Open Access Article
Open Access ArticleColorimetric and naked-eye detection of arsenic(III) using a paper-based microfluidic device decorated with silver nanoparticles†
Arezoo Saadatiabc,
Fatemeh Farshchid,
Mohammad Hasanzadeh *be,
Yuqian Liu
*be,
Yuqian Liu a and
Farzad Seidi
a and
Farzad Seidi *a
*a
aJiangsu Co-Innovation Center for Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing 210037, China. E-mail: f_seidi@njfu.edu.cn
bPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
cCentral European Institute of Technology, Brno University of Technology, Brno CZ-612 00, Czech Republic
dFundação Oswaldo Cruz, Instituto Oswaldo Cruz, Laboratório de Biologia Molecular e Doenças Endêmicas, Avenida Brasil No. 4365 – Manguinhos, Rio de Janeiro 21040-900, RJ, Brazil
eNutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
First published on 8th August 2022
Abstract
Arsenic (As) as a metal ion has long-term toxicity and its presence in water poses a serious threat to the environment and human health. So, rapid and accurate recognition of traces of As is of particular importance in environmental and natural resources. In this study, a fast and sensitive colorimetric method was developed using silver nano prisms (Ag NPrs), cysteine-capped Ag NPrs, and methionine-capped Ag NPrs for accurate detection of arsenic-based on transforming the morphology of silver nanoparticles (AgNPs). The generated Ag atoms from the redox reaction of silver nitrate and As(III) were deposited on the surface of Ag NPrs and their morphology changed to a circle. The morphological changes resulted in a change in the color of the nanoparticles from blue to purple, which was detectable by the naked eye. The rate of change was proportional to the concentration of arsenic. The changes were also confirmed using UV-Vis absorption spectra and showed a linear relationship between the change in adsorption peak and the concentration of arsenic in the range of 0.0005 to 1 ppm with a lower limit of quantification (LLOQ) of 0.0005 ppm. The proposed probes were successfully used to determine the amount of As(III) in human urine samples. In addition, modified microfluidic substrates were fabricated with Ag NPrs, Cys-capped Ag NPrs, and methionine-capped Ag NPrs nanoparticles that are capable of arsenic detection in the long-time and can be used in the development of on-site As(III) detection kits. In addition, silver nanowires (AgNWs) were used as a probe to detect arsenic, but good results were not obtained in human urine specimens and paper microfluidic platforms. In this study, for the first time, AgNPs were developed for optical colorimetric detection of arsenic using paper-based microfluidics. Ag NPrs performed best in both optical and colorimetric techniques. Therefore, they can be a promising option for the development of sensitive, inexpensive, and portable tools in the environmental and biomedical diagnosis of As(III).
1. Introduction
As a heavy metal, arsenic is the twentieth most plentiful element in the Earth's crust and the twelfth most plentiful element in the human body and is an ongoing pollutant owing to long-term toxicity. This substance is present in various forms with different oxidation states (0 in elemental arsenic, −3 in AsH3, (IV) in arsenic acid, (III) in arsenous acid, and (II) in As4S4).1 Arsenic in aqueous media is predominantly inorganic in the form of As5+ (pentavalent arsenate) or As3+ (oxyanions of trivalent arsenite).2 As3+ (as H3AsO3) is 60 times more toxic than As5+ (as H3AsO4), and its presence in drinking water poses a serious threat to the environment and human health. According to the World Health Organization, the permissible level of arsenic in drinking water is less than 10 ppb, and excessive consumption leads to increased kidney, brain, and heart disease and increases the risk of skin, lung, and bladder cancer.3,4Therefore, the detection of arsenic levels is of particular importance both in the environment and natural resources and in the human body. For this purpose, various analytical methods such as ICP-MS (inductively coupled plasma mass spectrometry),5 atomic absorption/emission spectroscopy,6 ion-selective electrode,7 fluorescence spectrometry,8 voltammetry,9 X-ray fluorescence,10 surface enhanced Raman scattering,11 spectrophotometry,12 and high-performance liquid chromatography13 have been developed. However, these techniques require expensive instruments, pure chemicals, trained labor, various treatment processes, and laboratory environments. On the other hand, developing countries do not have the proper infrastructure to perform complex tasks. So, researchers are using simple methods like colorimetric and UV-Vis spectroscopy as an alternative method.14 Although UV-Vis spectroscopy has potential advantages such as low cost and ease of use, they have limited use for on-site applications due to the need for electricity. Therefore, a cheap and easy method such as colorimetric detection in the solution phase can be a good alternative, which seems to be the most popular method among users due to time and cost savings.15 To increase the sensitivity, speed, accuracy, efficiency, and cost-effectiveness of colorimetric sensors, various types of advanced nanoparticles (NPs) have been utilized in this matter.16–23
Nanostructures have attracted much attention in the field of metal ion analysis due to their unique optical traits. The basic principle of nanoparticle-based detection of metal ions is the accumulation of NPs because of interaction with heavy metal ions and their color change. The change in color intensity of the nanoparticle solution is proportional to the analyte concentration, which is monitored using UV-Vis spectroscopy.24,25 Metal NPs such as silver and gold have attracted a lot of attention in this field due to their high extinction coefficient and unique optical properties. These properties are known as surface plasmon resonance (SPR) and are attributed to mass bipolar oscillation, making silver nanoparticles (AgNPs) desirable for colorimetric-based detection of heavy metal ions.26 Because the position and intensity of the absorption band in the visible spectrum change because of the interaction between the analyte and the NPs, this process can be seen even with the naked eye. The optical properties of NPs are proportional to their size and structure.27 Silver triangular NPs have strong SPR properties and can be adjusted across the visible area by adjusting the length and thickness of the edges and the tip morphology of the nanoplate. These structures have special optical, chemical, and electronic properties compared to spherical AgNPs due to their three sharp heads.28 However, colorimetric-based sensors in the solution phase have limitations such as the need for trained personnel and complex steps. To develop cheaper, more reliable, and disposable methods with on-site diagnostics without the need for electrical connections, researchers began modifying existing methods or exploring new diagnostic strategies.
The microfluidic systems were proposed by scientists as a suitable solution for the development of simple and inexpensive tools.29–31 Paper-based microfluidics are made by creating hydrophilic channels using hydrophobic barriers and are as portable as traditional microfluidic devices.32 In addition, they can perform multiple measurements and process small volumes of fluids. Traditional microfluidics is made of glass, silicon, and polymers (such as PDMS (polydimethylsiloxane) and poly(methyl methacrylate) (PMMA)) and requires a cleanroom to build. Interestingly paper-based microfluidics is made from affordable materials and does not require a cleanroom or computer-controlled pumps to operate. These features make paper microfluidics the ideal medium for developing point-of-care diagnostic tests in all areas.33,34 In this study, a novel diagnostic platform was presented for the rapid and accurate recognition of As(III) using UV-Vis spectroscopy technique and colorimetric assay. AgNPs containing Ag NPrs, Cys-capped Ag NPrs, Met-capped Ag NPrs, and AgNWs were first used as diagnostic probes. The change in color and absorption spectrum as a result of the interaction between the sensing probes and the arsenic ions indicates the ability of the developed method to sensitive recognition of arsenic in real samples. Therefore, the ability of probes to detect arsenic in human urine samples was evaluated, and except for AgNWs, other probes performed well. In addition, the ability of the designed microfluidics in the arsenic on-site analysis was also evaluated. This simple and inexpensive strategy has shown potential for the development of arsenic diagnostic kits.
2. Experimental
2.1. Instruments
U-3010 spectrophotometer (Hitachi, Japan) was employed for UVVis spectroscopy analysis. The particle size and morphology of NPs were assessed by transmission electron microscope (TEM) (Adelaide, Australia-with an operating voltage of 200 kV). The dynamic size of NPs was investigated using an atomic force microscope (AFM) with Nanosurf (AG Gräubernstrasse 12, 4410 Liestal Switzerland) in a tapping mode. High-resolution field-emission scanning electron microscopy (FE-SEM, Hitachi-Su8020, Czech-with a working voltage of 3 kV) was applied for assessment of synthesized NPs surface morphology. Energy-dispersive spectroscopy (EDS) was carried out for the appraisal of the elemental composition of NPs. Surface charge and size distribution were also evaluated by zeta potential measurement, Zetasizer Ver. 7.11 and dynamic light scattering (DLS) analysis (Malvern Instruments Ltd, MAL1032660, England).2.2. Materials and reagents
The solution of ions (As(III), Ti(II), Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mg(II), Se(IV), Ba(II), Sr(II), Te(II), Mo(II), K(I), Cr(II), Bi(III), Zn(II), Li(II), Mn(II), B(III), Na(I), Ca(II), Al(III), Si(II), Co(II), Cs(II), Ni(II)) with 1 ppm concentration were acquired from Chemlab Company (Zedelgem, Belgium). Tri-sodium citrate (TSC) (Na3C6H5O7) (99.0%), L-cysteine (97%), methionine (99%), methanol (≥99.8%), PVP K-30 (polyvinyl pyrrolidone), sodium borohydride (NaBH4), silver nitrate (AgNO3), hydrogen peroxide (H2O2, 30 wt%) and ethylene glycol (EG) were attained from Sigma-Aldrich (Ontario, Canada).2.3. Synthesis of optical probes
2.4. Optical determination of As(III)
Optical identification of arsenic(III) by using Ag NPs as a sensing probe was carried out at room temperature in the wavelength range of 200–800 nm. To do so, As(III) with an equal volume ratio was added to the sensing probe and the UV-Vis were recorded in a quartz cuvette.2.5. Colorimetric detection of As(III)
For the colorimetric recognition of As(III), the color change was monitored by a mobile phone camera under a natural light source in a solution and paper-based analytical device.2.6. µPCD manufacture
The paper-based microfluidic chip pattern was created by CorelDRAW software. The chips have substrate storage that is connected through eight channels with eight detection zones (Scheme S1 (see ESI†)). These chips were produced using the technique developed in our previous report.373. Results and discussion
3.1. Selection of Ag nanoparticle as a sensing probe for detection of As(III)
The interaction of NPs with heavy metal ions leads to the accumulation of NPs and their color change, which can be considered as the basic principle of colorimetric identification of metal ions using NPs.24,38 A variety of NPs have been used in colorimetric studies, among which metal NPs of Au and Ag have attracted the attention of researchers.24,39 These NPs have been widely used in the efficient analysis of toxic heavy metals due to their unique optical properties and high extinction coefficient.40 These properties are known as SP (surface plasmon) which is attributed to combined bipolar oscillation.41 This phenomenon has made AgNPs a very desirable and practical option for detecting different types of heavy metals due to interaction with the analyte and changes in the position and intensity of absorption of the visible spectrum. Even this process can be seen with the naked eye.42 Therefore, in this work, AgNPrs and AgNWs were used as sensing probes for the accurate monitoring of As(III).Because smaller colloidal particles can absorb visible light through SPR motion, capping or stabilizing agents can be used to prevent NPs from binding or accumulating with other particle components.43,44 Hence, cysteine (Cys) and methionine (Met) were used as additives. The additives are attached to the Ag NPrs surface via an Ag–S bond by thiol terminals and modify the sensing probes. The enclosure of NPs with additives leads to electrostatic repulsion between them. Silver atoms are strongly bonded to electron-rich sites due to their performance among the highest.38,45 These cumulative negative charges carried through the nanoparticle domain are separate and increase the stability of the NPs. Cysteine acts as an adsorbent due to its thiol, amine, and carboxylate sites.46 Arsenic has a positive charge and can strongly interact with the negative charge of oxygen (O–H) in the additive-sensing probe system and lead to aggregation which is necessary for the colorimetric recognition of the target.47,48
3.2. Characterization of sensing probes
Optimization of additive-probe ratio. As previously mentioned, arsenic as a semi-metal and toxic element is very dangerous to human health and can lead to a variety of diseases, including cancer.59 Therefore, the development of simple and inexpensive systems to monitor it is very valuable. So, several sensing probes were evaluated for rapid and accurate identification of As(III) in real samples. Due to the more favorable results obtained from Ag NPrs, various additives were used to cap them. The nature and number of additives affect the optical properties of NPs. Hence, different volume ratios of the amino acids cysteine and methionine (as additives) were mixed with Ag NPrs (1 to 6, including Ag NPrs, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 3
3 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Ag NPrs and additive, respectively). After 10 minutes of storage at room temperature, color changes were visually examined. The results are presented in Fig. S20 (see ESI†), which reveals that the best case is related to the 1
1 ratio of Ag NPrs and additive, respectively). After 10 minutes of storage at room temperature, color changes were visually examined. The results are presented in Fig. S20 (see ESI†), which reveals that the best case is related to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratios of additive and Ag NPrs. When a 1
3 ratios of additive and Ag NPrs. When a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio additives is combined with a probe, there is no significant change in the color of the probe, whereas, in the presence of the analyte, the color changes obtained from the capped probe are different from the non-capped probe. Then, arsenic (1 ppm) was added to the sensing probes in a 1
3 ratio additives is combined with a probe, there is no significant change in the color of the probe, whereas, in the presence of the analyte, the color changes obtained from the capped probe are different from the non-capped probe. Then, arsenic (1 ppm) was added to the sensing probes in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and the color change was evaluated in the presence of it. Under experimental conditions, the cysteine and methionine molecules attached to the surface of Ag NPrs are negatively charged. Electrostatic interactions between the positive charge of arsenic and the surface of modified sensing probes can lead to the accumulation of AgNPs in a mixture solution.
1 ratio, and the color change was evaluated in the presence of it. Under experimental conditions, the cysteine and methionine molecules attached to the surface of Ag NPrs are negatively charged. Electrostatic interactions between the positive charge of arsenic and the surface of modified sensing probes can lead to the accumulation of AgNPs in a mixture solution.
3.3. Optical detection of As(III) in solution
Optical examinations were performed using UV-Vis spectroscopic technique in solutions prepared from sensing probes based on AgNPs. First, the capability of the introduced probes in the detection of arsenic was evaluated. Then, the sensitivity and selectivity of the sensing probes in the standard and real samples were investigated.Due to their free electrons, Ag NPs produce a light effect known as the surface plasmon absorption band, which results from the combined vibration of the electron NPs in the light wave resonance at the aqueous suspension.58 Increasing the size of the NPs causes the plasmon absorption to move towards a lower energy wavelength and the color red. In addition, the peak of absorption becomes wider, which indicates the growth of the suspension and the formation of aggregation. The presence of an analyte leads to a constant dielectric change that appears as a change in color and a change in the location and intensity of absorption.66 So, UV-Vis spectroscopy of the sensing probes (AgNPrs, Cys-capped AgNPrs, Met-capped AgNPrs, and AgNWs) was recorded before and after interaction with the candidate analyte (with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
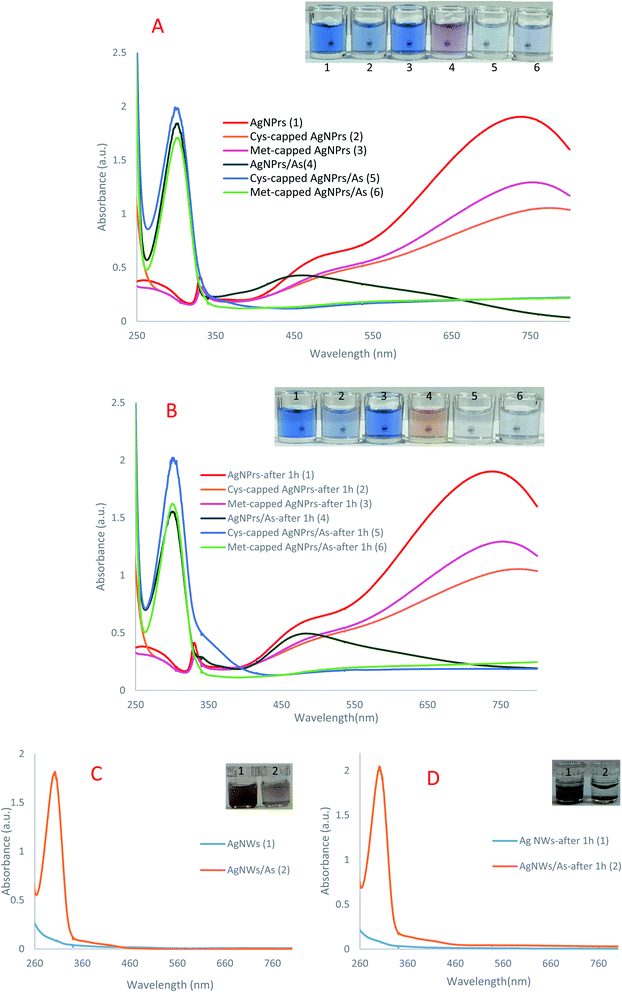
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In the UV-Vis absorption spectra recorded in the presence of arsenic (Fig. 1), an absorption peak of 305 nm is observed, which confirms the accumulation in the NP's presence of the analyte.
1). In the UV-Vis absorption spectra recorded in the presence of arsenic (Fig. 1), an absorption peak of 305 nm is observed, which confirms the accumulation in the NP's presence of the analyte.
In other words, the plasmon traits of the NPs are altered in the presence of analytes. As can be seen in colorimetric studies, Ag NPrs are blue with a negative surface charge (Scheme 1). As a result of the addition of positively charged arsenic, AgNO3 is reduced to Ag atoms deposited on the Ag NPrs surface, resulting in deformation from Ag NPrs to Ag NPs.67 As can be seen, the prepared Ag NPrs are blue and have UV-Vis absorption at wavelengths of 337, 477, and 705 nm. The first two absorption bands (337 and 477 nm) correspond to out-of-plane and in-plane quadrupole resonance of Ag NPrs, respectively.49,50 The 705 nm band can also be attributed to the Ag NPrs in-plane dipole resonance. The peak of in-plane bipolar resonance is strongly dependent on the Ag NPrs shape. This phenomenon, which is used in the design of sensors based on wavelength changes, can be attributed to changes in the morphology of Ag NPrs and the tendency of newly produced Ag atoms to deposit at the Ag NPrs surface.68
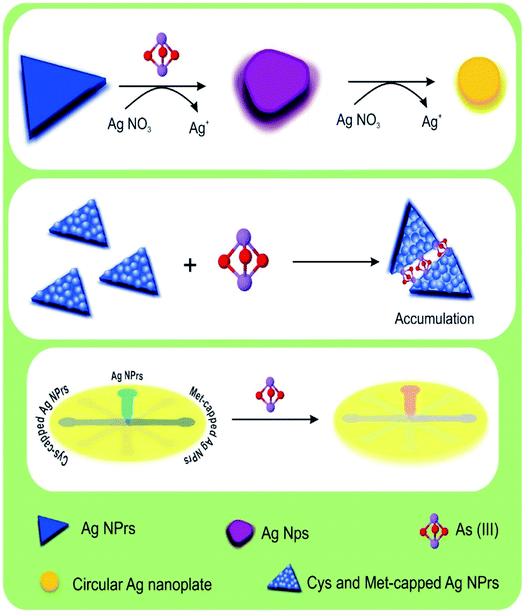 | ||
| Scheme 1 Schematic illustration of naked-eye detection of As(III) by colorimetric assay based on AgNPs. | ||
The resulting deformation is also seen visually in the color of the solution, allowing arsenic analysis in both the solution and leading to a change of UV-Vis spectrum wavelengths. As time goes on and the redox reaction between arsenic and silver ions is completed, the newly produced silver atoms on the Ag NPrs produce circular nanoplates that change the color of the solution to orange.28 Despite discoloration of the detection probe solutions after one hour in the presence of the analyte, no significant changes in the absorption peak intensity were observed at the 300 nm wavelength. In fact, at the beginning of the Ag NPrs interaction with As(III), AgNO3 is reduced to Ag atoms deposited on the surface of Ag NPrs, converting the Ag NPrs to Ag NPs. Morphological changes lead to a change in the color of NPs from blue to purple, which can be detected by the naked eye. Over time, the redox reaction between the arsenic and silver ions was completed, and the newly formed silver atoms on the Ag NPrs formed circular nanoplates, changing the color of the orange solution.
Under experimental conditions, the cysteine and methionine molecules attached to the surface of Ag NPrs are negatively charged. Electrostatic interactions between the positive charge of arsenic and the surface of modified detection probes can lead to the accumulation of Ag NPs in solution. After one hour, no change in the color of the methionine-modified sensing probe was observed, while the peak intensity of adsorption decreased. In the cysteine-modified probe, a slight discoloration was observed in the solution after one hour, but no significant changes were observed in the peak intensity of adsorption.
Ag NWs have an anisotropic form and induce two plasmon bands including a longitudinal plasmon band and a transverse plasmon band (corresponding to the electronic change along the short axis of the rod).58 This feature distinguishes them for use in a wide range of spectroscopic studies. As can be seen, no significant discoloration is seen in Ag NWs probes in the presence of analytes, while spectroscopic examination shows the ability of this probe to detect arsenic. Over time, no change in spectroscopic and colorimetric results was observed and only sediment was observed. These results indicate that the proposed sensing probes are capable of arsenic(III) detection by new mechanisms.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
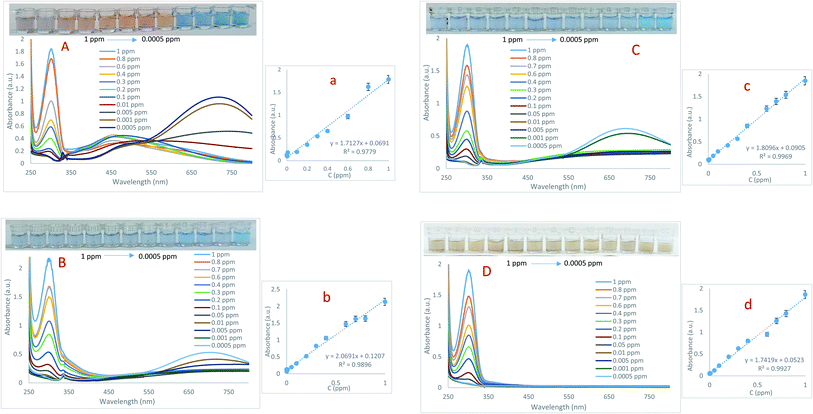
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The absorption spectra recorded in the range of 250 to 800 nm are exhibited in Fig. 2. In addition, the adsorption versus concentration ratio is also presented in Fig. 2. Then, important analytical parameters such as linear range and lower limit of quantification (LLOQ) were obtained from calibration curves plotted through absorption changes in the 304 nm band at different concentrations of As(III). As can be seen, there is a significant linear relationship between the absorbance of probes in the concentration range of 0.0005 to 1 ppm of As(III). At high concentrations of arsenic, in addition to adsorption in the 305 nm band, a displacement at the peak of in-plane dipolar resonance (705 nm) is also observed in the LSPR spectrum, which is accompanied by a change in solution color from blue to yellow. This phenomenon can be attributed to changes in the morphology of Ag NPrs and the tendency of silver atoms to deposit on their surface.28 It should be noted that the amount of adsorption in this band is not related to the concentration of the analyte. At low concentrations of arsenic, the adsorption decreases in the 304 nm band and increases in the 715 nm band, which can be attributed to the fact that the morphology of sensing probes (Ag NPrs, Cys-capped Ag NPrs, Met-capped AgNPrs) did not change at low concentrations of the analyte. A comparison of developed Ag NPs-based colorimetric biosensors for the detection of As(III) is presented in Table 1.
1. The absorption spectra recorded in the range of 250 to 800 nm are exhibited in Fig. 2. In addition, the adsorption versus concentration ratio is also presented in Fig. 2. Then, important analytical parameters such as linear range and lower limit of quantification (LLOQ) were obtained from calibration curves plotted through absorption changes in the 304 nm band at different concentrations of As(III). As can be seen, there is a significant linear relationship between the absorbance of probes in the concentration range of 0.0005 to 1 ppm of As(III). At high concentrations of arsenic, in addition to adsorption in the 305 nm band, a displacement at the peak of in-plane dipolar resonance (705 nm) is also observed in the LSPR spectrum, which is accompanied by a change in solution color from blue to yellow. This phenomenon can be attributed to changes in the morphology of Ag NPrs and the tendency of silver atoms to deposit on their surface.28 It should be noted that the amount of adsorption in this band is not related to the concentration of the analyte. At low concentrations of arsenic, the adsorption decreases in the 304 nm band and increases in the 715 nm band, which can be attributed to the fact that the morphology of sensing probes (Ag NPrs, Cys-capped Ag NPrs, Met-capped AgNPrs) did not change at low concentrations of the analyte. A comparison of developed Ag NPs-based colorimetric biosensors for the detection of As(III) is presented in Table 1.
As can be seen, there is no report on the detection of As(III) based on the sensing probes introduced in this study, which is one of the benefits of this work. The ability of proposed probes for arsenic examination was evaluated using UV-Vis spectroscopic technique. A comparison of the analytical performance of the presented sensor with previous studies shows that this sensor is more sensitive to them and can detect As(III) in a wider linear range. In addition, colorimetric studies were performed using paper-based microfluidics, which indicates the potential performance of these microfluidics in the development of As(III) diagnostic kits.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the prepared mixtures were mixed with a 1
1. Then, the prepared mixtures were mixed with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with candidate probes, and their interaction was evaluated using UV-Vis spectroscopic technique. Colorimetric analysis was also performed using a mobile camera. The recorded absorption spectra and the calibration curves resulting from the adsorption changes at different concentrations are obtained (Fig. S21 (see ESI†)). The results revealed that the AgNWs probe was not able to detect As(III) in the urine sample. Analytical parameters obtained from other probes are presented in Table S1 (see ESI†). As it turns out, the Cys-capped Ag NPrs probe detects a wide linear range of As(III), and compared to the other two probes (AgNPrs and Met-capped AgNPrs) it has a lower detection limit. Therefore, it can be said that for detection of As(III) in human urine samples, the Cys-capped Ag NPrs probe exhibits better performance.
1 ratio with candidate probes, and their interaction was evaluated using UV-Vis spectroscopic technique. Colorimetric analysis was also performed using a mobile camera. The recorded absorption spectra and the calibration curves resulting from the adsorption changes at different concentrations are obtained (Fig. S21 (see ESI†)). The results revealed that the AgNWs probe was not able to detect As(III) in the urine sample. Analytical parameters obtained from other probes are presented in Table S1 (see ESI†). As it turns out, the Cys-capped Ag NPrs probe detects a wide linear range of As(III), and compared to the other two probes (AgNPrs and Met-capped AgNPrs) it has a lower detection limit. Therefore, it can be said that for detection of As(III) in human urine samples, the Cys-capped Ag NPrs probe exhibits better performance.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
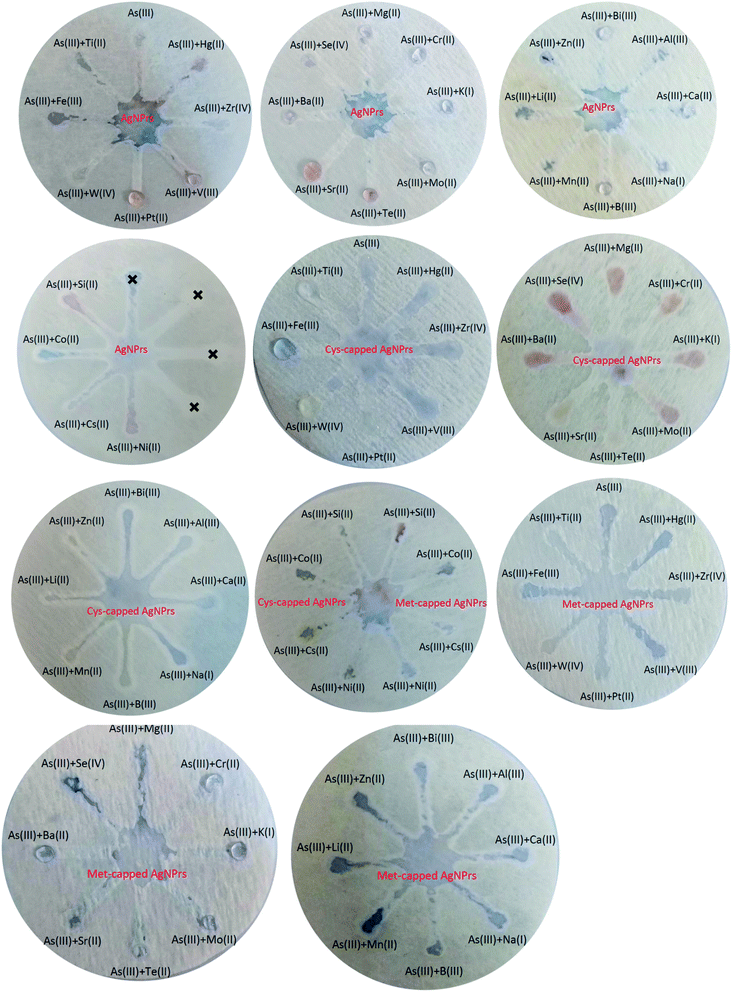
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). Colorimetric and optical examinations were performed. The results indicated that the probes utilized could detect the target analyte in the presence of other metal ions and most metal ions did not interfere with the detection of the target analyte. However, some ions interfere with the detection of arsenic by increasing the amount of adsorption (such as Mg(II), Cr(III), K(I), Mn(II), etc.) or decreasing it (such as Ti(II), Si(II), Cs(II), etc.). In both cases of interfering investigation (separately and in combination with the analyte) in the presence of some ions, a shift at the peak of the absorption band is observed. Changes in the absorption band in different optical probes are different. For example, in Ag NPrs optical probes, the adsorption band is shifted in the presence of Ti(II), Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mg(II), Se(IV), Mo(II), Cr(III), Bi(III), Zn(II) and Cs(II), as well as in the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mg(II), Mo(II), Bi(III) and Zn(II). Absorbance band shift was observed in the presence of Ti(II), Fe(III), Pt(II), V(III), Zr(IV), Hg(II), Se(IV), Te(II), Mo(II), Bi(III), Si(II) and Cs(II) and the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Zr(IV), Mg(II), Ba(II), Sr(II), Mo(II), K(I), Bi(III), Zn(II), Li(II) and Al(III) using a Cys-capped Ag NPrs probe. Also in the Met-capped Ag NPrs probe, the absorption band shift was observed in the presence of Ti(II), Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mo(II), Bi(III) and Cs(II) ions and the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Hg(II), Mo(II) and Bi(III). As shown in Fig. S22 and S23 (see ESI†), a shift in the absorption band is observed in the LSPR spectrum and is accompanied by discoloration of the solution. This phenomenon can be attributed to changes in the morphology of NPs and the tendency of silver atoms to deposit on their surface.28
1 v/v). Colorimetric and optical examinations were performed. The results indicated that the probes utilized could detect the target analyte in the presence of other metal ions and most metal ions did not interfere with the detection of the target analyte. However, some ions interfere with the detection of arsenic by increasing the amount of adsorption (such as Mg(II), Cr(III), K(I), Mn(II), etc.) or decreasing it (such as Ti(II), Si(II), Cs(II), etc.). In both cases of interfering investigation (separately and in combination with the analyte) in the presence of some ions, a shift at the peak of the absorption band is observed. Changes in the absorption band in different optical probes are different. For example, in Ag NPrs optical probes, the adsorption band is shifted in the presence of Ti(II), Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mg(II), Se(IV), Mo(II), Cr(III), Bi(III), Zn(II) and Cs(II), as well as in the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mg(II), Mo(II), Bi(III) and Zn(II). Absorbance band shift was observed in the presence of Ti(II), Fe(III), Pt(II), V(III), Zr(IV), Hg(II), Se(IV), Te(II), Mo(II), Bi(III), Si(II) and Cs(II) and the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Zr(IV), Mg(II), Ba(II), Sr(II), Mo(II), K(I), Bi(III), Zn(II), Li(II) and Al(III) using a Cys-capped Ag NPrs probe. Also in the Met-capped Ag NPrs probe, the absorption band shift was observed in the presence of Ti(II), Fe(III), W(IV), Pt(II), V(III), Zr(IV), Hg(II), Mo(II), Bi(III) and Cs(II) ions and the combination of As(III) with Fe(III), W(IV), Pt(II), V(III), Hg(II), Mo(II) and Bi(III). As shown in Fig. S22 and S23 (see ESI†), a shift in the absorption band is observed in the LSPR spectrum and is accompanied by discoloration of the solution. This phenomenon can be attributed to changes in the morphology of NPs and the tendency of silver atoms to deposit on their surface.283.4. µPAD development for colorimetric detection of As(III)
Fast, selective, proprietary, and inexpensive tools are especially meaningful for on-site environmental surveys.87 For the development of such devices, papers have been considered an appropriate substrate due to their advantages such as low cost, commercial availability, and easy design. In addition, papers are made of cellulosic fibers and are environmentally friendly, and due to their capillary properties, they can flow fluids into their channels without the need for a pump or external force.88 Efforts to develop paper-based microfluidics for the detection of heavy metal ions have attracted tremendous research attention due to the increasing environmental pollution of metals and their adverse effects on human health. These tools have all the features of the WHO-approved diagnostic devices such as sensitivity, cost-effectiveness, user-friendliness, and portability.89 Hence, µPADs have recently been used for the on-site monitoring of environment sites. Due to its excellent variability and hydrophilicity, the paper can facilitate the adsorption and stabilization of target analytes and prevent excessive separation and dispersion by creating clear detection zones. Probe–metal complexes are effectively detected by the naked eye and can be measured using inexpensive optical patterns.90 Therefore, in this part of the study, the efficiency of paper microfluidics in the diagnosis of As(III) has been evaluated.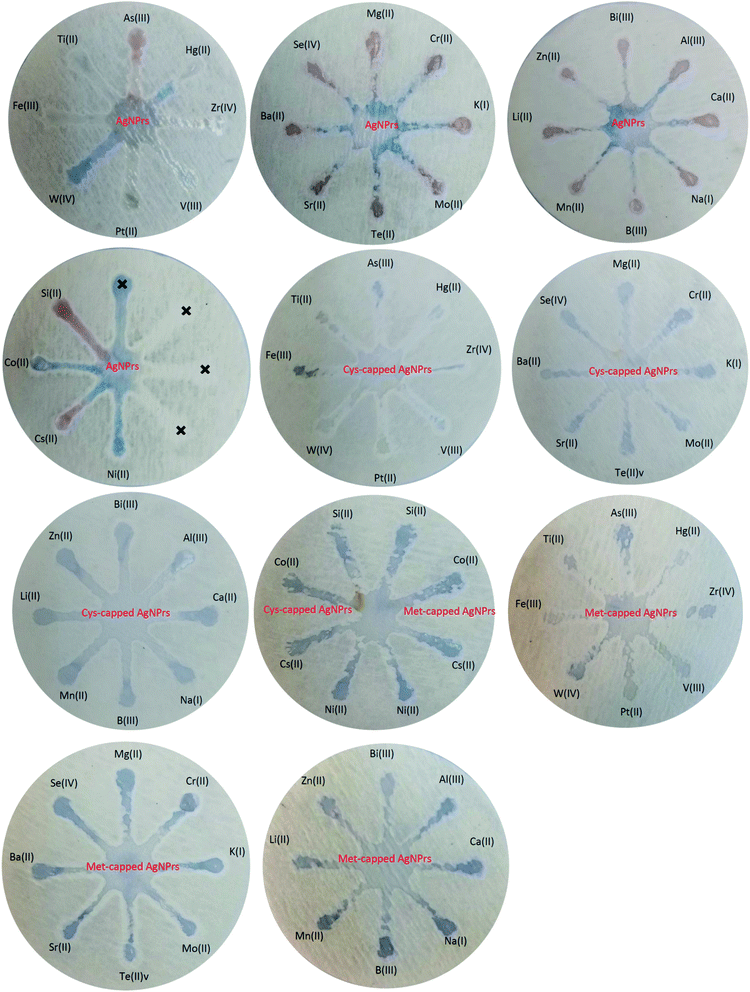 | ||
| Fig. 3 Specificity analysis of chemosensing performance of optical probes for individual identification of some metal ions using µPADs. | ||
4. Conclusion
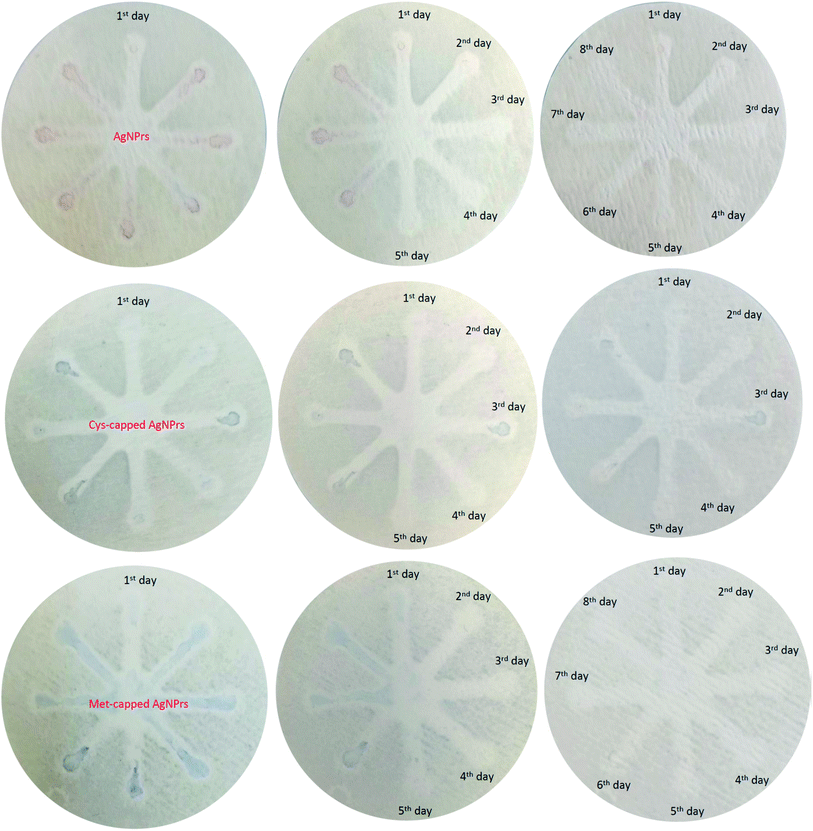
In summary, this study presents a novel As(III) detection method based on changing the morphology of Ag NPrs. The Ag NPrs changed to a spherical shape as a result of the reduction–oxidation reaction between silver nitrate and As(III), and consequently, the deformation resulted in a rapid color change. Based on this phenomenon, a detection strategy with the naked eye of As(III) was developed. By measuring the UV-Vis absorption wavelength in developed systems, a fast visual method with As(III) analysis capability in the range of 0.0005 to 1 ppm with an LLOQ of 0.0005 ppm was presented. In addition to the quantitative analysis, we also qualitatively evaluated the capabilities of the presented paper-based microfluidic in the detection of As(III). We also assessed the potential of µPADs to build on-site diagnostic kits. The results revealed that nanoparticle-modified µPADs have long-term stability and can be utilized for long-term As(III) monitoring. Examination of the interferers also showed that some ions interfere with the detection of As(III). Therefore, in future studies, it is necessary to control or eliminate all other interfering agents. In conclusion, an innovative, simple, inexpensive, and sensitive method was proposed for the monitoring of As(III) in real samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge Tabriz University of Medical Sciences for instrumental supporting of this research.References
- B. Chen, Q. Liu, A. Popowich, S. Shen, X. Yan, Q. Zhang, X.-F. Li, M. Weinfeld, W. R. Cullen and X. C. Le, Metallomics, 2015, 7, 39–55 CrossRef CAS PubMed.
- P. Nath, N. Priyadarshni and N. Chanda, ACS Appl. Nano Mater., 2017, 1, 73–81 CrossRef.
- J. Li, B. Zheng, Z. Zheng, Y. Li and J. Wang, Sensors and Actuators Reports, 2020, 2, 100013 CrossRef.
- B. Zheng, J. Li, Z. Zheng, C. Zhang, C. Huang, J. Hong, Y. Li and J. Wang, Opt. Laser Technol., 2021, 133, 106522 CrossRef CAS.
- S. H. Koli, B. V. Mohite, R. K. Suryawanshi, H. P. Borase and S. V. Patil, Bioprocess Biosyst. Eng., 2018, 41, 715–727 CrossRef CAS PubMed.
- L. Gong, B. Du, L. Pan, Q. Liu, K. Yang, W. Wang, H. Zhao, L. Wu and Y. He, Microchim. Acta, 2017, 184, 1185–1190 CrossRef CAS.
- P. K. Sarkar, A. Halder, N. Polley and S. K. Pal, Water, Air, Soil Pollut., 2017, 228, 1–11 CrossRef CAS.
- N. Yogarajah and S. S. Tsai, Environ. Sci.: Water Res. Technol., 2015, 1, 426–447 RSC.
- Z.-G. Liu and X.-J. Huang, TrAC, Trends Anal. Chem., 2014, 60, 25–35 CrossRef CAS.
- A. Rahman, S. Kumar, A. Bafana, S. A. Dahoumane and C. Jeffryes, Molecules, 2019, 24, 98 CrossRef PubMed.
- G. Vyas, S. Bhatt and P. Paul, ACS Omega, 2019, 4, 3860–3870 CrossRef CAS PubMed.
- H. Guan, X. Liu, W. Wang and J. Liang, Spectrochim. Acta, Part A, 2014, 121, 527–532 CrossRef CAS PubMed.
- P. C. Mane, M. D. Shinde, S. Varma, B. P. Chaudhari, A. Fatehmulla, M. Shahabuddin, D. P. Amalnerkar, A. M. Aldhafiri and R. D. Chaudhari, Sci. Rep., 2020, 10, 1–14 CrossRef PubMed.
- H. Revanasiddappa, B. Dayananda and T. Kumar, Environ. Chem. Lett., 2007, 5, 151–155 CrossRef CAS.
- G. P. Pincetti-Zúniga, L. A. Richards, Y. M. Tun, H. P. Aung, A. K. Swar, U. P. Reh, T. Khaing, M. M. Hlaing, T. A. Myint and M. L. Nwe, Appl. Geochem., 2020, 115, 104535 CrossRef.
- H. Li, Z. Cui and C. Han, Sens. Actuators, B, 2009, 143, 87–92 CrossRef.
- T. H. A. Nguyen, V.-C. Nguyen, T. N. H. Phan, Y. Vasseghian, M. A. Trubitsyn, A.-T. Nguyen, T. P. Chau and V.-D. Doan, Chemosphere, 2022, 287, 132271 CrossRef CAS PubMed.
- P. Prema, V. Veeramanikandan, K. Rameshkumar, M. K. Gatasheh, A. A. Hatamleh, R. Balasubramani and P. Balaji, Environ. Res., 2022, 204, 111915 CrossRef CAS PubMed.
- S. Bhatt, G. Vyas and P. Paul, ACS Omega, 2022, 7(1), 1318–1328 CrossRef CAS PubMed.
- J. Ji, H. Wu, D. Wang, D. Liu, X. Chen and S. Feng, Anal. Methods, 2022, 14(6), 643–651 RSC.
- Y. Yu, S. S. Naik, Y. Oh, J. Theerthagiri, S. J. Lee and M. Y. Choi, J. Hazard. Mater., 2021, 420, 126585 CrossRef CAS PubMed.
- T. T.-T. Ho, C.-H. Dang, T. K.-C. Huynh, T. K.-D. Hoang and T.-D. Nguyen, Carbohydr. Polym., 2021, 251, 116998 CrossRef CAS PubMed.
- F. Li, T. He, S. Wu, Z. Peng, P. Qiu and X. Tang, Microchem. J., 2021, 164, 105987 CrossRef CAS.
- E. Priyadarshini and N. Pradhan, Sens. Actuators, B, 2017, 238, 888–902 CrossRef CAS.
- C. Wang and C. Yu, Rev. Anal. Chem., 2013, 32, 1–14 CrossRef.
- F. Li, J. Wang, Y. Lai, C. Wu, S. Sun, Y. He and H. Ma, Biosens. Bioelectron., 2013, 39, 82–87 CrossRef CAS PubMed.
- M. Sabela, S. Balme, M. Bechelany, J. M. Janot and K. Bisetty, Adv. Eng. Mater., 2017, 19, 1700270 CrossRef.
- C. Liu, Q. Pang, T. Wu, W. Qi, W. Fu and Y. Wang, J. Anal. Test., 2021, 1–7 Search PubMed.
- M. A. Mahmud, E. J. Blondeel, M. Kaddoura and B. D. MacDonald, Analyst, 2016, 141, 6449–6454 RSC.
- M. M. Gong, P. Zhang, B. D. MacDonald and D. Sinton, Anal. Chem., 2014, 86, 8090–8097 CrossRef CAS PubMed.
- M. A. Chowdury, N. Walji, M. Mahmud and B. D. MacDonald, Micromachines, 2017, 8, 71 CrossRef.
- A. Tribhuwan Singh, D. Lantigua, A. Meka, S. Taing, M. Pandher and G. Camci-Unal, Sensors, 2018, 18, 2838 CrossRef PubMed.
- S. Nishat, A. T. Jafry, A. W. Martinez and F. R. Awan, Sens. Actuators, B, 2021, 129681 CrossRef CAS.
- P. Abdollahiyan, M. Hasanzadeh, F. Seidi and P. Pashazadeh-Panahi, J. Environ. Chem. Eng., 2021, 9, 106197 CrossRef CAS.
- Y. Sun, B. Mayers, T. Herricks and Y. Xia, Nano Lett., 2003, 3, 955–960 CrossRef CAS.
- A. Saadati, F. Farshchi, M. Hasanzadeh and F. Seidi, Anal. Methods, 2021, 13, 3909–3921 RSC.
- F. Farshchi, A. Saadati, M. Hasanzadeh and F. Seidi, RSC Adv., 2021, 11, 27298–27308 RSC.
- Y. Wang, C. Wang, L. Wang, L. Wang and F.-S. Xiao, Acc. Chem. Res., 2021, 54, 2579–2590 CrossRef CAS PubMed.
- C.-C. Wang, H.-T. Yau and C.-C. Wang, Math. Probl. Eng., 2013, 2013, 143–149 Search PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS PubMed.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389–458 CrossRef CAS PubMed.
- Y.-L. Hung, T.-M. Hsiung, Y.-Y. Chen, Y.-F. Huang and C.-C. Huang, J. Phys. Chem. C, 2010, 114, 16329–16334 CrossRef CAS.
- A. K. Jain, V. K. Gupta, B. B. Sahoo and L. P. Singh, Anal. Proc., 1995, 32, 99–101 RSC.
- Y. Li, P. Wang, X. Wang, M. Cao, Y. Xia, C. Cao, M. Liu and C. Zhu, Microchim. Acta, 2010, 169, 65–71 CrossRef CAS.
- A. Gutiérrez‐Blanco, C. Dobbe, A. Hepp, C. G. Daniliuc, M. Poyatos, F. E. Hahn and E. Peris, Eur. J. Inorg. Chem., 2021, 2021, 2442–2451 CrossRef.
- L. Xing, Q. Zhao, X. Zheng, M. Hui, Y. Peng, X. Zhu, L. Hu, W. Yao and Z. Yan, ACS Appl. Nano Mater., 2021, 4, 3639–3646 CrossRef CAS.
- V. K. Gupta, A. K. Singh and L. K. Kumawat, Sens. Actuators, B, 2014, 195, 98–108 CrossRef CAS.
- K. Siriwardana, A. Wang, M. Gadogbe, W. E. Collier, N. C. Fitzkee and D. Zhang, J. Phys. Chem. C, 2015, 119, 2910–2916 CrossRef CAS PubMed.
- G. S. Métraux and C. A. Mirkin, Adv. Mater., 2005, 17, 412–415 CrossRef.
- J. E. Millstone, S. J. Hurst, G. S. Métraux, J. I. Cutler and C. A. Mirkin, Small, 2009, 5, 646–664 CrossRef CAS PubMed.
- P. Abdollahiyan, M. Hasanzadeh, P. Pashazadeh-Panahi and F. Seidi, J. Mol. Liq., 2021, 338, 117020 CrossRef CAS.
- G. Wang, W. Wang, J. Wu, H. Liu, S. Jiao and B. Fang, Microchim. Acta, 2009, 164, 149–155 CrossRef CAS.
- N. Kumar and L. S. B. Upadhyay, Appl. Surf. Sci., 2016, 385, 225–233 CrossRef CAS.
- F. Bamdad, F. Khorram, M. Samet, K. Bamdad, M. R. Sangi and F. Allahbakhshi, Spectrochim. Acta, Part A, 2016, 161, 52–57 CrossRef CAS PubMed.
- J. Li, L. Chen, T. Lou and Y. Wang, ACS Appl. Mater. Interfaces, 2011, 3, 3936–3941 CrossRef CAS PubMed.
- P. Mulpur, A. Kurdekar, R. Podila, A. M. Rao and V. Kamisetti, Nanotechnol. Rev., 2015, 4, 393–400 CrossRef CAS.
- Y. Sun, X. Wang and H. Zhang, Biosensors, 2022, 12, 382 CrossRef CAS PubMed.
- P. Prosposito, L. Burratti and I. Venditti, Chemosensors, 2020, 8, 26 CrossRef CAS.
- T. Poonia, N. Singh and M. Garg, Int. J. Environ. Sci. Technol., 2021, 1–12 Search PubMed.
- D. Vilela, M. C. González and A. Escarpa, Anal. Chim. Acta, 2012, 751, 24–43 CrossRef CAS PubMed.
- C. V. Restrepo and C. C. Villa, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100428 CAS.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed.
- S. Szunerits and R. Boukherroub, Chem. Commun., 2012, 48, 8999–9010 RSC.
- K. Shiva Prasad, G. Shruthi and C. Shivamallu, Sensors, 2018, 18, 2698 CrossRef PubMed.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- V. N. Mehta, A. K. Mungara and S. K. Kailasa, Anal. Methods, 2013, 5, 1818–1822 RSC.
- S. Diamai and D. P. Negi, Spectrochim. Acta, Part A, 2019, 215, 203–208 CrossRef CAS PubMed.
- B. Malile and J. I. Chen, J. Am. Chem. Soc., 2013, 135, 16042–16045 CrossRef CAS PubMed.
- S.-H. Wen, R.-P. Liang, L. Zhang and J.-D. Qiu, ACS Sustainable Chem. Eng., 2018, 6, 6223–6232 CrossRef CAS.
- W. Siangproh, O. Chailapakul and K. Songsrirote, Talanta, 2016, 153, 197–202 CrossRef CAS PubMed.
- F. Divsar, K. Habibzadeh, S. Shariati and M. Shahriarinour, Anal. Methods, 2015, 7, 4568–4576 RSC.
- B. S. Boruah, N. K. Daimari and R. Biswas, Results Phys., 2019, 12, 2061–2065 CrossRef.
- S. Kapaj, H. Peterson, K. Liber and P. Bhattacharya, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 2399–2428 CrossRef CAS PubMed.
- Q. Y. Chen and M. Costa, Annu. Rev. Pharmacol. Toxicol., 2021, 61, 47–63 CrossRef CAS PubMed.
- S. N. Kales, K. L. Huyck and R. H. Goldman, J. Anal. Toxicol., 2006, 30, 80–85 CrossRef CAS PubMed.
- K. Yamanaka, H. Hayashi, M. Tachikawa, K. Kato, A. Hasegawa, N. Oku and S. Okada, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1997, 394, 95–101 CrossRef CAS.
- E. Shaji, M. Santosh, K. Sarath, P. Prakash, V. Deepchand and B. Divya, Geosci. Front., 2021, 12, 101079 CrossRef CAS.
- J. T. Hindmarsh, Clin. Biochem., 2002, 35, 1–11 CrossRef CAS.
- R. R. Lauwerys and P. Hoet, Industrial chemical exposure: guidelines for biological monitoring, CRC Press, 2001 Search PubMed.
- M. Ozturk, M. Metin, V. Altay, R. A. Bhat, M. Ejaz, A. Gul, B. T. Unal, M. Hasanuzzaman, L. Nibir and K. Nahar, Biol. Trace Elem. Res., 2021, 1–14 Search PubMed.
- H. Ismail, M. N. Ahmad and E. Normaya, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- P. W. Cheah, M. P. Heng, H. M. Saad, K. S. Sim and K. W. Tan, Opt. Mater., 2021, 114, 110990 CrossRef CAS.
- A. Sanfeld and A. Steinchen, Surf. Sci., 2000, 463, 157–173 CrossRef CAS.
- N. Yekeen, T. X. Kun, A. Al-Yaseri, F. Sagala and A. K. Idris, J. Mol. Liq., 2021, 338, 116658 CrossRef CAS.
- Y. Cao, Z. Chen, X. Li, Z. Li, G. Lin, T. Liu and Y. Wu, Anal. Chim. Acta, 2022, 339998 CrossRef CAS PubMed.
- E. A. Syukkalova, A. V. Sadetskaya, N. D. Demidova, N. P. Bobrysheva, M. G. Osmolowsky, M. A. Voznesenskiy and O. M. Osmolovskaya, Ceram. Int., 2021, 47, 2809–2821 CrossRef CAS.
- H. Doi, T. Watanabe, N. Nishizawa, T. Saito, H. Nagata, Y. Kameda, N. Maki, K. Ikeda and T. Fukuzawa, Mol. Ecol. Resour., 2021, 21, 2364–2368 CrossRef CAS PubMed.
- I. Jang, D. B. Carrão, R. F. Menger, A. R. Moraes de Oliveira and C. S. Henry, ACS Sens., 2020, 5, 2230–2238 CrossRef CAS PubMed.
- V. Abergel, K. Jacquot, L. De Luca and P. Veron, Disegnarecon, 2021, 14, 1310–1314 Search PubMed.
- H. Manisha, P. P. Shwetha and K. Prasad, in Environmental, Chemical and Medical Sensors, Springer, 2018, pp. 315–341 Search PubMed.
- X. Qin, J. Liu, Z. Zhang, J. Li, L. Yuan, Z. Zhang and L. Chen, TrAC, Trends Anal. Chem., 2021, 143, 116371 CrossRef CAS.
- W. Zhou, M. Dou, S. S. Timilsina, F. Xu and X. Li, Lab Chip, 2021, 21, 2658–2683 RSC.
- L. J. Loh, G. C. Bandara, G. L. Weber and V. T. Remcho, Analyst, 2015, 140, 5501–5507 RSC.
- K. M. Schilling, A. L. Lepore, J. A. Kurian and A. W. Martinez, Anal. Chem., 2012, 84, 1579–1585 CrossRef CAS PubMed.
- Y. H. Ngo, D. Li, G. P. Simon and G. Garnier, Adv. Colloid Interface Sci., 2011, 163, 23–38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02820d |
| This journal is © The Royal Society of Chemistry 2022 |