DOI:
10.1039/D2RA02388A
(Paper)
RSC Adv., 2022,
12, 13628-13638
Synthesis, SAR studies, and insecticidal activities of certain N-heterocycles derived from 3-((2-chloroquinolin-3-yl)methylene)-5-phenylfuran-2(3H)-one against Culex pipiens L. larvae†
Received
13th April 2022
, Accepted 20th April 2022
First published on 5th May 2022
Abstract
An acid hydrazide derivative was synthesized and transformed into a variety of valuable N-heterocycles such as pyridazinone, oxadiazole, triazolopyridazinone, and triazole derivatives via reactions with certain carbon electrophiles such as 4-methoxybenzaldehyde, indole-3-carbaldehyde, pentan-2,4-dione, and carbon disulfide. The chemical structures of all prepared compounds were verified via their analytical and spectroscopic data. The insecticidal activity of the N-heterocycles was evaluated against field and lab strains of the third larval instar of Culex pipiens. All tested compounds exhibited higher larvicidal activity against the lab strains compared to the field strains, with dissimilar ratios. The obtained results demonstrate that the high toxicity achieved by oxadiazole followed the order of furanone, pyridazinone and hydrazide, with lower LC50 values of the hydrazone and N-acetylpyridazinone derivatives compared to that of imidacloprid. Interestingly, these compounds are promising agents for insect pest control, especially since they are insoluble in water and can overcome the disadvantages of neonicotinoid applications in pest management programs.
Introduction
Heterocyclic compounds, especially nitrogen heterocycles, play an important role in the process of drug development and drug analysis (approximately two-thirds of the medicines available on the market include a heterocyclic skeleton).1–13 Accordingly, furanones are found to be easily converted to a variety of heterocyclic skeletons of biological importance.14–20 A broad range of pharmacological activities including insecticidal, antiviral, anticancer, antimicrobial, antimalarial, anti-inflammatory, and analgesic properties have been documented in quinoline (benzo[b]pyridine) derivatives (Fig. 1). The quinoline skeleton has been utilized for certain important engineered agrochemicals and to plan manufactured mixtures possessing many pharmacological effects.21–28
 |
| | Fig. 1 Some quinoline-based drugs. | |
Mosquitoes are the main vector of serious diseases that threaten human health and cause adverse socioeconomic impacts, particularly in subtropical and tropical climates.29,30 Culex pipiens Linnaeus (Diptera: Culicidae) is a widely distributed and epidemic mosquito found in Egypt.31 It is the vector of the West Nile virus, Rift Valley fever virus, and filariasis.32,33 Synthetic chemicals are a major, rapid, and effective control measure for mosquitoes, especially at the outbreak of diseases.34 However, the injudicious and intensive use of chemical insecticides for vector control is common malpractice, especially in developing countries.35 The accumulation of insecticide residues and the development of resistance in the target insect have adverse impacts on public health, ecological balance, and the environment, which gradually leads to the failure of the commercial products.36,37
Meanwhile, the widespread nature of mosquito breeding sites makes the application of insecticides for mosquito control inevitable.38 Consequently, the creation and evaluation of the toxicity of novel chemicals are important to develop new control recommendations. Neonicotinoids with a pyridine moiety have high efficacy against insects as well as distinctive characteristics such as a specific and selective mode of action for insect nicotinic acetylcholine receptors (nAChRs), thus exhibiting low mammalian toxicity in addition to a lack of cross-resistance with other insecticide groups and systemic properties.39–41 Even so, these systemic insecticides (soluble in water) may affect pollinators due to the accumulation of their traces in the plant nectar and pollen.54 Neonicotinoids possess molecular characteristics such as an aromatic heterocycle, hydroheterocycle, guanidine/amidine, flexible linkage, and electron-withdrawing segment.42 Thus, it is crucial to discover new hydrophobic heterocycles related to neonicotinoids with desired toxicological potencies to overcome this problem, target different insect pests and offer new methods of applications. The present study aims to synthesize and assess the activity of a few new N-heterocycles based on the benzopyridine group against lab and field strains of C. pipiens larvae.
Results and discussion
Synthesis
The requisite acid hydrazide derivative 2, obtained via hydrazinolysis of the corresponding furan-2(3H)-one derivative 1,43 was reacted with a few different carbon electrophiles to form a variety of valuable heterocycles including oxadiazole, triazole, and pyridazinone derivatives. First, its treatment with aromatic or heterocyclic aldehydes such as 4-methoxybenzaldehyde and indole-3-carbaldehyde in refluxing dioxane yielded the corresponding hydrazone derivatives 3 and 4 (Scheme 1). Alternatively, reacting 2 with pentan-2,4-dione in boiling dioxane achieved the pyridazinone derivative 5, which was confirmed by characterization.43 Second, the interaction of 2 with acetic anhydride under reflux conditions afforded the N-acetylpyridazinone derivative 6 (Scheme 1). The IR spectra of compounds 3, 4, and 6 exhibit NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption bands. The 1H NMR spectra of 3 and 4 reveal a singlet signal for the methine proton of the CH
O absorption bands. The 1H NMR spectra of 3 and 4 reveal a singlet signal for the methine proton of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group at δ 9.34 and 9.45 ppm, respectively, as well as a singlet signal for the methyl protons of the OCH3 group at δ 3.81 ppm for compound 3 and an exchangeable singlet signal for the NH of the indole ring at δ 11.95 ppm for compound 4. The 1H NMR spectrum of 6 showed an exchangeable singlet signal for NH at δ 12.09 ppm as well as signals for the
N group at δ 9.34 and 9.45 ppm, respectively, as well as a singlet signal for the methyl protons of the OCH3 group at δ 3.81 ppm for compound 3 and an exchangeable singlet signal for the NH of the indole ring at δ 11.95 ppm for compound 4. The 1H NMR spectrum of 6 showed an exchangeable singlet signal for NH at δ 12.09 ppm as well as signals for the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH of the pyridazinone ring at δ 7.13 ppm and methyl protons at δ 2.36 ppm. Their 13C NMR spectra provided additional evidence for the assigned structures. Further, the mass spectra of 3 and 6 exhibited their correct molecular ion and M + 2 peaks, as well as other important fragments (Experimental section).
CH of the pyridazinone ring at δ 7.13 ppm and methyl protons at δ 2.36 ppm. Their 13C NMR spectra provided additional evidence for the assigned structures. Further, the mass spectra of 3 and 6 exhibited their correct molecular ion and M + 2 peaks, as well as other important fragments (Experimental section).
 |
| | Scheme 1 Reactions of the hydrazide 2 with certain carbonyl compounds. | |
Third, the action of hydrazide 2 with carbon disulfide was investigated and studied under various conditions. The 5-mercapto-1,3,4-oxadiazole derivative 7 was produced by refluxing 2 in an ethanolic solution containing sodium hydroxide and carbon disulfide. Similarly, stirring the latter reaction mixture in ethanol with potassium hydroxide at room temperature yielded the corresponding potassium dithiocarbazinate 8, which was then treated with hydrazine hydrate in refluxing water or ethanol to construct the triazolopyridazine derivative 9 and 4-amino-5-mercapto-1,2,4-triazole derivative 10, respectively (Scheme 2).
 |
| | Scheme 2 Reactions of the hydrazide 2 with carbon disulfide under different conditions. | |
The IR spectra of compounds 7 and 9 show the absorption bands for the NH and CO moieties. Their 1H NMR spectra reveal two exchangeable broad singlet signals for NH and SH at δ 11.85; 13.20 and 11.81; 13.15 ppm for 7 and 9, respectively, as well as the signal for the methylene group. Further support for the proposed structure 7 was obtained from its 13C NMR and EIMS spectra (Experimental section). The amino and carbonyl groups were found in the IR spectrum of 10. Its 1H NMR spectrum indicated its existence as a mixture of two tautomeric forms: thiolactam and thiolactim in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Experimental section). Because of the stability of the aromatic triazole ring, the thiolactim form has a greater abundance. Furthermore, its mass spectrum showed the correct M+ peak at m/z 422 (33.43%) as well as the M + 2 peak at m/z 424 (17.89%), indicating the existence of the chlorine isotope.
3 (Experimental section). Because of the stability of the aromatic triazole ring, the thiolactim form has a greater abundance. Furthermore, its mass spectrum showed the correct M+ peak at m/z 422 (33.43%) as well as the M + 2 peak at m/z 424 (17.89%), indicating the existence of the chlorine isotope.
Insecticidal activity
The results of the larvicidal activity of the synthesized compounds and imidacloprid (IMI) exhibited good mortality against the third larval instar of lab and field strains of C. pipiens, indicating that the tested compounds can significantly control C. pipiens at the larval stage. Additionally, the tested compounds were more effective against the lab strain than the field population of C. pipiens larvae, as seen in Tables 1–4, with resistance ratios of 9.12, 11.06, 9.44, 13.14, 10.12, 9.49, and 8.62 for the compounds 1, 2, 3, 5, 6, 7 and IMI, respectively, at 48 h post-treatment (Table 2). Abou Rawash, Giza, where the field strain was collected, is a rural area, and many agricultural and public health insecticides are applied there. Thus, tolerance or reduction in the sensitivity of the field strain of C. pipiens larvae might be due to past insecticide application conferring cross-resistance as well as natural variations.44 The selection pressure of imidacloprid against Ae. aegypti larvae in the laboratory for several generations cause over-transcription of multiple genes encoding cuticle proteins, which reduce the penetration of the insecticide molecules through the insect cuticle.45 The mosquito could be affected by agricultural practices, pesticides used in agriculture and natural xenobiotics present in mosquito breeding sites, which confer cross-resistance to multiple insecticides.46
Table 1 Toxicity of the tested compounds against the field strain of the third instar of Culex pipiens larvae after 24 ha
| (F.l.) fiducial limits. Slope of the concentration–inhibition regression line ± standard error. (χ2) chi square value. (RR) resistance ratio. |
| Compounds (μg ml−1) |
1 |
2 |
3 |
5 |
6 |
7 |
IMI |
| LC25 (F.l. at 95%)a |
4.52 (1.52–8.014) |
12.08 (7.29–16.89) |
9.18 (1.8–16.78) |
7.69 (5.25–10.18) |
12.40 (5.77–19.19) |
6.51 (3.44–9.72) |
6.31 (3.16–9.64) |
| LC50 (F.l. at 95%)a |
18.58 (11.46–27.06) |
33.69 (25.21–45.49) |
38.87 (23.87–61.84) |
22.73 (17.83–29.66) |
53.19 (35.97–93.42) |
18.52 (13.06–24.77) |
19.07 (13.24–25.84) |
| LC90 (F.l. at 95%)a |
272.11 (136.42–1063.46) |
236.31 (141.77–561.1) |
603.21 (228.24–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 439.6) 439.6) |
177.91 (109.88–374.54) |
845.92 (322.59–6822.22) |
134.84 (86.457–278.31) |
155.69 (95.97–351.45) |
| Slope ± SEa |
1.09 ± 0.15 |
1.51 ± 0.16 |
1.07 ± 0.21 |
1.43 ± 0.13 |
1.06 ± 0.15 |
1.48 ± 0.16 |
1.405 ± 0.15 |
| P |
0.13 |
0.909 |
0.373 |
0.237 |
0.641 |
0.835 |
0.995 |
| χ2a |
7.105 |
1.001 |
3.11 |
5.53 |
2.51 |
1.44 |
2.206 |
| Toxicity index |
99.67 |
54.97 |
47.64 |
81.47 |
34.81 |
100 |
97.11 |
| Relative potency |
2.86 |
1.57 |
1.36 |
2.34 |
1 |
2.87 |
2.79 |
| RR |
7.74 |
10.43 |
9.74 |
8.26 |
14.26 |
9.9 |
9.5 |
Table 2 Toxicity of the tested compounds against the field strain of the third instar of Culex pipiens larvae after 48 h
| (F.l.) fiducial limits. Slope of the concentration–inhibition regression line ± standard error. (χ2) chi square value. |
| Compounds (μg ml−1) |
1 |
2 |
3 |
5 |
6 |
7 |
IMI |
| LC25 (F.l. at 95%)a |
2.94 (1.05–5.22) |
5.8 (2.83–8.96) |
4.64 (1.44–8.39) |
4.99 (2.47–7.68) |
6.94 (2.86–11.33) |
2.24 (0.64–4.29) |
2.59 (0.81–4.82) |
| LC50 (F.l. at 95%)a |
9.67 (5.54–13.99) |
17.59 (12.04–23.9) |
20.59 (12.64–30.59) |
14.07 (9.53–19.01) |
27.408 (18.43–40.14) |
7.69 (3.91–11.62) |
9.05 (4.88–13.41) |
| LC90 (F.l. at 95%)a |
92.49 (58.43–202.47) |
144.73 (89.85–322.29) |
348.94 (161.64–1711.96) |
101.04 (66.74–196.39) |
371.89 (178.57–1579.12) |
79.74 (49.23–188.9) |
97.19 (59.87–227.82) |
| Slope ± SEa |
1.307 ± 0.16 |
1.4 ± 0.15 |
1.042 ± 0.15 |
1.4973 ± 0.1635 |
1.13 ± 0.15 |
1.26 ± 0.17 |
1.24 ± 0.16 |
| P |
0.0695 |
0.37 |
0.149 |
0.0509 |
0.45 |
0.918 |
0.89 |
| χ2a |
8.68 |
4.27 |
6.7547 |
9.4461 |
3.68 |
5.945 |
1.108 |
| Toxicity index |
79.52 |
43.71 |
37.34 |
54.65 |
28.05 |
100 |
84.97 |
| Relative potency |
2.83 |
1.55 |
1.33 |
1.94 |
1 |
3.56 |
3.03 |
| RRa |
9.12 |
11.06 |
9.44 |
13.14 |
10.12 |
9.49 |
8.62 |
Table 3 Toxicity of the tested compounds against the lab strain of the third instar of Culex pipiens larvae after 24 h
| (F.l.) fiducial limits. Slope of the concentration–inhibition regression line ± standard error. (χ2) chi square value. |
| Compounds (μg ml−1) |
1 |
2 |
3 |
5 |
6 |
7 |
IMI |
| LC25 (F.l. at 95%)a |
0.76 (0.38–1.15) |
1.1 (0.63–1.57) |
1.05 (0.502–1.62) |
0.94 (0.53–1.36) |
1.07 (0.55–1.59) |
0.55 (0.24–0.89) |
0.52 (0.205–0.88) |
| LC50 (F.l. at 95%)a |
2.4 (1.69–3.28) |
3.23 (2.38–4.41) |
4.101 (2.85–6.37) |
2.75 (2.014–3.706) |
3.73 (2.65–5.47) |
1.87 (1.25–2.6) |
2.003 (1.29–2.86) |
| LC90 (F.l. at 95%)a |
21.29 (12.36–54.99) |
25.05 (14.58–63.73) |
54.003 (24.05–275.25) |
20.74 (12.51–48.808) |
40.01 (19.91–150.26) |
18.63 (10.77–49.25) |
25.16 (13.21–85.38) |
| Slope ± SEa |
1.35 ± 0.15 |
1.44 ± 0.16 |
1.14 ± 0.15 |
1.46 ± 0.16 |
1.24 ± 0.15 |
1.28 ± 0.15 |
1.16 ± 0.15 |
| P |
0.49 |
0.66 |
0.701 |
0.23 |
0.67 |
0.81 |
0.93 |
| χ2a |
3.41 |
2.409 |
2.18 |
5.502 |
2.34 |
1.58 |
2.92 |
| Toxicity index |
77.91 |
57.89 |
45.59 |
68 |
50.13 |
100 |
93.35 |
| Relative potency |
1.7 |
1.27 |
1 |
1.5 |
1.01 |
2.19 |
2.04 |
Table 4 Toxicity of the tested compounds against the lab strain of the third instar of Culex pipiens larvae after 48 h
| (F.l.) fiducial limits. Slope of the concentration–inhibition regression line ± standard error. (χ2) chi square value. |
| Compounds (μg ml−1) |
1 |
2 |
3 |
5 |
6 |
7 |
IMI |
| LC25 (F.l. at 95%)a |
0.3 (0.099–0.54) |
0.52 (0.25–0.82) |
0.57 (0.22–0.95) |
0.37 (0.16–0.603) |
0.74 (0.33–1.17) |
0.24 (0.079–0.45) |
0.31 (0.11–0.55) |
| LC50 (F.l. at 95%)a |
1.06 (0.601–1.55) |
1.59 (1.07–2.17) |
2.18 (1.43–3.14) |
1.07 (0.67–1.48) |
2.707 (1.86–3.87) |
0.81 (0.43–1.204) |
1.05 (0.61–1.52) |
| LC90 (F.l. at 95%)a |
11.75 (7.05–29.18) |
13.07 (8.21–28.42) |
28.09 (14.407–101.06) |
7.95 (5.31–15.16) |
31.304 (16.09–109.78) |
7.88 (5.038–16.91) |
10.69 (6.59–24.78) |
| Slope ± SEa |
1.22 ± 0.15 |
1.401 ± 0.15 |
1.15 ± 0.15 |
1.47 ± 0.16 |
1.205 ± 0.15 |
1.29 ± 0.16 |
1.27 ± 0.15 |
| P |
0.34 |
0.8459 |
0.38 |
0.28 |
0.56 |
0.28 |
0.53 |
| χ2a |
4.46 |
1.39 |
4.14 |
5.06 |
2.98 |
5.003 |
3.13 |
| Toxicity index |
76.41 |
50.94 |
37.15 |
75.7 |
29.92 |
100 |
77.14 |
| Relative potency |
2.55 |
1.7 |
1.24 |
2.53 |
1 |
3.34 |
2.57 |
The larval mortality showed a concentration and time-dependent behavior, as larval mortality increased with increasing concentration of the tested compounds and time of exposure. The obtained results showed that different levels of mortality were achieved by different tested compounds for both the susceptible and field strains, as shown in Fig. 2–5. For the field strain, great variation in the susceptibility of C. pipiens larvae towards the tested compounds was observed, whereas 7, 1, and IMI showed high activity with toxicity indexes of 100%, 99.67%, and 97.11% 24 h post-treatment (Table 1). The toxicities of 1, IMI, and 7 are about 3, 3, and 4 times that of 6, respectively, after 48 h with low LC50 values of 7.69, 9.05, and 9.67 μg mL−1, respectively (Table 2). For the lab strain, the toxicity indexes of 1, 2, 3, 5, 6, and IMI were 77.91%, 57.89%, 45.59%, 68%, 50.13%, and 93.35%, respectively, compared to 7, which showed 100% after 24 h of treatment (Table 3). At 48 h post-treatment, the toxicity of most tested compounds was convergent; however, 1, 5, 7, and IMI displayed high potencies 2.55, 2.53, 3.34, and 2.57 times that of 6, respectively, with LC50 values of 0.81, 1.06, 1.07 and 1.05 μg mL−1, respectively (Table 4). The chi-square values were significant at P < 0.01. The low slope values of all treatments revealed the homogeneity of the tested populations. In conclusion, the obtained results demonstrate that the high toxicities achieved by 7 followed by 1 then 5 make them promising agents for the control of insect pests.
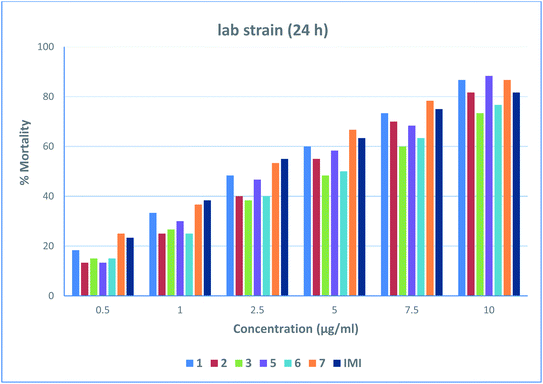 |
| | Fig. 2 % Mortality of the lab strain of Culex pipiens larvae observed after 24 h of treatment at different concentrations of 1, 2, 3, 5, 6, 7, and IMI. | |
 |
| | Fig. 3 % Mortality of the lab strain of Culex pipiens larvae observed after 48 h of treatment at different concentrations of 1, 2, 3, 5, 6, 7, and IMI. | |
 |
| | Fig. 4 % Mortality of a field strain of Culex pipiens larvae observed after 24 h of treatment at different concentrations of 1, 2, 3, 5, 6, 7, and IMI. | |
 |
| | Fig. 5 % Mortality of a field strain of Culex pipiens larvae observed after 48 h of treatment at different concentrations of 1, 2, 3, 5, 6, 7, and IMI. | |
Structure–action relationship
Neonicotinoids act against nicotinic acetylcholine receptors (nAChRs) through electrostatic interactions and H-bonding, which elevates the toxicity of neonicotinoids.47 Neonicotinoids have common molecular features such as an electron-withdrawing group, flexible linkage, aromatic heterocycle, and guanidine or amidine moiety.42 The synthesized compounds based on the benzopyridine moiety possess an electron-withdrawing Cl, a halogen, and an electron-withdrawing head that plays a substantial role in binding interactions with the receptor subsites via van der Waals and H-bonding interactions, as well as facilitates π-stacking interactions of the loop C aromatic residue with the amidine moiety.48 It was observed that the highest efficacy associated with compound 7 may be due to the presence of the oxadiazole moiety with the SH group that forms two H-bonds with the receptor. This agrees with,40 who reported that the presence of sulfanyl acetophenone, sulfanyl acetanilide and phenyl moieties cause the insecticidal activity against Aphis craccivora. The furanone derivative 1 contains two electron-withdrawing O atoms that increase the positivity of the C-2 position. In previous simulations with the insect receptor model, the electronegative moieties of neonicotinoids cause electronic conjugation that facilitates the flow of partial negative charge toward the tips, resulting in π-stacking and hydrogen bonding with the loop D Trp indole of nAChR.49 The receptor forms H-bonds between the pyridinyl nitrogen and loop E amino acids and the carbonylimino oxygen with the loop DW79 indole NH.50a Compound 5 (pyridazinone derivative) exhibited relatively good activity, which was interpreted by,50b who demonstrated that the nitroimino moiety can be replaced by phenoxycarbonylimino and acylimino variants. The C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–Ph analogs with an electron-donating group on the phenyl ring had higher affinity than those with an electron-withdrawing substituent, suggesting that the benzene plane forms a face-to-edge aromatic interaction with the loop D Trp indole, which was confirmed by39 who proved the activity of certain heterocyclic derivatives containing a pyridine moiety (aromatic heterocycle) against Aphis craccivora Koch.
O)–Ph analogs with an electron-donating group on the phenyl ring had higher affinity than those with an electron-withdrawing substituent, suggesting that the benzene plane forms a face-to-edge aromatic interaction with the loop D Trp indole, which was confirmed by39 who proved the activity of certain heterocyclic derivatives containing a pyridine moiety (aromatic heterocycle) against Aphis craccivora Koch.
The presence of the aromatic moiety in the synthesized compounds increased their hydrophobicity, which improves their permeability into the insect integument, thereby enhancing insecticidal activity. The intrinsic insecticidal efficacy and binding affinity of hydrophobic neonicotinoid derivatives against the housefly nicotinic receptor were higher than that of nitro or cyano neonicotinoids via topical application.48 Consequently, the increased hydrophobicity of neonicotinoids derivatives increases their penetrability through the insect integument and consequently their insecticidal effectiveness, which allows for diverse methods of exposure and makes them safe for aquatic habitats.39
Conclusion
A new series of N-heterocycles including pyridazinone, oxadiazole, triazolopyridazinone, and triazole derivatives were synthesized from the acid hydrazide via its reaction with certain carbon electrophiles. The potency of the synthesized compounds compared to that of IMI was estimated against lab and field strains of C. pipiens larvae. The tested compounds possess good toxicity, and the furanone, pyridazinone, and oxadiazole derivatives revealed the best insecticidal toxicity relative to IMI against both lab and field strains. The SAR study demonstrated that the halogen or electron-withdrawing substituents play an effective role in binding interactions with the nAChR subunits through van der Waals and H-bonding interactions, and enhance the π-stacking interactions, which effectively increase the activity. The aromatic moiety in the synthesized compounds imparts increased hydrophobicity, which improves their permeability into the insect integument and their binding affinity with the target receptor, thereby increasing their intrinsic insecticidal efficacy. These results are valuable for additional studies on the improvement of novel, effective, and safe insecticides.
Experimental
Instrumentation
Melting points were measured in open capillary tubes on an electrothermal melting point apparatus and were uncorrected. The infrared spectra (ν, cm−1) were recorded using the KBr wafer technique on a Fourier transform infrared Thermo Electron Nicolet iS10 spectrometer (Thermo Fisher Scientific Inc., Waltham, MA) at the Faculty of Science, Ain Shams University. The 1H and 13C NMR spectra (δ, ppm) were measured on a Varian GEMINI (GEMINI, Manufacturing & Engineering Inc., Anaheim, CA, USA) at the Faculty of Science, Cairo University, with tetramethyl silane (TMS) as an internal standard in DMSO-d6 as a solvent. The mass spectra were recorded on a direct probe controller inlet part to single quadrupole mass analyzer (Thermo Scientific GCMS MODEL (ISQ LT)) using the Thermo X-CALIBUR software at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Elemental analyses were performed on a PerkinElmer 2400 CHN elemental analyzer (PerkinElmer, Waltham, MA) at the Faculty of Science, Ain Shams University. Thin-layer chromatography (TLC) was run using TLC aluminum sheets silica gel F254 (Merck, Whitehouse Station, NJ). The 2(3H)-furanone derivative 1 and its corresponding acid hydrazide 2 were synthesized as previously reported.43
2-((2-Chloroquinolin-3-yl)methylene)-N′-(4-methoxybenzylidene)-4-oxo-4-phenylbutanehydrazide (3). A solution of the hydrazide 2 (1 g, 3 mmol) and 4-methoxybenzaldehyde (0.3 mL, 3 mmol) in dioxane (10 mL) was heated under reflux for 8 h. After cooling, the solid obtained was collected and recrystallized from a benzene/ethanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide orange crystals, mp. 250–252 °C. IR (KBr, ν, cm−1): 3300 (NH), 1700 (C
1) to provide orange crystals, mp. 250–252 °C. IR (KBr, ν, cm−1): 3300 (NH), 1700 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone), 1658 (C
O ketone), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1607 (C
O amide), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 9.34 (s, 1H, CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 9.34 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.64 (s, 1H, C4–H quinoline), 7.92 (s, 1H, CH
N), 8.64 (s, 1H, C4–H quinoline), 7.92 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.83–6.99 (m, 13H, Ar–H), 3.81 (s, 3H, OCH3), 3.35 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 160.87 (C
), 7.83–6.99 (m, 13H, Ar–H), 3.81 (s, 3H, OCH3), 3.35 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 160.87 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 156.57 (C
O), 156.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.27 (C
O), 143.27 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 140.05 (C
N), 140.05 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 139.15, 132.09, 130.88, 130.19, 129.34, 129.11, 128.37, 128.33, 127.91, 127.70, 126.27, 125.44, 122.43, 119.41, 115.19, 114.46 (Ar–C + CH
N), 139.15, 132.09, 130.88, 130.19, 129.34, 129.11, 128.37, 128.33, 127.91, 127.70, 126.27, 125.44, 122.43, 119.41, 115.19, 114.46 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 63.08 (CH3), 40.33 (CH2). MS, m/z (%): 486 (M + 2, 12.09), 484 (M+, 17.5), 371 (17.46), 367 (23.37), 292 (17.15), 275 (94.52), 183 (15.09), 141 (18.55), 103 (27.02), 86 (33.7), 76 (70.97), 71 (73.14), 63 (41.18), 43 (96.2), 40 (100). Anal. calcd for C28H22ClN3O3 (483.95): C, 69.49; H, 4.58; N, 8.68. Found: C, 69.32; H, 4.44; N, 8.70%.
), 63.08 (CH3), 40.33 (CH2). MS, m/z (%): 486 (M + 2, 12.09), 484 (M+, 17.5), 371 (17.46), 367 (23.37), 292 (17.15), 275 (94.52), 183 (15.09), 141 (18.55), 103 (27.02), 86 (33.7), 76 (70.97), 71 (73.14), 63 (41.18), 43 (96.2), 40 (100). Anal. calcd for C28H22ClN3O3 (483.95): C, 69.49; H, 4.58; N, 8.68. Found: C, 69.32; H, 4.44; N, 8.70%.
N′-((1H-Indol-3-yl)methylene)-2-((2-chloroquinolin-3-yl)methylene)-4-oxo-4-phenylbutanehydrazide (4). A solution of the hydrazide 2 (1 g, 3 mmol) and 3-formylindole (3 mmol) in dioxane (10 mL) was refluxed for 8 h. After cooling, the solid obtained was collected and recrystallized from dioxane to provide orange crystals, mp. 310–312 °C. IR (KBr, ν, cm−1): 3245 (NH), 1685 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone), 1650 (C
O ketone), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1634 (C
O amide), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.14 (br.s, 1H, NH, exchangeable), 11.95 (br.s, 1H, NH, exchangeable), 9.45 (s, 1H, CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.14 (br.s, 1H, NH, exchangeable), 11.95 (br.s, 1H, NH, exchangeable), 9.45 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.64 (s, 1H, C4–H quinoline), 7.97–6.96 (m, 15H, Ar–H + CH
N), 8.64 (s, 1H, C4–H quinoline), 7.97–6.96 (m, 15H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.55 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 166.61 (C
), 3.55 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 166.61 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.18 (C
O), 161.18 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 153.78 (C
O), 153.78 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 149.01 (C
N), 149.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 139.74, 138.92, 137.22, 132.52, 131.57, 130.41, 129.61, 129.29, 128.91, 128.55, 128.14, 126.86, 125.07, 124.09, 122.68, 122.24, 121.77, 120.66, 119.58, 115.09, 112.06, 111.40 (Ar–C + CH
N), 139.74, 138.92, 137.22, 132.52, 131.57, 130.41, 129.61, 129.29, 128.91, 128.55, 128.14, 126.86, 125.07, 124.09, 122.68, 122.24, 121.77, 120.66, 119.58, 115.09, 112.06, 111.40 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 40.33 (CH2). Anal. calcd for C29H21ClN4O2 (492.96): C, 70.66; H, 4.29; N, 11.37. Found: C, 70.49; H, 4.11; N, 11.40%.
), 40.33 (CH2). Anal. calcd for C29H21ClN4O2 (492.96): C, 70.66; H, 4.29; N, 11.37. Found: C, 70.49; H, 4.11; N, 11.40%.
4-((2-Chloroquinolin-3-yl)methyl)-6-phenylpyridazin-3(2H)-one (5). A solution of the hydrazide 2 (3 mmol) and pentan-2,4-dione (3 mmol) in dioxane (10 mL) was refluxed for 6 h. After cooling, the solid obtained was filtered off and recrystallized from an ethanol/dioxane mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and found to be the pyridazinone 6, which was identical in all respects (IR, mp, mixed mp, and TLC) with an authentic sample prepared by boiling the hydrazide 2 in n-butanol for 3 h.43
1) and found to be the pyridazinone 6, which was identical in all respects (IR, mp, mixed mp, and TLC) with an authentic sample prepared by boiling the hydrazide 2 in n-butanol for 3 h.43
1-Acetyl-4-((2-chloroquinolin-3-yl)methylene)-6-phenyl-1,4-dihydropyridazin-3(2H)-one (6). A suspension of the hydrazide 2 (1 g, 3 mmol) in acetic anhydride (10 mL) was refluxed for 3 h. After cooling, the solid obtained was collected and recrystallized from benzene to provide orange crystals, mp. 240−242 °C. IR (KBr, ν, cm−1): 3157 (NH), 1731, 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1608 (C
O amide), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 8.67 (s, 1H, C4–H quinoline), 7.92–7.21 (m, 10H, Ar–H + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 8.67 (s, 1H, C4–H quinoline), 7.92–7.21 (m, 10H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.13 (s, 1H, C5–H pyridazine), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6): 170.95 (C
), 7.13 (s, 1H, C5–H pyridazine), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6): 170.95 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.52 (C
O), 168.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.01 (C
O), 161.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 146.35, 140.56, 139.14, 131.97, 130.29, 129.43, 129.06, 128.35, 127.22, 127.01, 126.37, 126.33, 122.33, 119.45, 115.10 (Ar–C + CH
N), 146.35, 140.56, 139.14, 131.97, 130.29, 129.43, 129.06, 128.35, 127.22, 127.01, 126.37, 126.33, 122.33, 119.45, 115.10 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 24.57 (CH3). MS, m/z (%): 392 (M + 2, 43.80), 390 (M+, 15.11), 361 (40.57), 293 (16.71), 250 (10.17), 234 (22.31), 225 (49.47), 183 (37.24), 161 (42.10), 123 (41.57), 111 (51.43), 87 (33.95), 76 (64.72), 69 (40.25), 41 (100). Anal. calcd for C22H16ClN3O2 (389.84): C, 67.78; H, 4.14; N, 10.78. Found: C, 67.60; H, 4.01; N, 10.81%.
), 24.57 (CH3). MS, m/z (%): 392 (M + 2, 43.80), 390 (M+, 15.11), 361 (40.57), 293 (16.71), 250 (10.17), 234 (22.31), 225 (49.47), 183 (37.24), 161 (42.10), 123 (41.57), 111 (51.43), 87 (33.95), 76 (64.72), 69 (40.25), 41 (100). Anal. calcd for C22H16ClN3O2 (389.84): C, 67.78; H, 4.14; N, 10.78. Found: C, 67.60; H, 4.01; N, 10.81%.
3-(2-(5-Mercapto-1,3,4-oxadiazol-2-yl)-4-oxo-4-phenylbut-1-en-1-yl)quinolin-2(1H)-one (7). Carbon disulfide (5.5 mmol) was added to a solution of hydrazide 2 (5 mmol) in alc. NaOH (17 mL) and the reaction mixture was heated at ∼90 °C for 12 h. The reaction mixture was left overnight. The deposited solid was collected and recrystallized from a benzene/ethanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford off-white crystals, mp. 270–272 °C. IR (KBr, ν, cm−1): 3162 (NH), 1656 (C
1) to afford off-white crystals, mp. 270–272 °C. IR (KBr, ν, cm−1): 3162 (NH), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1607 (C
O), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.20 (br.s, 1H, SH, exchangeable), 11.85 (br.s, 1H, NH, exchangeable), 7.83–7.11 (m, 11H, Ar–H + C4–H quinoline + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.20 (br.s, 1H, SH, exchangeable), 11.85 (br.s, 1H, NH, exchangeable), 7.83–7.11 (m, 11H, Ar–H + C4–H quinoline + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.77 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 161.70 (C
), 3.77 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 161.70 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.69 (C
O), 160.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.93 (C
O), 143.93 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 140.81 (C
N), 140.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 138.07, 137.46, 134.95, 129.63, 129.60, 129.06, 128.88, 128.50, 128.31, 127.51, 125.64, 121.76, 119.28, 114.86 (Ar–C + CH
N), 138.07, 137.46, 134.95, 129.63, 129.60, 129.06, 128.88, 128.50, 128.31, 127.51, 125.64, 121.76, 119.28, 114.86 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 30.02 (CH2). MS, m/z (%): 389 (M+, 34.88), 388 (M −- 1, 11.97), 361 (M–CO, 41.43), 306 (40.71), 299 (45.09), 286 (25.33), 261 (30.7), 249 (34.31), 243 (37.62), 205 (27.49), 155 (18.41), 75 (100), 56 (67.43), 56 (67.43), 44 (33.32). Anal. calcd for C21H15N3O3S (389.43): C, 64.77; H, 3.88; N, 10.79. Found: C, 64.61; H, 3.93; N, 10.76%.
), 30.02 (CH2). MS, m/z (%): 389 (M+, 34.88), 388 (M −- 1, 11.97), 361 (M–CO, 41.43), 306 (40.71), 299 (45.09), 286 (25.33), 261 (30.7), 249 (34.31), 243 (37.62), 205 (27.49), 155 (18.41), 75 (100), 56 (67.43), 56 (67.43), 44 (33.32). Anal. calcd for C21H15N3O3S (389.43): C, 64.77; H, 3.88; N, 10.79. Found: C, 64.61; H, 3.93; N, 10.76%.
3-((3-Mercapto-6-phenyl-[1,2,4]triazolo[4,3-b]pyridazin-8(7H)-ylidene)methyl)quinolin-2(1H)-one (9). To a stirred solution of hydrazide 2 (5 mmol) in ethanol (30 mL), potassium hydroxide (5.5 mmol in 5 mL ethanol) was added at ambient temperature, and carbon disulfide (5.5 mmol) was then added dropwise. The reaction mixture was further stirred at ambient temperature for 24 h. The solid obtained was collected by filtration, washed with dry ether, and dried to afford the potassium salt 8 that was used without further purification. A solution of potassium salt 8 (5 mmol) and hydrazine hydrate (5 mmol, 80%) in water (15 mL) was heated under reflux until all hydrogen sulfide evolved (∼8 h). The precipitated solid was collected and crystallized from an ethanol/dioxane mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford yellow crystals, mp. 320–322 °C (decomp.). IR (KBr, ν, cm−1): 3160 (NH), 1656 (C
1) to afford yellow crystals, mp. 320–322 °C (decomp.). IR (KBr, ν, cm−1): 3160 (NH), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.15 (br.s, 1H, SH, exchangeable), 11.81 (br.s, 1H, NH, exchangeable), 7.80–7.14 (m, 11H, Ar–H + C4–H quinoline + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.15 (br.s, 1H, SH, exchangeable), 11.81 (br.s, 1H, NH, exchangeable), 7.80–7.14 (m, 11H, Ar–H + C4–H quinoline + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.76 (s, 2H, CH2). Anal. calcd for C21H15N5OS (325.39): C, 65.44; H, 3.92; N, 18.17. Found: C, 65.31; H, 3.85; N, 18.20%.
), 3.76 (s, 2H, CH2). Anal. calcd for C21H15N5OS (325.39): C, 65.44; H, 3.92; N, 18.17. Found: C, 65.31; H, 3.85; N, 18.20%.
3-(4-Amino-5-mercapto-4H-1,2,4-triazol-3-yl)-4-(2-chloroquinolin-3-yl)-1-phenylbut-3-en-1-one (10). A solution of potassium salt 8 (5 mmol), prepared from the previous step, and hydrazine hydrate (5 mmol, 80%) in ethanol (15 mL) was heated under reflux until all hydrogen sulfide evolved (∼8 h). The precipitated solid was collected and crystallized from dioxane to afford yellow crystals, mp. 252–254 °C. IR (KBr, ν, cm−1): 3250, 3161 (NH2), 1705 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 11.96 (br.s, 1H, NH, thiolactam form, exchangeable), 8.05 (s, 1H, C4–H quinoline), 7.77–7.12 (m, 10H, Ar–H + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 11.96 (br.s, 1H, NH, thiolactam form, exchangeable), 8.05 (s, 1H, C4–H quinoline), 7.77–7.12 (m, 10H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.71 (s, 1H, SH, thiolactim form, exchangeable), 4.43 (br.s, 2H, NH2, exchangeable), 3.27 (s, 2H, CH2). MS, m/z (%): 424 (M + 2, 17.89), 422 (M+, 33.43), 419 (40.37), 393 (22.67), 354 (34.02), 331 (49.44), 315 (75.39), 300 (46.12), 286 (45.06), 246 (40.65), 221 (39.56), 193 (40.51), 142 (61.61), 97 (39.40), 69 (57.66), 43 (100). Anal. calcd for C21H16ClN5OS (421.90): C, 59.78; H, 3.82; N, 16.60. Found: C, 59.64; H, 3.71; N, 16.70%.
), 6.71 (s, 1H, SH, thiolactim form, exchangeable), 4.43 (br.s, 2H, NH2, exchangeable), 3.27 (s, 2H, CH2). MS, m/z (%): 424 (M + 2, 17.89), 422 (M+, 33.43), 419 (40.37), 393 (22.67), 354 (34.02), 331 (49.44), 315 (75.39), 300 (46.12), 286 (45.06), 246 (40.65), 221 (39.56), 193 (40.51), 142 (61.61), 97 (39.40), 69 (57.66), 43 (100). Anal. calcd for C21H16ClN5OS (421.90): C, 59.78; H, 3.82; N, 16.60. Found: C, 59.64; H, 3.71; N, 16.70%.
Insecticidal activity
Breeding of mosquitoes. The laboratory strain of Culex pipiens was reared in the mosquito insectary at the Entomology Department, Faculty of Science, Ain Shams University. The insectary was maintained at 70 ± 10%, relative humidity 27 ± 3 °C, and a cycle of dark and light (10 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 h).51 C. pipiens breeding went through three aquatic stages: egg, larva, and pupa, in addition to flying adult. Egg rafts were placed in enamel bowls containing dechlorinated water. The newly hatched larvae were fed on a mixture of dog biscuits and yeast powder (in a ratio of 3
14 h).51 C. pipiens breeding went through three aquatic stages: egg, larva, and pupa, in addition to flying adult. Egg rafts were placed in enamel bowls containing dechlorinated water. The newly hatched larvae were fed on a mixture of dog biscuits and yeast powder (in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The pupae were collected daily and transferred to glass jars containing dechlorinated water and then placed in 25 × 25 × 25 screened cages for adult breeding. Adults were provided daily with cotton pads soaked in a 10% sugar solution. Females were fed pigeons for a blood meal.55 The field strain of C. pipiens larvae was collected from Mansoura Canal, Abou Rawash, Giza, Egypt.
1). The pupae were collected daily and transferred to glass jars containing dechlorinated water and then placed in 25 × 25 × 25 screened cages for adult breeding. Adults were provided daily with cotton pads soaked in a 10% sugar solution. Females were fed pigeons for a blood meal.55 The field strain of C. pipiens larvae was collected from Mansoura Canal, Abou Rawash, Giza, Egypt.
Larvicidal bioassay. The larvicidal activities of the synthesized compounds (1, 2, 3, 5, 6, 7) and the reference insecticide imidacloprid (IMI) were assessed against C. pipiens larvae according to ref. 52 with some modifications. For the preparation of a 1000 ppm stock solution, the tested compounds were dissolved in dimethyl sulfoxide (DMSO). Six concentrations of each compound were prepared by diluting them with dechlorinated water, as follows: 10, 7.5, 5, 2.5, 1, and 0.5 μg mL−1 for the lab strain treatments, and each concentration was replicated three times. For control, three replicates of a DMSO and dechlorinated water mixture were used. Twenty third instar C. pipiens larvae were placed in each concentration replicate, and likewise for the control.41 In the same manner, the field strain third instar C. pipiens larvae were subjected to six concentrations of the tested compounds viz., 100, 75, 50, 25, 10, and 5 μg mL−1 and the control replicates. Mortality rates were recorded after exposure times of 24 and 48 h.
Statistical analysis. The data of larval mortality were analyzed using probit analysis53 via a statistics (LDP-line) package to calculate the LC values with 95% fiducial limits of lower and upper confidence limit, slope, standard error, chi-square, and correlation coefficient.
Conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Research Groups Program under grant number RGP.2/183/43.
References
- K. N. M. Halim, S. A. Rizk, M. A. El-Hashash and S. K. Ramadan, Straightforward synthesis, antiproliferative screening, and density functional theory study of some pyrazolylpyrimidine derivatives, J. Heterocycl. Chem., 2021, 58(2), 636 CrossRef CAS.
- S. K. Ramadan, E. Z. Elrazaz, K. A. M. Abouzid and A. M. El-Naggar, Design, synthesis and: in silico studies of new quinazolinone derivatives as antitumor PARP-1 inhibitors, RSC Adv., 2020, 10(49), 29475 RSC.
- S. K. Ramadan, N. A. Ibrahim, S. A. El-Kaed and E. A. E. El-Helw, New potential fungicides pyrazole-based heterocycles derived from 2-cyano-3-(1,3-diphenyl-1H-pyrazol-4-yl)acryloyl isothiocyanate, J. Sulfur Chem., 2021, 42, 529 CrossRef CAS.
- F. Gao, P. Wang, H. Yang, Q. Miao, L. Ma and G. Lu, Eur. J. Med. Chem., 2018, 157, 1223 CrossRef CAS PubMed.
- D. S. A. Haneen, R. S. Gouhar, H. E. Hashem and A. S. A. Youssef, Synthesis and reactions of 4H-3,1-benzoxazin-4-one derivative bearing pyrazolyl moiety as antimicrobial and antioxidant agents, Synth. Commun., 2019, 49(21), 2840 CAS.
- M. K. Abou-Elregal, A. T. A. Mohamed, A. S. A. Youssef, M. M. Hemdan, S. S. Samir and W. S. I. Abou-Elmagd, Synthesis and antitumor activity evaluation of some 1,2,4-triazine and fused triazine derivatives, Synth. Commun., 2018, 48(18), 2347 CrossRef CAS.
- W. S. I. Abou-Elmagd and A. I. Hashem, Novel synthesis of some isatin hydrazones and pyridazinophthalazines, Synth. Commun., 2013, 43(8), 1083 CrossRef CAS.
-
(a) K. N. M. Halim, S. K. Ramadan, S. A. Rizk and M. A. El-Hashash, Synthesis, DFT study, molecular docking and insecticidal evaluation of some pyrazole-based tetrahydropyrimidine derivatives, Synth. Commun., 2020, 50(8), 1159 CrossRef CAS;
(b) S. K. Ramadan, K. N. M. Halim, S. A. Rizk and M. A. El-Hashash, Cytotoxic activity and density functional theory studies of some 1,3-diphenylpyrazolyltetrahydropyrimidine derivatives, J. Iran. Chem. Soc., 2020, 17(7), 1575 CrossRef CAS.
- M. R. Mahmoud, M. M. El-Shahawi, W. S. I. Abou-Elmagd and M. H. Hekal, Novel Synthesis of Isoquinoline Derivatives Derived from (Z)-4-(1,3-Diphenylpyrazol-4-yl)isochromene-1,3-dione, Synth. Commun., 2015, 45(14), 1632 CrossRef CAS.
- M. M. Kaddah, A. A. Fahmi, M. M. Kamel, S. K. Ramadan and S. A. Rizk, Synthesis, characterization, computational chemical studies and antiproliferative activity of some heterocyclic systems derived from 3-(3-(1,3-diphenyl-1H-pyrazol-4-yl)acryloyl)-2H-chromen-2-one, Synth. Commun., 2021, 51, 1798 CrossRef CAS.
-
(a) S. K. Ramadan, A. K. El-Ziaty and R. S. Ali, Synthesis, antiproliferative activity, and molecular docking of some N-heterocycles bearing a pyrazole scaffold against liver and breast tumors, J. Heterocycl. Chem., 2021, 58(1), 290 CrossRef CAS;
(b) S. K. Ramadan, A. K. El-Ziaty and E. A. E. El-Helw, Synthesis and antioxidant evaluation of some heterocyclic candidates from 3-(1,3-diphenyl-1H-pyrazol-4-yl)-2-(4-oxo-4H-benzo[d][1,3]oxazin-2-yl)propenonitrile, Synth. Commun., 2021, 51, 1272 CAS.
- M. R. Mahmoud, W. S. I. Abou-Elmagd, H. A. Derbala and M. H. Hekal, Synthesis and spectral characterization of some phthalazinone derivatives, J. Chem. Res., 2012, 36(2), 75 CrossRef CAS.
-
(a) A. S. A. Youssef, K. A. Kandeel, W. S. I. Abou-Elmagd and D. S. A. Haneen, Action of Some Nitrogen and Carbon Nucleophiles on 4-Arylidene-1,3-oxazolones, J. Heterocycl. Chem., 2016, 53(1), 175 CrossRef CAS;
(b) D. S. A. Haneen, W. S. I. Abou-Elmagd and A. S. A. Youssef, 5(4H)-oxazolones: Synthesis and biological activities, Synth. Commun., 2021, 51(2), 215 CrossRef CAS.
- A. R. I. Morsy, S. K. Ramadan and M. M. Elsafty, Synthesis and antiviral activity of some pyrrolonyl substituted heterocycles as additives to enhance inactivated Newcastle disease vaccine, Med. Chem. Res., 2020, 29(6), 979 CrossRef CAS.
- S. K. Ramadan, S. S. Shaban and A. I. Hashem, Facile and
expedient synthesis and anti-proliferative activity of diversely pyrrolones bearing 1,3-diphenylpyrazole moiety, Synth. Commun., 2020, 50(2), 185 CrossRef CAS.
- A. I. Hashem, W. S. I. Abou-Elmagd, A. K. El-Ziaty and S. K. Ramadan, Ring Transformation of a 2(3H)-furanone Derivative into Oxazinone and Pyrimidinone Heterocycles, J. Heterocycl. Chem., 2017, 54(6), 3711 CrossRef CAS.
- A. I. Hashem, A. S. A. Youssef, K. A. Kandeel and W. S. I. Abou-Elmagd, Conversion of some 2(3H)-furanones bearing a pyrazolyl group into other heterocyclic systems with a study of their antiviral activity, Eur. J. Med. Chem., 2007, 42, 934 CrossRef CAS PubMed.
-
(a) E. A. E. El-Helw, A. R. I. Morsy and A. I. Hashem, Evaluation of some new heterocycles bearing 2-oxoquinolyl moiety as immunomodulator against highly pathogenic avian influenza virus (H5N8), J. Heterocycl. Chem., 2021, 58(4), 1003 CrossRef CAS;
(b) E. A. E. El-Helw and A. I. Hashem, Synthesis and antitumor activity evaluation of some pyrrolone and pyridazinone heterocycles derived from 3-((2-oxo-5-(p-tolyl)furan-3(2H)-ylidene)methyl)quinolin-2(1H)-one, Synth. Commun., 2020, 50(7), 1046 CrossRef CAS.
- M. Alam, P. Sarkar, A. Husain, A. Marella, M. S. Zaman, M. Akhter, M. Shaharyar, O. Alam and F. Azam, J. Serb. Chem. Soc., 2011, 76, 1617 CrossRef CAS.
- S. K. Ramadan, W. S. I. Abou-Elmagd and A. I. Hashem, Reactions of 2(3H)-furanones. A review, Synth. Commun., 2019, 49(22), 3031 CrossRef CAS.
- C. Fernandez-Galleguillos, L. A. Saavedra and M. Gutierrez, J. Braz. Chem. Soc., 2014, 25, 365 CAS.
- M. M. Kaddah, A. R. I. Morsy, A. A. Fahmi, M. M. Kamel, M. M. Elsafty, S. A. Rizk and S. K. Ramadan, Synthesis and biological activity on IBD virus of diverse heterocyclic systems derived from 2-cyano-N′-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)acetohydrazide, Synth. Commun., 2021, 51(22), 3366 CrossRef CAS.
- I. Radini, T. Elsheikh, E. El-Telbani and R. Khidre, Molecules, 2016, 21, 909 CrossRef.
- A. M. El-Naggar and S. K. Ramadan, Efficient synthesis of some pyrimidine and thiazolidine derivatives bearing quinoline scaffold under microwave irradiation, Synth. Commun., 2020, 50(14), 2188 CrossRef CAS.
- R. Musiol, J. Jampilek, V. Buchta, L. Silva, H. Niedbala, B. Podeszwa, A. Palka, K. Majerz-Maniecka, B. Oleksyn and J. Polanski, Bioorg. Med. Chem., 2006, 14, 3592 CrossRef CAS.
- A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman and M. M. Alam, Saudi Pharm. J., 2013, 21(1), 1 CrossRef PubMed.
- E. A. Ghareeb, N. F. H. Mahmoud, E. A. El-Bordany and E. A. E. El-Helw, Synthesis, DFT, and eco-friendly insecticidal activity of some N-heterocycles derived from 4-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)-2-phenyloxazol-5(4H)-one, Bioorg. Chem., 2021, 112, 104945 CrossRef CAS PubMed.
- V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488 CrossRef CAS PubMed.
- Z. Shu, M. Shahen, M. Hegazi, I. Al-Sharkaw and A. Seif, Physiological response of Culex pipiens larvae to sublethal concentrations of sodium and calcium hypochlorite, J. Environ. Biol., 2018, 39, 314 CrossRef CAS.
- Y. Ma, M. Li, H. Zhang, H. Sun, H. Su, Y. Wang and Z. Du, Bioassay-guided isolation of active compounds from Adenosma buchneroides essential oil as mosquito repellent against Aedes albopictus, J. Ethnopharm., 2019, 231(1), 386 CrossRef CAS PubMed.
- M. Shahen, H. M. El-Wahsh, C. H. Ramadan, M. A. M. Hegazi, I. M. Al-Sharkawi and A. I. Seif, A Comparison of the Toxicity of Calcium
and Sodium Hypochlorite against Culex pipiens (Diptera: Culicidae) Larvae, J. Environ. Sci. Curr. Res., 2020, 3, 21 Search PubMed.
- M. A. Fouda, M. I. Hassan, K. M. Hammad and A. I. Hasaballah, The Effects of Midgut Bacteria and Protease Inhibitors on the Reproductive Potential and Midgut Enzymes of Culex pipiens, mosquito infected with Wuchereria Bancrofti filaria, J. Egypt. Soc. Parasitol., 2013, 43, 537 Search PubMed.
- A. Outammassine, S. Zouhair and S. Loqman, Rift Valley Fever and West Nile virus vectors in Morocco: Current situation and future anticipated scenarios, Transbound. Emerg. Dis., 2021, 33876581, DOI:10.1111/tbed.14113.
- T. Chareonviriyaphap, M. J. Bangs, W. Suwonkerd, M. Kongmee, V. Corbel and R. Ngoen-Klan, Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand, Parasite Vectors, 2013, 6(280), 1 Search PubMed.
- H. R. Rathor, G. Nadeem and I. A. Khan, Pesticide susceptibility status of Anopheles mosquitoes in four flood-affected districts of South Punjab, Pakistan, Vector-Borne Zoonotic Dis., 2013, 13(1), 60 CrossRef PubMed.
- R. F. A. Bakr, A. M. Kamel, S. A. Sheba and D. R. Abdel-Haleem, A mathematical model for estimating the LC50 (or LD50) among an insect life cycle. Egypt, Acad. J. Biol. Sci., 2010, 3(2), 75 Search PubMed.
- N. Abbas, S. A. Shad and M. Ismail, Resistance to conventional and new insecticides in house flies (Diptera: Muscidae) from poultry facilities in Punjab, Pakistan, J. Econom. Entomol., 2015, 108(2), 826 CrossRef CAS PubMed.
- R. M. Shah, M. Alam, D. Ahmad, M. Waqas, Q. Ali, M. Binyamin and S. A. Shad, Toxicity of 25 synthetic insecticides to the field population of Culex quinquefasciatus Say, Parasit. Res., 2016, 115(11), 4345 CrossRef PubMed.
- E. A. Bakhite, A. A. Abd-Ella, M. E. A. El-Sayed and S. A. A. Abdel-Raheem, Pyridine Derivatives as Insecticides. Part 1: Synthesis and Toxicity of Some Pyridine Derivatives Against Cowpea Aphid, Aphis craccivora Koch (Homoptera: Aphididae), J. Agric. Food Chem., 2014, 62, 9982 CrossRef CAS PubMed.
- A. M. Kamal El-Dean, A. A. Abd-Ella, R. Hassanien, M. E. A. El-Sayed, R. M. Zaki and S. A. A. Abdel-Raheem, Chemical design and toxicity evaluation of new pyrimidothienotetrahydroisoquinolines as potential insecticidal agents, Toxicol. Rep., 2019, 6, 100 CrossRef PubMed.
- D. R. Abdel-Haleem, A. A. Gad and S. M. Farag, Larvicidal, biochemical and physiological effects of acetamiprid and thiamethoxam against Culex pippin’s L. (Diptera: Culicidae), Egypt. J. Aquatic Biol. Fish., 2020, 24(3), 271 CrossRef.
- Z. Zian, X. Shao, Z. Li, X. Qian and Q. Huang, Synthesis, insecticidal activity and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification, J. Agric. Food Chem., 2007, 55(6), 2288 CrossRef.
- N. M. Gad, W. S. I. Abou-Elmagd, D. S. A. Haneen and S. K. Ramadan, Reactivity of 5-phenyl-3-[(2-chloroquinolin-3-yl)methylene] furan-2(3H)-one towards hydrazine and benzylamine: a comparative study, Synth. Commun., 2021, 51(9), 1384 CrossRef CAS.
- D. R. Abdel-Haleem, N. A. Genidy, A. R. Fahmy, F. S. Abu-El Azm and N. S. M. Ismail, Comparative Modelling, Toxicological and Biochemical Studies of Imidacloprid and Thiamethoxam Insecticides on the House Fly, Musca Domestica L. (Diptera: Muscidae), Egypt. Acad. J. Biolog. Sci., 2018, 11(1), 33 Search PubMed.
- M. A. Riaz, A. Chandor-Proust, C. Dauphin-Villemant, R. Poupardin, C. M. Jones, C. Strode, M. Regent-Kloeckner, J. P. David and S. Reynaud, Molecular mechanisms associated with increased tolerance to the neonicotinoid insecticide imidacloprid in the dengue vector Aedes aegypti, Aquat. Toxicol., 2013, 126, 326 CrossRef CAS PubMed.
- T. E. Nkya, I. Akhouayri, W. Kisinza and J.-P. David, Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects, Insect Biochem. Mol. Biol., 2013, 43(4), 407 CrossRef CAS PubMed.
- M. Tomizawa and J. E. Casida, Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian
nicotinic receptors, Annu. Rev. Entomol., 2003, 48, 339 CrossRef CAS PubMed.
- I. Ohno, M. Tomizawa, A. Aoshima, S. Kumazawa and S. Kagabu, Trifluoroacetyl Neonicotinoid Insecticides with Enhanced Hydrophobicity and Effectiveness, J. Agric. Food Chem., 2010, 58, 4999 CrossRef CAS PubMed.
- M.-J. Wang, X.-B. Zhao, D. Wu, Y.-Q. Liu, Y. Zhang, X. Nan, H. Liu, H.-T. Yu, G.-F. Hu and L.-T. Yan, Design, synthesis, crystal structure, insecticidal activity, molecular docking, and QSAR studies of novel N3-substituted imidacloprid derivatives, J. Agric. Food Chem., 2014, 62, 5429 CrossRef CAS PubMed.
-
(a) M. Tomizawa, K. A. Durkin, I. Ohno, K. I. Nagura, M. Manabe, S. Kumazawa and S. Kagabu, N-Haloacetylimino neonicotinoids: Potency and molecular recognition at the insect nicotinic receptor, Bioorg. Med. Chem. Lett., 2011, 21, 3583 CrossRef CAS PubMed;
(b) M. Tomizawa, S. Kagabu and J. E. Casida, Receptor Structure-Guided Neonicotinoid Design, J. Agric. Food Chem., 2011, 59, 2918 CrossRef CAS PubMed.
- M. Emam, D. R. Abdel-Haleem, S. M. Farag, M. A. El-Ansari and M. Sobeh, Larvicidal activity of pentagalloyl glucose and mangiferin isolated from the waste of mango kernel against Culex pipiens L, Waste Biomass Valorization, 2022, 13(1), 83 CrossRef CAS.
- M.-X. Li, Y.-P. Ma, H.-X. Zhang, H.-Z. Sun, H.-H. Su, S.-J. Pei and Z.-Z. Du, Repellent, larvicidal and adulticidal activities of essential oil from Dai medicinal plant Zingiber cassumunar against Aedes albopictus, Plant Div., 2021, 43, 317e323 Search PubMed.
- D. J. Finney, Probit analysis, Cambridge University Press, Cambridge, 3rd edn, 1971 Search PubMed.
- T. Blacquière, G. Smagghe, C. A. M. van Gestel and V. Mommaerts, Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment, Ecotoxicology, 2012, 21, 973 CrossRef PubMed.
- D. R. Abdel Haleem, N. H. El Tablawy, L. A. Alkeridis, S. Sayed, A. M. Saad, M. T. El-Saadony and S. M. Farag, Screening and evaluation of different algal extracts and prospects for controlling the disease vector mosquito Culex pipiens L., Saudi J. Biol. Sci., 2022, 29, 933 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Doaa R. Abdel Haleem
*a,
Doaa R. Abdel Haleem *b,
Hisham S. M. Abd-Rabbohc,
Nourhan M. Gada,
Wael S. I. Abou-Elmagd
*b,
Hisham S. M. Abd-Rabbohc,
Nourhan M. Gada,
Wael S. I. Abou-Elmagd a and
David S. A. Haneen
a and
David S. A. Haneen a
a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption bands. The 1H NMR spectra of 3 and 4 reveal a singlet signal for the methine proton of the CH
O absorption bands. The 1H NMR spectra of 3 and 4 reveal a singlet signal for the methine proton of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group at δ 9.34 and 9.45 ppm, respectively, as well as a singlet signal for the methyl protons of the OCH3 group at δ 3.81 ppm for compound 3 and an exchangeable singlet signal for the NH of the indole ring at δ 11.95 ppm for compound 4. The 1H NMR spectrum of 6 showed an exchangeable singlet signal for NH at δ 12.09 ppm as well as signals for the
N group at δ 9.34 and 9.45 ppm, respectively, as well as a singlet signal for the methyl protons of the OCH3 group at δ 3.81 ppm for compound 3 and an exchangeable singlet signal for the NH of the indole ring at δ 11.95 ppm for compound 4. The 1H NMR spectrum of 6 showed an exchangeable singlet signal for NH at δ 12.09 ppm as well as signals for the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH of the pyridazinone ring at δ 7.13 ppm and methyl protons at δ 2.36 ppm. Their 13C NMR spectra provided additional evidence for the assigned structures. Further, the mass spectra of 3 and 6 exhibited their correct molecular ion and M + 2 peaks, as well as other important fragments (Experimental section).
CH of the pyridazinone ring at δ 7.13 ppm and methyl protons at δ 2.36 ppm. Their 13C NMR spectra provided additional evidence for the assigned structures. Further, the mass spectra of 3 and 6 exhibited their correct molecular ion and M + 2 peaks, as well as other important fragments (Experimental section).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Experimental section). Because of the stability of the aromatic triazole ring, the thiolactim form has a greater abundance. Furthermore, its mass spectrum showed the correct M+ peak at m/z 422 (33.43%) as well as the M + 2 peak at m/z 424 (17.89%), indicating the existence of the chlorine isotope.
3 (Experimental section). Because of the stability of the aromatic triazole ring, the thiolactim form has a greater abundance. Furthermore, its mass spectrum showed the correct M+ peak at m/z 422 (33.43%) as well as the M + 2 peak at m/z 424 (17.89%), indicating the existence of the chlorine isotope.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 439.6)
439.6)



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–Ph analogs with an electron-donating group on the phenyl ring had higher affinity than those with an electron-withdrawing substituent, suggesting that the benzene plane forms a face-to-edge aromatic interaction with the loop D Trp indole, which was confirmed by39 who proved the activity of certain heterocyclic derivatives containing a pyridine moiety (aromatic heterocycle) against Aphis craccivora Koch.
O)–Ph analogs with an electron-donating group on the phenyl ring had higher affinity than those with an electron-withdrawing substituent, suggesting that the benzene plane forms a face-to-edge aromatic interaction with the loop D Trp indole, which was confirmed by39 who proved the activity of certain heterocyclic derivatives containing a pyridine moiety (aromatic heterocycle) against Aphis craccivora Koch.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide orange crystals, mp. 250–252 °C. IR (KBr, ν, cm−1): 3300 (NH), 1700 (C
1) to provide orange crystals, mp. 250–252 °C. IR (KBr, ν, cm−1): 3300 (NH), 1700 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone), 1658 (C
O ketone), 1658 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1607 (C
O amide), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 9.34 (s, 1H, CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 9.34 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.64 (s, 1H, C4–H quinoline), 7.92 (s, 1H, CH
N), 8.64 (s, 1H, C4–H quinoline), 7.92 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.83–6.99 (m, 13H, Ar–H), 3.81 (s, 3H, OCH3), 3.35 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 160.87 (C
), 7.83–6.99 (m, 13H, Ar–H), 3.81 (s, 3H, OCH3), 3.35 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 160.87 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 156.57 (C
O), 156.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.27 (C
O), 143.27 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 140.05 (C
N), 140.05 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 139.15, 132.09, 130.88, 130.19, 129.34, 129.11, 128.37, 128.33, 127.91, 127.70, 126.27, 125.44, 122.43, 119.41, 115.19, 114.46 (Ar–C + CH
N), 139.15, 132.09, 130.88, 130.19, 129.34, 129.11, 128.37, 128.33, 127.91, 127.70, 126.27, 125.44, 122.43, 119.41, 115.19, 114.46 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 63.08 (CH3), 40.33 (CH2). MS, m/z (%): 486 (M + 2, 12.09), 484 (M+, 17.5), 371 (17.46), 367 (23.37), 292 (17.15), 275 (94.52), 183 (15.09), 141 (18.55), 103 (27.02), 86 (33.7), 76 (70.97), 71 (73.14), 63 (41.18), 43 (96.2), 40 (100). Anal. calcd for C28H22ClN3O3 (483.95): C, 69.49; H, 4.58; N, 8.68. Found: C, 69.32; H, 4.44; N, 8.70%.
), 63.08 (CH3), 40.33 (CH2). MS, m/z (%): 486 (M + 2, 12.09), 484 (M+, 17.5), 371 (17.46), 367 (23.37), 292 (17.15), 275 (94.52), 183 (15.09), 141 (18.55), 103 (27.02), 86 (33.7), 76 (70.97), 71 (73.14), 63 (41.18), 43 (96.2), 40 (100). Anal. calcd for C28H22ClN3O3 (483.95): C, 69.49; H, 4.58; N, 8.68. Found: C, 69.32; H, 4.44; N, 8.70%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone), 1650 (C
O ketone), 1650 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1634 (C
O amide), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.14 (br.s, 1H, NH, exchangeable), 11.95 (br.s, 1H, NH, exchangeable), 9.45 (s, 1H, CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.14 (br.s, 1H, NH, exchangeable), 11.95 (br.s, 1H, NH, exchangeable), 9.45 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.64 (s, 1H, C4–H quinoline), 7.97–6.96 (m, 15H, Ar–H + CH
N), 8.64 (s, 1H, C4–H quinoline), 7.97–6.96 (m, 15H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.55 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 166.61 (C
), 3.55 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 166.61 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.18 (C
O), 161.18 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 153.78 (C
O), 153.78 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 149.01 (C
N), 149.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 139.74, 138.92, 137.22, 132.52, 131.57, 130.41, 129.61, 129.29, 128.91, 128.55, 128.14, 126.86, 125.07, 124.09, 122.68, 122.24, 121.77, 120.66, 119.58, 115.09, 112.06, 111.40 (Ar–C + CH
N), 139.74, 138.92, 137.22, 132.52, 131.57, 130.41, 129.61, 129.29, 128.91, 128.55, 128.14, 126.86, 125.07, 124.09, 122.68, 122.24, 121.77, 120.66, 119.58, 115.09, 112.06, 111.40 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 40.33 (CH2). Anal. calcd for C29H21ClN4O2 (492.96): C, 70.66; H, 4.29; N, 11.37. Found: C, 70.49; H, 4.11; N, 11.40%.
), 40.33 (CH2). Anal. calcd for C29H21ClN4O2 (492.96): C, 70.66; H, 4.29; N, 11.37. Found: C, 70.49; H, 4.11; N, 11.40%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and found to be the pyridazinone 6, which was identical in all respects (IR, mp, mixed mp, and TLC) with an authentic sample prepared by boiling the hydrazide 2 in n-butanol for 3 h.43
1) and found to be the pyridazinone 6, which was identical in all respects (IR, mp, mixed mp, and TLC) with an authentic sample prepared by boiling the hydrazide 2 in n-butanol for 3 h.43![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide), 1608 (C
O amide), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 8.67 (s, 1H, C4–H quinoline), 7.92–7.21 (m, 10H, Ar–H + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 12.09 (br.s, 1H, NH, exchangeable), 8.67 (s, 1H, C4–H quinoline), 7.92–7.21 (m, 10H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 7.13 (s, 1H, C5–H pyridazine), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6): 170.95 (C
), 7.13 (s, 1H, C5–H pyridazine), 2.36 (s, 3H, CH3). 13C NMR (75 MHz, DMSO-d6): 170.95 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.52 (C
O), 168.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 161.01 (C
O), 161.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 146.35, 140.56, 139.14, 131.97, 130.29, 129.43, 129.06, 128.35, 127.22, 127.01, 126.37, 126.33, 122.33, 119.45, 115.10 (Ar–C + CH
N), 146.35, 140.56, 139.14, 131.97, 130.29, 129.43, 129.06, 128.35, 127.22, 127.01, 126.37, 126.33, 122.33, 119.45, 115.10 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 24.57 (CH3). MS, m/z (%): 392 (M + 2, 43.80), 390 (M+, 15.11), 361 (40.57), 293 (16.71), 250 (10.17), 234 (22.31), 225 (49.47), 183 (37.24), 161 (42.10), 123 (41.57), 111 (51.43), 87 (33.95), 76 (64.72), 69 (40.25), 41 (100). Anal. calcd for C22H16ClN3O2 (389.84): C, 67.78; H, 4.14; N, 10.78. Found: C, 67.60; H, 4.01; N, 10.81%.
), 24.57 (CH3). MS, m/z (%): 392 (M + 2, 43.80), 390 (M+, 15.11), 361 (40.57), 293 (16.71), 250 (10.17), 234 (22.31), 225 (49.47), 183 (37.24), 161 (42.10), 123 (41.57), 111 (51.43), 87 (33.95), 76 (64.72), 69 (40.25), 41 (100). Anal. calcd for C22H16ClN3O2 (389.84): C, 67.78; H, 4.14; N, 10.78. Found: C, 67.60; H, 4.01; N, 10.81%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford off-white crystals, mp. 270–272 °C. IR (KBr, ν, cm−1): 3162 (NH), 1656 (C
1) to afford off-white crystals, mp. 270–272 °C. IR (KBr, ν, cm−1): 3162 (NH), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1607 (C
O), 1607 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.20 (br.s, 1H, SH, exchangeable), 11.85 (br.s, 1H, NH, exchangeable), 7.83–7.11 (m, 11H, Ar–H + C4–H quinoline + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.20 (br.s, 1H, SH, exchangeable), 11.85 (br.s, 1H, NH, exchangeable), 7.83–7.11 (m, 11H, Ar–H + C4–H quinoline + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.77 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 161.70 (C
), 3.77 (s, 2H, CH2). 13C NMR (75 MHz, DMSO-d6): 161.70 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.69 (C
O), 160.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 143.93 (C
O), 143.93 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 140.81 (C
N), 140.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 138.07, 137.46, 134.95, 129.63, 129.60, 129.06, 128.88, 128.50, 128.31, 127.51, 125.64, 121.76, 119.28, 114.86 (Ar–C + CH
N), 138.07, 137.46, 134.95, 129.63, 129.60, 129.06, 128.88, 128.50, 128.31, 127.51, 125.64, 121.76, 119.28, 114.86 (Ar–C + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 30.02 (CH2). MS, m/z (%): 389 (M+, 34.88), 388 (M −- 1, 11.97), 361 (M–CO, 41.43), 306 (40.71), 299 (45.09), 286 (25.33), 261 (30.7), 249 (34.31), 243 (37.62), 205 (27.49), 155 (18.41), 75 (100), 56 (67.43), 56 (67.43), 44 (33.32). Anal. calcd for C21H15N3O3S (389.43): C, 64.77; H, 3.88; N, 10.79. Found: C, 64.61; H, 3.93; N, 10.76%.
), 30.02 (CH2). MS, m/z (%): 389 (M+, 34.88), 388 (M −- 1, 11.97), 361 (M–CO, 41.43), 306 (40.71), 299 (45.09), 286 (25.33), 261 (30.7), 249 (34.31), 243 (37.62), 205 (27.49), 155 (18.41), 75 (100), 56 (67.43), 56 (67.43), 44 (33.32). Anal. calcd for C21H15N3O3S (389.43): C, 64.77; H, 3.88; N, 10.79. Found: C, 64.61; H, 3.93; N, 10.76%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford yellow crystals, mp. 320–322 °C (decomp.). IR (KBr, ν, cm−1): 3160 (NH), 1656 (C
1) to afford yellow crystals, mp. 320–322 °C (decomp.). IR (KBr, ν, cm−1): 3160 (NH), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609 (C
O), 1609 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.15 (br.s, 1H, SH, exchangeable), 11.81 (br.s, 1H, NH, exchangeable), 7.80–7.14 (m, 11H, Ar–H + C4–H quinoline + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 13.15 (br.s, 1H, SH, exchangeable), 11.81 (br.s, 1H, NH, exchangeable), 7.80–7.14 (m, 11H, Ar–H + C4–H quinoline + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.76 (s, 2H, CH2). Anal. calcd for C21H15N5OS (325.39): C, 65.44; H, 3.92; N, 18.17. Found: C, 65.31; H, 3.85; N, 18.20%.
), 3.76 (s, 2H, CH2). Anal. calcd for C21H15N5OS (325.39): C, 65.44; H, 3.92; N, 18.17. Found: C, 65.31; H, 3.85; N, 18.20%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 11.96 (br.s, 1H, NH, thiolactam form, exchangeable), 8.05 (s, 1H, C4–H quinoline), 7.77–7.12 (m, 10H, Ar–H + CH
N). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 11.96 (br.s, 1H, NH, thiolactam form, exchangeable), 8.05 (s, 1H, C4–H quinoline), 7.77–7.12 (m, 10H, Ar–H + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.71 (s, 1H, SH, thiolactim form, exchangeable), 4.43 (br.s, 2H, NH2, exchangeable), 3.27 (s, 2H, CH2). MS, m/z (%): 424 (M + 2, 17.89), 422 (M+, 33.43), 419 (40.37), 393 (22.67), 354 (34.02), 331 (49.44), 315 (75.39), 300 (46.12), 286 (45.06), 246 (40.65), 221 (39.56), 193 (40.51), 142 (61.61), 97 (39.40), 69 (57.66), 43 (100). Anal. calcd for C21H16ClN5OS (421.90): C, 59.78; H, 3.82; N, 16.60. Found: C, 59.64; H, 3.71; N, 16.70%.
), 6.71 (s, 1H, SH, thiolactim form, exchangeable), 4.43 (br.s, 2H, NH2, exchangeable), 3.27 (s, 2H, CH2). MS, m/z (%): 424 (M + 2, 17.89), 422 (M+, 33.43), 419 (40.37), 393 (22.67), 354 (34.02), 331 (49.44), 315 (75.39), 300 (46.12), 286 (45.06), 246 (40.65), 221 (39.56), 193 (40.51), 142 (61.61), 97 (39.40), 69 (57.66), 43 (100). Anal. calcd for C21H16ClN5OS (421.90): C, 59.78; H, 3.82; N, 16.60. Found: C, 59.64; H, 3.71; N, 16.70%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 h).51 C. pipiens breeding went through three aquatic stages: egg, larva, and pupa, in addition to flying adult. Egg rafts were placed in enamel bowls containing dechlorinated water. The newly hatched larvae were fed on a mixture of dog biscuits and yeast powder (in a ratio of 3
14 h).51 C. pipiens breeding went through three aquatic stages: egg, larva, and pupa, in addition to flying adult. Egg rafts were placed in enamel bowls containing dechlorinated water. The newly hatched larvae were fed on a mixture of dog biscuits and yeast powder (in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The pupae were collected daily and transferred to glass jars containing dechlorinated water and then placed in 25 × 25 × 25 screened cages for adult breeding. Adults were provided daily with cotton pads soaked in a 10% sugar solution. Females were fed pigeons for a blood meal.55 The field strain of C. pipiens larvae was collected from Mansoura Canal, Abou Rawash, Giza, Egypt.
1). The pupae were collected daily and transferred to glass jars containing dechlorinated water and then placed in 25 × 25 × 25 screened cages for adult breeding. Adults were provided daily with cotton pads soaked in a 10% sugar solution. Females were fed pigeons for a blood meal.55 The field strain of C. pipiens larvae was collected from Mansoura Canal, Abou Rawash, Giza, Egypt.


