are much larger than the lipids, will likely reduce local fluidity and add another negative consequence on top of the induced changes in lateral organization.
We have previously studied GNPs and reported a negative effect on the lateral organization and stability of a simpler 5 lipid model system. These results were confirmed in the more complex system presented here. The elasticity as well as the lateral organization were significantly altered by NPs in all systems analyzed, including the most complex 8 lipid/peptide system.
The appearance of lipid-NP clusters at surface pressures below the monolayer–multilayer transition of lung surfactant indicates that key factors consider for NP drug delivery design such as the material’s biodegradability, biocompatibility, and particle size limit49,90–93 are not sufficient to assess the safety of the drug delivery application as shown here for materials considered as safe.
This ultimately indicates the benefits of having a cheaper, easily assembled and at the same time representative in vitro model for initial screening on NP toxicity. This concept was previously shown using polysorbate 80 coated NPs and DPPC models where a drastic decrease in collapse pressure corresponded to acute pulmonary toxicity in mice.94 As advances in nanomedicine continue to grow, more formulations will need to be evaluated for their potential to impair lung surfactant function. Ultimately, a broader bases screening would allow for correlation between in vitro and in vivo models95 which can be used to quickly verify potential toxicity of nanoparticles.
Abbreviations
| NP | Nanoparticle |
| LE | Liquid expanded |
| LC | Liquid condensed |
| BAM | Brewster angle microscopy |
| SP | Surfactant proteins |
| DPPC | Dipalmitoyl phosphatidylcholine |
| POPC | Palmitoyloleoyl phosphatidylcholine |
| 16-1PC | Palmitoylpalmitoleoyl phosphatidylcholine |
| DPPG | Dipalmitoyl phosphatidylglycerol |
| POPG | Palmitoyloleoyl phosphatidylglycerol |
| SOPG | Stearoyloleoyl phosphatidylglycerol |
| 16-1PG | Palmitoylpalmitoleoyl phosphatidylglycerol |
| Cm | Compressibility |
Author contributions
WD conducted the experimental work. WD and EJP were involved in experimental planning. PL and KS contributed to data analysis. WD, KS, PL, and EJP contributed to the writing of the final manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.Acknowledgements
This work was supported by a NSERC Discovery Grant to EJP and Queen Elizabeth II scholarship (QEII) to WD.References
- R. K. Harishchandra, M. Saleem and H. J. Galla, J. R. Soc., Interface, 2010, 7(1), S15–S26 CAS.
- J. Kreuter, Int. J. Pharm., 2007, 331, 1–10 CrossRef CAS PubMed.
- K. Strebhardt and A. Ullrich, Nat. Rev. Cancer, 2008, 8, 473–480 CrossRef CAS PubMed.
- R. Singh and J. W. Lillard, Exp. Mol. Pathol., 2009, 86, 215–223 CrossRef CAS PubMed.
- W. H. De Jong and P. J. A. Borm, Int. J. Nanomed., 2008, 3, 133–149 CrossRef CAS PubMed.
- E. Rytting, J. Nguyen, X. Wang and T. Kissel, Expert Opin. Drug Deliv., 2008, 5, 629–639 CrossRef CAS PubMed.
- E. R. Weibel, Physiol. Rev., 1973, 53, 419–495 CrossRef CAS.
- J. C. Sung, B. L. Pulliam and D. A. Edwards, Trends Biotechnol., 2007, 25, 563–570 CrossRef CAS PubMed.
- G. Scheuch, M. J. Kohlhaeufl, P. Brand and R. Siekmeier, Adv. Drug Deliv. Rev., 2006, 58, 996–1008 CrossRef CAS.
- A. Steimer, E. Haltner and C. M. Lehr, J. Aerosol Med., 2005, 18, 137–182 CrossRef CAS PubMed.
- T. Hussell and T. J. Bell, Nat. Rev. Immunol., 2014, 14, 81–93 CrossRef CAS PubMed.
- E. R. Weibel, Clin. Respir. Physiol., 1979, 15, 999–1013 CAS.
- M. Paranjpe and C. Müller-Goymann, Int. J. Mol. Sci., 2014, 15, 5852–5873 CrossRef CAS PubMed.
- M. M. Bailey and C. J. Berkland, Med. Res. Rev., 2009, 29, 196–212 CrossRef CAS PubMed.
- S. Schurch, J. Goerke and J. A. Clements, Proc. Natl. Acad. Sci. U. S. A., 1976, 73, 4698–4702 CrossRef CAS PubMed.
- R. Veldhuizen, K. Nag, S. Orgeig and F. Possmayer, Biochim. Biophys. Acta, Mol. Basis Dis., 1998, 1408, 90–108 CrossRef CAS.
- L. A. J. M. Creuwels, L. M. G. Van Golde and H. P. Haagsman, Lung, 1997, 175, 1–39 CrossRef CAS.
- M. Agassandian and R. K. Mallampalli, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2013, 1831, 612–625 CrossRef CAS PubMed.
- D. Salgado, R. Fischer, S. Schillberg, R. M. Twyman and S. Rasche, Front. Immunol., 2014, 5, 623 Search PubMed.
- E. Lopez-Rodriguez and J. Pérez-Gil, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 1568–1585 CrossRef CAS PubMed.
- R. L. Biltonen and D. Lichtenberg, Chem. Phys. Lipids, 1993, 64, 129–142 CrossRef CAS.
- M. Hallman, B. H. Feldman, E. Kirkpatrick and L. Gluck, Pediatr. Res., 1977, 11, 714–720 CrossRef CAS PubMed.
- C. Schleh and J. M. Hohlfeld, Inhalation Toxicol., 2009, 21, 97–103 CrossRef CAS PubMed.
- J. Pérez-Gil, J. Tucker, G. Simatos and K. M. Keough, Biochem. Cell Biol., 1992, 70, 332–338 CrossRef PubMed.
- S. Schurch, D. Schurch, T. Curstedt and B. Robertson, J. Appl. Physiol., 1994, 77, 974–986 CrossRef CAS PubMed.
- J. Perez-Gil and T. E. Weaver, Physiology, 2010, 25, 132–141 CrossRef CAS PubMed.
- J. R. Wright, Nat. Rev. Immunol., 2005, 5, 58–68 CrossRef CAS PubMed.
- E. Crouch and J. R. Wright, Annu. Rev. Physiol., 2001, 63, 521–554 CrossRef CAS PubMed.
- S. Orgeig, P. S. Hiemstra, E. J. A. Veldhuizen, C. Casals, H. W. Clark, A. Haczku, L. Knudsen and F. Possmayer, Respir. Physiol. Neurobiol., 2010, 173, S43–S54 CrossRef CAS PubMed.
- M. Dipasquale, O. Gbadamosi, M. H. L. Nguyen, S. R. Castillo, B. W. Rickeard, E. G. Kelley, M. Nagao and D. Marquardt, Chem. Res. Toxicol., 2020, 33, 2432–2440 Search PubMed.
- P. Lai, S. Nathoo, T. Ku, S. Gill, S. Azarmi, W. Roa, R. Löbenberg and E. J. Prenner, J. Biomed. Nanotechnol., 2010, 6, 145–152 Search PubMed.
- W. Daear, P. Lai, M. Anikovskiy and E. J. Prenner, J. Phys. Chem. B, 2015, 119, 5356–5366 CrossRef CAS.
- A. D. Postle, E. L. Heeley and D. C. Wilton, in Comparative Biochemistry and Physiology – A Molecular and Integrative Physiology, Elsevier Inc., 2001, vol. 129, pp. 65–73 Search PubMed.
- M. Amrein, A. Von Nahmen and M. Sieber, Eur. Biophys. J., 1997, 26, 349–357 CrossRef CAS PubMed.
- A. Kramer, A. Wintergalen, M. Sieber, H. J. Galla, M. Amrein and R. Guckenberger, Biophys. J., 2000, 78, 458–465 CrossRef CAS PubMed.
- W. Bernhard, H. P. Haagsman, T. Tschernig, C. F. Poets, A. D. Postle, M. E. Van Eijk and H. Von Der Hardt, Am. J. Respir. Cell Mol. Biol., 1997, 17, 41–50 CrossRef CAS PubMed.
- L. M. Nogee, G. Garnier, H. C. Dietz, L. Singer, A. M. Murphy, D. E. DeMello and H. R. Colten, J. Clin. Invest., 1994, 93, 1860–1863 CrossRef CAS.
- J. C. Clark, S. E. Wert, C. J. Bachurski, M. T. Stahlman, B. R. Stripp, T. E. Weaver and J. A. Whitsett, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7794–7798 CrossRef CAS PubMed.
- F. Bringezu, J. Ding, G. Brezesinski, A. J. Waring and J. A. Zasadzinski, Langmuir, 2002, 18, 2319–2325 CrossRef CAS.
- M. M. Lipp, K. Y. C. Lee, J. A. Zasadzinski and A. J. Waring, Science, 1996, 273, 1196–1199 CrossRef CAS.
- P. Lai, W. Daear, R. Löbenberg and E. J. Prenner, Colloids Surf., B, 2014, 118, 154–163 CrossRef CAS PubMed.
- E. Locatelli and M. C. Franchini, J. Nanoparticle Res., 2012, 14, 1–17 CrossRef.
- F. Danhier, E. Ansorena, J. M. Silva, R. Coco, A. Le Breton and V. Préat, J. Control. Release, 2012, 161, 505–522 CrossRef CAS PubMed.
- J. M. Anderson and M. S. Shive, Adv. Drug Deliv. Rev., 1997, 28, 5–24 CrossRef CAS.
- D. Abrams, Y. Huang, S. McQuarrie, W. Roa, H. Chen, R. Löbenberg, S. Azarmi, G. G. Miller and W. H. Finlay, J. Pharm. Pharm. Sci., 2006, 9(1), 124–132 Search PubMed.
- K. S. Birdi, in Lipid and Biopolymer Monolayers at Liquid Interfaces, Springer US, 1989, pp. 57–147 Search PubMed.
- R. E. Brown and H. L. Brockman, Methods Mol. Biol., 2007, 398, 41–58 CrossRef CAS PubMed.
- M. Broniatowski, M. Flasiński, P. Dynarowicz-Ł
![[A with combining cedilla]](https://www.rsc.org/images/entities/char_0041_0327.gif) tka and J. Majewski, J. Phys. Chem. B, 2010, 114, 9474–9484 CrossRef CAS PubMed.
tka and J. Majewski, J. Phys. Chem. B, 2010, 114, 9474–9484 CrossRef CAS PubMed. - S. Gill, R. Löbenberg, T. Ku, S. Azarmi, W. Roa and E. J. Prenner, J. Biomed. Nanotechnol., 2007, 3, 107–119 CrossRef CAS.
- E. Keating, Y. Y. Zuo, S. M. Tadayyon, N. O. Petersen, F. Possmayer and R. A. W. Veldhuizen, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 1225–1234 CrossRef CAS.
- J. Pérez-Gil, Biochim. Biophys. Acta, Biomembr., 2008, 1778, 1676–1695 CrossRef PubMed.
- J. Ding, D. Y. Takamoto, A. Von Nahmen, M. M. Lipp, K. Y. C. Lee, A. J. Waring and J. A. Zasadzinski, Biophys. J., 2001, 80, 2262–2272 CrossRef CAS PubMed.
- T. Gröger, S. Nathoo, T. Ku, C. Sikora, R. J. Turner and E. J. Prenner, Chem. Phys. Lipids, 2012, 165, 216–224 CrossRef.
- G. D. Fidelio, B. Maggio and F. A. Cumar, Biochem. J., 1982, 203, 717–725 CrossRef CAS PubMed.
- D. Y. Takamoto, M. M. Lipp, A. Von Nahmen, K. Y. C. Lee, A. J. Waring and J. A. Zasadzinski, Biophys. J., 2001, 81, 153–169 CrossRef CAS.
- J. Pérez-Gil, C. Casals and D. Marsh, Biochemistry, 1995, 34, 3964–3971 CrossRef PubMed.
- M. L. Longo, A. M. Bisagno, J. A. N. Zasadzinski, R. Bruni and A. J. Waring, Science, 1993, 261, 453–456 CrossRef CAS PubMed.
- K. Nag, S. G. Taneva, J. Perez-Gil, A. Cruz and K. M. W. Keough, Biophys. J., 1997, 72, 2638–2650 CrossRef CAS PubMed.
- J. Liekkinen, G. Enkavi, M. Javanainen, B. Olmeda, J. Pérez-Gil and I. Vattulainen, J. Mol. Biol., 2020, 432, 3251–3268 CrossRef CAS PubMed.
- Z. Leonenko, S. Gill, S. Baoukina, L. Monticelli, J. Doehner, L. Gunasekara, F. Felderer, M. Rodenstein, L. M. Eng and M. Amrein, Biophys. J., 2007, 93, 674–683 CrossRef CAS.
- S. Taneva and K. M. Keough, Biophys. J., 1994, 66, 1137–1148 CrossRef CAS PubMed.
- M. A. Oosterlaken-Dijksterhuis, H. P. Haagsman, L. M. G. van Golde and R. A. Demel, Biochemistry, 1991, 30, 10965–10971 CrossRef CAS PubMed.
- S. Schürch, F. H. Y. Green and H. Bachofen, Biochim. Biophys. Acta, Mol. Basis Dis., 1998, 1408, 180–202 CrossRef.
- S. L. Duncan and R. G. Larson, Biochim. Biophys. Acta, Biomembr., 2010, 1798, 1632–1650 CrossRef CAS PubMed.
- S. G. Taneva and K. M. W. Keough, Biochemistry, 1994, 33, 14660–14670 CrossRef CAS.
- Y. Y. Zuo, R. A. W. Veldhuizen, A. W. Neumann, N. O. Petersen and F. Possmayer, Biochim. Biophys. Acta, Biomembr., 2008, 1778, 1947–1977 CrossRef CAS.
- R. Koynova and M. Caffrey, Biochim. Biophys. Acta, Rev. Biomembr., 1998, 1376, 91–145 CrossRef CAS.
- A. K. Soutar, H. J. Pownall, A. S. Hu and L. C. Smith, Biochemistry, 1974, 13, 2828–2836 CrossRef CAS PubMed.
- C. Vilchèze, T. P. W. McMullen, R. N. McElhaney and R. Bittmana, Biochim. Biophys. Acta, Biomembr., 1996, 1279, 235–242 CrossRef.
- J. R. Silvius and R. N. McElhaney, Chem. Phys. Lipids, 1979, 25, 125–134 CrossRef CAS.
- J. Li, X. Wang, T. Zhang, C. Wang, Z. Huang, X. Luo and Y. Deng, Asian J. Pharm. Sci., 2015, 10, 81–98 CrossRef.
- A. K. Panda, K. Nag, R. R. Harbottle, K. Rodriguez-Capote, R. A. W. Veldhuizen, N. O. Petersen and F. Possmayer, Am. J. Respir. Cell Mol. Biol., 2004, 30, 641–650 CrossRef CAS PubMed.
- H. Zhang, Y. E. Wang, Q. Fan and Y. Y. Zuo, Langmuir, 2011, 27, 8351–8358 CrossRef CAS PubMed.
- J. Goerke, Biochim. Biophys. Acta, Mol. Basis Dis., 1998, 1408, 79–89 CrossRef CAS.
- J. C. Watkins, Biochim. Biophys. Acta, Lipids Lipid Metab., 1968, 152, 293–306 CrossRef CAS.
- A. D. Bangham, C. J. Morley and M. C. Phillips, Biochim. Biophys. Acta, Lipids Lipid Metab., 1979, 573, 552–556 CrossRef CAS.
- O. Borozenko, M. Faral, S. Behyan, A. Khan, J. Coulombe, C. Dewolf and A. Badia, ACS Appl. Nano Mater., 2018, 1, 5268–5278 CrossRef CAS.
- N. van Bavel, P. Lai, R. Loebenberg and E. J. Prenner, Nanomedicine, 2022, 17, 827–843 CrossRef CAS PubMed.
- M. M. Hossain and T. Kato, Langmuir, 2000, 16, 10175–10183 CrossRef CAS.
- H. M. McConnell and V. T. Moy, J. Phys. Chem., 1988, 92, 4520–4525 CrossRef CAS.
- J. Miones, J. M. Rodríguez Patino, O. Conde, C. Carrera and R. Seoane, Colloids Surf., A, 2002, 203, 273–286 CrossRef.
- R. I. S. Romao, Q. Ferreira, J. Morgado, J. M. G. Martinho and A. M. P. S. G. Da Silva, Langmuir, 2010, 26, 17165–17177 CrossRef CAS PubMed.
- J. Ding, I. Doudevski, H. E. Warriner, T. Alig, J. A. Zasadzinski, A. J. Waring and M. A. Sherman, Langmuir, 2003, 19, 1539–1550 CrossRef CAS.
- P. Dhar, E. Eck, J. N. Israelachvili, D. W. Lee, Y. Min, A. Ramachandran, A. J. Waring and J. A. Zasadzinski, Biophys. J., 2012, 102, 56–65 CrossRef CAS PubMed.
- J. Meunier, Colloids Surf., A, 2000, 171, 33–40 CrossRef CAS.
- M. Patterson, H. J. Vogel and E. J. Prenner, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 403–414 CrossRef CAS PubMed.
- E. J. Cabré, L. M. S. Loura, A. Fedorov, J. Perez-Gil and M. Prieto, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 1717–1725 CrossRef.
- F. Possmayer, S. B. Hall, T. Haller, N. O. Petersen, Y. Y. Zuo, J. Bernardino de la Serna, A. D. Postle, R. A. W. Veldhuizen and S. Orgeig, Respir. Physiol. Neurobiol., 2010, 173, S55–S64 CrossRef CAS.
- M. Al-Saiedy, A. Tarokh, S. Nelson, K. Hossini, F. Green, C. C. Ling, E. J. Prenner and M. Amrein, Biochim. Biophys. Acta, Biomembr., 2017, 1859, 1372–1380 CrossRef CAS.
- G. Oberdörster, A. Elder and A. Rinderknecht, J. Nanosci. Nanotechnol., 2009, 9, 4996–5007 CrossRef PubMed.
- E. Guzmán, L. Liggieri, E. Santini, M. Ferrari and F. Ravera, Colloids Surf., A, 2012, 413, 280–287 CrossRef.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef.
- D. Q. Arick, Y. H. Choi, H. C. Kim and Y. Y. Won, Adv. Colloid Interface Sci., 2015, 225, 218–228 CrossRef CAS PubMed.
- M. H. D. K. Al-Hallak, S. Azarmi, C. Sun, P. Lai, E. J. Prenner, W. H. Roa and R. Löbenberg, AAPS J., 2010, 12, 294–299 CrossRef CAS PubMed.
- M. A. Selo, J. A. Sake, K. J. Kim and C. Ehrhardt, Adv. Drug Delivery Rev., 2021, 177, 113862 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02859j |
| ‡ Both contributed equally and are listed in alphabetical order. |
| § Current address: Rane Pharmaceutical, Edmonton, AB, Canada. |
| This journal is © The Royal Society of Chemistry 2022 |