 Open Access Article
Open Access ArticleSynthesis of MoS2-based nanostructures and their applications in rechargeable ion batteries, catalysts and gas sensors: a review
Wei Sun ac,
Yaofang Zhang*ac,
Weimin Kang
ac,
Yaofang Zhang*ac,
Weimin Kang ad,
Nanping Dengad,
Xiaoxiao Wangad,
Xiaoying Kangac,
Zirui Yanac,
Yingwen Panac and
Jian Nib
ad,
Nanping Dengad,
Xiaoxiao Wangad,
Xiaoying Kangac,
Zirui Yanac,
Yingwen Panac and
Jian Nib
aState Key Laboratory of Separation Membranes and Membrane Processes, Tiangong University, Tianjin 300387, PR China
bDepartment of Electronic Science and Technology, College of Electronic Information and Optical Engineering, Nankai University, Tianjin, 300350, China
cSchool of Physical Science and Technology, Tiangong University, Tianjin 300387, PR China
dSchool of Textile Science and Engineering, Tiangong University, Tianjin 300387, China
First published on 6th July 2022
Abstract
Molybdenum disulfide (MoS2) is a two-dimensional (2D) layered material with a graphene-like structure that has attracted attention because of its large specific surface area and abundant active sites. In addition, the compounding of MoS2 with other materials can enhance the performance in applications such as batteries, catalysts, and optoelectronic devices, etc. MoS2 is prepared by various methods, among which chemical deposition and hydrothermal methods are widely used. In this review, we focus on summarizing the applications of MoS2 and MoS2 composite nanomaterials in rechargeable ion batteries, catalysts for water splitting and gas sensors, and briefly outline the preparation methods.
1. Introduction
Nanomaterials have attracted increasing research interest as a result of its fascinating physicochemical properties, such as the nano-size effect and large specific surface area. In 2005, the emergence of monolayer graphene set off a research boom in 2D materials.1–3 Many novel 2D materials have also been developed, such as hexagonal boron nitride (hBN) and transition metal dichalcogenides (TMDs). They are widely used in energy, sensing and other applications due to their excellent physical and chemical properties.4–9 Notably, MoS2, a member of TMDs, is a promising 2D material among compounds with graphene-like structures.It is well known that MoS2 materials have a wide range of applications, and we found that it has a high proportion of catalysts, batteries and gas sensors applications by searching the Web of Science for articles related to the applications of MoS2 in the last decade (Fig. 1b). Fig. 1a summarizes the number of published SCI papers on MoS2 over the last decade (up to May 2022) in the batteries, catalysts, and gas sensors. It is clear that MoS2 is attracting more and more attention in these applications.
 | ||
| Fig. 1 (a) Statistics of MoS2 core publications in batteries, catalysts, and gas sensors. (b) Percentage of core publications of MoS2 in different applications in the last decade (up to May 2022). | ||
MoS2 exhibits unique advantages over graphene-based or hBN-based nanomaterials in these applications. In detail, in batteries, MoS2 is used as an electrode material due to its high specific surface area and unique layer-like structure.10 In catalysts, MoS2 is a promising alternative to the precious metal Pt catalysts for hydrogen reaction evolution (HER) and photocatalytic water splitting, while MoS2 can be used in combination with other materials to improve visible light catalytic activity for the degradation of organic pollutants in industrial wastewater.11,12 In terms of gas-sensitive properties, MoS2 has good responsiveness and selectivity to some gases at room temperature (RT), which has led to widespread research and application of MoS2 materials in gas sensors.13
Recent years, many reviews about MoS2 nanomaterials were published. Some researchers have reviewed the application and preparation of MoS2 in energy (such as batteries and catalysts),14–16 some have reviewed the application and preparation of MoS2 in electronic components (such as memristors and field-effect transistors),17–20 some focus on MoS2 for detection and sensing applications,21–23 some have listed in detail the synthesis and application of 1T MoS2,24–26 and others have focused on the synthesis method of MoS2.27 Based on the previous researches and summaries, in this review, we comprehensively and systematically describe the applications of MoS2 and MoS2-based composites in rechargeable ion batteries, catalysts and gas sensors in recent years, and summarize the corresponding preparation schemes.
2. Applications and synthesis strategies of MoS2 in rechargeable ion batteries
To meet future energy storage needs, rechargeable ion batteries based on Li+, Na+, Al3+ and Zn2+ have been widely studied and prepared.28–31 MoS2 has a layered structure, which are connected by van der Waals forces with weak interlayer interactions and large layer spacing.32 High theoretical capacity, high charging rate and excellent stability make MoS2 become a promising electrode material. In this work, we will focus on the application and preparation of MoS2 as electrode materials.2.1 Lithium-ion batteries
Using MoS2 or composites of MoS2 for the anode materials is beneficial to lithium-ion batteries (LIBs). Wei et al.33 studied the electrochemical reactions of MoS2 nanosheets in LIBs. Their study represented that intercalation of Li ions into MoS2 anode contributes the electrochemical charge storage. However, the low conductive of MoS2 and its aggregation during the electrode manufacturing process greatly hinder the development of LIBs.34,35 In order to improve the performance of MoS2 as an electrode material for LIBs, there are two main options, one is to change the structure of MoS2 material, and the other is to prepare MoS2 composites.On the one hand, Zhao et al.36 studied MoS2 materials with nanotube structures to improve electrochemical performance. They reported a facile wet etching method for the preparation of low crystalline MoS2 nanotubes. First, MoO3 nanobelts (MoO3 NBs) were prepared by hydrothermal method. Sodium molybdate dihydrate (Na2MoO4·2H2O) and nitric acid were used in this step. Second, 3D MoS2 nanomasks were grown in situ on MoO3 NBs, which was obtained by the chemical reaction of sublimed sulfur with MoO3 NBs in CVD quartz tube. Finally, MoS2 nanotubes (MoS2 NTs) were synthesized by mixing the previously obtained MoOx/MoS2 NBs with concentrated hydrochloric acid. As demonstrated in Fig. 2a, the inner MoOx are etched with concentrated hydrochloric acids to yield low crystalline MoS2 NTs. With the increase of etching times, the molybdenum oxide is gradually removed which allowed the internal cavity of MoS2 NTs to be emptied. After the fourth etching process, most of the molybdenum oxides were removed to give MoS2 NTs (Fig. 2b).
 | ||
| Fig. 2 (a) MoS2 NTs are obtained after etching MoOx/MoS2 NBs with concentrated hydrochloric acid.36 (b) MoS2 NTs obtained from the fourth etching.36 (c) Schematic illustration of the preparation process of MoS2/C/C fiber.34 (d) SEM images of MoS2/C/C fiber. The inset is a magnified TEM image of the sample.34 (e) Schematic diagram of the synthesis of TiO2/C/MoS2 microsphere.35 (f) SEM images of TiO2/C/MoS2 microsphere.35 (g) Capacity retention of the MoS2, MoS2/C, and MoS2/C/C fiber electrodes at a current density of 0.2 A g−1 for the subsequent 150 cycles.36 (h) Comparative cycling performance of MoS2, TiO2/C and the TiO2/C/MoS2 microsphere at a current density of 100 mA g−1.35 | ||
The electrochemical test results illustrated that MoS2 NTs, as the anode material for LIBs, reached a specific capacity of 1253 mA g−1 at a current rate of 200 mA g−1 and was stabilized after 250 cycles. Obviously, the low crystalline MoS2 NTs have even higher specific capacity and cyclic performance than the reported electrode materials.37,38
On the other hand, some researchers have investigated MoS2 nanocomposites to improve the electrochemical properties of MoS2 in LIBs.
Wu et al.34 reported an electrode material of two-layer carbon-coated MoS2/carbon nanofiber (MoS2/C/C fiber) which prepared by hydrothermal and electrospinning method. First, MoS2 spheres were obtained by hydrothermal. Hexaammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O), thiourea (NH2CSNH2), and polyvinylpyrrolidone (PVP) were used in this step. Second, MoS2/C spheres were fabricated by using glucose and the above-obtained MoS2 spheres. Finally, they synthesized MoS2/C/C nanofiber by electrospinning method. Polyacrylonitrile (PAN), N,N-dimethylformamide (DMF) and the above obtained MoS2/C spheres were used. The preparation process of MoS2/C/C fiber is shown in Fig. 2c.
Meanwhile, Zhang et al.35 synthesized TiO2/C/MoS2 microspheres as anodes for LIBs. TiO2/C/MoS2 microspheres were prepared by solvent-thermal method and calcination. First of all they used PVP, acetic acid and tetrabutyltitanate (TBT) in a Teflon-lined autoclave for the reaction to prepare TiO2/C materials. Secondly, TiO2/C/MoS2 was synthesized by the obtained TiO2/C, ammonium molybdatetetrahydrate ((NH4)6Mo7O24·4H2O) and thiourea (CH4N2S). The preparation process of TiO2/C/MoS2 microsphere is shown in Fig. 2e.
No matter MoS2 is compounded with carbon materials or TiO2 materials, the electrochemical properties of MoS2 materials have been improved. On the one hand, for the MoS2/C/C electrode, the double-layer carbon coating (Fig. 2d) could not only suppress the irreversible reaction, but also confine the volume change during the lithiation/delithiation process.34 Moreover, MoS2/C/C fiber has better cycling performance than MoS2 spheres (Fig. 2g). On the other hand, the unique structure with flower-shaped of TiO2/C/MoS2 (Fig. 2f) could not only enlarge the electrolyte–electrode interface area but also shorten the diffusion length of Li+ intercalation/deintercalation.35 Compared with MoS2 materials, the cycling performance of TiO2/C/MoS2 are enhanced (Fig. 2h).
2.2 Sodium-ion batteries
Sodium ion batteries (SIBs) are considered as an alternative to LIBs because of their abundant reserves and low cost. However, Na+ has larger radius than Li+,39 which hingers the development of SIBs. As a highly promising electrode material, MoS2 has not only a layered structure but also a large interlayer spacing, which promises to solve the inherent defects of SIBs. However, MoS2 also has inherent limitations, such as low intrinsic electron conductivity. In response to these characteristics, some researchers have prepared composites of MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–43 and others have improved the structure of MoS2 by doping or inserting molecules to achieve improved electrochemical properties.40–45
40–43 and others have improved the structure of MoS2 by doping or inserting molecules to achieve improved electrochemical properties.40–45
Pan et al.42 reported a simple template method to prepared MoS2/amorphous carbon (C) microtubes (MTs) composed of heterostructured MoS2/C nanosheets. The synthesis of MoS2/C MTs was achieved by a three-step procedure: first, obtaining Sb2S3 microrods by a simple hydrothermal method, second, MoS2/C nanosheets were grown on the outer surface of Sb2S3 microrods by using sodium molybdate dehydrate (Na2MoO4·2H2O), N2H4CS, and glucose (C6H12O6) in a Teflon-lined stainless steel autoclave for chemical reaction, and third, MoS2/C MTs were obtained by removing Sb2S3 microrods via annealing. The synthesis schematic is shown in Fig. 3a. Electrochemical measurements demonstrated that MoS2/C MTs possessed high specific capacity and excellent stability, improving the electrochemical performance of SIBs.
 | ||
| Fig. 3 (a) Schematic illustration of the synthesis process of MoS2/C MTs.42 (b) Schematic diagram of the fabrication process of MoS2–C hollow rhomboids.41 (c) FESEM images of OMSCF calcined in air at 400 °C (OMSCF-400).43 (d) Schematic illustration of the synthesis of MoS2−xSex/G.45 (e) Capacity of all intercalated MoS2 at 50 mA g−1 arranged according to the interlayer distance, respectively.44 (f) Capacity of all intercalated MoS2 50 mA g−1 arranged according to conductivity, respectively.44 (g) Schematic of intercalation of molecules into MoS2.44 | ||
Similarly, some researchers have also reported composites of MoS2 for enhancing the electrochemical performance of SIBs.
The MoS2/carbon nanofibers (MoS2/CNFs) were prepared by a two-step procedures: first, obtaining ammonium tetrathiomolybdate (AMT), and second, synthesizing MoS2/CNFs by electrospinning and high temperature carbonization. MoS2/CNFs have a large specific surface area and high electrical conductivity, which enhances Na storage performance.40
The MoS2–C hollow rhomboids (MCHRs) were fabricated by a sample one-pot solvothermal reaction (Fig. 3b). First of all, manganese(II)acetylacetonate (Mn(acac)2), molybdenyl acetylacetonate (MoO2(acac)2) were dispersed in distilled water and isopropanol. And then, glucose and thiourea were incorporated into the mixture. Finally, the mixture was annealed after reaction in a Teflon-lined autoclave and washed several times with dilute hydrochloric acid and deionized water to obtain MCHRs. Electrochemical measurements revealed that MCHRs had better Na storage performance, higher rate capability, more stable cycling performance and superior reversible specific capacity.41
The vertically oxygen-incorporated MoS2 nanosheets coated on carbon fiber (OMSCF) were synthesized by hydrothermal process and calcination reaction in air. First, carbon fiber was extracted from commercial wet tissue (Vinda Paper Group) with concentrated hydrochloric acid. Second, graphite oxide (GO) was synthesized through the modified Hummers' method. Finally, MoS2/carbon fibers (MSCF) were obtained by hydrothermal method. The FESEM images of OMSCF are shown in Fig. 3c. Oxygen atoms are incorporated into MoS2 by the MSCF calcined in air. The incorporation of oxygen not only creates more defects, but also expands the interlayer spacing. The composite of carbon fiber and MoS2 nanosheets not only improves electronic conductivity, but also enhances structural stability.43
In addition, Zhang et al.45 prepared ternary MoS2−xSex alloy/graphene (MoS2−xSex/G) composite though hydrothermal reaction and selenization treatment (Fig. 3d). The interlayer spacing of MoS2 is expanded due to the doping of Se atoms which facilitates Na+ fast transfer. Meanwhile, the electronic conductivity of composite is enhanced due to graphene, which boosts the electrochemical performance for NIBs.
Dai et al.44 reported a series of molecule-intercalated MoS2 as anode materials for SIBs. The molecular intercalation method expands the interlayer spacing as well as increases the electrical conductivity of MoS2 (Fig. 3e and f). The interlayer spacing can be varied in the range of 0.62 to 1.26 nm precisely by inserting different molecules (Fig. 3g).
In the example of the dimethylacetamide–MoS2 (DAM–MoS2) construct, MoS2 was synthesized by hydrothermal method firstly. Second, squeezing small DAM molecules into the layers.46 Specifically, DAM, (NH4)6Mo7O24·4H2O, Na2S·9H2O, N2H4·H2O and deionized water were mixed. Then, the mixture was placed in Teflon-liner autoclave and heated at 230 °C for 24 h. Finally, dark powders were collected after naturally cooled to RT. Benefiting from the expanded interlayer spacing and improved conductivity, the electrochemical performance of SIBs with MoS2 as the electrode material has been enhanced.
2.3 Other rechargeable batteries
Aluminum ion batteries (AIBs) are also members of energy storage systems. MoS2 and its composites can be used as cathode materials for AIBs. Li et al.47 prepared MoS2 microspheres structure by hydrothermal method. The preparation process is shown in the Fig. 4a. First, the MoS2 microsphere precursor was synthesized by using (NH4)6Mo7O24·4H2O and (NH4)2CS in a hydrothermal method. And then, MoS2 microsphere was obtained by heat treatment in a nitrogen atmosphere.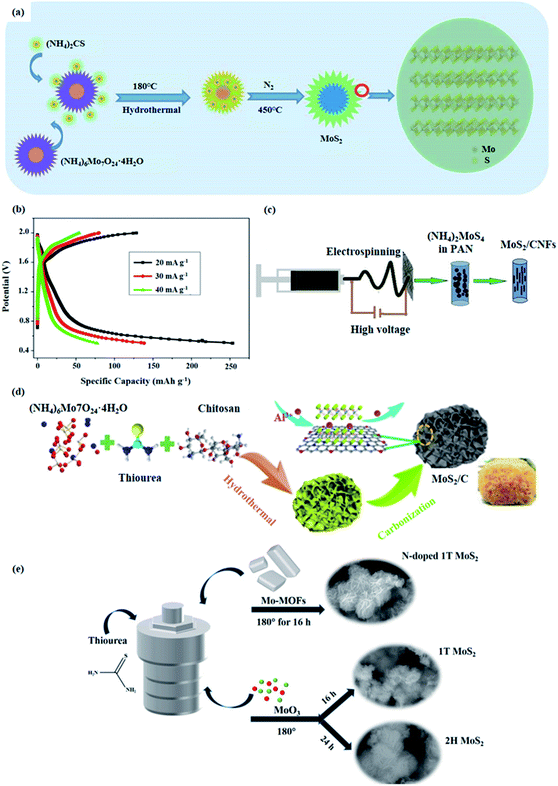 | ||
| Fig. 4 (a) Schematic illustration of MoS2 microspheres prepared by hydrothermal method.47 (b) The first discharge–charge curves at different current densities.47 (c) Schematic illustration of the preparation process of MoS2/CNFs.48 (d) The preparation process of MNC.49 (e) The synthesis of N-doped 1T MoS2, pure 1T MoS2, and 2H MoS2.50 | ||
Fig. 4b depicts the electrochemical performance of MoS2 microspheres. Obviously, the electrochemical performance of AIBs with MoS2 microsphere cathode material is not excellent. The reason for this can be attributed to the inherent defects of MoS2. Therefore, future research focusing on enhancing the electrochemical properties of MoS2 electrode materials is needed.
Yang et al.48 reported a flexible free-standing MoS2/CNFs cathode for rechargeable AIBs. As shown in Fig. 4c, the MoS2/CNFs are prepared by electrospinning and annealing treatment. As electrode materials for AIBs, MoS2/CNFs exhibit better cycling stability and higher rate capacity than MoS2 microspheres.
In order to overcome the defects of MoS2 and achieve the improved electrochemical performance of AIBs, another method is to use N-doped carbon materials compounded with MoS2 as a cathode material for AIBs. Guo et al.49 synthesized interlayer-expanded MoS2/N-doped carbon (MNC) with a three-dimensional (3D) hierarchical structure by a hydrothermal method and calcination. Fig. 4d represents the synthesis of MNC. Electrochemical test results illustrated that MNC had excellent cycling ability and high discharge capacity, which were owing to the unique 3D structure provides a large specific surface area and the N-doped carbon expands the interlayer spacing of MoS2.
Aqueous zinc ion batteries (ZIBs) are one of the rechargeable batteries based on divalent cations. Nevertheless, Zn2+ has strong interactions with water molecules, increasing the difficulty of Zn2+ diffusion and intercalation,54 which hinders the development of ZIBs. To address these problems, researchers used MoS2 as an electrode material to improve the electrochemical performance of ZIBs by increasing its interlayer spacing through doping with nitrogen or oxygen.50,54
In the example of the N-doped MoS2, Mo–organic framework (Mo–MOF) served as the nitrogen source. Basing on the one-step hydrothermal sulfurization, N-doped MoS2 was prepared.50 Ideally, the 1T and 2H phases of MoS2 can be obtained by different reaction conditions (Fig. 4e).
The electrochemical test results illustrated that N-doped 1T MoS2 has not only high multiplicative performance but also superior cycling stability, which greatly improves the electrochemical performance of ZIBs.
In order to better display the synthesis and application of MoS2-based nanomaterials in electrode materials, the preparation methods and batteries performance are summarized in Table 1. In addition, we also collected some typical nanomaterials for battery applications to compare with MoS2.51–53
| No. | Materials | Preparation | Mo source | S source | Morphology of MoS2 | Battery electrodes | Specific capacity (mA h g−1) | Cycling number | Current rate (mA g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MoS2 | Wet etching method | Na2MoO4·2H2O | Sulfur | Nanotube | LIBs cathode | 1150 | 250 | 200 | 36 |
| 2 | MoS2/C/C | Hydrothermal and electrospinning method | (NH4)6Mo7O24·4H2O | N2H4CS | Sphere | LIBs anode | 1062 | 150 | 200 | 34 |
| 3 | TiO2/C/MoS2 | Solvent-thermal method and calcination | (NH4)6Mo7O24·4H2O | N2H4CS | Fish-scale-shaped (10 nm in size) | LIBs anode | 621 | 100 | 100 | 35 |
| 4 | MoS2/C | Template method | Na2MoO4·2H2O | N2H4CS | Nanosheet | SIBs anode | 484.9 | 1500 | 2000 | 42 |
| 5 | MoS2/CNFs | Electrospinning and high temperature carbonization | AMT | AMT | Single-layer structure | SIBs anode | 485 | 100 | 100 | 40 |
| 6 | MCHRs | One-pot solvothermal reaction | MoO2(acac)2 | N2H4CS | Nanosheet | SIBs anode | 265 | 3000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
41 |
| 7 | OMSCF | Hydrothermal process and calcination reaction | Na2MoO4 | N2H4CS | Nanosheet | SIBs anode | 330 | 100 | 100 | 43 |
| 8 | MoS2−xSex/G | Hydrothermal reaction and selenization treatment | (NH4)6Mo7O24·4H2O | N2H4CS | — | SIBs anode | 178 | 700 | 2000 | 45 |
| 9 | DAM–MoS2 | Hydrothermal method | (NH4)6Mo7O24·4H2O | Na2S·9H2O | Layered structure (0.62–1.24 nm in size) | SIBs anode | 420 | 600 | 100 | 44 |
| 10 | MoS2 | Hydrothermal method | (NH4)6Mo7O24·4H2O | N2H4CS | Microsphere | AIBs cathode | 66.7 | 100 | 40 | 47 |
| 11 | MoS2/CNFs | Electrospinning and annealing treatment | (NH4)2MoS4 | (NH4)2MoS4 | Nanosheet | AIBs cathode | 130 | 200 | 100 | 48 |
| 12 | MNC | Hydrothermal method and calcination | (NH4)6Mo7O24·4H2O | N2H4CS | Nanosheet | AIBs cathode | 127.5 | 1700 | 1000 | 49 |
| 13 | N-doped MoS2 | One-step hydrothermal sulfurization | Mo–MOF | N2H4CS | Nanoflower | ZIBs cathode | 98.1 | 1000 | 3000 | 50 |
| 14 | hBN/C | Liquid-phase shear exfoliation method | — | — | — | LIBs separators | 158 | 100 | — | 51 |
| 15 | rGO/Al | Electrospraying | — | — | — | LiNi0.5Mn1.5O4 cathode | 109.5 | 840 | — | 52 |
| 16 | P4Nb2O15@CNTs | Solvothermal method | — | — | — | LIBs anode | 250 | 500 | — | 53 |
3. Applications and synthesis strategies of MoS2 in catalyst for water splitting
The use of large amounts of fossil fuels has led to increasing environmental degradation, therefore, it is essential to produce clean, renewable energy. Hydrogen energy, as one of the clean energy sources, has been widely researched in recent years. Electrocatalytic water splitting and photocatalytic water splitting are recognized as efficient methods for the preparation of hydrogen.55–60 The water splitting reaction requires an efficient catalyst. It is well known that MoS2 is a lamellar structure with abundant active sites at the edges. This property makes it a promising non-precious metal catalyst with large numbers of applications in catalysis. However, the defects of MoS2 with low bulk conductivity and anisotropic electrical transport restrict the catalytic efficiency. Therefore, researchers have developed amount of MoS2 composite catalysts to improve the catalytic efficiency.3.1 Electrocatalyst
According to previous reports, either 1T-phase MoS2 catalysts or MoS2 composites catalysts have efficient catalytic performance in the HER. In detail, 1T-phase MoS2 has higher catalytic performance than 2H-phase MoS2, benefiting from the fast charge transfer rate in the metal phase.61 The compounding of MoS2 with MoN can not only improve the electrical conductivity of MoS2, but also make MoS2 have good stability in acidic and alkaline environments.62 The compounding of MoS2 with CNFs can improve the electrical conductivity of MoS2 and restrict the growth of MoS2 nanosheets.63 In addition, MoS2 composites can be used as bifunctional and efficient electrocatalysts for water splitting. For example, CoS2–C@MoS2 exhibits both excellent HER catalytic performance and good oxygen evolution reaction (OER) catalytic performance.64 MoS2 compounded with Mo2N-containing multichannel hollow CNFs (Mo2N–MoS2 MCNFs) also possesses excellent HER and OER catalytic properties.65 Subsequently, the preparation of these materials will be described.1T-MoS2 was synthesized by hydrothermal reaction. Specifically, (NH4)6Mo7O24·4H2O and N2H4CS were dissolved in distilled water to form a homogeneous solution, and then the solution was put into a Teflon-lined stainless steel autoclave for reaction. The formation of 1T phase or 2H phase depends on the reaction temperature.
Hierarchical MoS2/MoN heterostructures were obtained by a simple hydrothermal reaction and nitridation treatment. MoS2 nanospheres were synthesized from N2H4CS and hexaammonium molybdate in a hydrothermal reaction. Subsequently, the layered MoS2/MoN heterostructures were synthesized by nitriding under ammonia atmosphere.
MoS2–carbon CNFs were prepared by electrospinning and graphitization treatment. First, (NH4)2MoS4 was dissolved in PAN solution and used for electrospinning to prepare PAN/(NH4)2MoS4 (PANAMo) nanofibers. Afterwards, the precursor nanofibers were graphitized to obtain MoS2–CNFs hybrids.
CoS2–C@MoS2 core–shell nanofibers were fabricated by electrospinning method, carbonization treatment and hydrothermal synthesis. First, a certain amount of PAN and Co(Ac)2·4H2O were dissolved in DMF to prepare Co(Ac)2/PAN membranes by electrospinning method. Later, the Co–C nanofibers were obtained by carbonization under Ar atmosphere. Second, CoS2–C@MoS2 core–shell nanofibers were prepared by a simple hydrothermal method using (NH4)2MoS4 as the S source.
As depicted in Fig. 5a, the synthesis of Mo2N–MoS2 MCNFs was achieved by a four-step procedure. First, a certain amount of PAN and polystyrene (PS) were dissolved in DMF, stirred well and then MoO2(acac)2 was added to form a precursor solution for electrospinning to obtain MoO2(acac)2@PAN/PS fiber. Second, previously obtained fiber was pre-oxidized in air. Third, Mo2N/C MCFs were prepared by calcination of the pre-oxidation fiber under NH3 atmosphere. During calcination, PS gradually decomposed, leading to the formation of channels in the fibers. Finally, Mo2N–MoS2 MCNFs were successfully prepared by hydrothermal method to grow MoS2 nanosheets on the surface of Mo2N/Carbon MCNFs.
 | ||
| Fig. 5 The diagrammatic sketch for the preparation of (a) Mo2N–MoS2 MCNFs,65 (b) MoS2@TiO2 composites,68 (c) TiO2/MoS2/CdS tandem heterojunction,69 (d) 2D–2D MoS2/g-C3N4 composites70 and (e) g-C3N4/Co3O4/MoS2 heterojunction.71 | ||
3.2 Photocatalyst
Photocatalytic water splitting reaction is considered as one of the effective ways to prepare green, renewable energy, due to its ability to convert solar energy into hydrogen energy. In recent years, with the development of hydrogen preparation reaction by photocatalytic water splitting, more and more photocatalysts have been studied and prepared, including those prepared with graphite carbon nitride (g-C3N4), TiO2 or CdS as materials. It has been shown that the compound of MoS2 with the above materials can improve the catalytic activity of the photocatalyst and promote the preparation of hydrogen by water splitting.68–71,73Hu et al.68 prepared MoS2@TiO2 composites by using combination of hydrothermal/annealing treatment with subsequent photoreduction method. It is noted that MoS2 nanosheets can be selectively deposited on the (101) facets of TiO2, allowing for increased photocatalytic hydrogen production activity of the MoS2@TiO2 composites. Sun et al.69 fabricated a hollow TiO2/MoS2/CdS tandem heterojunction via three main steps. First, the hollow mesoporous TiO2 spheres were synthesized by a template-free solvothermal approach. Second, MoS2 nanosheets were coated on the surface of TiO2 by a solvothermal approach. Finally, CdS nanoparticles were selectively deposited on the edges of MoS2 nanosheets though a wet chemical method. MoS2 not only serves as an excellent cocatalyst, but also promotes charge separation and effectively inhibits the complexation of photogenerated electrons and holes. Yuan et al.70 obtained 2D–2D MoS2/g-C3N4 photocatalyst though a simple probe sonication assisted liquid exfoliation method and a solvent-thermal method. The large surface area of g-C3N4 nanosheets and the large 2D nanointerface between MoS2 and g-C3N4 nanosheets greatly enhance the catalytic hydrogen production activity of the photocatalyst. Zhao et al.71 synthesized g-C3N4/Co3O4/MoS2 heterojunction via chemical deposition and photo-deposition method. Co3O4 and MoS2 were used as co-catalysts with efficient photocatalytic activity under visible light irradiation. Their synthesis schematic is demonstrated in Fig. 5b–e.
In order to better display the synthesis and application of MoS2-based nanomaterials in catalysis, the preparation methods and catalytic performance are summarized in Table 2. Furthermore, for comparison, we summarize performance parameters of some typical nanomaterials in electrocatalysis and photocatalysis at the end of the table.66,67,72
| No. | Materials | Preparation | Mo source | S source | Morphology of MoS2 | Electrocatalyst | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Tafel slope (mV dec−1) | Overpotential (mV vs. RHE) at J = 10 mA cm−2 | |||||||
| 1 | 1T-MoS2 | Hydrothermal reaction | (NH4)6Mo7O24·4H2O | Thiourea | Nanosheet | 54 (HER) | 214 (HER) | 61 |
| 2 | MoS2/MoN | Hydrothermal reaction and nitridation treatment | Hexaammonium molybdate | Thiourea | Nanosphere | 98 (HER, KOH); 87 (HER, H2SO4) | 132 (HER, KOH); 117 (HER, H2SO4) | 62 |
| 3 | MoS2/CNFs | Electrospinning and graphitization treatment | (NH4)2MoS4 | (NH4)2MoS4 | Nanoplate | 42 (HER) | 93 (HER) | 63 |
| 4 | CoS2–C@MoS2 | Electrospinning method, carbonization treatment and hydrothermal synthesis | (NH4)2MoS4 | (NH4)2MoS4 | Nanosheet | 61 (HER); 46 (OER) | 173 (HER); 391 (OER) | 64 |
| 5 | Mo2N–MoS2 MCNFs | Electrospinning method, NH3 calcination and hydrothermal synthesis | (NH4)6Mo7O24·4H2O | Thiourea | Nanosheet | 68.9 (HER); 57.2 (OER) | 131 (HER); 270 (OER) | 65 |
| 6 | Graphene–hBN | Exfoliation and Hummer's method | — | — | — | — | 390 (HER) | 66 |
| 7 | Cobalt- and nitrogen-codoped graphene | Annealing strategy | — | — | — | 73 (OER) | 210 (OER) | 67 |
| No. | Materials | Preparation | Mo source | S source | Morphology of MoS2 | Photocatalyst | Ref. |
|---|---|---|---|---|---|---|---|
| H2 evolution rate (mmol h−1 g−1) | |||||||
| 8 | MoS2@TiO2 | Hydrothermal/annealing treatment and subsequent photoreduction method | (NH4)2MoS4 | (NH4)2MoS4 | Nanosheet | 2.16 | 68 |
| 9 | TiO2/MoS2/CdS | Template-free solvothermal approach, solvothermal approach and wet chemical method | MoO3 | Thiourea | Nanosheet | 9 | 69 |
| 10 | MoS2/g-C3N4 | Direct heating of urea and a solvent-thermal method | (NH4)2MoS4 | (NH4)2MoS4 | Nanosheet | 1.155 | 70 |
| 11 | g-C3N4/Co3O4/MoS2 | Two-step thermal treatment, coprecipitation-calcination strategy and in situ photo-deposition | (NH4)2MoS4 | (NH4)2MoS4 | MoS2 nanocrystal | 5.25 | 71 |
| 12 | Sulfur-doped h-BN | CVD | — | — | — | 1.3485 | 72 |
4. Applications and synthesis strategies of MoS2 in gas sensors
Important factors affecting the performance of gas sensors have been reported to include specific surface area, semiconductor properties, and redox reaction active sites.74 As mentioned earlier, MoS2 is a graphene-like material possessing a 2D layer structure with a large specific surface area and excellent semiconductor properties. In addition, it has been pointed out that MoS2 has different affinities for different molecules,75 which makes MoS2 one of the promising materials for the preparation of gas sensors.4.1 MoS2-based gas sensors toward nitrogen dioxide
Nitrogen dioxide (NO2) is one of the prevalent pollutants in the air, as well as a toxic gas that endangers human health, causing great damage to human eyes and respiratory tracts even when exposed to concentrations as low as 3 ppm.76 Therefore, it is urgent to develop gas sensors that can detect NO2 effectively and rapidly. The detection of NO2 by pure MoS2 or MoS2 composites as gas-sensitive elements is one of the main focuses of gas sensors research in recent years.Using pure MoS2 as gas sensitive element, some researches have prepared MoS2 by chemical vapor deposition (CVD) method. For instance, Kumar et al.77 obtained 2D MoS2 by CVD with MoO3 powder and sulfur as precursors. The test results revealed that the MoS2 gas sensor had a response time of 29 s and a recovery time of 350 s for 100 ppm concentration of NO2 when operating in a RT environment irradiated by UV lamps (∼365 nm). Similarly, Kim et al.13 fabricated layer-controlled MoS2 by CVD with molybdenum hexacarbonyl (Mo(CO)6) and hydrogen sulfide (H2S). It is found that the Schottky barrier changes due to the change in the number of MoS2 layers, which results in an improved response of the gas sensor. Zheng et al.75 synthesized n-type and p-type MoS2 films by CVD and soft-chemistry route, respectively. In CVD process, MoO3 and sulfur were used as precursors, while in the soft-chemistry route, molybdate sol–gel (contain 1% W) was used as precursors. Uniquely, they prepared a novel p–n junction gas sensor by stacking n-type and p-type MoS2 atomic layers. The results represented that compared with n-type MoS2 gas sensor, the p-type MoS2 has a faster response to NO2. More importantly, the p–n junction sensor not only has a 20-fold increase in sensitivity to 20 ppm NO2, but also has a lower detection limit of 8 ppb.
Just as pure MoS2 gas sensors exhibit gas-sensitive performance on NO2 gas, MoS2 composite gas sensors also have excellent gas-sensitive properties. For example, the PbS quantum dots modified MoS2 (MoS2/PbS) composite gas sensor prepared by Xin et al.78 has excellent gas-sensitive performance for NO2 due to the high response of PbS quantum dots to NO2 and the prevention of MoS2 oxidation. MoS2/PbS was prepared by hydrothermal and chemical precipitation methods, and the specific preparation is shown in Fig. 6a. First of all, pure MoS2 was prepared from Na2MoO4·2H2O and CH3CSNH2 by hydrothermal reaction under an Teflon-lined autoclave at 200 °C. Secondly, the doping of PbS quantum dots was achieved by chemical precipitation using Na2S·9H2O and Pb(NO3)2 as precursors. Compared with pure MoS2, the MoS2/PbS gas sensor has higher response and recovery performance for 100 ppm NO2 gas at RT (Fig. 6b).
 | ||
| Fig. 6 (a) Preparation process of MoS2/PbS composites.78 (b) Transient response characteristic of MoS2/PbS gas sensor at 100 ppm NO2.78 | ||
Composites of MoS2 nanosheets with SnO2 nanotubes were prepared for gas-sensitive properties by Bai et al. MoS2@SnO2 heterostructure exhibits impressive sensitivity and selectivity for the detection of NO2 gas at RT. Tests illustrated that the MoS2@SnO2 gas sensor had a fast response time (2.2 s), a short recovery time (10.54 s), a low detection limit (10 ppb) and excellent stability (20 weeks) (Fig. 7a).79 Another reported composite is MoS2 nanoflowers modified with Au nanoparticles prepared by Chen et al. Surprisingly, the Au–MoS2 gas sensor exhibits an extremely low detection limit (10 ppb) for NO2 at RT with strong resistance to moisture interference under 530 nm light illumination (Fig. 7b).80
 | ||
| Fig. 7 (a) Response and response time of MoS2@SnO2 sensor to 0.01–100 ppm NO2.79 (b) Real-time sensing response curves of the 530 nm-light-assisted Au–MoS2 sensor at 1–50 ppm NO2.80 (c) Schematic diagram of the synthesis of MoS2@SnO2.79 | ||
The preparation of MoS2@SnO2 was achieved by electrostatic spinning and hydrothermal methods, as presented in Fig. 7c. First, stannous chloride (SnCl2·2H2O) was mixed with anhydrous ethanol, DMF and PVP to make electrospinning solution, and SnO2 NTs were obtained by spinning technique and subsequent high-temperature calcination treatment. Second, N2H4CS and Na2MoO4·2H2O were used as the S and Mo sources, respectively, to mix with the previously prepared SnO2 NTs, and the reaction was carried out in an autoclave at 200 °C to realize MoS2 on SnO2 NTs growth.79
The fabrication of Au–MoS2 composites was achieved by a two-step hydrothermal method. Firstly, MoS2 was obtained by reacting Na2MoO4·2H2O and thioacetamide (CH3CSNH2) in a Teflon-lined autoclave at 200 °C for 36 h. Secondly, Au–MoS2 was synthesized by mixing sodium citrate tribasic dihydrate (C6H5Na3O7·2H2O), tannic acid (C76H52O46) and previously prepared MoS2, then adding gold chloride trihydrate (HAuCl4·3H2O) solution dropwise and stirring well, and then reacting in a Teflon-lined autoclave.80
4.2 MoS2-based gas sensors for other gases
The MoS2-based gas sensors not only detect NO2 gas extremely well, but also reveal excellent gas sensitivity to NH3, SO2 and alcohol gases.Dattatray J. Late et al.74 prepared layered MoS2 films by micromechanical exfoliation method in 2013 for the preparation of gas sensors to detect NH3 gas. The experimental results demonstrated that the 2-layer MoS2 and 5-layer MoS2 have excellent gas-sensitive performance to NH3, and the 5-layer MoS2 is more sensitive to detect NH3. In addition, when the MoS2 gas sensor is applied with a positive gate voltage, the electric field formed at the interface will repel the electrons given by NH3 as an electron donor, resulting in a decrease in the sensitivity of MoS2 to NH3. Fig. 8a shows the curves of sensitivity with NH3 concentration for 2-layer and 5-layer MoS2 with and without gate voltage.
 | ||
| Fig. 8 (a) Sensitivity of 2-layer and 5-layer MoS2 as a function of NH3 concentration.74 (b) Schematic diagram of the fabrication of MoS2 sensors and MoS2/SnO2 sensors.81 (c) Response of MoS2/SnO2 sensors to different concentrations of SO2 gas at different operating temperatures.81 | ||
Nguyen Ngoc Viet et al.81 prepared MoS2/SnO2 sensors for SO2 gas detection by on-chip electrostatic spinning and subsequently dropping MoS2 nanosheets-dispersed solution, and the fabrication is depicted in Fig. 8b. The test results indicated that the MoS2/SnO2 gas sensor had good gas-sensitive performance for 10 ppm SO2 gas at 150 °C (Fig. 8c).
Sukhwinder Singh et al.82 prepared MoS2/TiO2 composite for the detection of methanol and ethanol. As shown in Fig. 9a, MoS2/TiO2 hybrid was obtained by two steps: first, pure TiO2 powder was mixed with ethanol and other solvents for probe sonication, and second, (NH4)2MoS4 was mixed with the produced TiO2 suspension to prepare MoS2/TiO2 composites by hydrothermal method. The test results revealed that the best working temperatures of MoS2/TiO2 composites for methanol and ethanol were 240 °C and 300 °C, respectively, and more importantly, the MoS2/TiO2 sensor had good response and better stability (Fig. 9b and c).
 | ||
| Fig. 9 (a) Schematic diagram of the synthesis of MoS2/TiO2 composite.82 (b) Repeatability testing of 200 ppm methanol for six consecutive cycles at an operating temperature of 240 °C.82 (c) Repeatability testing of 300 ppm methanol for six consecutive cycles at an operating temperature of 300 °C.82 | ||
The gas sensing performance of MoS2-based nanomaterials and the preparation methods are listed in Table 3. As a comparison, the gas-sensitive properties of some typical materials are collected at the end of the table.83–86
| No. | Materials | Preparation | Mo source | S source | Morphology | Target Gas | Res/Rec (s) | Response (Rg/Ra) | T (°C) | Detection limits | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MoS2 | CVD | MoO3 | Sulfur | Film | 100 ppm of NO2 | 29/350 | 1.3516 | RT (UV) | — | 77 |
| 2 | MoS2 | CVD | Mo(CO)6 | H2S | Film | 10 ppm of NO2 | —/— | 1.6 | RT | — | 13 |
| 3 | MoS2 | CVD | MoO3 | Sulfur | Film | 20 ppm of NO2 | 150/30 | — | RT (UV) | 8 ppb | 75 |
| 4 | MoS2/PbS | Hydrothermal method combined with chemical precipitation | Na2MoO4·2H2O | CH3CSNH2 | Fluffy ball-like structure | 100 ppm of NO2 | 30/235 | — | RT | — | 78 |
| 5 | MoS2@SnO2 | Electrospinning and hydrothermal growth | Na2MoO4·2H2O | N2H4CS | Nanoflake | 100 ppm of NO2 | 2.2/10.54 | 0.02884 | RT | 10 ppb | 79 |
| 6 | Au–MoS2 | Hydrothermal method | Na2MoO4·2H2O | CH3CSNH2 | Fluffy flower-like structure | 1 ppm of NO2 | —/27 | 8.1 | RT (530 nm LED) | 10 ppb | 80 |
| 7 | MoS2 | Micromechanical exfoliation method | Bulk MoS2 crystal | Bulk MoS2 crystal | Layered | 1000 ppm of NO2 | —/— | 14.72 | RT | — | 74 |
| 8 | MoS2 | Micromechanical exfoliation method | Bulk MoS2 crystal | Bulk MoS2 crystal | Layered | 1000 ppm of NH3 | —/— | 1.86 | RT | — | 74 |
| 9 | SnO2/MoS2 | Electrospinning and drop-coated process | MoS2 powder | MoS2 powder | Nanosheet | 10 ppm of SO2 | —/— | 11.1 | 150 | 5 ppt (parts-per-trillion) | 81 |
| 10 | MoS2/TiO2 | Low-cost hydrothermal method | (NH4)2MoS4 | (NH4)2MoS4 | Layered | 500 ppm of ethanol | 50/100 | nearly 0 | 300 | — | 82 |
| 11 | MoS2/TiO2 | Low-cost hydrothermal method | (NH4)2MoS4 | (NH4)2MoS4 | Layered | 500 ppm of methanol | —/— | 0.15 | 240 | — | 82 |
| 12 | CuO/rGO | LbL self-assemble | — | — | — | 1 ppm of CO | 70/160 | 1.0256 | RT | — | 83 |
| 13 | Single-walled carbon nanotubes | — | — | — | — | 100 ppb of NO | —/— | 0.7136 | RT | — | 84 |
| 14 | Graphene oxide | Thermal reduction | — | — | — | 5 ppm of NO2 | —/— | 0.83 | RT | — | 85 |
| 15 | DETA doped graphene | CVD and vapor-phase molecular doping | — | — | — | 50 ppm of NO2 | —/— | 0.23 | RT | 0.83 ppq (parts per quadrillion) | 86 |
5. Conclusion
This review highlights recent advances in MoS2-based materials synthesis and their applications toward batteries, catalysts and gas sensors. First of all, MoS2, due to the large specific surface area and abundant active sites, has become one of the most popular electrode materials. In addition, the compound of MoS2 with CNFs and TiO2 materials overcomes the inherent defects of MoS2 and greatly improves the electrochemical performance of the battery. Second, MoS2 has catalytic active sites on the edges, which makes it one of the most popular candidates to replace noble metal catalysts. The composite of MoS2 with MoN, CoS2 and C3N4 improved the catalytic performance of the catalyst. Finally, MoS2 can be used in gas sensors due to the semiconductor properties and non-zero forbidden bandwidth. The compound of MoS2 with materials such as SnO2 and PbS can enhance the sensitivity of the gas sensor to the gas to be detected and reduce the detection limit.It is worth noting that while MoS2 has made good progress in these areas, challenges remain in its future development. First, MoS2 has low electrical conductivity and multilayer MoS2 tends to accumulate and aggregate in the preparation, which is not conducive to electron transport. Second, the active sites of MoS2 are mainly at the edges but not at the basal plane, which has a significant impact on both the sensing performance and catalytic performance. Therefore, it is necessary to further explore the compounding of MoS2 with other materials or to optimize the structure of MoS2 (e.g., preparation of MoS2 NTs, etc.). In addition, 1T-MoS2 has better electrical conductivity compared with 2H-MoS2, and there are also interesting electrical properties using 1T-MoS2 compounded with other materials.
In a word, MoS2 has promising applications in energy and gas sensors due to its excellent and unique physicochemical properties. We believe that with the joint efforts of researchers in the future, better progress will be made in the applications and synthesis of MoS2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (No. 61904123), the Natural Science Foundation of Tianjin (No. 18JCQNJC71800), Scientific Research Project of Tianjin Educational Committee (No. 2018KJ220), Tianjin Technical and Engineering Center of Nonwovens (No. KF202103).References
- K. S. Novoselov, D. Jiang, F. Schedin, T. Booth, V. Khotkevich, S. Morozov and A. K. Geim, Proc. Natl. Acad. Sci., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang and A. F. Ismach, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS.
- G. R. Bhimanapati, Z. Lin, V. Meunier, Y. Jung, J. Cha, S. Das, D. Xiao, Y. Son, M. S. Strano and V. R. Cooper, ACS Nano, 2015, 9, 11509–11539 CrossRef CAS.
- Z. Wang and B. Mi, Environ. Sci. Technol., 2017, 51, 8229–8244 CrossRef CAS PubMed.
- H. Wang, C. Li, P. Fang, Z. Zhang and J. Z. Zhang, Chem. Soc. Rev., 2018, 47, 6101–6127 RSC.
- U. Krishnan, M. Kaur, K. Singh, M. Kumar and A. Kumar, Superlattices Microstruct., 2019, 128, 274–297 CrossRef CAS.
- T. Nawz, A. Safdar, M. Hussain, D. Sung Lee and M. Siyar, Crystals, 2020, 10 Search PubMed.
- D. Saha and P. Kruse, J. Electrochem. Soc., 2020, 167 Search PubMed.
- O. Samy and A. El Moutaouakil, Energies, 2021, 14 Search PubMed.
- Y. Zhang, H. Tao, S. Du and X. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 11327–11337 CrossRef CAS.
- R. Bose, Z. Jin, S. Shin, S. Kim, S. Lee and Y. S. Min, Langmuir, 2017, 33, 5628–5635 CrossRef CAS.
- Y. Wang, J. Sunarso, F. Wang, B. Zhao, X. Liu and G. Chen, Ceram. Int., 2017, 43, 11028–11033 CrossRef CAS.
- Y. Kim, S. K. Kang, N. C. Oh, H. D. Lee, S. M. Lee, J. Park and H. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 38902–38909 CrossRef CAS PubMed.
- Z. Liu, L. Zhao, Y. Liu, Z. Gao, S. Yuan, X. Li, N. Li and S. Miao, Appl. Catal., B, 2019, 246, 296–302 CrossRef CAS.
- E. Singh, K. S. Kim, G. Y. Yeom and H. S. Nalwa, ACS Appl. Mater. Interfaces, 2017, 9, 3223–3245 CrossRef CAS.
- X. Li and H. Zhu, J. Materiomics, 2015, 1, 33–44 CrossRef.
- Y. Qiao, T. Hirtz, F. Wu, G. Deng, X. Li, Y. Zhi, H. Tian, Y. Yang and T.-L. Ren, ACS Appl. Electron. Mater., 2019, 2, 346–370 CrossRef.
- Y. Liu and F. Gu, Nanoscale Adv., 2021, 3, 2117–2138 RSC.
- O. Samy, S. Zeng, M. D. Birowosuto and A. El Moutaouakil, Crystals, 2021, 11 Search PubMed.
- Y. P. Venkata Subbaiah, K. J. Saji and A. Tiwari, Adv. Funct. Mater., 2016, 26, 2046–2069 CrossRef CAS.
- W. Zhang, P. Zhang, Z. Su and G. Wei, Nanoscale, 2015, 7, 18364–18378 RSC.
- H. S. Nalwa, RSC Adv., 2020, 10, 30529–30602 RSC.
- S. Barua, H. S. Dutta, S. Gogoi, R. Devi and R. Khan, ACS Appl. Nano Mater., 2017, 1, 2–25 CrossRef.
- S. Shi, Z. Sun and Y. H. Hu, J. Mater. Chem. A, 2018, 6, 23932–23977 RSC.
- L. Lei, D. Huang, G. Zeng, M. Cheng, D. Jiang, C. Zhou, S. Chen and W. Wang, Coord. Chem. Rev., 2019, 399, 213020 CrossRef CAS.
- Y. Jiao, A. M. Hafez, D. Cao, A. Mukhopadhyay, Y. Ma and H. Zhu, Small, 2018, 14, e1800640 CrossRef.
- J. Sun, X. Li, W. Guo, M. Zhao, X. Fan, Y. Dong, C. Xu, J. Deng and Y. Fu, Crystals, 2017, 7(7), 198 CrossRef.
- Z. Liu, X. Wang, Z. Liu, S. Zhang, Z. Lv, Y. Cui, L. Du, K. Li, G. Zhang, M. C. Lin and H. Du, ACS Appl. Mater. Interfaces, 2021, 13, 28164–28170 CrossRef CAS.
- N. Qiu, Z. Yang, R. Xue, Y. Wang, Y. Zhu and W. Liu, Nano Lett., 2021, 21, 2738–2744 CrossRef CAS.
- S. G. Stolyarova, A. A. Kotsun, Y. V. Shubin, V. O. Koroteev, P. E. Plyusnin, Y. L. Mikhlin, M. S. Mel’gunov, A. V. Okotrub and L. G. Bulusheva, ACS Appl. Energy Mater., 2020, 3, 10802–10813 CrossRef CAS.
- Z. Yuan, L. Wang, D. Li, J. Cao and W. Han, ACS Nano, 2021, 15, 7439–7450 CrossRef CAS.
- C. Zhu, X. Mu, P. A. van Aken, Y. Yu and J. Maier, Angew. Chem., Int. Ed. Engl., 2014, 53, 2152–2156 CrossRef CAS PubMed.
- C.-Y. Wei, P.-C. Lee, C.-W. Tsao, L.-H. Lee, D.-Y. Wang and C.-Y. Wen, ACS Appl. Energy Mater., 2020, 3, 7066–7072 CrossRef CAS.
- H. Wu, C. Hou, G. Shen, T. Liu, Y. Shao, R. Xiao and H. Wang, Nano Res., 2018, 11, 5866–5878 CrossRef CAS.
- J. Zhang, Y. Li, T. Gao, X. Sun, P. Cao and G. Zhou, Ceram. Int., 2018, 44, 8550–8555 CrossRef CAS.
- X. Zhao, Z. Liu, W. Xiao, H. Huang, L. Zhang, Y. Cheng and J. Zhang, ACS Appl. Nano Mater., 2020, 3, 7580–7586 CrossRef CAS.
- S. Ding, D. Zhang, J. S. Chen and X. W. Lou, Nanoscale, 2012, 4, 95–98 RSC.
- Y. Lu, X. Yao, J. Yin, G. Peng, P. Cui and X. Xu, RSC Adv., 2015, 5, 7938–7943 RSC.
- K. Yao, Z. Xu, J. Huang, M. Ma, L. Fu, X. Shen, J. Li and M. Fu, Small, 2019, 15, e1805405 CrossRef PubMed.
- A. Cheng, H. Zhang, W. Zhong, Z. Li, Y. Tang and Z. Li, J. Electroanal. Chem., 2019, 843, 31–36 CrossRef CAS.
- L. Han, S. Wu, Z. Hu, M. Chen, J. Ding, S. Wang, Y. Zhang, D. Guo, L. Zhang, S. Cao and S. Chou, ACS Appl. Mater. Interfaces, 2020, 12, 10402–10409 CrossRef CAS PubMed.
- Q. Pan, Q. Zhang, F. Zheng, Y. Liu, Y. Li, X. Ou, X. Xiong, C. Yang and M. Liu, ACS Nano, 2018, 12, 12578–12586 CrossRef CAS PubMed.
- Y. Zhang, H. Tao, T. Li, S. Du, J. Li, Y. Zhang and X. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 35206–35215 CrossRef CAS.
- H. Dai, M. Tang, J. Huang and Z. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 10870–10877 CrossRef CAS.
- Y. Zhang, H. Tao, S. Du and X. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 11327–11337 CrossRef CAS PubMed.
- H. Dai, J. Sun, Y. Zhou, Z. Zhou, W. Luo, G. Wei and H. Deng, ACS Sustainable Chem. Eng., 2020, 8, 8102–8110 CrossRef CAS.
- Z. Li, B. Niu, J. Liu, J. Li and F. Kang, ACS Appl. Mater. Interfaces, 2018, 10, 9451–9459 CrossRef CAS.
- W. Yang, H. Lu, Y. Cao, B. Xu, Y. Deng and W. Cai, ACS Sustainable Chem. Eng., 2019, 7, 4861–4867 CrossRef CAS.
- S. Guo, H. Yang, M. Liu, X. Feng, H. Xu, Y. Bai and C. Wu, ACS Appl. Energy Mater., 2021, 4, 7064–7072 CrossRef CAS.
- Z. Sheng, P. Qi, Y. Lu, G. Liu, M. Chen, X. Gan, Y. Qin, K. Hao and Y. Tang, ACS Appl. Mater. Interfaces, 2021, 13, 34495–34506 CrossRef CAS PubMed.
- A. C. M. de Moraes, W. J. Hyun, N. S. Luu, J. M. Lim, K. Y. Park and M. C. Hersam, ACS Appl. Mater. Interfaces, 2020, 12, 8107–8114 CrossRef CAS PubMed.
- G. Zhang, K. Lin, X. Qin, L. Zhang, T. Li, F. Lv, Y. Xia, W. Han, F. Kang and B. Li, ACS Appl. Mater. Interfaces, 2020, 12, 37034–37046 CrossRef CAS PubMed.
- P. Hei, S. Luo, K. Wei, J. Zhou, Y. Zhao and F. Gao, ACS Sustainable Chem. Eng., 2020, 9, 216–223 CrossRef.
- H. Liang, Z. Cao, F. Ming, W. Zhang, D. H. Anjum, Y. Cui, L. Cavallo and H. N. Alshareef, Nano Lett., 2019, 19, 3199–3206 CrossRef CAS PubMed.
- L. Jia, B. Liu, Y. Zhao, W. Chen, D. Mou, J. Fu, Y. Wang, W. Xin and L. Zhao, J. Mater. Sci., 2020, 55, 16197–16210 CrossRef CAS.
- D. Wang, X. Zhang, S. Bao, Z. Zhang, H. Fei and Z. Wu, J. Mater. Chem. A, 2017, 5, 2681–2688 RSC.
- X. Han, X. Tong, X. Liu, A. Chen, X. Wen, N. Yang and X.-Y. Guo, ACS Catal., 2018, 8, 1828–1836 CrossRef CAS.
- Y.-J. Yuan, P. Wang, Z. Li, Y. Wu, W. Bai, Y. Su, J. Guan, S. Wu, J. Zhong, Z.-T. Yu and Z. Zou, Appl. Catal., B, 2019, 242, 1–8 CrossRef CAS.
- X.-L. Yin, G.-Y. He, B. Sun, W.-J. Jiang, D.-J. Xue, A.-D. Xia, L.-J. Wan and J.-S. Hu, Nano Energy, 2016, 28, 319–329 CrossRef CAS.
- Q. Li, W. Liu, L. Xiao, X. Chen and X. Xu, Mater. Lett., 2021, 285 Search PubMed.
- J. Wang, W. Fang, Y. Hu, Y. Zhang, J. Dang, Y. Wu, H. Zhao and Z. Li, Catal. Sci. Technol., 2020, 10, 154–163 RSC.
- A. Wu, Y. Gu, Y. Xie, H. Yan, Y. Jiao, D. Wang and C. Tian, J. Alloys Compd., 2021, 867 Search PubMed.
- H. Zhu, F. Lyu, M. Du, M. Zhang, Q. Wang, J. Yao and B. Guo, ACS Appl. Mater. Interfaces, 2014, 6, 22126–22137 CrossRef CAS PubMed.
- Y. Zhu, L. Song, N. Song, M. Li, C. Wang and X. Lu, ACS Sustainable Chem. Eng., 2019, 7, 2899–2905 CrossRef CAS.
- D. Xie, G. Yang, D. Yu, Y. Hao, S. Han, Y. Cheng, F. Hu, L. Li, H. Wei, C. Ji and S. Peng, ACS Sustainable Chem. Eng., 2020, 8, 14179–14189 CrossRef.
- S. Bawari, N. M. Kaley, S. Pal, T. V. Vineesh, S. Ghosh, J. Mondal and T. N. Narayanan, Phys. Chem. Chem. Phys., 2018, 20, 15007–15014 RSC.
- Q. Zhang, Z. Duan, M. Li and J. Guan, Chem. Commun., 2020, 56, 794–797 RSC.
- X. Hu, S. Lu, J. Tian, N. Wei, X. Song, X. Wang and H. Cui, Appl. Catal., B, 2019, 241, 329–337 CrossRef CAS.
- B. Sun, W. Zhou, H. Li, L. Ren, P. Qiao, W. Li and H. Fu, Adv. Mater., 2018, 30, e1804282 CrossRef PubMed.
- Y.-J. Yuan, Z. Shen, S. Wu, Y. Su, L. Pei, Z. Ji, M. Ding, W. Bai, Y. Chen, Z.-T. Yu and Z. Zou, Appl. Catal., B, 2019, 246, 120–128 CrossRef CAS.
- H. Zhao, Z. Jiang, K. Xiao, H. Sun, H. S. Chan, T. H. Tsang, S. Yang and P. K. Wong, Appl. Catal., B, 2021, 280 Search PubMed.
- G. Zhao, A. Wang, W. He, Y. Xing and X. Xu, Adv. Mater. Interfaces, 2019, 6, 1900062 CrossRef CAS.
- N. Qin, J. Xiong, R. Liang, Y. Liu, S. Zhang, Y. Li, Z. Li and L. Wu, Appl. Catal., B, 2017, 202, 374–380 CrossRef CAS.
- D. J. Late, Y. K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare and V. P. Dravid, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed.
- W. Zheng, Y. Xu, L. Zheng, C. Yang, N. Pinna, X. Liu and J. Zhang, Adv. Funct. Mater., 2020, 30, 2000435 CrossRef CAS.
- T. Pham, G. Li, E. Bekyarova, M. E. Itkis and A. Mulchandani, ACS Nano, 2019, 13, 3196–3205 CrossRef CAS PubMed.
- R. Kumar, N. Goel and M. Kumar, ACS Sens., 2017, 2, 1744–1752 CrossRef CAS PubMed.
- X. Xin, Y. Zhang, X. Guan, J. Cao, W. Li, X. Long and X. Tan, ACS Appl. Mater. Interfaces, 2019, 11, 9438–9447 CrossRef CAS PubMed.
- X. Bai, H. Lv, Z. Liu, J. Chen, J. Wang, B. Sun, Y. Zhang, R. Wang and K. Shi, J. Hazard. Mater., 2021, 416, 125830 CrossRef CAS PubMed.
- P. Chen, J. Hu, M. Yin, W. Bai, X. Chen and Y. Zhang, ACS Appl. Nano Mater., 2021, 4, 5981–5991 CrossRef CAS.
- N. N. Viet, L. V. Thong, T. K. Dang, P. H. Phuoc, N. H. Chien, C. M. Hung, N. D. Hoa, N. Van Duy, N. Van Toan, N. T. Son and N. Van Hieu, Anal. Chim. Acta, 2021, 1167, 338576 CrossRef CAS PubMed.
- S. Singh and S. Sharma, Sens. Actuators, B, 2022, 350 Search PubMed.
- D. Zhang, C. Jiang, J. Liu and Y. Cao, Sens. Actuators, B, 2017, 247, 875–882 CrossRef CAS.
- D.-W. Jeong, K. H. Kim, B. S. Kim and Y. T. Byun, Appl. Surf. Sci., 2021, 550 Search PubMed.
- Y. R. Choi, Y.-G. Yoon, K. S. Choi, J. H. Kang, Y.-S. Shim, Y. H. Kim, H. J. Chang, J.-H. Lee, C. R. Park, S. Y. Kim and H. W. Jang, Carbon, 2015, 91, 178–187 CrossRef CAS.
- B. Kwon, H. Bae, H. Lee, S. Kim, J. Hwang, H. Lim, J. H. Lee, K. Cho, J. Ye, S. Lee and W. H. Lee, ACS Nano, 2022, 16, 2176–2187 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |
