Open Access Article
Open Access ArticleCopper-catalyzed three-component reaction to synthesize polysubstituted imidazo[1,2-a]pyridines†
Zitong Zhoua,
Danyang Luoa,
Guanrong Lia,
Zhongtao Yanga,
Liao Cui*a and
Weiguang Yang *abc
*abc
aPublic Service Platform of South China Sea for R&D Marine Biomedicine Resources, The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, 524023, China. E-mail: cuiliao@163.com; 09ywg@163.com
bThe Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong 524023, China
cSouthern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, Guangdong 524023, China
First published on 14th July 2022
Abstract
An efficient three-component one-pot and operationally simple cascade of 2-aminopyridines with sulfonyl azides and terminal ynones is reported, providing a variety of polysubstituted imidazo[1,2-a]pyridine derivatives in moderate to excellent yields. In particular, the reaction goes a through CuAAC/ring-cleavage process and forms a highly active intermediate α-acyl-N-sulfonyl ketenimine with base free.
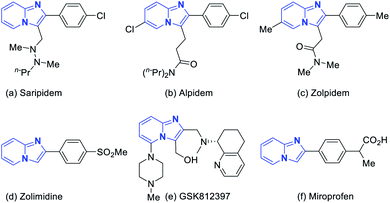
Polysubstituted imidazo[1,2-a]pyridines are well established as privileged scaffolds which are commonly encountered in many bioactive natural products and biological molecules that may be good drug candidates.1 Most imidazo[1,2-a]pyridines possess various biological activities, like antibacterial,2 antiinflammatory,3 antiviral,4 and anticancer.5 Some of the imidazo[1,2-a]pyridine derivatives are commercially available drugs, including Saripidem,6 Alpidem,7 Zolpidem,8 Zolimidine,9 Miroprofen10 and drug candidates GSK812397 (Fig. 1).11 Therefore, the development of novel methods for the synthesis of these imidazo[1,2-a]pyridines is important in the field of synthetic organic and pharmaceutical chemistry.
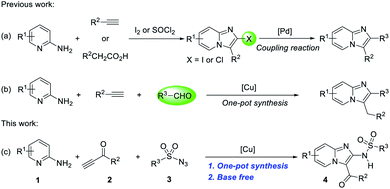
In the past few years, reactions utilizing Cu,12 Pd,13 Mn,14 TEMPO-mediated,15 I2 (ref. 16) and a few other catalysts17 have provided attractive and valuable routes for the construction of imidazo[1,2-a]pyridines. However, most reactions can only produce monosubstituted imidazo[1,2-a]pyridines or halogenated intermediates (Scheme 1a)18 which can undergo one more steps of coupling reaction leading to polysubstituted products. Therefore, developing one-pot synthetic reactions will provide a direct and powerful tool to meet these challenges. To the best of our knowledge, imidazo[1,2-a]pyridines can be synthesized from 2-aminopyridines, terminal alkyne and aldehyde in a three-component coupling reaction, catalyzed by copper, in one pot (Scheme 1b).19 However, aldehydes are unstable and easily oxidized. They are environmentally unfriendly for synthesis or complex procedures. Under this background, the development of multicomponent one-pot synthetic strategies for the preparation of polysubstituted imidazo[1,2-a]pyridines still remains highly desirable.
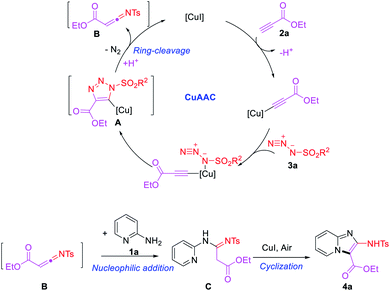
Previous studies reported that the copper-catalyzed multicomponent reactions (MCRs) of sulfonyl azides, terminal alkynes and other components (CuAAC/ring-cleavage reaction) has been applied to synthesize numerous oxygen- and nitrogen-containing heterocyclic compounds.20 However, the reaction generally carried out under strong base conditions, and limited the application of some substrates, such as terminal ynones, which will take a self-condensation under the base conditions.21 Thus, the neutral or weak acidic conditions have developed by our group and the terminal ynones successfully used in CuAAC/ring-cleavage reaction to form a highly active intermediate α-acyl-N-sulfonyl ketenimines.22 Accordingly, an efficient one-pot and operationally three-component reaction of 2-aminopyridines, sulfonyl azides and terminal ynones is reported (Scheme 1c).
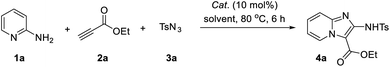
Our initial study began with an examination of the synthesis of imidazo[1,2-a]pyridine 4a from 2-aminopyridine (1a), ethyl propiolate (2a) and p-tosyl azide (3a). Initial screenings involved using CuI as catalyst and no additive with a variety of solvents in a range of standard solvents. These results revealed that the desired conversion could be effected in most solvents (Table 1, entries 1–9), with EtOH delivering product 4a in highest yield (83%). The other solvents give a comparable yields, such as DCE, toluene, MeCN and THF, while the DMSO and DMF gave the 4a lowest yield of 26% and 35%. Thus, the optimal solvent was determined to be EtOH. Encouraged by this promising result, variety of catalysts were screened. Among the copper catalysts used, most Cu-catalysts exhibited the high catalytic reactivity in this reaction whether it's CuI-catalysts or CuII-catalysts (Table 1, entries 10–13). However, Cu(OTf)2 exhibited low efficiencies for this reaction, and other catalysts, such as AgOAc failed to produce the desired product (Table 1, entries 14 and 15). Lastly, the effect of temperature was evaluated. Screening results revealed that the reaction temperature above or below 80 °C decreased the reaction yield (Table 1, entries 16 and 17).
| Entry | Cat. | Solvent | Yieldb (%) 4a |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), cat. (10 mol%) in the solvent (3 mL) was added 2a (1.5 mmol) and 3a (1.5 mmol) stirring at 80 °C for 6 h.b Isolated yields.c nd = not detected the target product.d The reaction temperature was 70 °C.e The temperature was 90 °C. | |||
| 1 | CuI | CHCl3 | 74 |
| 2 | CuI | DCE | 77 |
| 3 | CuI | Toluene | 78 |
| 4 | CuI | MeCN | 80 |
| 5 | CuI | THF | 62 |
| 6 | CuI | 1,4-Dioxane | 44 |
| 7 | CuI | DMSO | 26 |
| 8 | CuI | DMF | 35 |
| 9 | CuI | EtOH | 83 |
| 10 | CuCl | EtOH | 75 |
| 11 | CuBr | EtOH | 73 |
| 12 | CuBr2 | EtOH | 70 |
| 13 | Cu(OAc)2 | EtOH | 50 |
| 14 | Cu(OTf)2 | EtOH | 32 |
| 15 | AgOAc | EtOH | ndc |
| 16 | CuI | EtOH | 80d |
| 17 | CuI | EtOH | 76e |
Under the optimized conditions (Table 1, entries 9), the capacity of this reaction to affect the coupling of a range of different substrates was investigated. Agreeably, as shown in Table 2, various 2-aminopyridines, with an alkyl group or methoxy group, all exhibited good functional group tolerance to obtain the desired products (4a–4c, 4e). However, the 2-aminopyridines with electron-withdrawing nature can't obtain the desire products. In addition, due to steric hindrance, some 2-aminopyridines obtained the products with low yield or cannot be separated to obtain the desired products. Such as 6-ethyl-2-aminopyridine (1d) obtained the product 4d with low yield and 6-methyl-2-aminopyridine (1f) or 6-methoxy-2-aminopyridine (1g) obtained the uncyclized products 4s and 4t.
| a Unless otherwise noted, the reaction conditions were as follow: 1 (1.0 mmol), CuI (10 mol%) in the MeCN (3 mL) was added 2 (1.5 mmol), 3 (1.5 mmol) stirring at 80 °C for 6 h. |
|---|
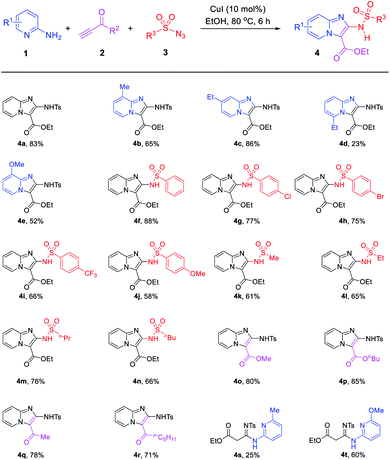 |
Next, the scope and limitation of the terminal ynone 2 and sulfonyl azide 3 substrates were tested. It is noteworthy that the sulfonyl azide substrates showed slight influences on this reaction. With R3 changed by aromatic or aliphatic substituents, such as –Ph, –(4-ClC6H4), –(4-CF3C6H4), –(4-OMeC6H4), –Me and –n-Bu, the reaction could smoothly give the anticipated products (4f–4n) in comparable yields. The substrates R2 bearing the –OMe, –OtBu, –Me and other alkyl group also can obtain 4o–4r in good yields.
In order to broaden the suitability of substrates, we also investigated other terminal alkynes, such as aryl acetylenes. The experiments revealed that some aryl acetylene such as 3-methyl phenylacetylene can obtain imidazo[1,2-a]pyridine 4u with low yield of 26% and an uncyclized linear product 4v (Scheme 2a). Most aryl acetylenes such as 4-methoxy phenylacetylene only obtain uncyclized products (Scheme 2b). It shows that the reactivity of terminal ynones is higher than that of traditional terminal alkyne.
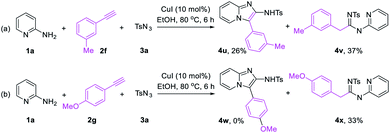 | ||
| Scheme 2 Investigation the reaction of 2-aminopyridine (1a), aryl acetylenes (2f, 2g) and p-tosyl azide (3a). | ||
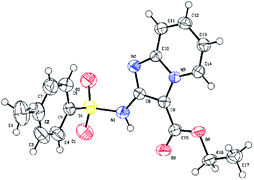
None of the product imidazo[1,2-a]pyridines 4a–4r have been reported previously, which were subject to full spectroscopic characterization (see ESI† for details) and the derived data were in complete accord with the assigned structures. And 4a was confirmed by single-crystal X-ray analysis (Fig. 2).
A possible reaction pathway for the formation of imidazo[1,2-a]pyridine (4a) from precursors 1a, 2a and 3a is shown in Scheme 3. Thus, in keeping with earlier proposals,19,21 the substrates 2a and 3a are expected to react, in the presence of the copper(I) catalyst to form the metallated triazole A through the CuAAC procedure. Then, the complex A undergo a ring-cleavage rearrangement leading to a highly active intermediate N-sulfonyl-α-acylketenimine B. This last species B is captured by 1a via nucleophilic addition to generate the intermediate C, which deliver the observed product 4a by intramolecular oxidative coupling similar to literature.23 Otherwise, due to the poor activity, most of the traditional terminal alkynes involved in the reaction will stop in the intermediate C leading the uncyclized products.
In summary, we have developed an original approach for the synthesis of polysubstituted imidazo[1,2-a]pyridines from a mixture of the corresponding 2-aminopyridines, sulfonyl azides and terminal ynones, through CuAAC/ring-cleavage process and generated a highly active intermediate α-acyl-N-sulfonyl ketenimines. More detailed novel reactions and the investigation of new applications of this intermediate are now being undertaken in our laboratory.
Experimental
General
All melting points were determined on a Yanaco melting point apparatus and were uncorrected. IR spectra were recorded as KBr pellets on a Nicolet FT-IR 5DX spectrometer. All spectra of 1H NMR (400 MHz) and 13C NMR (100 MHz) were recorded on a JEOL JNM-ECA 400 spectrometer in DMSO-d6 or CDCl3 (otherwise as indicated) with TMS was used as an internal reference and J values are given in Hz. HRMS were obtained on a Thermo Scientific Q Exactive Focus Orbitrap LC-MS/MS spectrometer.Preparation and characterizations of compounds 4a–4x
Ethyl-2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4a). To a solution of CuI (19.5 mg, 0.10 mmol) in EtOH (3 mL) was added pyridin-2-amine (1a, 94.2 mg, 1 mmol), ethyl propiolate (2a, 147 mg, 1.5 mmol), TsN3 (295.8 mg, 1.5 mmol). After the mixture was stirred at 80 °C for 6 h (monitored by TLC), the solvent was removed. The residue was purified via flash chromatography (silica gel, 25% EtOAc in petroleum ether) to give of product 4a (298.2 mg, 83%) as a white solid, m.p. = 155–157 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 8.80 (s, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.59 (d, J = 8.8 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.27 (d, J = 7.2 Hz, 2H), 6.95 (t, J = 6.8 Hz, 1H), 4.48–4.43 (m, 2H), 2.37 (s, 3H), 1.44 (t, J = 7.2, 3H); 13C NMR (100 MHz, CDCl3) δ 160.4, 149.0, 146.0, 144.2, 136.8, 129.4 (2C), 128.5, 128.2 (2C), 127.9, 117.0, 114.1, 100.3, 61.0, 21.6, 14.7; IR (KBr) ν 3257, 2308, 1656, 1550, 1435, 1336, 1220, 1165, 1089 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H17N3O4S, [M + H]+ 360.1013, found 360.1006.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 8.80 (s, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.59 (d, J = 8.8 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.27 (d, J = 7.2 Hz, 2H), 6.95 (t, J = 6.8 Hz, 1H), 4.48–4.43 (m, 2H), 2.37 (s, 3H), 1.44 (t, J = 7.2, 3H); 13C NMR (100 MHz, CDCl3) δ 160.4, 149.0, 146.0, 144.2, 136.8, 129.4 (2C), 128.5, 128.2 (2C), 127.9, 117.0, 114.1, 100.3, 61.0, 21.6, 14.7; IR (KBr) ν 3257, 2308, 1656, 1550, 1435, 1336, 1220, 1165, 1089 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H17N3O4S, [M + H]+ 360.1013, found 360.1006.
The products 4b–4x were prepared by the similar procedure
Ethyl-8-methyl-2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4b) (242.7 mg, 65%), white solid, m.p. = 151–152 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.68 (s, 1H), 8.14 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 6.8 Hz, 1H), 6.83 (t, J = 6.8 Hz, 1H), 4.40–4.46 (m, 2H), 2.55 (s, 3H), 2.37 (s, 3H), 1.43 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 148.4, 146.0, 144.2, 136.7, 129.1 (2C), 128.9 (2C), 127.6, 126.7, 125.6, 113.9, 100.5, 60.9, 21.7, 16.7, 14.7; IR (KBr) ν 2974, 1654, 1544, 1446, 1359, 1236, 1163, 1087, 1056 cm−1; HRMS (ESI) (m/z). Calcd for C18H19N3O4S, [M + H]+ 374.1169, found 374.1162.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.68 (s, 1H), 8.14 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 6.8 Hz, 1H), 6.83 (t, J = 6.8 Hz, 1H), 4.40–4.46 (m, 2H), 2.55 (s, 3H), 2.37 (s, 3H), 1.43 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 148.4, 146.0, 144.2, 136.7, 129.1 (2C), 128.9 (2C), 127.6, 126.7, 125.6, 113.9, 100.5, 60.9, 21.7, 16.7, 14.7; IR (KBr) ν 2974, 1654, 1544, 1446, 1359, 1236, 1163, 1087, 1056 cm−1; HRMS (ESI) (m/z). Calcd for C18H19N3O4S, [M + H]+ 374.1169, found 374.1162.
Ethyl-7-ethyl-2-((4-methylphenyl) sulfonamido) imidazo[1,2-a] pyridine-3-carboxylate (4c) (333.0 mg, 86%), yellow solid, m.p. = 109–111 °C (Rf = 0.33 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.81 (s, 2H), 8.06 (d, J = 7.6 Hz, 2H), 7.39 (s, 1H), 7.27 (d, J = 7.6 Hz, 2H), 6.80 (d, J = 6.8 Hz, 1H), 4.46–4.41 (m, 2H), 2.72–2.67 (m, 2H), 2.37 (s, 3H), 1.43 (t, J = 7.2 Hz, 3H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.8, 146.5, 144.2, 136.8, 129.5 (3C), 128.1 (2C), 127.3, 115.7, 114.4, 100.2, 61.0, 28.5, 21.7, 14.7, 14.2; IR (KBr) ν 2970, 1656, 1544, 1436, 1384, 1220, 1165, 1085, 864 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1326.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.81 (s, 2H), 8.06 (d, J = 7.6 Hz, 2H), 7.39 (s, 1H), 7.27 (d, J = 7.6 Hz, 2H), 6.80 (d, J = 6.8 Hz, 1H), 4.46–4.41 (m, 2H), 2.72–2.67 (m, 2H), 2.37 (s, 3H), 1.43 (t, J = 7.2 Hz, 3H), 1.26 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.8, 146.5, 144.2, 136.8, 129.5 (3C), 128.1 (2C), 127.3, 115.7, 114.4, 100.2, 61.0, 28.5, 21.7, 14.7, 14.2; IR (KBr) ν 2970, 1656, 1544, 1436, 1384, 1220, 1165, 1085, 864 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1326.
Ethyl-5-ethyl-2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4d) (89.5 mg, 23%), yellow solid, m.p. = 105–107 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.84 (s, 1H), 8.05 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 8.8 Hz, 1H), 7.38 (t, J = 7.8 Hz, 1H), 7.27 (d, J = 7.2 Hz, 2H), 6.80 (d, J = 6.8 Hz, 1H), 4.43–4.38 (m, 2H), 3.13–3.07 (m, 2H), 2.37 (s, 3H), 1.43 (t, J = 6.8 Hz, 3H), 1.20 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.7, 150.1, 148.3, 145.3, 144.1, 136.8, 129.5, 129.4 (2C), 128.3 (2C), 114.6, 113.5, 101.9, 61.3, 27.4, 21.7, 14.7, 11.3; IR (KBr) ν 3263, 2978, 1597, 1519, 1440, 1327, 1159, 1089, 812, 663 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1317.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.84 (s, 1H), 8.05 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 8.8 Hz, 1H), 7.38 (t, J = 7.8 Hz, 1H), 7.27 (d, J = 7.2 Hz, 2H), 6.80 (d, J = 6.8 Hz, 1H), 4.43–4.38 (m, 2H), 3.13–3.07 (m, 2H), 2.37 (s, 3H), 1.43 (t, J = 6.8 Hz, 3H), 1.20 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.7, 150.1, 148.3, 145.3, 144.1, 136.8, 129.5, 129.4 (2C), 128.3 (2C), 114.6, 113.5, 101.9, 61.3, 27.4, 21.7, 14.7, 11.3; IR (KBr) ν 3263, 2978, 1597, 1519, 1440, 1327, 1159, 1089, 812, 663 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1317.
Ethyl-8-methoxy-2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4e) (202.4 mg, 52%), white solid, m.p. = 162–164 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 8.57 (d, J = 6.4 Hz, 1H), 8.11 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 6.83 (t, J = 7.4 Hz, 1H), 6.70 (d, J = 7.6 Hz, 1H), 4.46–4.41 (m, 2H), 3.99 (s, 3H), 2.37 (s, 3H), 1.43 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 148.3, 148.0, 144.2, 139.9, 137.0, 129.4 (2C), 128.6 (2C), 120.6, 114.1, 106.5, 101.4, 61.1, 56.5, 21.8, 14.7; IR (KBr) ν 2983, 1656, 1544, 1452, 1267, 1159, 1089, 1012, 665 cm−1; HRMS (ESI) (m/z). Calcd for C18H19N3O5S, [M + H]+ 390.1118, found 390.1112.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 8.57 (d, J = 6.4 Hz, 1H), 8.11 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 6.83 (t, J = 7.4 Hz, 1H), 6.70 (d, J = 7.6 Hz, 1H), 4.46–4.41 (m, 2H), 3.99 (s, 3H), 2.37 (s, 3H), 1.43 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 148.3, 148.0, 144.2, 139.9, 137.0, 129.4 (2C), 128.6 (2C), 120.6, 114.1, 106.5, 101.4, 61.1, 56.5, 21.8, 14.7; IR (KBr) ν 2983, 1656, 1544, 1452, 1267, 1159, 1089, 1012, 665 cm−1; HRMS (ESI) (m/z). Calcd for C18H19N3O5S, [M + H]+ 390.1118, found 390.1112.
Ethyl-2-(phenylsulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4f) (303.8 mg, 88%), white solid, m.p. = 116–118 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 2H), 8.19 (d, J = 7.6 Hz, 2H), 7.59 (d, J = 8.8 Hz, 1H), 7.54 (t, J = 7.2 Hz, 1H), 7.47 (t, J = 7.4 Hz, 2H), 7.37 (t, J = 8.0 Hz, 1H), 6.95 (t, J = 6.8 Hz, 1H), 4.48–4.42 (m, 2H), 1.44 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 149.0, 145.9, 139.7, 133.3, 128.8 (2C), 128.6, 128.2 (2C), 127.9, 117.0, 114.1, 100.3, 61.0, 14.7; IR (KBr) ν 3273, 2983, 1660, 1546, 1440, 1332, 1220, 1166, 1087 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O4S, [M + H]+ 346.0856, found 346.0851.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 2H), 8.19 (d, J = 7.6 Hz, 2H), 7.59 (d, J = 8.8 Hz, 1H), 7.54 (t, J = 7.2 Hz, 1H), 7.47 (t, J = 7.4 Hz, 2H), 7.37 (t, J = 8.0 Hz, 1H), 6.95 (t, J = 6.8 Hz, 1H), 4.48–4.42 (m, 2H), 1.44 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 149.0, 145.9, 139.7, 133.3, 128.8 (2C), 128.6, 128.2 (2C), 127.9, 117.0, 114.1, 100.3, 61.0, 14.7; IR (KBr) ν 3273, 2983, 1660, 1546, 1440, 1332, 1220, 1166, 1087 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O4S, [M + H]+ 346.0856, found 346.0851.
Ethyl-2-((4-chlorophenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4g) (292.4 mg, 77%), white solid, m.p. = 141–143 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 2H), 8.15 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 9.2 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 8.0 Hz, 1H), 6.97 (t, J = 6.6 Hz, 1H), 4.49–4.44 (m, 2H), 1.45 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.8, 145.9, 139.8, 138.2, 129.8 (2C), 129.1 (2C), 128.7, 127.9, 117.0, 114.3, 100.4, 61.1, 14.7; IR (KBr) ν 3273, 2981, 1660, 1546, 1438, 1334, 1219, 1166, 1082 cm−1; HRMS (ESI) (m/z). Calcd for C16H14ClN3O4S, [M + H]+ 380.0467, found 380.0460.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 2H), 8.15 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 9.2 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 8.0 Hz, 1H), 6.97 (t, J = 6.6 Hz, 1H), 4.49–4.44 (m, 2H), 1.45 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.8, 145.9, 139.8, 138.2, 129.8 (2C), 129.1 (2C), 128.7, 127.9, 117.0, 114.3, 100.4, 61.1, 14.7; IR (KBr) ν 3273, 2981, 1660, 1546, 1438, 1334, 1219, 1166, 1082 cm−1; HRMS (ESI) (m/z). Calcd for C16H14ClN3O4S, [M + H]+ 380.0467, found 380.0460.
Ethyl-2-((4-bromophenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4h) (318.2 mg, 75%), white solid, m.p. = 135–137 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) 8.94 (s, 2H), 8.07 (d, J = 8.0 Hz, 2H), 7.61 (t, J = 8.2 Hz, 3H), 7.40 (t, J = 7.8 Hz, 1H), 6.98 (t, J = 6.6 Hz, 1H), 4.49–4.44 (m, 2H), 1.45 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.6, 143.9, 138.8, 132.1 (2C), 129.9 (2C), 128.7, 128.4, 127.9, 117.0, 114.3, 100.4, 61.2, 14.7; IR (KBr) ν 2964, 1658, 1546, 1438, 1330, 1217, 1147, 1085, 873, 759 cm−1; HRMS (ESI) (m/z). Calcd for C16H14BrN3O4S, [M–H]− 421.9815, found 421.9816.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) 8.94 (s, 2H), 8.07 (d, J = 8.0 Hz, 2H), 7.61 (t, J = 8.2 Hz, 3H), 7.40 (t, J = 7.8 Hz, 1H), 6.98 (t, J = 6.6 Hz, 1H), 4.49–4.44 (m, 2H), 1.45 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.6, 143.9, 138.8, 132.1 (2C), 129.9 (2C), 128.7, 128.4, 127.9, 117.0, 114.3, 100.4, 61.2, 14.7; IR (KBr) ν 2964, 1658, 1546, 1438, 1330, 1217, 1147, 1085, 873, 759 cm−1; HRMS (ESI) (m/z). Calcd for C16H14BrN3O4S, [M–H]− 421.9815, found 421.9816.
Ethyl-2-((4-(trifluoromethyl)phenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4i) (272.8 mg, 66%), yellow solid, m.p. = 160–162 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (d, J = 6.4 Hz, 1H), 8.34 (d, J = 8.0 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.8 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H), 7.00 (t, J = 6.6 Hz, 1H), 4.50–4.45 (m, 2H), 1.46 (t, J = 7.0 Hz, 3H) (N–H signals obscured); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.5, 145.8, 143.2, 135.0 (q, J = 32.8 Hz, 1C), 129.0, 128.8 (2C), 128.0, 126.0 (q, J = 3.8 Hz, 2C), 121.9 (q, J = 271.3 Hz,1C), 117.0, 114.5, 100.6, 61.3, 14.7; IR (KBr) ν 3273, 2985, 1664, 1546, 1438, 1321, 1166, 1128, 1087, 1060 cm−1; HRMS (ESI) (m/z). Calcd for C17H14F3N3O4S, [M + H]+ 414.0730, found 414.0730.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.94 (d, J = 6.4 Hz, 1H), 8.34 (d, J = 8.0 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.8 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H), 7.00 (t, J = 6.6 Hz, 1H), 4.50–4.45 (m, 2H), 1.46 (t, J = 7.0 Hz, 3H) (N–H signals obscured); 13C NMR (100 MHz, CDCl3) δ 160.5, 148.5, 145.8, 143.2, 135.0 (q, J = 32.8 Hz, 1C), 129.0, 128.8 (2C), 128.0, 126.0 (q, J = 3.8 Hz, 2C), 121.9 (q, J = 271.3 Hz,1C), 117.0, 114.5, 100.6, 61.3, 14.7; IR (KBr) ν 3273, 2985, 1664, 1546, 1438, 1321, 1166, 1128, 1087, 1060 cm−1; HRMS (ESI) (m/z). Calcd for C17H14F3N3O4S, [M + H]+ 414.0730, found 414.0730.
Ethyl-2-((4-methoxyphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4j) (217.6 mg, 58%), yellow solid, m.p. = 135–137 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.95 (s, 1H), 8.79 (s, 1H), 8.12 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 8.8 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 6.97–6.92 (m, 3H), 4.48–4.43 (m, 2H), 3.82 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.4, 160.5, 149.0, 146.0, 131.3, 130.5 (2C), 128.5, 127.9, 117.0, 114.1, 113.9 (2C), 100.3, 61.0, 55.6, 14.7; IR (KBr) ν 3363, 1685, 1546, 1442, 1325, 1219, 1159, 1085, 773 cm−1; HRMS (ESI) (m/z). Calcd for C17H17N3O5S, [M + H]+ 376.0962, found 376.0956.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.95 (s, 1H), 8.79 (s, 1H), 8.12 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 8.8 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 6.97–6.92 (m, 3H), 4.48–4.43 (m, 2H), 3.82 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.4, 160.5, 149.0, 146.0, 131.3, 130.5 (2C), 128.5, 127.9, 117.0, 114.1, 113.9 (2C), 100.3, 61.0, 55.6, 14.7; IR (KBr) ν 3363, 1685, 1546, 1442, 1325, 1219, 1159, 1085, 773 cm−1; HRMS (ESI) (m/z). Calcd for C17H17N3O5S, [M + H]+ 376.0962, found 376.0956.
Ethyl-2-(methylsulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4k) (172.8 mg, 61%), yellow solid, m.p. = 145–147 °C (Rf = 0.22 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 8.49 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.02 (t, J = 7.0 Hz, 1H), 4.50–4.45 (m, 2H), 3.51 (s, 3H), 1.46 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 149.1, 146.0, 128.9, 128.0, 116.8, 114.3, 100.3, 61.1, 42.1, 14.7; IR (KBr) ν 3294, 2983, 1662, 1546, 1438, 1328, 1219, 1153, 1085, 758 cm−1; HRMS (ESI) (m/z). Calcd for C11H13N3O4S, [M + H]+ 284.0700, found 284.0693.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 8.49 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.02 (t, J = 7.0 Hz, 1H), 4.50–4.45 (m, 2H), 3.51 (s, 3H), 1.46 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 149.1, 146.0, 128.9, 128.0, 116.8, 114.3, 100.3, 61.1, 42.1, 14.7; IR (KBr) ν 3294, 2983, 1662, 1546, 1438, 1328, 1219, 1153, 1085, 758 cm−1; HRMS (ESI) (m/z). Calcd for C11H13N3O4S, [M + H]+ 284.0700, found 284.0693.
Ethyl-2-(ethylsulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4l) (193.2 mg, 65%), brown solid, m.p. = 114–116 °C (Rf = 0.20 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 8.37 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 7.8 Hz, 1H), 7.02 (t, J = 6.8 Hz, 1H), 4.51–4.46 (m, 2H), 3.75–3.69 (m, 2H), 1.47–1.43 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 160.4, 149.3, 146.0, 128.8, 128.0, 116.8, 114.3, 100.3, 61.1, 48.2, 14.7, 8.2; IR (KBr) ν 3363, 1685, 1546, 1440, 1325, 1219, 1157, 1085, 773 cm−1; HRMS (ESI) (m/z). Calcd for C12H15N3O4S, [M + H]+ 298.0856, found 298.0850.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 8.37 (s, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 7.8 Hz, 1H), 7.02 (t, J = 6.8 Hz, 1H), 4.51–4.46 (m, 2H), 3.75–3.69 (m, 2H), 1.47–1.43 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 160.4, 149.3, 146.0, 128.8, 128.0, 116.8, 114.3, 100.3, 61.1, 48.2, 14.7, 8.2; IR (KBr) ν 3363, 1685, 1546, 1440, 1325, 1219, 1157, 1085, 773 cm−1; HRMS (ESI) (m/z). Calcd for C12H15N3O4S, [M + H]+ 298.0856, found 298.0850.
Ethyl-2-(propylsulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4m) (236.5 mg, 76%), white solid, m.p. = 129–130 °C (Rf = 0.30 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 1H), 8.40 (s, 1H), 7.63 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 7.01 (t, J = 6.8 Hz, 1H), 4.50–4.44 (m, 2H), 3.66 (t, J = 7.8 Hz, 2H),1.99–1.90 (m, 2H), 1.45 (t, J = 7.2 Hz, 3H), 1.07 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 149.2, 146.0, 128.8, 128.0, 116.8, 114.2, 100.2, 61.1, 55.4, 17.2, 14.2, 12.9; IR (KBr) ν 3363, 1685, 1544, 1440, 1365, 1325, 1274, 1219, 1157, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C13H17N3O4S, [M + H]+ 312.1013, found 312.1006.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.04 (s, 1H), 8.40 (s, 1H), 7.63 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 7.01 (t, J = 6.8 Hz, 1H), 4.50–4.44 (m, 2H), 3.66 (t, J = 7.8 Hz, 2H),1.99–1.90 (m, 2H), 1.45 (t, J = 7.2 Hz, 3H), 1.07 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 149.2, 146.0, 128.8, 128.0, 116.8, 114.2, 100.2, 61.1, 55.4, 17.2, 14.2, 12.9; IR (KBr) ν 3363, 1685, 1544, 1440, 1365, 1325, 1274, 1219, 1157, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C13H17N3O4S, [M + H]+ 312.1013, found 312.1006.
Ethyl-2-((2-methylpropyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4n) (214.6 mg, 66%), white solid, m.p. = 114–116 °C (Rf = 0.30 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 9.30 (s, 1H), 9.10 (d, J = 6.8 Hz, 1H), 7.71 (d, J = 9.2 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.22 (t, J = 6.8 Hz, 1H), 4.41–4.36 (m, 2H), 3.57 (d, J = 6.4 Hz, 2H), 2.29–2.19 (m, 1H), 1.36 (t, J = 6.8 Hz, 3H), 1.05 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 159.8, 147.8, 144.8, 129.3, 127.8, 116.2, 114.7, 101.0, 60.6, 60.4, 24.2, 22.1 (2C), 14.3; IR (KBr) ν 2964, 1658, 1546, 1438, 1330, 1217, 1147, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C14H19N3O4S, [M + H]+ 326.1169, found 326.1163.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 9.30 (s, 1H), 9.10 (d, J = 6.8 Hz, 1H), 7.71 (d, J = 9.2 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.22 (t, J = 6.8 Hz, 1H), 4.41–4.36 (m, 2H), 3.57 (d, J = 6.4 Hz, 2H), 2.29–2.19 (m, 1H), 1.36 (t, J = 6.8 Hz, 3H), 1.05 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 159.8, 147.8, 144.8, 129.3, 127.8, 116.2, 114.7, 101.0, 60.6, 60.4, 24.2, 22.1 (2C), 14.3; IR (KBr) ν 2964, 1658, 1546, 1438, 1330, 1217, 1147, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C14H19N3O4S, [M + H]+ 326.1169, found 326.1163.
Methyl-2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4o) (276.2 mg, 80%), white solid, m.p. = 144–146 °C (Rf = 0.30 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.69 (s, 1H), 8.03 (d, J = 7.6 Hz, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 7.6 Hz, 2H), 6.90 (t, J = 7.0 Hz, 1H), 3.93 (s, 3H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.7, 149.0, 146.0, 144.2, 136.8, 129.4 (2C), 128.6, 128.3 (2C), 127.9, 117.0, 114.1, 100.2, 51.8, 21.6; IR (KBr) ν 3282, 2954, 1691, 1664, 1544, 1450, 1332, 1222, 1163, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O4S, [M + H]+ 346.0856, found 346.0850.
5 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.69 (s, 1H), 8.03 (d, J = 7.6 Hz, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 7.6 Hz, 2H), 6.90 (t, J = 7.0 Hz, 1H), 3.93 (s, 3H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.7, 149.0, 146.0, 144.2, 136.8, 129.4 (2C), 128.6, 128.3 (2C), 127.9, 117.0, 114.1, 100.2, 51.8, 21.6; IR (KBr) ν 3282, 2954, 1691, 1664, 1544, 1450, 1332, 1222, 1163, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O4S, [M + H]+ 346.0856, found 346.0850.
Tert-butyl 2-((4-methylphenyl)sulfonamido)imidazo[1,2-a]pyridine-3-carboxylate (4p) (329.3 mg, 85%), white solid, m.p. = 145–147 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 2H), 8.01 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 9.2 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 8.4 Hz, 2H), 6.88 (t, J = 6.8 Hz, 1H), 2.32 (s, 3H), 1.60 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 160.1, 148.7, 145.7, 144.1, 137.0, 129.8, 129.5 (2C), 128.2 (2C), 127.7, 117.0, 113.9, 101.2, 83.4, 28.7 (3C), 21.7; IR (KBr) ν 2978, 1658, 1544, 1438, 1334, 1263, 1165, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1317.
6 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 2H), 8.01 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 9.2 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 8.4 Hz, 2H), 6.88 (t, J = 6.8 Hz, 1H), 2.32 (s, 3H), 1.60 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 160.1, 148.7, 145.7, 144.1, 137.0, 129.8, 129.5 (2C), 128.2 (2C), 127.7, 117.0, 113.9, 101.2, 83.4, 28.7 (3C), 21.7; IR (KBr) ν 2978, 1658, 1544, 1438, 1334, 1263, 1165, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C19H21N3O4S, [M + H]+ 388.1326, found 388.1317.
N-(3-acetylimidazo[1,2-a]pyridin-2-yl)-4-methylbenzene sulfonamide (4q) (256.8 mg, 78%), yellow solid, m.p. = 176–178 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.36 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.86 (t, J = 7.8 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H), 7.41 (s, 1H), 7.27 (d, J = 7.6 Hz, 3H), 2.54 (s, 3H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.5, 152.0, 149.5, 142.5, 140.0, 137.0, 129.3 (2C), 128.5, 126.6 (2C), 126.4, 117.3, 103.2, 25.1, 21.5; IR (KBr) ν 3051, 1598, 1552, 1513, 1261, 1139, 1080, 827 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O3S, [M + H]+ 330.0907, found 330.0902.
1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.36 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.86 (t, J = 7.8 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H), 7.41 (s, 1H), 7.27 (d, J = 7.6 Hz, 3H), 2.54 (s, 3H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.5, 152.0, 149.5, 142.5, 140.0, 137.0, 129.3 (2C), 128.5, 126.6 (2C), 126.4, 117.3, 103.2, 25.1, 21.5; IR (KBr) ν 3051, 1598, 1552, 1513, 1261, 1139, 1080, 827 cm−1; HRMS (ESI) (m/z). Calcd for C16H15N3O3S, [M + H]+ 330.0907, found 330.0902.
N-(3-Hexanoylimidazo[1,2-a]pyridin-2-yl)-4-methylbenzene sulfonamide (4r) (273.6 mg, 71%), brown solid, m.p. = 112–114 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.84 (t, J = 7.8 Hz, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.39 (s, 1H), 7.27 (d, J = 7.2 Hz, 3H), 2.74 (t, J = 7.6 Hz, 2H), 2.39 (s, 3H), 1.76–1.67 (m, 2H), 1.32 (s, 4H), 0.89 (t, J = 6.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 169.4, 152.2, 149.6, 142.5, 140.0, 136.8, 129.3 (2C), 128.6, 126.7 (2C), 126.6, 117.2, 102.7, 38.7, 31.5, 28.4, 22.5, 21.6, 14.0; IR (KBr) ν 3118, 2926, 1598, 1550, 1415, 1280, 1139, 1080 cm−1; HRMS (ESI) (m/z). Calcd for C20H23N3O3S, [M + H]+ 386.1533, found 386.1525.
1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 7.6 Hz, 2H), 7.84 (t, J = 7.8 Hz, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.39 (s, 1H), 7.27 (d, J = 7.2 Hz, 3H), 2.74 (t, J = 7.6 Hz, 2H), 2.39 (s, 3H), 1.76–1.67 (m, 2H), 1.32 (s, 4H), 0.89 (t, J = 6.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 169.4, 152.2, 149.6, 142.5, 140.0, 136.8, 129.3 (2C), 128.6, 126.7 (2C), 126.6, 117.2, 102.7, 38.7, 31.5, 28.4, 22.5, 21.6, 14.0; IR (KBr) ν 3118, 2926, 1598, 1550, 1415, 1280, 1139, 1080 cm−1; HRMS (ESI) (m/z). Calcd for C20H23N3O3S, [M + H]+ 386.1533, found 386.1525.
Ethyl-3-((6-methylpyridin-2-yl)amino)-3-(tosylimino)propanoate (4s) (93.9 mg, 25%), yellow solid, m.p. = 149–151 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 10.94 (s, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.73–7.68 (m, 3H), 7.37 (d, J = 7.6 Hz, 2H), 7.06 (d, J = 7.2 Hz, 1H), 4.11–4.06 (m, 2H), 4.02 (s, 2H), 2.41 (s, 3H), 2.36 (s, 3H), 1.17 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.8, 158.3, 156.9, 150.0, 142.4, 139.9, 138.6, 129.4 (3C), 125.9 (2C), 120.2, 112.3, 60.9, 23.4, 20.9, 13.9; IR (KBr) ν 3286, 2983, 1737, 1597, 1541, 1452, 1280, 1145, 1087 cm−1; HRMS (ESI) (m/z). Calcd for C18H21N3O4S, [M + H]+ 376.1326, found 376.1319.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 10.94 (s, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.73–7.68 (m, 3H), 7.37 (d, J = 7.6 Hz, 2H), 7.06 (d, J = 7.2 Hz, 1H), 4.11–4.06 (m, 2H), 4.02 (s, 2H), 2.41 (s, 3H), 2.36 (s, 3H), 1.17 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.8, 158.3, 156.9, 150.0, 142.4, 139.9, 138.6, 129.4 (3C), 125.9 (2C), 120.2, 112.3, 60.9, 23.4, 20.9, 13.9; IR (KBr) ν 3286, 2983, 1737, 1597, 1541, 1452, 1280, 1145, 1087 cm−1; HRMS (ESI) (m/z). Calcd for C18H21N3O4S, [M + H]+ 376.1326, found 376.1319.
Ethyl-3-((6-methoxypyridin-2-yl)amino)-3-(tosylimino)propanoate (4t) (234.6 mg, 60%), white solid, m.p. = 123–125 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H), 7.71 (t, J = 9.2 Hz, 3H), 7.63 (d, J = 6.8 Hz, 1H), 7.37 (d, J = 7.6 Hz, 2H), 6.61 (d, J = 7.6 Hz, 1H), 4.12–4.05 (m, 4H), 3.83 (s, 3H), 2.37 (s, 3H), 1.18 (t, J = 6.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.9, 162.3, 158.3, 148.4, 142.5, 141.1, 139.8, 129.4 (3C), 126.0 (2C), 107.5, 106.9, 60.9, 53.2, 20.9, 13.9; IR (KBr) ν 3288, 2983, 1739, 1597, 1543, 1463, 1423, 1145, 1089, 1024 cm−1; HRMS (ESI) (m/z). Calcd for C18H21N3O5S, [M + H]+ 392.1275, found 392.1268.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H), 7.71 (t, J = 9.2 Hz, 3H), 7.63 (d, J = 6.8 Hz, 1H), 7.37 (d, J = 7.6 Hz, 2H), 6.61 (d, J = 7.6 Hz, 1H), 4.12–4.05 (m, 4H), 3.83 (s, 3H), 2.37 (s, 3H), 1.18 (t, J = 6.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.9, 162.3, 158.3, 148.4, 142.5, 141.1, 139.8, 129.4 (3C), 126.0 (2C), 107.5, 106.9, 60.9, 53.2, 20.9, 13.9; IR (KBr) ν 3288, 2983, 1739, 1597, 1543, 1463, 1423, 1145, 1089, 1024 cm−1; HRMS (ESI) (m/z). Calcd for C18H21N3O5S, [M + H]+ 392.1275, found 392.1268.
4-Methyl-N-(3-(m-tolyl)imidazo[1,2-a]pyridin-2-yl)benzene sulfonamide (4u) (98.0 mg, 26%), brown solid, m.p. = 192–194 °C (Rf = 0.25 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.09–8.03 (m, 2H), 7.66 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 8.8 Hz, 1H), 7.26–7.25 (m, 3H), 7.21 (d, J = 7.2 Hz, 1H), 7.02 (t, J = 6.4 Hz, 1H), 6.98 (d, J = 7.2 Hz, 1H),6.90 (s, 1H), 2.34 (s, 3H), 2.24 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 178.0, 162.5, 146.5, 141.4, 140.1, 138.0, 137.5, 135.4, 130.1, 128.62, 128.60 (2C), 126.9 (2C), 125.3, 122.1, 116.1, 115.7, 95.9, 21.0, 20.9; IR (KBr) ν 3057, 1747, 1566, 1467, 1336, 1278, 1145, 1082 cm−1; HRMS (ESI) (m/z). Calcd for C21H19N3O2S, [M + H]+ 378.1271, found 378.1267.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.09–8.03 (m, 2H), 7.66 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 8.8 Hz, 1H), 7.26–7.25 (m, 3H), 7.21 (d, J = 7.2 Hz, 1H), 7.02 (t, J = 6.4 Hz, 1H), 6.98 (d, J = 7.2 Hz, 1H),6.90 (s, 1H), 2.34 (s, 3H), 2.24 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 178.0, 162.5, 146.5, 141.4, 140.1, 138.0, 137.5, 135.4, 130.1, 128.62, 128.60 (2C), 126.9 (2C), 125.3, 122.1, 116.1, 115.7, 95.9, 21.0, 20.9; IR (KBr) ν 3057, 1747, 1566, 1467, 1336, 1278, 1145, 1082 cm−1; HRMS (ESI) (m/z). Calcd for C21H19N3O2S, [M + H]+ 378.1271, found 378.1267.
N-(Pyridin-2-yl)-2-(m-tolyl)-N′-tosylacetimidamide (4v) (141.2 mg, 37%), yellow solid, m.p. = 103–105 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 2H), 7.90 (d, J = 7.6 Hz, 2H), 7.59 (t, J = 7.6 Hz, 2H), 7.32–7.26 (m, 3H), 7.15–6.99 (m, 4H), 4.42 (s, 2H), 2.43 (s, 3H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.8, 150.2, 147.9, 142.9, 140.0, 139.5, 138.4, 132.5, 130.8, 129.6, 129.5 (2C), 129.3, 127.1, 126.6 (2C), 120.8, 115.4, 40.5, 21.6, 21.4; IR (KBr) ν 3358, 3278, 1597, 1566, 1527, 1435, 1280, 1143, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C21H21N3O2S, [M + H]+ 380.1427, found 380.1421.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 2H), 7.90 (d, J = 7.6 Hz, 2H), 7.59 (t, J = 7.6 Hz, 2H), 7.32–7.26 (m, 3H), 7.15–6.99 (m, 4H), 4.42 (s, 2H), 2.43 (s, 3H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.8, 150.2, 147.9, 142.9, 140.0, 139.5, 138.4, 132.5, 130.8, 129.6, 129.5 (2C), 129.3, 127.1, 126.6 (2C), 120.8, 115.4, 40.5, 21.6, 21.4; IR (KBr) ν 3358, 3278, 1597, 1566, 1527, 1435, 1280, 1143, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C21H21N3O2S, [M + H]+ 380.1427, found 380.1421.
2-(4-Methoxyphenyl)-N-(pyridin-2-yl)-N′-tosylacetimidamide (4x) (130.5 mg, 33%), yellow solid, m.p. = 137–139 °C (Rf = 0.3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 8.11 (s, 1H), 7.90 (d, J = 7.6 Hz, 2H), 7.60 (t, J = 8.0 Hz, 1H), 7.50 (s, 1H), 7.32 (d, J = 7.6 Hz, 2H), 7.24 (t, J = 7.0 Hz, 2H),7.01 (t, J = 5.8 Hz, 1H), 6.94 (d, J = 6.8 Hz, 2H),4.42 (s, 2H), 3.81 (s, 3H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 159.7, 150.2, 148.0, 142.9, 140.0, 138.4, 131.5, 129.5 (3C), 126.6 (3C), 124.2, 120.9, 115.3, 115.2, 55.4, 39.9, 21.6; IR (KBr) ν 3358, 2837, 1597, 1512, 1433, 1247, 1143, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C21H21N3O3S, [M + H]+ 396.1377, found 396.1369.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 8.11 (s, 1H), 7.90 (d, J = 7.6 Hz, 2H), 7.60 (t, J = 8.0 Hz, 1H), 7.50 (s, 1H), 7.32 (d, J = 7.6 Hz, 2H), 7.24 (t, J = 7.0 Hz, 2H),7.01 (t, J = 5.8 Hz, 1H), 6.94 (d, J = 6.8 Hz, 2H),4.42 (s, 2H), 3.81 (s, 3H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 159.7, 150.2, 148.0, 142.9, 140.0, 138.4, 131.5, 129.5 (3C), 126.6 (3C), 124.2, 120.9, 115.3, 115.2, 55.4, 39.9, 21.6; IR (KBr) ν 3358, 2837, 1597, 1512, 1433, 1247, 1143, 1085 cm−1; HRMS (ESI) (m/z). Calcd for C21H21N3O3S, [M + H]+ 396.1377, found 396.1369.
All NMR spectra please see ESI Section 3.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Foundation and Applied Basic Research Fund project of Guangdong Province of China (2019A1515110918); Science and Technology Planning Program of Zhanjiang (2021A05247); Medical Scientific Research Foundation of Guangdong Province (A2021037 and A2020202); Key Discipline Construction Project of Guangdong Medical University (4SG22004G); Innovation and Entrepreneurship Team Leads the Pilot Program of Zhanjiang (2020LHJH005) and the Science and technology program of Guangdong Province (2019B090905011) for support.Notes and references
- (a) U. B. Karale, A. U. Shinde, D. A. Babar, K. G. Sangu, S. K. Vagolu, V. K. Eruva, S. S. Jadav, S. Misra, S. Dharmarajan and H. B. Rode, Arch. Pharm., 2021, 354, e2000419 CrossRef PubMed; (b) A. Muthengi, V. K. Wimalasena, H. O. Yosief, M. J. Bikowitz, L. H. Sigua, T. Wang, D. Li, Z. Gaieb, G. Dhawan, S. Liu, J. Erickson, R. E. Amaro, E. Schönbrunn, J. Qi and W. Zhang, J. Med. Chem., 2021, 64, 5787 CrossRef CAS PubMed; (c) A. K. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. Commun., 2015, 51, 1555 RSC; (d) F. Zeng and M. M. Goodman, Curr. Top. Med. Chem., 2013, 13, 909 CrossRef CAS PubMed; (e) J. M. Monti, D. W. Spence, S. R. Pandi-Perumal, S. Z. Langer and R. Hardeland, Clin. Med.: Ther., 2009, 1, 123 CAS; (f) C. EnguehardGueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS PubMed; (g) H. Heitsch, Curr. Med. Chem., 2002, 9, 913 CrossRef CAS PubMed.
- (a) C. Wei, J. Huang, Y. Luo, S. Wang, S. Wu, Z. Xing and J. Chen, Pestic. Biochem. Physiol., 2021, 175, 104857 CrossRef CAS PubMed; (b) O. Ebenezer, P. Awolade, N. Koorbanally and P. Singh, Chem. Biol. Drug Des., 2019, 1 Search PubMed; (c) N. M. Shukla, D. B. Salunke, E. Yoo, C. A. Mutz, R. Balakrishna and S. A. David, Bioorg. Med. Chem., 2012, 20, 5850 CrossRef CAS PubMed; (d) T. H. Al-Tel, R. A. Al-Qawasmeh and R. Zaarour, Eur. J. Med. Chem., 2011, 46, 1874 CrossRef CAS PubMed.
- (a) S. R. Sagar, D. P. Singh, R. D. Das, N. B. Panchal, V. Sudarsanam, M. Nivsarkar and K. K. Vasu, Bioorg. Med. Chem., 2021, 36, 116091 CrossRef CAS PubMed; (b) R. N. Rao, B. Mm, B. Maiti, R. Thakuria and K. Chanda, ACS Comb. Sci., 2018, 20, 164 CrossRef CAS PubMed; (c) R. B. Lacerda, C. K. F. de Lima, L. L. da Silva, N. C. Romeiro, A. L. P. Miranda, E. J. Barreiro and C. A. M. Fraga, Bioorg. Med. Chem., 2009, 17, 74 CrossRef CAS PubMed.
- (a) A. Hartwich, N. Zdzienicka, D. Schols, G. Andrei, R. Snoeck and I. E. Głowacka, Nucleosides, Nucleotides Nucleic Acids, 2020, 39, 542 CrossRef CAS PubMed; (b) G. C. Moraski, L. D. Markley, P. A. Hipskind, H. Boshoff, S. Cho, S. G. Franzblau and M. J. Miller, ACS Med. Chem. Lett., 2011, 2, 466 CrossRef CAS PubMed; (c) I. Vliegen, J. Paeshuyse, T. D. Burghgraeve, L. S. Lehman, M. Paulson, I.-H. Shih, E. Mabery, N. Boddeker, E. D. Clercq, H. Reiser, D. Oare, W. A. Lee, W. Zhong, S. Bondy, G. Pürstinger and J. Neyts, J. Hepatol., 2009, 50, 999 CrossRef CAS PubMed; (d) K. S. Gudmundsson, J. D. Williams, J. C. Drach and L. B. Townsend, J. Med. Chem., 2003, 46, 1449 CrossRef CAS PubMed.
- (a) D. C. D. Santos, J. Rafique, S. Saba, G. M. Almeida, T. Siminski, C. Pádua, D. W. Filho, A. Zamoner, A. L. Braga, R. C. Pedrosa and F. Ourique, J. Biochem. Mol. Toxicol., 2021, 35, e22663 CrossRef PubMed; (b) D. K. Sigalapalli, G. Kiranmai, G. P. Devi, R. Tokala, S. Sana, C. Tripura, G. S. Jadhav, M. Kadagathur, N. Shankaraiah, N. Nagesh, B. N. Babu and N. D. Tangellamudi, Bioorg. Med. Chem., 2021, 43, 116277 CrossRef CAS PubMed; (c) M. Matsumura, T. Takahashi, H. Yamauchi, S. Sakuma, Y. Hayashi, T. Hyodo, T. Obata, K. Yamaguchi, Y. Fujiwara and S. Yasuike, Beilstein J. Org. Chem., 2020, 16, 1075 CrossRef CAS PubMed; (d) J.-B. Xi, Y.-F. Fang, B. Frett, M.-L. Zhu, T. Zhu, Y.-N. Kong, F.-J. Guan, Y. Zhao, X.-W. Zhang, H.-Y. Li, M.-L. Ma and W. Hu, Eur. J. Med. Chem., 2017, 126, 1083 CrossRef CAS PubMed; (e) O. Kim, Y. Jeong, H. Lee, S.-S. Hong and S. Hong, J. Med. Chem., 2011, 54, 2455 CrossRef CAS PubMed.
- D. Dheer, K. R. Reddy, S. K. Rath, P. L. Sangwan, P. Das and R. Shankar, RSC Adv., 2016, 6, 38033 RSC.
- N. Chernyak and V. Gevorgyan, Angew. Chem., 2010, 122, 2803 CrossRef.
- (a) A. N. Edinoff, N. Wu, Y. T. Ghaffar, R. Prejean, R. Gremillion, M. Cogburn, A. A. Chami, A. M. Kaye and A. D. Kaye, Health. Psychol. Res., 2021, 9, 24927 Search PubMed; (b) G. Richter, V. W. Y. Liao, P. K. Ahring and M. Chebib, Front. Neurosci., 2020, 14, 599812 CrossRef PubMed; (c) R. Rosenberg, P. Murphy, G. Zammit, D. Mayleben, D. Kumar, S. Dhadda, G. Filippov, A. LoPresti and M. Moline, JAMA Netw. Open, 2019, 2, e1918254 CrossRef PubMed.
- K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef.
- (a) A. Heidari, J. Data Min. Genomics Proteomics, 2016, 7, e125 Search PubMed; (b) Y. Maruyama, K. Anami, M. Terasawa, K. Goto, T. Imayoshi, Y. Kadobe and Y. Mizushima, Arzneimittelforschung, 1981, 31, 1111 CAS.
- Y. Abe, H. Kayakiri, S. Satoh, T. Inoue, Y. Sawada, N. Inamura, M. Asano, I. Aramori, C. Hatori, H. Sawai, T. Oku and H. Tanaka, J. Med. Chem., 1998, 41, 4587 CrossRef CAS PubMed.
- (a) Y. Wu, L. Li, K. Wen, J. Deng, J. Chen, J. Shi, T. Wu, J. Pang and X. Tang, J. Org. Chem., 2021, 86, 12394 CrossRef CAS PubMed; (b) R. Semwal, C. Ravi, S. Saxena and S. Adimurthy, J. Org. Chem., 2019, 84, 14151 CrossRef CAS PubMed; (c) K. Sun, S. Mu, Z. Liu, R. Feng, Y. Li, K. Pang and B. Zhang, Org. Biomol. Chem., 2018, 16, 6655 RSC.
- (a) R. Semwal, G. Badhani and S. Adimurthy, Chem. Commun., 2022, 58, 1585 RSC; (b) X. Chen, P. Sun, B. Mo, C. Chen and J. Peng, J. Org. Chem., 2021, 86, 352 CrossRef CAS PubMed; (c) J. A. Tali, G. Kumar, D. Singh and R. Shankar, Org. Biomol. Chem., 2021, 19, 9401 RSC; (d) A. Joshi, R. Semwal, E. Suresh and S. Adimurthy, Chem. Commun., 2019, 55, 10888 RSC.
- (a) H. Yao, X. Zhong, B. Wang, S. Lin, L. Liu and Z. Yan, Org. Biomol. Chem., 2021, 19, 3479 RSC; (b) J. Rakhtshah and F. Yaghoobi, Int. J. Biol. Macromol., 2019, 139, 904 CrossRef CAS PubMed.
- D. S. Nipate, S. Jaspal, V. N. Shinde, K. Rangan and A. Kumar, Org. Lett., 2021, 23, 1373 CrossRef CAS PubMed.
- Z. Hu, J. Hou, J. Liu, W. Yu and J. Chang, Org. Biomol. Chem., 2018, 16, 5653 RSC.
- (a) F. Vuillermet, J. Bourret and G. Pelletier, J. Org. Chem., 2021, 86, 388 CrossRef CAS PubMed; (b) Y. Yuan, Z. Zhou, L. Zhang, L. S. Li and A. Lei, Org. Lett., 2021, 23, 5932 CrossRef CAS PubMed.
- (a) R. Semwal, C. Ravi, R. Kumar, R. Meena and S. Adimurthy, J. Org. Chem., 2019, 84, 792 CrossRef CAS PubMed; (b) R. Q. Tran, S. A. Jacoby, K. E. Roberts, W. A. Swann, N. W. Harris, L. P. Dinh, E. L. Denison and L. Yet, RSC Adv., 2019, 9, 17778 RSC; (c) Y. Liu, W. Wang, J. Han and J. Sun, Org. Biomol. Chem., 2017, 15, 9311 RSC; (d) S. Samanta, S. Jana, S. Mondal, K. Monir, S. K. Chandra and A. Hajra, Org. Biomol. Chem., 2016, 14, 5073 RSC; (e) D. Dheer, K. R. Reddy, S. K. Rath, P. L. Sangwan, P. Das and R. Shankar, RSC Adv., 2016, 6, 38033 RSC; (f) X. Xiao, Y. Xie, S. Bai, Y. Deng, H. Jiang and W. Zeng, Org. Lett., 2015, 17, 3998 CrossRef CAS PubMed.
- (a) H. D. de Salles, T. L. da Silva, C. S. Radatz, R. F. Affeldt, E. V. Benvenutti and P. H. Schneider, J. Braz. Chem. Soc., 2019, 30, 1825 CAS; (b) J. B. Bharate, S. K. Guru, S. K. Jain, S. Meena, P. P. Singh, S. Bhushan, B. Singh, S. B. Bharate and R. A. Vishwakarma, RSC Adv., 2013, 3, 20869 RSC; (c) S. K. Guchhait, A. L. Chandgude and G. Priyadarshani, J. Org. Chem., 2012, 77, 4438 CrossRef CAS PubMed; (d) H. Yan, R. Yan, S. Yang, X. Gao, Y. Wang, G. Huang and Y. Liang, Chem.–Asian J., 2012, 7, 2028 CrossRef CAS PubMed; (e) T. Palani, K. Park, M. R. Kumar, H. M. Jung and S. Lee, Eur. J. Org. Chem., 2012, 5038 CrossRef CAS; (f) S. Mishra and R. Ghosh, Synlett, 2011, 3463 CAS; (g) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (h) P. Liu, L. Fang, X. Lei and G. Lin, Tetrahedron Lett., 2010, 51, 4605 CrossRef CAS.
- (a) S. Bahadorikhalili, M. Divar, T. Damghani, F. Moeini, S. Ghassamipour, A. Iraji, M. A. Miller, B. Larijani and M. Mahdavi, J. Organomet. Chem., 2021, 939, 121773 CrossRef CAS; (b) S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem.–Asian J., 2011, 6, 2618 CrossRef CAS PubMed; (c) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038 CrossRef CAS PubMed; (d) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046 CrossRef CAS PubMed.
- (a) C. Ńajera, L. K. Sydnes and M. Yus, Chem. Rev., 2019, 119, 11110 CrossRef PubMed; (b) M. Nallagangula and K. Namitharan, Org. Lett., 2017, 19, 3536 CrossRef CAS PubMed; (c) C. Shao, Q. Zhang and G. Cheng, Eur. J. Org. Chem., 2013, 2013, 6443 CrossRef CAS; (d) P. V. Ramachandran, M. T. Rudd and M. V. R. Reddy, Tetrahedron Lett., 2005, 46, 2547 CrossRef CAS.
- (a) W. Yang, Y. Zhao, Q. Bu, L. Li, B. Zhou and Z. Huang, Org. Lett., 2022, 24, 457 CrossRef CAS PubMed; (b) Y. Zhao, L. Li, Z. Zhou, M. Chen, W. Yang and H. Luo, Org. Biomol. Chem., 2021, 19, 3868 RSC; (c) X. Luo, Y. Zhao, S. Tao, Z.-T. Yang, H. Luo and W. Yang, RSC Adv., 2021, 11, 31152 RSC; (d) W. Yang, D. Huang, X. Zeng, J. Zhang, X. Wang and Y. Hu, Tetrahedron, 2019, 75, 381 CrossRef CAS; (e) W. Yang, D. Huang, X. Zeng, D. Luo, X. Wang and Y. Hu, Chem. Commun., 2018, 54, 8222 RSC.
- X. Li, J. Chem. Res., 2012, 36, 525 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2121234. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra02722d |
| This journal is © The Royal Society of Chemistry 2022 |