Open Access Article
Open Access ArticleAmino acids with fluorescent tetrazine ethers as bioorthogonal handles for peptide modification†
Enric Ros a,
Marina Bellidoa,
Joan A. Matarinab,
Albert Gallen
a,
Marina Bellidoa,
Joan A. Matarinab,
Albert Gallen c,
Manuel Martínez
c,
Manuel Martínez cd,
Laura Rodríguezcd,
Xavier Verdaguer
cd,
Laura Rodríguezcd,
Xavier Verdaguer ae,
Lluís Ribas de Pouplanaaf and
Antoni Riera
ae,
Lluís Ribas de Pouplanaaf and
Antoni Riera *ae
*ae
aInstitute for Research in Biomedicine (IRB Barcelona), Barcelona Institute of Science and Technology, Baldiri Reixac 10, 08028 Barcelona, Spain. E-mail: antoni.riera@irbbarcelona.org
bBCN Peptides S.A., Pol. Ind. Els Vinyets-Els Fogars, Sector II, Ctra. Comarcal 244, Km. 22, 08777 Sant Quintí de Mediona, Barcelona, Spain
cDepartament de Química Inorgànica i Orgànica, Secció Química Inorgànica. Universitat de Barcelona, Martí i Franquès 1, 08028 Barcelona, Spain
dInstitut de Nanociència i Nanotecnologia (IN2UB), Universitat de Barcelona, 08028. Barcelona, Spain
eDepartament de Química Inorgànica i Orgànica, Secció Química Orgànica. Universitat de Barcelona, Martí i Franquès 1, 08028 Barcelona, Spain
fInstitució Catalana de Recerca i Estudis Avançats (ICREA), Passeig Lluís Companys 23, 08010 Barcelona, Spain
First published on 12th May 2022
Abstract
A set of 3-bromo-1,2,4,5-tetrazines with three distinct substitutions have been used as reagents for late-stage functionalization of small molecules through nucleophilic aromatic substitution. Spectroscopic studies of the products obtained proved that tetrazine ethers are intrinsically fluorescent. This fluorescence is lost upon inverse Electron-Demand Diels–Alder (iEDDA) cycloaddition with strained alkenes. Tetrazine-phenol ethers are rather interesting because they can undergo rapid iEDDA reactions with a second order rate constant (k2) compatible with bioorthogonal ligations. As a showcase, L-tyrosine was derivatized with 3-bromo-6-methyl-1,2,4,5-tetrazine and coupled to the peptide drug octreotide. This peptide was detected in cellular flow cytometry, and its fluorescence turned off through a bioorthogonal iEDDA cycloaddition with a strained alkene, showing for the first time the detection and reactivity of intrinsically fluorescent tetrazines in a biologically relevant context. The synthesis and characterization of fluorescent tetrazine ethers with bioorthogonal applicability pave the way for the generation of useful compounds for both detection and bioconjugation in vivo.
Introduction
Since the advent of “click chemistry”1 at the beginning of the century, bioconjugation has experienced exponential growth. Click reactions fulfill the criteria of being simple and non-sensitive to oxygen and/or water and they yield clean final products. They can be used in biological contexts if other requirements are met, namely they must be unreactive towards the functional groups found in nature, display fast kinetics and not require catalysis or generate toxic entities. These click reactions are known as bioorthogonal.2 In recent years, bioorthogonal reactions have found increasing applications in research and biomedical settings because they offer unique control over the reactivity of small molecules when applied in biological contexts.Of the handful of bioorthogonal reactions reported to date, the most widely used is probably the tetrazine ligation with strained alkenes or alkynes through an inverse Electron-Demand Diels–Alder (iEDDA) cycloaddition.3,4 These reactions have been used for drug delivery strategies,5,6 in vivo prodrug activation,7 novel drug discovery techniques8 and pre-targeted PET imaging,9 amongst many others.
In particular, one application that has attracted a huge interest is the development of fluorescent probes.10 Indeed, over recent years, several tetrazine-conjugates with a range of fluorophores such as BODIPY,11,12 coumarin,13 xanthene,14 phenoxazine,15 siliconrhodamine,16,17 naphthalene18 or cyanine19 derivatives have been developed. The fluorogenic character of these compounds after iEDDA reaction of the tetrazine core makes them advantageous for in vivo imaging experiments as they do not require washing steps, thus resulting in a “turn-on” fluorescent system.20,21
In contrast, although intrinsically fluorescent tetrazines are among the smallest organic fluorophores,22 their use is far less common. To date, fluorescent tetrazines, initially reported in the 1960's,23 are prepared mostly by nucleophilic aromatic substitution (SNAr) on 3,6-dichlorotetrazine.24 In this regard, both symmetrical and unsymmetrical ether-bridged tetrazines have been reported as well as their fluorescent and electrochemical behaviour (Fig. 1, top).25–27 However, there are no precedents in the exploitation of the emissive behavior and bioorthogonal reactivity of fluorescent tetrazines in vivo. This may be due to the slow reaction kinetics shown by the bis-substituted products.
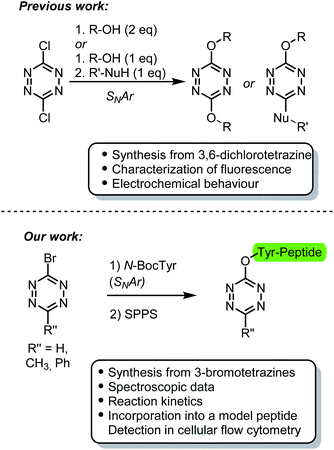 | ||
| Fig. 1 Comparison between the characterization and application of tetrazine ethers previously reported and those presented herein. | ||
When considering the reaction kinetics in iEDDA-type cycloadditions, the predominant factor is the energy gap between the HOMO (highest occupied molecular orbital) of the dienophile and LUMO (lowest unoccupied molecular orbital) of the diene.28,29 Consequently, the presence of electron-donating groups (EDGs) in the tetrazine ring, which acts as the diene, decreases their reactivity by increasing the energy of the LUMO.29 For SNAr products of 3,6-dichlorotetrazine, the presence of two EDGs on the tetrazine probably leads to sluggish kinetics in the iEDDA cycloaddition, thus limiting their applicability in bioorthogonal reactions.
We and others, have previously reported the synthesis and use of 3-bromotetrazines as excellent electrophiles for SNAr reactions.30–32 We envisaged that we could exploit the reactivity of these compounds under mild conditions to easily generate asymmetrically substituted fluorescent tetrazine ethers. We anticipated that the reaction products would display faster kinetics in the iEDDA cycloaddition compared with those synthesized using 3,6-dichlorotetrazine as precursor. To this end, here we describe the synthesis of tyrosine derivatives bearing a tetrazine ether moiety. One of these amino acids was introduced into a model peptide, thereby showcasing its potential use as fluorescent probe capable to undergo bioorthogonal reactions in vivo (Fig. 1, bottom).
Results and discussion
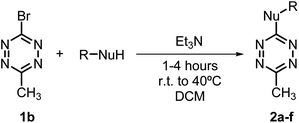
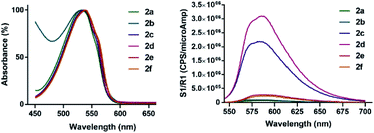
Recent years have witnessed increasing interest in the use of different bromotetrazines for late-stage functionalization of small molecules or macromolecules, either through SNAr reactions30,31 or via Pd-catalyzed cross-coupling reactions (such as Stille21 or Sonogashira33). Here we prepared the monosubstituted 3-bromo-1,2,4,5-tetrazine (1a), and the asymmetrically substituted 3-bromo-6-methyl-1,2,4,5-tetrazine (1b) and 3-bromo-6-phenyl-1,2,4,5-tetrazine (1c). We used a recently described and convenient synthetic route, that gave the highest overall yields (12–15%) reported to date.31,33 We selected 1b for the first screening of nucleophiles. As expected, tetrazine 1b reacted rapidly and in quantitative yields with aliphatic and aromatic amines to give the secondary amines 2a and 2b; with alcohols to give the ethers 2c and 2d; and with thiols to give the sulfides 2e and 2f (Table 1).To determine the emission behaviour of the heteroatom-substituted tetrazines resulting from these reactions, we registered the UV-Vis spectra of compounds 2a–f. They all showed an absorption band (λAbs) in the 530–540 nm region, which is characteristic of tetrazines (Table 2; Fig. 2). Interestingly, only the tetrazine ethers prepared from alcohols or phenols (2c and 2d) were found moderately emissive with quantum yields over 12% (Table 2) and emission band at 580–590 nm (Fig. 2). On the contrary, the substituted amines 2a–b and sulfides 2e–f were virtually non-emissive. As expected, the emission signals of 2c and 2d were lost when they were reacted with a strained dienophile, resulting in a fluorescence “turn-off” effect upon iEDDA reaction.
| λmax (nm) | εmax (M−1 cm−1) | λem (nm) | ΦF (%) | |
|---|---|---|---|---|
| 2a | 531 | 416 | 587 | 0.3 |
| 2b | 533 | 303 | 572 | 0.6 |
| 2c | 536 | 696 | 581 | 12.2 |
| 2d | 539 | 688 | 588 | 12.4 |
| 2e | 537 | 550 | 586 | 1.6 |
| 2f | 537 | 554 | 593 | 1.0 |
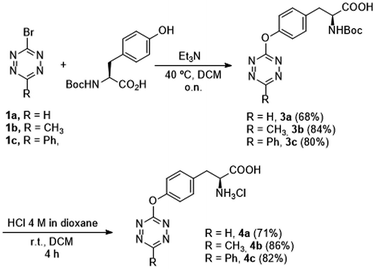
Although some intrinsically fluorescent tetrazines have been reported,25–27 they have never been used as bioorthogonal probes in vivo. We envisaged that tetrazines similar to products 2c–d could fulfill the criteria of fluorescence intensity, reactivity in the iEDDA cycloaddition, and stability in biological media, to be used under biocompatible conditions. To study these parameters, we synthesized a set of unnatural amino acids containing the tetrazine ether functionality. These compounds were easily prepared by SNAr reaction of 1a–c with N-Boc-tyrosine, followed by Boc-deprotection with HCl 4 M to obtain amino acids 4a–c as hydrochloride salts (Scheme 1). With the available tyrosine analogues, we characterized the emission intensity of compounds 4a–c (Table 3). Somewhat surprisingly, compound 4b, bearing a 4-methyltetrazinyl substituent, showed higher fluorescence than analogues 4a and 4c. We speculate that this difference may be due to the geometries of the products and the donor/acceptor properties of the different substituents in the tetrazine ether ring.
| λexc (nm) | λem (nm) | ΦF (%) | |
|---|---|---|---|
| 4a | 520 | 572 | 7.58 ± 0.02 |
| 4b | 531 | 587 | 11.9 ± 0.3 |
| 4c | 532 | 581 | 7.08 ± 0.01 |
We then studied the reaction kinetics of amino acids 4a–c in the iEDDA cycloaddition with the strained dienophile (E)-cyclooct-4-enol (TCO-OH) under pseudo first-order conditions in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (4
DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 37 °C (Scheme 5). The second-order rate constants (k2) are shown in Fig. 3. As expected, the monosubstituted tetrazine 4a undergoes cycloaddition 14 times faster than the methyl analogue 4b, whereas the phenyl substituted tetrazine 4c was three times faster than 4b. The rate enhancements are clearly attributed to a combination of electronic and steric effects34,35 and confirms the high reactivity of monosubstituted tetrazines. Despite the moderate values obtained, when compared with certain tetrazines substituted with electron-withdrawing groups (EWGs), the second-order rate constants are in the range of some of the most commonly used bioorthogonal reactions.
1) at 37 °C (Scheme 5). The second-order rate constants (k2) are shown in Fig. 3. As expected, the monosubstituted tetrazine 4a undergoes cycloaddition 14 times faster than the methyl analogue 4b, whereas the phenyl substituted tetrazine 4c was three times faster than 4b. The rate enhancements are clearly attributed to a combination of electronic and steric effects34,35 and confirms the high reactivity of monosubstituted tetrazines. Despite the moderate values obtained, when compared with certain tetrazines substituted with electron-withdrawing groups (EWGs), the second-order rate constants are in the range of some of the most commonly used bioorthogonal reactions.
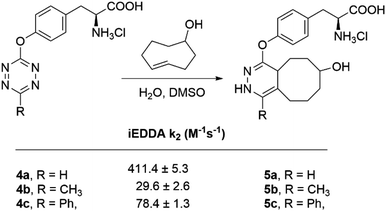 | ||
Fig. 3 Second-order rate constants for the iEDDA cycloaddition between L-tyrosine derivatives (4a–c) and TCO-OH in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (4 DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 37 °C. 1) at 37 °C. | ||
Given the moderate but significant fluorescence quantum yield (11.89 ± 0.25) for 4b, we decided to proceed our studies with this compound, despite showing the slowest k2 of all three compounds (29.6 M−1 s−1). Consequently, we determined the stability of 4b in fetal bovine serum (FBS) at 37 °C, thus mimicking physiological conditions. By monitoring the absorbance decay at the specific wavelength of the tetrazine ring over time, we found that more than 50% of tetrazine 4b remained intact after 48 h of incubation (see ESI†). The excitation wavelength of 4b (λExc = 531 nm) ensured minimal absorption overlap with endogenous chromophores and the absence of major scattering effects, both events known to interfere with fluorophores excited at shorter wavelengths (<500 nm) when used in a biological context.36,37 We hypothesized that this advantage could counterbalance the moderate fluorescence quantum yield displayed by 4b. We concluded that 4b presents the necessary trade-off between stability and reactivity to be used in vivo.38
Octreotide is a marketed drug with high affinity for Somatostatin receptor type 2 (SSTR2) that is used to treat several endocrine disorders.39 To showcase the potential of 4b in a biologically relevant setting, we synthesized an octreotide (OCT)40 derivative by coupling the N-Boc protected intermediate 3b to its N-terminal residue by solid-phase peptide synthesis (SPPS). After cleavage of the corresponding resin and removal of the protecting groups, OCT-4b (Fig. 4a) was obtained and purified by preparative HPLC. As expected, the spectroscopic characteristics of OCT-4b (λAbs = 513 nm and λEm = 579 nm) matched those observed for 4b alone, and the fluorescence was equally lost after iEDDA reaction with TCO-PEG, a water soluble version of TCO-OH (Fig. 4b).
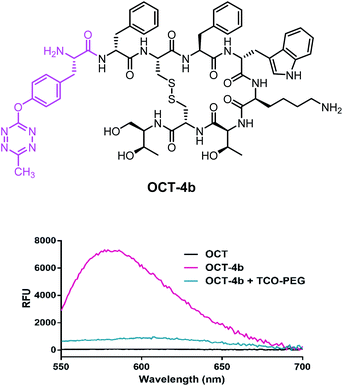 | ||
| Fig. 4 (a) Structure of OCT-4b; (b) emission spectra of OCT, OCT-4b and the iEDDA cycloaddition product of OCT-4b with TCO-PEG. | ||
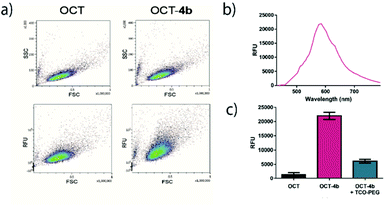
To study whether the intrinsic tetrazine fluorescence can be detected in a biologically relevant context, OCT-4b was then incubated with CHO–K1 cells overexpressing SSTR2. After the necessary time for internalization, the excess culture medium was removed and cells were monitored by flow cytometry (Fig. 5a). The density plots obtained showed a clear increase in the total relative fluorescence of the samples treated with OCT-4b compared to the control cells treated with OCT alone. Moreover, no differences were observed between the side scatter vs. forward scatter plots in both conditions, therby indicating that there was no toxicity related to the use of 4b.
To demonstrate that the increased fluorescence was caused by the tetrazine ether in 4b, the averaged emission spectra of cells were calculated and plotted (Fig. 5b). Indeed, the emission maximum of OCT-4b treated cells (λem = 585 nm) were consistent with the emission of tetrazine ethers. Finally, to validate that the reaction kinetics of 4b are suitable to undergo in vivo iEDDA cycloadditions, we incubated OCT-4b treated cells with TCO-PEG for 30 min. Gratifyingly, we observed that the fluorescence at the maximum emission wavelength returned to near-basal levels, thereby indicating that the reaction was taking place, and fluorescence was subsequently lost after iEDDA cycloaddition in vivo (Fig. 5c).
Experimental
1. Synthesis of 3-bromotetrazines
3-Bromotetrazines 1a–c were prepared following the described procedures.31,332. General procedure for the SNAr reaction with aliphatic and aromatic nucleophiles
Bromo tetrazine 1a (30 mg, 0.171 mmol, 1.0 eq.) and the corresponding R-NuH (0.206 mmol, 1.2 eq.) were placed in a sealed vial, which was degassed and placed under N2 atmosphere. Anhydrous DCM (1 mL, 0.17 M) was then added, followed by Et3N (24 μL, 0.17 mmol, 1.0 eq.). The mixture was stirred for 1–4 h until completion of the reaction was confirmed by TLC. For alcohol substitutions, the reaction mixture was heated at 40 °C to reach completion.The reaction mixtures were then concentrated under reduced pressure, diluted in water and extracted with DCM three times. The combined organic layers were dried over MgSO4 and evaporated under vacuum. Purification by flash column chromatography was performed using hexanes with increasing ethyl acetate (EtOAc) concentrations to afford 2a–f.
3. Synthesis of tetrazine-containing tyrosine derivatives
The reaction mixtures were concentrated under reduced pressure, diluted in HCl 0.1 M aqueous solution and extracted with DCM three times. The combined organic layers were dried over MgSO4 and evaporated under vacuum. Purification by flash column chromatography was performed in DCM with 1% acetic acid, with increasing concentrations of MeOH, to afford 3a–c.
4. Synthesis of OCT-4b by SPPS
OCT-resin was prepared by standard SPPS on a 2-chlorotrityl resin following the Fmoc/tBu strategy.Starting from 5.0 g of 2-Cl-Trt resin (1.60 mmol g−1) and using Fmoc-L-threoninol (1 eq.) as the first amino acid. After Fmoc-removal of the last amino acid (Fmoc-D-Phe-OH) the resin was dried. Peptidyl resin (11.6 g, f = 0.338 mmol g−1) was obtained and OCT was cleaved from the resin using a deprotection cocktail of TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecanethiol
dodecanethiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thioanisole
thioanisole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triisopropylsilane
triisopropylsilane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (85
H2O (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v/v/v/v) for 2 h. The formation of the disulfide bridge was achieved by air oxidation dissolving the crude peptide in a mixture of H2O
3 v/v/v/v/v) for 2 h. The formation of the disulfide bridge was achieved by air oxidation dissolving the crude peptide in a mixture of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO
DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (80
AcOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v/v) and stirring overnight. DMSO was removed through a polyaromatic adsorbent resin column chromatography.
10 v/v/v) and stirring overnight. DMSO was removed through a polyaromatic adsorbent resin column chromatography.
OCT-4b was synthesized from peptidyl resin (OCT-resin) (1 eq., 0.14 mmol, 0.42 g) and 3b (1.5 eq., 0.21 mmol, 0.08 g) using PyBOP/HOBt/DIEA (1.5eq./1.5eq./3eq.) as coupling reagents in DMF for 24 h. Reactivation with PyBOP/HOBt/DIEA was carried out for 20 h to achieve reaction completion. Peptidyl resin (OCT-4b-resin) (0.46 g) was obtained and subsequently cleaved and deprotected with TFA cocktail to obtain the reduced version of the peptide, which was air-oxidized in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO
DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH for 15 h. Then, DMSO was removed to finally obtain OCT-4b.
AcOH for 15 h. Then, DMSO was removed to finally obtain OCT-4b.
ESI-MS: calculated for C49H69N10O10S2 [M]+: 1021.3; found: 1021.3.
5. Absorbance spectra acquisition
Absorption spectra of compounds 2a–f (in DCM) and 4a–c (in methanol) at a final concentration of 1 mM were recorded on a Varian Cary 100 Bio UV spectrophotometer.6. Fluorescence spectra acquisition
Fluorescence emission spectra of compounds 2a–f (in DCM) and 4a–c (in methanol) were recorded on a Horiba-Jobin-Ybon SPEX Nanolog spectrofluorimeter from dilute solutions with an absorbance value of 0.2 AU at the excitation wavelength (λEx).7. Quantum yield determination
Quantum yields of compounds 2a–f (in DCM) and 4a–c (in methanol) were acquired on a Quantaurus-QY Absolute PL spectrometer at the excitation wavelength (λEx), using the specified solvent in each case as the reference solution.8. Determination of iEDDA rate constants
The observed reaction rate constants were measured at 37 °C varying concentrations of TCO-OH under pseudo first-order conditions (TCO-OH being in a 10 to 50 fold excess). The kinetic full spectra profiles were monitored by time-resolved UV-Vis spectroscopy in the 700–200 nm range. The time-resolved spectral changes have been quantified using ReactLab or Specfit software as a single step kinetics.41,42 Second-order rate constants were standardly derived43 from the slopes of the kobs vs. [TCO-OH] plots (see ESI†).9. Stability of 4b in FBS via UV-Vis spectroscopy
4b was dissolved in FBS (Merck) at a final concentration of 1 mM, and incubated at 37 °C for a total of 48 h in a flat-bottomed 96-well plate. Absorbance measurements were taken at λ = 510 nm at different time points (t = 0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h, 24 h and 48 h).10. Cellular flow cytometry
Chinese Hamster Ovary K1 (CHO-K1) cells overexpressing SSTR2 cells were grown in Kaighn's modification of Ham's F-12K medium (F-12K, ThermoFisher Scientific) supplemented with 10% FBS, 25 mM HEPES, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin and 200 μg mL−1 geneticin at 37 °C and 5% CO2.For flow cytometry studies, cells at 70–80% confluence were incubated with a solution of OCT, OCT-4b or OCT-4b-TCO-PEG in water at a final concentration of 250 μM for 14 h in flat-bottomed 24-well plates with 500 μL of culture medium. The medium was then removed and cells were washed with PBS once and fresh medium was added. In the cases were TCO-PEG was added (OCT-4b + TCO-PEG), 10 μL from a 50 mM solution in water was added (final concentration of 1 mM) for 30 minutes.
Cells were then trypsinized, quantified (1.1 × 106 total cells), washed with PBS once and resuspended in PBS at a final concentration of 1.5 × 106 cells per mL. They were then processed in a SA3800 Spectral Cell Analyzer (Sony Biotechnology) with excitation at 488 nm (Window Extension Normal, threshold CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) FSC, threshold value 16.7%, FSC gain = 10, SSC voltage 27%, fluorescence PMT voltage 85%).
FSC, threshold value 16.7%, FSC gain = 10, SSC voltage 27%, fluorescence PMT voltage 85%).
Conclusions
We have reported that 3-bromotetrazines are excellent electrophilic reagents to undergo SNAr reactions with heteroatoms acting as nucleophiles. The previously reported improved syntheses of 1a–c provides a facile and efficient method for late-stage tetrazine functionalization of small molecules.The corresponding oxygen-substituted tetrazines derived from alcohols or phenols showed a remarkable fluorescent behavior that is not present in the nitrogen or sulphur analogues. Such fluorescence is lost after the corresponding iEDDA reaction.
To showcase the applicability of tetrazine ethers in biologically relevant settings, we have functionalized the phenol contained in L-Tyrosine with 1a–c. Quantum yield determination of the obtained products revealed that the 6-methyltetrazin-3-yl analog 4b showed the strongest emissive properties, with an excitation wavelength that ensured minimal interference with endogenous chromophores. In addition, its second order rate constant for the subsequent iEDDA cycloaddition with a strained alkene was in line with some of the commonly used bioorthogonal ligations. Moreover, we demonstrated that 4b is also stable under physiological conditions for several days.
Consequently, we used the 6-methyltetrazin-3-yl tyrosine ether to modify a therapeutically relevant peptide (OCT-4b) and used it in cell flow cytometry, where we could “turn-off” its fluorescence upon reaction with a strained dienophile. Thus, we demonstrate for the first time that the intrinsic fluorescence of tetrazine ethers can be detected in a biological relevant context, and that the reaction kinetics of such compounds is suitable to undergo bioorthogonal iEDDA cycloadditions in vivo.
In summary, herein we report a strategy to achieve fluorescent tetrazine ethers that can be used as bioconjugation handles in vivo. The straightforward synthesis of tetrazine ethers from the reaction of 1a–c with alcohols or phenols, together with their fluorescent behaviour, bioorthogonal reactivity, stability in physiological conditions and small size are clear advantages for the generation of useful chemical biology tools with multiple applications.
Our future efforts will focus on incorporating the reported tetrazine ethers into other biologically relevant molecules, such as proteins through genetic code expansion methodologies. Alternatively, we anticipate that these compounds could serve to generate quantifiable fluorescent probes able to switch off their emissive properties upon iEDDA cycloaddition in vivo.
Author contributions
Conceptualization and writing: E. R. and A. R. Supervision and funding acquisition: A. R., L. RdP., X. V.; experimental: E. R., M. B., J. A. M. and A. G. Reaction kinetics: A. G., M. M.; optical measurements: E. R., L. R. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Spanish Ministerio de Ciencia e Innovación (MCINN), grant numbers PID2020-115074GB-I00 to A. R. and X. V., PID2019-104121GB-I00 to L. R. and PID2019-108037RB-100 to L. R.dP. We also acknowledge institutional funding from MINECO through the Centers of Excellence Severo Ochoa Award given to IRB Barcelona, as well as from the CERCA Program of the Generalitat de Catalunya. L.R.dP is an ICREA Programme Investigator.Notes and references
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS PubMed.
- B. L. Oliveira, Z. Guo and G. J. L. Bernardes, Chem. Soc. Rev., 2017, 46, 4895–4950 RSC.
- H. Wu and N. K. Devaraj, Acc. Chem. Res., 2018, 51, 1249–1259 CrossRef CAS PubMed.
- R. M. Versteegen, R. Rossin, W. Ten Hoeve, H. M. Janssen and M. S. Robillard, Angew. Chem., Int. Ed., 2013, 52, 14112–14116 CrossRef CAS PubMed.
- R. Rossin, S. M. J. Van Duijnhoven, W. Ten Hoeve, H. M. Janssen, L. H. J. Kleijn, F. J. M. Hoeben, R. M. Versteegen and M. S. Robillard, Bioconjugate Chem., 2016, 27, 1697–1706 CrossRef CAS PubMed.
- H. Lebraud, D. J. Wright, C. N. Johnson and T. D. Heightman, ACS Cent. Sci., 2016, 2, 927–934 CrossRef CAS PubMed.
- A. Rutkowska, D. W. Thomson, J. Vappiani, T. Werner, K. M. Mueller, L. Dittus, J. Krause, M. Muelbaier, G. Bergamini and M. Bantscheff, ACS Chem. Biol., 2016, 11, 2541–2550 CrossRef CAS PubMed.
- C. Denk, D. Svatunek, T. Filip, T. Wanek, D. Lumpi, J. Fröhlich, C. Kuntner and H. Mikula, Angew. Chem., Int. Ed., 2014, 53, 9655–9659 CrossRef CAS PubMed.
- G. B. Cserép, A. Herner and P. Kele, Methods Appl. Fluoresc., 2015, 3, 042001 CrossRef PubMed.
- N. K. Devaraj, S. Hilderbrand, R. Upadhyay, R. Mazitschek and R. Weissleder, Angew. Chem., Int. Ed., 2010, 49, 2869–2872 CrossRef CAS PubMed.
- A. Wieczorek, T. Buckup and R. Wombacher, Org. Biomol. Chem., 2014, 12, 4177–4185 RSC.
- L. G. Meimetis, J. C. T. Carlson, R. J. Giedt, R. H. Kohler and R. Weissleder, Angew. Chem., Int. Ed., 2014, 53, 7531–7534 CrossRef CAS PubMed.
- H. Wu, J. Yang, J. Šečkute and N. K. Devaraj, Angew. Chem., Int. Ed., 2014, 53, 5805–5809 CrossRef CAS PubMed.
- G. Knorr, E. Kozma, A. Herner, E. A. Lemke and P. Kele, Chem.–Eur. J., 2016, 22, 8972–8979 CrossRef CAS PubMed.
- E. Kozma, G. Estrada Girona, G. Paci, E. A. Lemke and P. Kele, Chem. Commun., 2017, 53, 6696–6699 RSC.
- P. Werther, K. Yserentant, F. Braun, N. Kaltwasser, C. Popp, M. Baalmann, D. P. Herten and R. Wombacher, Angew. Chem., Int. Ed., 2020, 59, 804–810 CrossRef CAS PubMed.
- D. Kim, J. H. Lee, J. Y. Koo, H. M. Kim and S. B. Park, Bioconjugate Chem., 2020, 31, 1545–1550 CrossRef CAS PubMed.
- G. Knorr, E. Kozma, J. M. Schaart, K. Németh, G. Török and P. Kele, Bioconjugate Chem., 2018, 29, 1312–1318 CrossRef CAS PubMed.
- A. Vázquez, R. Dzijak, M. Dračínský, R. Rampmaier, S. J. Siegl and M. Vrabel, Angew. Chem., Int. Ed., 2017, 56, 1334–1337 CrossRef PubMed.
- A. Wieczorek, P. Werther, J. Euchner and R. Wombacher, Chem. Sci., 2017, 8, 1506–1510 RSC.
- F. Miomandre and P. Audebert, J. Photochem. Photobiol., C, 2020, 44, 100372 CrossRef CAS.
- M. A. El-Sayed, J. Chem. Phys., 1963, 38, 2834–2838 CrossRef CAS.
- P. Audebert, F. Miomandre, G. Clavier, M. C. Verniéres, S. Badré and R. Méallet-Renault, Chem.–Eur. J., 2005, 11, 5667–5673 CrossRef CAS PubMed.
- Q. Zhou, P. Audebert, G. Clavier, F. Miomandre and J. Tang, RSC Adv., 2014, 4, 7193–7195 RSC.
- Z. Qing, P. Audebert, G. Clavier, F. Miomandre, J. Tang, T. T. Vu and R. Méallet-Renault, J. Electroanal. Chem., 2009, 632, 39–44 CrossRef CAS.
- E. Jullien-Macchi, V. Alain-Rizzo, C. Allain, C. Dumas-Verdes and P. Audebert, RSC Adv., 2014, 4, 34127–34133 RSC.
- J. M. J. M. Ravasco and J. A. S. Coelho, J. Am. Chem. Soc., 2020, 142, 4235–4241 CrossRef CAS PubMed.
- R. A. A. Foster and M. C. Willis, Chem. Soc. Rev., 2013, 42, 63–76 RSC.
- S. D. Schnell, L. V. Hoff, A. Panchagnula, M. H. H. Wurzenberger, T. M. Klapötke, S. Sieber, A. Linden and K. Gademann, Chem. Sci., 2020, 11, 3042–3047 RSC.
- E. Ros, M. Bellido, X. Verdaguer, L. Ribas de Pouplana and A. Riera, Bioconjugate Chem., 2020, 31, 933–938 CrossRef CAS PubMed.
- A. Counotte-Potman and H. C. Van Der Plas, J. Heterocycl. Chem., 1981, 18, 123–127 CrossRef CAS.
- E. Ros, A. Prades, D. Forson, J. Smyth, X. Verdaguer, L. R. de Pouplana and A. Riera, Chem. Commun., 2020, 56, 11086–11089 RSC.
- J. Yang, Y. Liang, J. Šečkutė, K. N. Houk and N. K. Devaraj, Chem.–Eur. J., 2014, 20, 3365–3375 CrossRef CAS PubMed.
- J. A. Wagner, D. Mercadante, I. Nikić, E. A. Lemke and F. Gräter, Chem.–Eur. J., 2015, 21, 12431–12435 CrossRef CAS PubMed.
- B. F. Buksh, S. D. Knutson, J. V. Oakley, N. B. Bissonnette, D. G. Oblinsky, M. P. Schwoerer, C. P. Seath, J. B. Geri, F. P. Rodriguez-Rivera, D. L. Parker, G. D. Scholes, A. Ploss and D. W. C. MacMillan, J. Am. Chem. Soc., 2022, 144, 6154–6162 CrossRef CAS PubMed.
- C. Ash, M. Dubec, K. Donne and T. Bashford, Lasers Med. Sci., 2017, 32, 1909–1918 CrossRef PubMed.
- A. C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131–5142 RSC.
- C. Bruns, F. Raulf, D. Hoyer, J. Schloos, H. Lübbert and G. Weckbecker, Metabolism, 1996, 45, 17–20 CrossRef CAS PubMed.
- S. W. J. Lamberts, A.-J. van der Lely, W. W. de Herder and L. J. Hofland, N. Engl. J. Med., 1996, 334, 246–254 CrossRef CAS PubMed.
- M. Maeder and P. King, ReactLab, Jplus Consulting Pty Ltd: East Fremantle, WA. Australia, 2009 Search PubMed.
- R. A. Binstead, A. D. Zuberbuhler and B. Jung, SPECFIT32, 3.0.34; Spectrum, Software Associates, Marlborough, MA, USA, 2005 Search PubMed.
- M. A. Gonzálvez, A. Gallen, M. Ferrer and M. Martínez, Inorg. Chem., 2020, 59, 1582–1587 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of new compounds and details of kinetic measurement. See https://doi.org/10.1039/d2ra02531k |
| This journal is © The Royal Society of Chemistry 2022 |