Open Access Article
Open Access ArticleDetermination of aflatoxin B1 in Pixian Douban based on aptamer magnetic solid-phase extraction†
Chaoyi Zeng‡
ad,
Chi Xu‡a,
Hongyun Tianc,
Kun Shaoa,
Yaning Songa,
Xiao Yang a,
Zhenming Cheab and
Yukun Huang
a,
Zhenming Cheab and
Yukun Huang *ab
*ab
aSchool of Food and Biological Engineering, Chongqing Key Laboratory of Speciality Food Co-Built by Sichuan and Chongqing, Xihua University, Chengdu 610039, China. E-mail: hyk_diana@163.com
bKey Laboratory of Food Non Thermal Processing, Engineering Technology Research Center of Food Non Thermal Processing, Yibin Xihua University Research Institute, Yibin 644004, China
cShandong Institute of Food and Drug Control, Jinan 250101, China
dDepartment of Food Biotechnology, Faculty of Biotechnology, Assumption University, Bangkok 10240, Thailand
First published on 6th July 2022
Abstract
Aflatoxin B1 (AFB1) is considered as the most prevalent and toxic mycotoxin in food, and is the indispensable index in the monitoring of Pixian Douban, a traditional chinese fermented bean paste from Sichuan. However, the effeciency of AFB1 detection in Pixian Douban is influenced by the traditional extraction, which is usually complex and time consuming. Therefore, an aptamer-based magnetic solid-phase extraction method was designed for the pretreatment of AFB1 in this sample, for which Fe3O4 was synthesized via the solvothermal method and then a Fe3O4@SiO2–NH2 with a core–shell structure was prepared, followed by an AFB1-aptamer attachment. The validation was performed via an enzyme-linked immunosorbent assay and compared with HPLC-MS/MS. The linearity range of this method was 0.5–2.0 ng mL −1 with R2 of 0.981, and recoveries of AFB1 ranged from 80.19% to 113.92% with RSDs below 7.28% with no significant differences compared to HPLC-MS/MS. The three-time reusability efficiencies of aptamer-MNPs were averaged at 78.24%. The results proved that aptamer-MNPs were high-performance adsorbents for extracting and enriching AFB1, facilitating quick and effective detection of AFB1 in Pixian DouBan samples.
1. Introduction
Pixian Douban is a fermented condiment with chinese regional characteristics. Its fermentation was recorded to be accompanied with a process called “sun and night dew” treatment. The complex changes of temperature to a certain extent can affect the various Aspergillus and enzymes that play a biochemical role, and form unique flavour characteristics widely used in Sichuan Cuisine. However, due to the relatively extensive process of traditional production, Douban's fermentation is liable to be contaminated by other Aspergillus and subsequently cause aflatoxin pollution.1,2 The evidence shows that AFB1 has the highest pollution frequency in this food.3 It has been reported that AFB1 can lead to liver cancer and other diseases in mammals,2,4 which is classified as type I carcinogen by international cancer research institutions.1,5 In order to reduce food safety risk of Pixian Douban, AFB1 has been regarded as a necessary item in Pixian Douban's Geographical Indication Product Standard of GB/T 20560-2006. Traditional detection methods mainly include thin layer chromatography, high performance liquid chromatography, gas chromatography, capillary electrophoresis and immunoassay.3,6,7 Generally speaking, these detection methods are relatively wide-applied in the practical detection right now. However, the broad bean is rich in nutrients such as protein, amino acids, enzymes, pigments, lipids and other components,8 which is too complex to be easily detected with high efficiency so far. The shortcomings of traditional detection methods, such as long detection time, high detection cost and poor repeatability as well as not environment friendly, pose great challenges to the effective monitoring of AFB1 in Douban. Therefore, it is necessary to establish an efficient, simple, and robust method for detection of AFB1 in Pixian Douban.Methanol solvent extraction is adopted in the commercial ELISA kit method, which is a solid–liquid extraction for AFB1. Then a quantitative analysis can be performed according to the colour depth after coloration. However, the blending of chilli in Pixian Douban brings bright red colouring matter into the extract, which shows the background interference in ELISA determination. Aptamers have several advantages, such as strong specificity, high affinity, wide target range, in vitro chemical synthesis, low molecular weight, and good stability. As a result, aptamers have been widely used in the fields of analytical chemistry, medicine, environmental monitoring, and food safety control.9–12 In addition, magnetic solid-phase extraction (MSPE) is a new solid-phase extraction (SPE) method based on the use of magnetic adsorbents, in which suitable adsorbents are combined with magnetic materials.13,14 In the MSPE method, magnetic adsorbents are critical for the efficient enrichment of analytes. Ferrosoferric oxide (Fe3O4) is widely used in sample pretreatment because of its characteristics of magnetic adsorption, low price, easy synthesis, and surface modification.15–17 Nevertheless, Fe3O4 is unstable and easily oxidized by air. Surface modification of Fe3O4 with appropriate functional groups can prevent oxidation and improve its durability and adsorption.13 The modification and functionalization of Fe3O4 are conducted mainly by using graphene,18 graphene oxide,19 carbon quantum dots,20 the polymer,21 and so on. Silica is one of the best coating materials for Fe3O4 magnetic nanoparticles (MNPs), because it owns characteristics of easy surface modification, high chemical stability, and environmental compatibility.17,22 Currently, aptamer-Fe3O4-based composite adsorption materials are gradually applied to various samples pretreatments, such as metal elements,23 bacteria,21,24 pesticides,25 veterinary drug,26 toxins,27 nucleotides,28 and other samples. Recently, the extraction method of AFB1 from dairy products and edible vegetable oils,29–31 such as SPE and MSPE, have been extensively studied in a fast, simple, inexpensive and safe method.
The deep eutectic solvent–based matrix solid-phase dispersion (DES-MSPD) method has currently been established for extracting and enriching aflatoxins (AFB1, AFB2, AFG1, and AFG2) in various crops.32 A simple modified SPE combined with high-performance liquid chromatography (HPLC)-fluorescence detection was established for detecting AFB1 and AFB2, and amine-functionalized MNPs were successfully employed in SPE for the first time for adsorbing AFB1 and AFB2 in drugs33 In addition, aptasensors are considered as an emerging strategy for quantifying AFB1 with high selectivity and sensitivity.34
In this work, the functionalization of Fe3O4@SiO2–NH2 using ssDNA aptamers (referred to as aptamer-MNPs) was designed to prepare AFB1-specific nanoparticles with excellent magnetic responses. Moreover, various adsorption conditions were tested to determine the optimal conditions for the adsorption of AFB1 in Pixian Douban to the prepared nanomaterials. The variables tested included the amount of aptamer-MNPs, extraction time, elution time, elution types, and elution volumes. An efficient method for extracting AFB1 from Pixian Douban was established, which lays the foundation for the subsequent analysis of AFB1 in food.
2. Materials and methods
2.1. Materials and reagents
The main text of the article should appear here with headings as appropriate. Ferric chloride hexahydrate (FeCl3·6H2O) and AFB1 standard were purchased from Shanghai Yuanye Biological Co., Ltd. (Shanghai, China). Poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA) was purchased from Huaxia Reagent Co., Ltd. (Harbin, China). Tetraethyl silicate (TEOS), 3-aminopropyltriethoxysilane (APTES), and glutaraldehyde were purchased from Shanghai McLean Company (Shanghai, China). The AFB1 kit was purchased from Jiangsu Suwei Co., Ltd. (Jiangsu, China). Hydroxymethylaminomethane (Tris) and streptavidin were purchased from Shanghai Shenggong Co., Ltd. Pixian Douban was purchased from the local supermarket. Biotinylated aptamer (5′-GT TGG GCA CGT GTT GTC TCT CTG TGT CTC GTG CCC TTC GCT AGG CCC ACA-Biotin-3′) was synthesized by Shanghai Shenggong Bioengineering Co., Ltd. (Shanghai, China). Further, NaAc, NaOH, ethanol, NH3·H2O, KCl, CaCl2 were purchased from Cologne Chemicals Co., Ltd. (Chengdu, China).2.2. Methods
The preparation of aptamer-MNPs comprises two steps: the preparation of Fe3O4@SiO2–NH2 nanoparticles and the functionalization of AFB1-aptamer, as shown in Scheme 1a. | ||
| Scheme 1 Schematic illustration of the synthesis process of aptamer-MNPs nanocomposite (a); a schematic diagram for the extraction and detection of AFB1 in Pixian Douban samples (b). | ||
2.2.1.1. Preparation of Fe3O4 nanoparticles. The preparation method for Fe3O4 nanoparticles reported by previous researchers35 was slightly modified. 1.08 g of FeCl3·6H2O, 3.0 g of NaAc, and 1.2 g of PSSMA were added in 40 mL of ethylene glycol. The mixture was uniformly stirred in an oil bath at 50 °C. Then, 0.6 g of NaOH was added to the mixture and continuously stirred until the solution became dark. Subsequently, the mixture was transferred to a stainless-steel autoclave, and Fe3O4 nanoparticles were obtained after being heated at 190 °C for 9 h. Fe3O4 nanoparticles were uniformly dispersed in 30 mL of distilled water (Fe3O4 concentration was approximately 0.8 wt%) and stored at 4 °C.
2.2.1.2. Preparation of Fe3O4 @SiO2. 12 mL of dispersion were dispersed in 70 mL of ethanol and transferred to a round-bottomed flask. After stirring for 15 min, 0.4 mL of TEOS and 4 mL of NH3·H2O were added twice at 20 min intervals into the reaction mixture. Subsequently, Fe3O4@SiO2 nanoparticles were obtained after stirring for 80 min.
2.2.1.3. Preparation of Fe3O4 @SiO2–NH2. 50 mg of Fe3O4@SiO2, 6 mL of distilled water, and 40 mL of anhydrous ethanol were added into the flask for ultrasonic dispersion. Then, 3 mL of ammonia was added to the mixture and stirred for 15 min. Furthermore, 10 mL of anhydrous ethanol and 2 mL of the APTES mixture were added and stirred for 10 h to obtain Fe3O4@SiO2–NH2. The upper liquid was discarded by magnetic separation, washed using PBS (2.7 mmol L−1 of KCl, 137 mmol L−1 of NaCl, 2 mmol L−1 of KH2PO4 and 10 mmol L−1 of Na2HPO4, and pH = 7.4) thrice, dispersed in 10 mL of PBS, and stored at 4 °C.
2.2.4.1. Aptamer-MNPs extraction of AFB1. 10.0 g of Pixian Douban, 50 ml of 50% aqueous methanol solution, and 20 mL of n-hexane were subjected to oscillation for 15 min and transferred to the separator funnel to stratify the solution. 10 mL of the solution at the lower layer was collected and concentrated via rotary evaporation. 10% of methanol-Tris-HCl was added to redissolve and obtain a crude sample extract. After 1 mL of crude extract and 5 mg of aptamer-MNPs were incubated with shaking for 30 min, the aptamer-MNPs were separated using a magnet. The separated aptamer-MNPs were washed with Tris–HCl, and 1 mL was eluted with methanol for 10 min. The eluate was collected and concentrated to near dryness, and then redissolved in 1 mL of PBS solution. 50 μL of the solution was used for ELISA.
2.2.4.2. Detection of AFB1 by ELISA. 200 μL of detergent was added to each hole of the ELISA plate. After 1 min, the detergent was shake off and patted dry. Repeat the washing plate once. In each well, 50 μL of the standard solution or sample solution, and 50 μL of enzyme-labeled antigen solution were added, shaken and mixed well, incubated for 30 min at 37 °C, the detergent was shaken off the reaction solution, and patted dry. 200 μL of detergent was added and placed for 2 min, patted dry and repeat the washing plate for four times. 50 μL of the chromogenic substrate a and 50 μL of chromogenic substrate b were added, shaken and mixed, incubated at 37 °C for 15 min, and 50 μL of terminating solution was added. After shaking well, the absorbance at a wavelength of 450 nm was determined via ELISA.
2.2.4.3. Comparison detection of AFB1 by HPLC-MS/MS. HPLC-MS/MS analysis was compared to the present work as the way of replacing ELISA test in the spike recovery of Pixian Douban samples. After Aptamer-MNPs treatment, the extractive was redissolved with acetonitrile-water (60 + 40 v/v). HPLC-MS/MS system (Agilent 6460) detection conditions:38 AFB1 were chromatographed on ACQUITY BEH-18 column (1.7 μm particle size, 110 × 2.1 mm i. d., waters) and separated using gradient elution with 2 mmol L−1 ammonium acetate aqueous solution and acetonitrile as mobile phase A and B, respectively (both acidified with 0.2% formic acid). The gradient program was as follows: at time zero 10% solvent B; at 2 min 35% solvent B; linear gradient to 60% solvent B within 7 min, at 9 min 90% solvent B then 10% B isocratic for 3 min. The flow rate was 0.3 mL min−1. The ionization was carried out with an ESI interface in positive mode as follows: spray capillary voltage was 4.0 kV; sheath gas flow rate 11 L min−1, respectively; temperature of sheath gas 350 °C. The mass spectrometric analysis was performed in MRM. For fragmentation of AFB1 [M + H]+ ions is 313 m/z., the detected and quantified fragment ions were: 241 and 269 m/z for AFB1. Quantitative determination was performed by MassHunter software (Agilent). All analyses were performed with the SPSS 23.0 software (SPSS Incorporated, USA). Statistical significance was analyzed using ANOVA. In all statistical comparisons, values of P ≤ 0.05 were considered as of significance, and values of P ≤ 0.01 were considered to be markedly significant.
3. Results and discussion
3.1. Characterization
The XRD pattern of Fe3O4 samples is shown in Fig. S3.† The XRD spectra of Fe3O4 show spinel ferrites, which fully matches with the diffraction card JCPDS (PDF-#76-1849). The diffraction peaks at 2θ = 18.06°, 30.13°, 35.47°, 43.03°, 53.19°, 56.89°, and 62.58°, corresponding to 111, 220, 311, 400, 422, 511, and 440 plates, respectively, indicate a magnetic phase and the crystalline cubic spinel structure of Fe3O4.23
The magnetic separation of Fe3O4@SiO2 from deionized water was performed to test the practical magnetic response-ability (Fig. S4†). Fe3O4@SiO2 particles were uniformly dispersed in deionized water. Under the presence of magnetic field, Fe3O4@SiO2 was rapidly separated from the solution and assembled in the corner near the magnet.
The FT-IR spectra of Fe3O4 and Fe3O4@SiO2–NH2 are depicted in Fig. S5.† The absorption peak at 3381.39 cm−1 was attributed to the stretching vibration of the hydroxyl group, indicating that a certain number of hydroxyl groups exist on the surface of Fe3O4. In the FT-IR spectra of Fe3O4@SiO2–NH2, the absorption intensity of the Fe–O group decreased with the addition of silica. The Si–O–Si group exhibited a strong absorption intensity at 1100.21 cm−1 owing to the silica coating, indicating that Fe3O4 had been successfully coated with SiO2.36 In addition, the Absorption peaks at 1627.94 and 3328.89 cm−1 correspond to bending and stretching vibrations of the–NH2 group,16 respectively. The peaks at 2856.35 and 2923.24 cm−1 correspond to symmetric and asymmetric stretching vibrations of the –CH2 group, respectively, which are attributed to the hydrolysis of the amination reagent APTES. These absorption peaks confirmed the successful modification of Fe3O4. The absorption peaks at 588.63 and 583.22 cm−1 are attributed to the characteristic absorption peak of the Fe–O group. After the surface modification of Fe3O4, the characteristic absorption peak slightly shifted, but the properties of Fe3O4 did not change.
3.2. The establishment of detection standard curve
In this experiment, a commercial AFB1 ELISA kit was used for performing the quantitative analysis of AFB1 extracted using aptamer-MNPs. The standard curve of ELISA was obtained after step 2.2.4.2. As shown in Fig. 1, the linearity of the developed method was observed over a range of 0.5–2.0 ng mL−1, with a correlation coefficient of 0.981.3.3. Optimization of sample preparation conditions
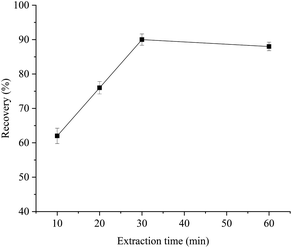
In the MSPE procedure, the extraction and elution processes are crucial in realizing satisfactory recovery of analytes. To obtain higher extraction recoveries, AFB1 in Pixian Douban was enriched with aptamer-MNPs. The main experimental parameters that affect the recovery of the extraction were optimized, such as the amount of aptamer-MNPs, extraction time, and elution time of the sample.3.4. Method performance validation
| Tagged value/(ng mL−1) | ELISA Detected value/(ng mL−1) | Recovery/(%) | RSD/(%) | HPLC-MS/MS Detected value/(ng mL−1) | Recovery/(%) | RSD/(%) |
|---|---|---|---|---|---|---|
| 0.5 | 0.42 | 84.29 | 3.27 | 0.44 | 88.63 | 1.37 |
| 1.0 | 1.14 | 113.92 | 7.28 | 0.89 | 89.88 | 2.91 |
| 2.0 | 1.60 | 80.19 | 2.34 | 1.87 | 93.50 | 1.32 |
| 10.0 | 9.37 | 93.70 | 2.30 | 10.11 | 101.10 | 3.44 |
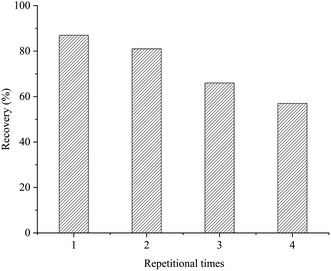 | ||
| Fig. 6 Reusability in four cycles of the aptamer-MNPs SPME adsorbents for extraction of AFB1 in Pixian Douban samples. | ||
Compared with traditional SPE, MSPE affords enhanced extraction efficiencies and avoids time-consuming and labor-intensive extraction steps, which are particularly important when considering large sample sets.13 Table 2 shows a comparison of sample preparation procedures with other methods in the literature. The LOD and LOQ obtained from the present method are comparable to or lower than those obtained from other methods. The MSPE method simplifies the sample preparation procedure because the adsorption processes are fast and the magnetic adsorbents can be easily separated from the sample solution under the applied external magnetic field.30 The MSPE method established in this study can identify target molecules with high specificity and realize rapid separation and enrichment under an external magnetic field, which saves the operation of centrifugation and filtration in other extraction methods, greatly simplifying the experimental steps, and reducing the experiment time. In addition, aptamer has strong chemical stability and storage stability; therefore, it can be used in various reaction systems and can be preserved for a long time without any reduction in its functional activity. Aptamer and nanomagnetic beads have the advantages of simple preparation, easy modification, and good stability.39 Therefore, the specific recognition of aptamer and the rapid separation as well as enrichment of nanomagnetic beads are integrated to prepare a new type of extraction material, and the pretreatment method for the detection of AFB1 is demonstrated to have great significance and value.
| Method/(ng mL−1) | Sample | Adsorbent | LOD (ng mL−1) | LOQ (ng mL−1) | Linear range (ng mL−1) | Ref. |
|---|---|---|---|---|---|---|
| a Note: “-” means the sample preparation procedures without aptamer@Fe3O4@SiO2–NH2. | ||||||
| MSPE-aptamer-ELISA | Pixian Douban | Aptamer@Fe3O4@SiO2–NH2 | 0.17 | 0.48 | 0.5–2 | Present work |
| ELISA | Pixian Douban | — | 0.41 | 1.26 | 0.5–2 | Comparison 1 |
| MSPE-aptamer-HPLC-MS/MS | Pixian Douban | Aptamer@Fe3O4@SiO2–NH2 | 0.07 | 0.22 | 0.5–10 | Comparison 2 |
| MSPE-UHPLC | Milk | PEG-MWCNTs-MNP | 0.01 | 0.03 | 1.25–40 | 30 |
| MSPE-HPLC | Vegetable oils | PDA@Fe3O4-MWCNTs | 0.20 | 0.60 | 1–50 | 31 |
| MSPD-HPLC | Crops | TBAC-hexyl alcohol DES | 0.10 | 0.33 | 0.1–100 | 32 |
Conclusions
In this study, aptamer-MNPs were prepared and adopted as the adsorbents for AFB1 in Pixian Douban samples. Aptamer-MNPs effective in extracting and enriching the targeted compound AFB1 and exhibited a good magnetic response. A new detection method for AFB1 in Pixian Douban samples was developed by combining aptamer-MNPs with ELISA. The linearity of the method was 0.5–2 ng mL−1 with a correlation coefficient of 0.981, and the content of detections for AFB1 was 3.4 μg kg−1. The recoveries of AFB1 ranged from 80.19% to 113.92% with RSDs lower than 7.28%, and the reusability of the selected membrane for aptamer-MNPs indicated efficiency of 87.34%, 81.20%, and 66.17% for the 1st, 2nd, and 3rd cycles, respectively. The results proved that aptamer-MNPs were high-performance adsorbents for extracting and enriching AFB1, which can quickly and effectively detect AFB1 in Pixian Douban samples.Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, analysis, and revision were performed by Chi Xu, Kun Shao, Yaning Song and Hongyun Tian. The first draft of the manuscript was written by Chaoyi Zeng and the previous versions of the manuscript were commented by Xiao Yang. The resources and supervision were provided by Zhenming Che. The conceptualization and writing – review and editing were done by Yukun Huang. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (31801647), Sichuan Science and Technology Program (2020YFN0153, 2020YFN0151, 2022JDTD0028), Research project of Sichuan Cuisine Development research Center (CC22Z27).References
- A. Vaz, A. C. Cabral Silva, P. Rodrigues and A. Venâncio, Detection Methods for Aflatoxin M1 in Dairy Products, Microorganisms, 2020, 8, 246 CrossRef CAS PubMed.
- O. Ketney, A. Santini and S. Oancea, Recent aflatoxin survey data in milk and milk products: A review, Int. J. Dairy Technol., 2017, 70, 320–331 CrossRef CAS.
- L. Zhang, W. Xu, P. Yue, Q. Wang, Y. Li, X. Pei and P. Zeng, High occurrence of aflatoxin B1 in Pixian Doubanjiang, a typical condiment in chinese cuisine, Food Control, 2020, 110, 107034 CrossRef CAS.
- A. C Manetta, Aflatoxins: Their Measure and Analysis, InTech, 2011 Search PubMed.
- M. Heinrich, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene, J. Ethnopharmacol., 2002, 88, 299–300 Search PubMed.
- N. W. Turner, S. Subrahmanyam and S. A. Piletsky, Analytical methods for determination of mycotoxins: A review, Anal. Chim. Acta, 2009, 632, 168–180 CrossRef CAS PubMed.
- P. Li, Z. Zhang, X. Hu and Q. Zhang, Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects, Mass Spectrom. Rev., 2013, 32, 420–452 CrossRef CAS PubMed.
- Y. Huang, Y. Song, F. Chen, Z. J. Jiang, Z. M. Che, X. Yang and X. G. Chen, Simultaneous determination of eight biogenic amines in the traditional Chinese condiment Pixian Douban using UHPLC-MS/MS, Food Chemistry, 2021, 353, 129423 CrossRef CAS PubMed.
- Z. Lu, X. Chen and W. Hu, A fluorescence aptasensor based on semiconductor quantum dots and MoS2 nanosheets for ochratoxin A detection, Sens. Actuators, B, 2017, 246, 61–67 CrossRef CAS.
- S. Zhang, L. Ma, K. Ma, B. Xu, L. Liu and W. Tian, Label-Free Aptamer-Based Biosensor for Specific Detection of Chloramphenicol Using AIE Probe and Graphene Oxide, ACS Omega, 2018, 3, 12886–12892 CrossRef CAS PubMed.
- S. Kim and H. J. Lee, Gold Nanostar Enhanced Surface Plasmon Resonance Detection of an Antibiotic at Attomolar Concentrations via an Aptamer-Antibody Sandwich Assay, Anal. Chem., 2017, 89, 6624–6630 CrossRef CAS PubMed.
- H. Gao, N. Gan, D. Pan, Y. Chen, T. Li, Y. Cao and T. Fu, A sensitive colorimetric aptasensor for chloramphenicol detection in fish and pork based on the amplification of a nano-peroxidase-polymer, Anal. Methods, 2015, 7, 6528–6536 RSC.
- Z. Liu, P. Qi, J. Wang, Z. Wang, S. Di, H. Xu, H. Zhao, Q. Wang, X. Wang, and X. Wang, Development, validation, comparison, and implementation of a highly efficient and effective method using magnetic solid-phase extraction with hydrophilic-lipophilic-balanced materials for LC-MS/MS analysis of pesticides in seawater, Science of the Total Environment, 2020, p. 708 Search PubMed.
- L. Kubíčková, J. Koktan, T. Kořínková, M. Klementova, T. Kmjec, J. Kohout, A. Weidenkaff and O. Kaman, Zn-substituted iron oxide nanoparticles from thermal decomposition and their thermally treated derivatives for magnetic solid-phase extraction, J. Magn. Magn. Mater., 2020, 498 Search PubMed.
- Z. Liu, P. Qi, X. Wang, Z. Wang, X. Xu, W. Chen, L. Wu, H. Zhang, Q. Wang and X. Wang, Multi-pesticides residue analysis of grains using modified magnetic nanoparticle adsorbent for facile and efficient cleanup, Food Chemistry, 2017, 230, 423–431 CrossRef CAS PubMed.
- Z. Yuan, R. Xu, J. Li, Y. Chen, B. Wu, J. Feng and Z. Chen, Biological responses to core-shell-structured Fe 3 O 4 @SiO 2 -NH 2 nanoparticles in rats by a nuclear magnetic resonance-based metabonomic strategy, Int. J. Nanomed., 2018, 13, 2447–2462 CrossRef CAS PubMed.
- J. Li, Z. Yuan, H. Liu, J. Feng and Z. Chen, Size-dependent tissue-specific biological effects of core–shell structured Fe3O4@SiO2–NH2 nanoparticles, J. Nanobiotechnol., 2019, 17, 1–14 CrossRef CAS PubMed.
- Q. Han, Z. Wang, J. Xia, S. Chen, X. Zhang and M. Ding, Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples, Talanta, 2012, 101, 388–395 CrossRef CAS PubMed.
- S. Alilou, M. Amirzehni and P. A. Eslami, A simple fluorometric method for rapid screening of aflatoxins after their extraction by magnetic MOF-808/graphene oxide composite and their discrimination by HPLC, Talanta, 2021, 235, 122709 CrossRef CAS PubMed.
- J. Ren, G. Liang, Y. Man, A. Li, X. Jin, Q. Liu and L. Pan, Aptamer-based fluorometric determination of Salmonella Typhimurium using Fe3O4 magnetic separation and CdTe quantum dots, PLoS One, 2019, 14, 1–13 Search PubMed.
- R. Binaymotlagh, F. Hajareh Haghighi, F. Aboutalebi, S. Z. Mirahmadi-Zare, H. Hadadzadeh and M.-H. Nasr-Esfahani, Selective chemotherapy and imaging of colorectal and breast cancer cells by a modified MUC-1 aptamer conjugated to a poly(ethylene glycol)-dimethacrylate coated Fe3O4–AuNCs nanocomposite, New J. Chem., 2019, 43, 238–248 RSC.
- F. Ghorbani and S. Kamari, Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments, Environ. Technol. Innovation, 2019, 14, 100333 CrossRef.
- X. X. Liang, X.-K. Ouyang, S. Wang, L.-Y. Yang, F. Huang, C. Ji and X. Chen, Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads, Int. J. Biol. Macromol., 2019, 137, 741–750 CrossRef CAS PubMed.
- G. Bayramoglu, V. C. Ozalp, M. Oztekin and M. Y. Arica, Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor, Talanta, 2019, 200, 263–271 CrossRef CAS PubMed.
- J. Fu, H. Dong, Q. Zhao, S. Cheng, Y. Guo and X. Sun, Fabrication of refreshable aptasensor based on hydrophobic screen-printed carbon electrode interface, Sci. Total Environ., 2020, 712, 136410 CrossRef CAS PubMed.
- W. Wang, S. Liu, C. Li, Y. Wang and C. Yan, Dual-target recognition sandwich assay based on core-shell magnetic mesoporous silica nanoparticles for sensitive detection of breast cancer cells, Talanta, 2018, 182, 306–313 CrossRef CAS PubMed.
- Y. Su, C. Shao, X. Huang, J. Qi, R. Ge, H. Guan and Z. Lin, Extraction and detection of bisphenol A in human serum and urine by aptamer-functionalized magnetic nanoparticles, Anal. Bioanal. Chem., 2018, 410, 1885–1891 CrossRef CAS PubMed.
- N. Yadav, A. Singh and M. Kaushik, Synthesis and characterization of hydrothermally synthesized superparamagnetic APTS–ZnFe2O4 nanoparticles: DNA binding studies for exploring biomedical applications, Chem. Pap., 2020, 74, 1177–1188 CrossRef CAS.
- M. H. Iha, C. B. Barbosa, R. M. D. Favaro and M. W. Trucksess, Chromatographic method for the determination of aflatoxin M1 in cheese, yogurt, and dairy beverages, J. AOAC Int., 2011, 94, 1513–1518 CrossRef CAS.
- Y. Zhao, Y.-C. Yuan, X.-L. Bai, Y.-M. Liu, G.-F. Wu, F.-S. Yang and X. Liao, Multi-mycotoxins analysis in liquid milk by UHPLC-Q-Exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes, Food Chemistry, 2020, 305, 125429 CrossRef CAS PubMed.
- H. Xu, J. Sun, H. Wang, Y. Zhang and X. Sun, Adsorption of aflatoxins and ochratoxins in edible vegetable oils with dopamine-coated magnetic multi-walled carbon nanotubes, Food Chemistry, 2021, 365, 130409 CrossRef CAS PubMed.
- X. Wu, X. Zhang, Y. Yang, Y. Liu and X. Chen, Development of a deep eutectic solvent-based matrix solid phase dispersion methodology for the determination of aflatoxins in crops, Food Chemistry, 2019, 291, 239–244 CrossRef CAS PubMed.
- Q. Li, L. Jiang, H. Zhang, M. Wei, C. Chu and J. Yan, Modified Magnetic Nanoparticle-Based Solid-Phase Extraction for the Determination of Trace Amounts of Aflatoxins B1 and B2 in Chinese Patent Medicines: The Use of Fupuganmao Granules as a Case Study, J. AOAC Int., 2019, 102, 761–766 CrossRef CAS PubMed.
- Y. Jia, G. Zhou, P. Liu, Z. Li and B. Yu, Recent Development of Aptamer Sensors for the Quantification of Aflatoxin B1, Appl. Sci., 2019, 9, 2364 CrossRef CAS.
- Y. Dong, B. Wen, Y. Chen, P. Cao and C. Zhang, Autoclave-free facile approach to the synthesis of highly tunable nanocrystal clusters for magnetic responsive photonic crystals, RSC Adv., 2016, 6, 64434–64440 RSC.
- X. Zhang, H. Niu, Y. Pan, Y. Shi and Y. Cai, Modifying the surface of Fe3O4/SiO2 magnetic nanoparticles with C-18/NH2 mixed group to get an efficient sorbent for anionic organic pollutants, J. Colloid Interface Sci., 2011, 362, 107–112 CrossRef CAS PubMed.
- F. Liu, X. Yang, X. Wu, X. Xi, H. Gao, S. Zhang, W. Zhou and R. Lu, A dispersive magnetic solid phase microextraction based on ionic liquid-coated and cyclodextrin-functionalized magnetic core dendrimer nanocomposites for the determination of pyrethroids in juice samples, Food Chem., 2018, 268, 485–491 CrossRef CAS PubMed.
- A. Pietri, S. Rastelli, A. Mulazzi and T. Bertuzzi, Aflatoxins and ochratoxin A in dried chestnuts and chestnut flour produced in Italy, Food Control, 2012, 25, 601–606 CrossRef CAS.
- R. Luo, X. Zhou, Y. Chen, S. Tuo, F. Jiang, X. Niu, F. Pan and H. Wang, Lysozyme Aptamer-Functionalized Magnetic Nanoparticles for the Purification of Lysozyme from Chicken Egg White, Foods, 2019, 8, 67–80 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02763a |
| ‡ The authors contributed equally in this work. |
| This journal is © The Royal Society of Chemistry 2022 |