Open Access Article
Open Access ArticleSolid-state molecular oxygen activation using ball milling and a piezoelectric material for aerobic oxidation of thiols†
Gefei Wang,
Jiajia Jia,
Yu He,
Diandian Wei,
Mingyu Song,
Lei Zhang,
Ganzhong Li,
Heng Li and
Bingxin Yuan
and
Bingxin Yuan *
*
Green Catalysis Center, College of Chemistry, Zhengzhou University, Zhengzhou, Henan 450001, China. E-mail: bxyuan@zzu.edu.cn
First published on 22nd June 2022
Abstract
The agitation of BaTiO3 via ball milling converts mechanical energy into electrical energy, leading to the reduction of molecular oxygen via a single electron transfer pathway analogous to the photocatalytic reaction. This mechanoredox strategy for the oxidative coupling of thiols could eliminate waste and develop a recyclable methodology to accomplish organic transformations in a greener fashion, exhibiting promising potential for large-scale chemical manufacturing.
Mechanochemical synthesis using ball milling has become an emerging field of research in organic synthesis as reactions under ball milling could be performed more sustainably and practically.1–8 The mechanochemical organic transformations exhibit many advantages over liquid-phase reactions in terms of the elimination of environmentally harmful organic solvents, ambient reaction temperatures, milder reaction conditions, shorter reaction times, and simpler work-up procedures.
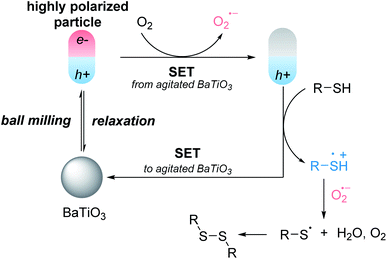
BaTiO3 as one of the most typical piezoelectric perovskite oxides has a dipole moment and produces a polarized electric field to drive the separation and migration of bulk charges to the surface under stress.9,10 Li's group applied the piezo-electrochemical effect (conversion of mechanical energy to chemical energy) of BaTiO3 for water splitting under ultrasonic vibration.11 BaTiO3 was also used to realize the decolorization of Rhodamine B in a vibration-catalytic process.12 BaTiO3 nanoparticles could reduce Cu(II) catalyst with ultrasound as an external stimulus and thereby generated an ATPR activator to initiate radical polymerization.13,14 Although effective, those strategies are limited to the use of acoustic cavitation by ultrasonication in solution. Recently, Ito's group developed a mechanoredox system to activate small organic molecules, in which the agitation of piezoelectric materials in response to ball milling generates temporarily highly polarized particles and thus serves as a charge-transfer catalyst. The electrons could be transferred from the distorted piezoelectric materials to substrates, generating radical intermediates. They applied this mechanoredox system to arylation or borylation reactions of aryl diazonium salts (Fig. 1A),15 and C–H trifluoromethylation of aryl compounds.16 In a more recent example, mechanical ball milling could be harnessed to induce electrical polarization of BaTiO3, leading to reduction of Cu(II) catalyst precursor into the active ligand-stabilized Cu(I) species for atom transfer radical cyclizations.17 Mechanically ball milling of BaTiO3 could merge electric and mechanical activation modes, extending the applicability of BaTiO3 not only as additives but also as a charge-transfer catalyst. Although the mechanical stimulation of piezoelectric BaTiO3 represents an attractive strategy to reduce the environmental impact of organic synthesis and leads to practical applications in industrial manufacturing, the studies in this realm are still highly limited.18
 | ||
| Fig. 1 Radical generation using ball milling and piezoelectric catalyst in the solid-state for organic synthesis. | ||
Organodisulfides possessing S–S bonds are commonly encountered moieties of great significance and synthetic interest.19 Applications of organic disulfides range from synthetic tools, anti-oxidants, pharmaceuticals, agricultural chemicals, and rubber vulcanization reagents. S–S bonds are widely found in naturally bioactive products and protein structures. The formation of disulfide units is essential for protein folding and the stability of secondary structures. Considering the great importance of disulfide compounds, extensive efforts have been made to assess the oxidative coupling of thiols with oxidants, such as molecular oxygen, peroxides, metal oxidants, halogens, halogenating agents, and so on. Among those, the aerobic oxidation of thiols to produce disulfides is preferred since molecular oxygen is much cheaper and more environmentally benign. The aerobic oxidative coupling of S–H bonds has been realized in a solvent by transition metal catalysts20–23 and metal-free catalysts.24–26
Inspired by piezoelectricity of BaTiO3 under ball milling for the generation of radical intermediates, we set to investigate whether this piezoelectric charge-transfer catalyst could be able to activate molecular oxygen and generate thiyl radical (Fig. 1B). If achievable, the agitated BaTiO3 under ball milling could enable the development of a mechanoredox system for oxidative coupling reaction with milder reaction conditions, shorter reaction times, solvent-free system, operational simplicity, recyclability of piezoelectric charge-transfer catalyst. While we were preparing our manuscript, Cao's group reported the mechanochemical synthesis of 1,2-diketoindolizines from indolizines and epoxides using BaTiO3 as a piezoelectric catalyst.27 In the reaction mechanism, they proposed that the highly polarized piezoelectric particles led to radical intermediate and superoxide radical anion, which could be supportive evidence for our hypothesis.
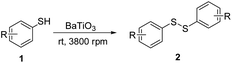
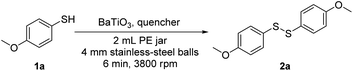
To explore this hypothesis, we applied the mechanoredox system to the aerobic oxidative homocoupling of thiols to synthesize disulfides under ball milling. 4-Methoxybenzenethiol was used as the model substrate, and the model reaction was carried out in an MSK-SFM-12M mixer mill (2 mL PE milling jar with 4 mm stainless-steel balls) in the presence of commercially available BaTiO3 under air. Initially, we used zirconia balls as milling media, unfortunately, only 15% product was observed (Table 1, entry 1). We speculated that the lightweight of zirconia balls subjected BaTiO3 to lower-impact mechanical collisions thus failed to provide sufficient mechanical impact to activate the BaTiO3. This result also suggests ball milling is not only mixing reactants but also agitating the piezoelectric material. Next, we switched the milling media to 4 mm stainless-steel balls. To our delight, the desired disulfide 2a was produced in quantitative yield in 6 min (entry 2). This result demonstrated that a piezoelectric potential to initiate the oxidative coupling reaction was generated by the mechanical agitation under suitable ball milling conditions. Other typical piezoelectric materials, such as Pb(Zr, Ti)O3 (PZT) ceramic powder, could also convert the starting thiol into the target product in 95% yield (entry 3). Considering its low cost, high efficiency, and environmental benignity, BaTiO3 was used as the optimal charge-transfer catalyst for further exploration. In addition, when anhydrous Na2SO4 was used as solid grinding aid while no BaTiO3 was added, the product yield dropped substantially (entry 4). Once we change the solid grinding aid to NaCl, the product yield decreased to 16% (entry 5). When the reaction was performed in the absence of piezoelectric material, product 3a was formed in low yield (entry 6). These results illustrate that the piezoelectric material is necessary for the transformation of thiols into the corresponding disulfides. The higher yield resulting from anhydrous Na2SO4 compare to that from NaCl should be caused by its water absorptivity, since anhydrous Na2SO4 is a typical drying agent and the byproduct of this oxidative disulfide formation is water. To test and verify the effect of molecular oxygen on the disulfide formation, we performed the model reaction under a nitrogen atmosphere as a control experiment (entry 7). The only trace amount of disulfide was observed by GCMS, which might be the impurity in the commercially purchased starting material or result from the presence of traces of air. We tried commercially available BaTiO3 with different particle sizes (0.6–1 μm, 200 nm). All particle sizes led to product 2a in quantitative yields with 6 min, showing no considerable size effect on the reaction performance (entries 8, 9). Even though there was no big difference in their prices, BaTiO3 (<3 μm) is the cheapest. Thus, we choose BaTiO3 (<3 μm) for the following experiments. When the reaction was carried out in CH3CN (0.1 M) with an air balloon under stirring for 20 min, only a trace amount of product 2a was observed (entry 10). This result states that mechanical agitation of BaTiO3 induced by ball milling is essential for the piezoelectric potential that is sufficient to activate molecular oxygen and thiol. The acoustic cavitation by ultrasonication in solution has been used to activate piezoelectric materials for catalysis.28–31 When the reaction was performed in acetone (0.1 M) with an air ballon and ultrasonication, the target product 2a was obtained in 20% yield (entry 11). This result illustrates that the piezoelectric potential generated by ball milling has a different reactivity profile compared to the solution version using ultrasonic agitation. Conclusively, these experimental results are consistent with our theory that the agitation of BaTiO3 under mechanochemical ball milling conditions could activate molecular oxygen for a successful oxidative transformation of thiols into disulfides.
| Entry | Piezoelectric material | Milling ball | Yield [%] |
|---|---|---|---|
| a 1a (0.2 mmol) and BaTiO3 (<3 μm, 0.2 mmol) in a 2 mL PE milling jar, five 4 mm stainless-steel balls, 3800 rmp.b Isolated yields.c Five 4 mm ZrO2 balls, 3800 rpm.d Anhydrous Na2SO4 (1.9 mmol) as solid grinding aid in the absence of BaTiO3.e NaCl (1.9 mmol) as solid grinding aid in the absence of BaTiO3.f The reaction was performed under N2 in a glovebox.g The reaction was carried out in CH3CN (0.1 M) with a stirring bar at room temperature for 20 min with an air balloon.h The reaction was carried out in acetone (0.1 M) under ultrasonic agitation at room temperature for 20 min with an air balloon. | |||
| 1c | BaTiO3 (<3 μm) | ZrO2 | 15 |
| 2 | BaTiO3 (<3 μm) | Steel | >99 |
| 3 | PZT | Steel | 95 |
| 4d | None (Na2SO4) | Steel | 22 |
| 5e | None (NaCl) | Steel | 16 |
| 6 | None | Steel | 12 |
| 7f | BaTiO3 (<3 μm) | Steel | Trace |
| 8 | BaTiO3 (0.6–1 μm) | Steel | 99 |
| 9 | BaTiO3 (200 nm) | Steel | 99 |
| 10g | BaTiO3 (<3 μm) | Steel | Trace |
| 11h | BaTiO3 (<3 μm) | Steel | 20 |
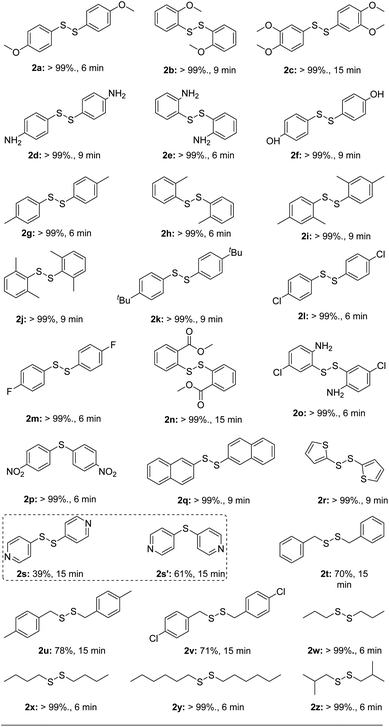
With the optimal reaction condition in hand, we started to explore the substrate scope of this mechanochemically aerobic oxidation with a wide range of aryl- and alkyl thiols (Table 2). With regard to arylthiols, a large variety of electron-donating groups on the phenyl ring, such as methoxyl groups at the para-, meta-, or ortho-position (2a–c), amino groups at the para- or ortho-position (2d, 2e), hydroxy (2f), methyl groups at the para-, meta-, or ortho-position (2g–j), t-butyl (2k), and electron-withdrawing groups, including chloro (2l, 2o), fluoro (2m), carboxymethyl (2n), could be converted into the target disulfides in quantitative yields in 6–15 min. Interestingly, when 4-nitrobenzenethiol was used as the substrate, diarylsulfide 2p was produced in quantitative yield in 6 min instead of the corresponding disulfide. 2-Naphthalenethiol with extended π-framework furnished the desired disulfide 2q in 6 min. In the case of heteroaryl thiols, the reaction of thiophene-2-thiol also underwent mechanochemical oxidative coupling to afford pure product 2r successfully in a very short reaction time. However, when we used 4-pyridinethiol as substrate, a mixture of the corresponding disulfide 2s and diarylsulfide 2s′ were produced in a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 while the starting material conversion was complete. Benzyl mercaptan and derivatives could be wholly converted in 15 min resulting in good yields as well (2t–v). We also obtained successful results with alkyl thiols as substrates in 6 min.
6 while the starting material conversion was complete. Benzyl mercaptan and derivatives could be wholly converted in 15 min resulting in good yields as well (2t–v). We also obtained successful results with alkyl thiols as substrates in 6 min.
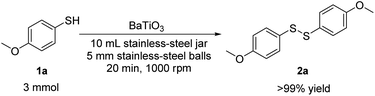
To be highlighted in most cases, the reactions were clean, fast, and quantitatively yielding with no byproduct. Once the reaction was accomplished (monitored by TLC/GCMS), the reaction mixture was washed by CH2Cl2, followed by evaporation of the solvent under vacuum. Such a simple work-up procedure was able to afford pure products (purity identified by NMR) without further purification by column chromatography on silica gel. Since the piezocatalyst BaTiO3 could be easily recovered by filtration, we tried the reuse of BaTiO3 for the aerobic oxidative coupling of 4-methoxybenzenethiol 1a. The piezoelectric BaTiO3 could be reused 20 times with no decrease in product yield or increase in reaction time (Fig. 2), indicating the catalytic activity of BaTiO3 was not attenuated at all. It is reasonable to assume that the piezoelectric material could be recycled more times before the recovery loss of BaTiO3 sets a limit to the recycling times. However, we found that the product yield started to drop rapidly after the 21st run. We did an SEM analysis of the piezoelectric catalyst to find out if the ball milling process would affect the size and morphology of BaTiO3 (Fig. S1–S4,† see the ESI), which might affect the reaction performance as well. Compared to the commercially available BaTiO3 (<3 μm) before use (Fig. S1†), the SEM image of recycled BaTiO3 from the 15th run showed that the particle size distribution got narrower along with the milling process. Most amorphous particles presented in the commercially available BaTiO3 disappeared during the milling process, which might be a result of agglomeration and recrystallization under the current ball milling conditions. The SEM image of recycled BaTiO3 from the 24th run showed that the BaTiO3 particles started to agglomerate into bigger particle sizes, which might cause the product yield reduction (the product yield of the 24th run is 90%). After 30th recycle run, the size and morphology of BaTiO3 showed a big difference, demonstrating the occurrence of BaTiO3 recrystallization during the milling process. Thus, the recovery loss and morphology change caused by the milling process of BaTiO3 would both affect the recycle limit of the piezoelectric material. Besides, we performed a gram-scale aerobic oxidative coupling of 1a in an AM100S mixer mill (10 mL stainless-steel milling jar with five 5 mm stainless-steel milling balls). During the reaction, we opened the mill jar every five minutes to ensure there was enough molecular oxygen for the reaction. To our delight, this gram-scale synthesis successfully afforded the desired product 2a in quantitative yield in 20 min (Scheme 1). The reaction is as clean and efficient as the 0.2 mmol-scale. Hence, we have developed a highly sufficient and recyclable strategy to eliminate waste and simplify the workup procedure, which is an essential aspect for performing the reaction in a green fashion.
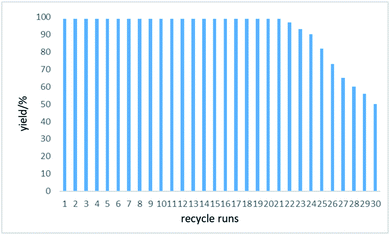 | ||
| Fig. 2 Recycling efficiency of piezocatalyst BaTiO3 for the oxidative coupling of 4-methoxybenzenethiol 1a as measured by yields of the corresponding disulfide 2a. Each run was conducted with 0.2 mmol 1a under the optimal reaction condition (entry 2, Table 1). | ||
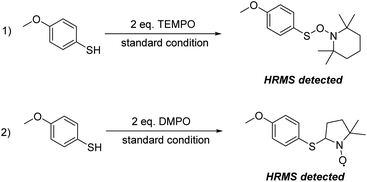
To probe the reaction mechanism, several mechanistic experiments were conducted with various scavengers (Table 3). When the reaction was performed in the presence of (2,2,6,6-tetramethyl piperidine-1-yl)oxidanyl (TEMPO) or 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a radical scavenger (entries 1 and 2), the product yields were sufficiently suppressed, indicating radical intermediates were involved in the reaction pathway. Then, we analyzed both reaction mixtures via HRMS (Scheme 2). The corresponding adducts of the thiyl radical with TEMPO or DMPO were identified respectively, illustrating that thiyl radical was produced during the reaction process. These results demonstrate our hypothesis that the piezoelectric material BaTiO3 under ball milling is able to generate a thiyl radical. When the reaction of benzoquinone as superoxide radical anion scavenger with 1a was conducted under the standard reaction condition, the product yield decreased to 10% (entry 3), suggesting superoxide radical anion takes part in the reaction.
Based on previous reports15,16,27 and mechanistic studies, a plausible reaction mechanism is proposed in Scheme 3. When subjected to ball milling, the BaTiO3 particles generate a temporary electrochemical potential that would be suitably persistent for the piezoelectric reduction of molecular oxygen to produce a superoxide radical anion via a single electron transfer (SET) mechanism analogous to the photoredox system. Subsequently, the thiol substrate could be oxidized by the hole in the agitated BaTiO3, generating a cationic-radical-type intermediate. Then, the superoxide radical anion abstracts a proton from the cationic-radical intermediate to form the corresponding thiyl radical. The homocoupling of thiyl radical resulted in the corresponding disulfide as the final product.
Conclusions
In conclusion, we described here the application of piezo-electric-effect for the activation of molecular oxygen and generation of thiyl radicals. This piezochemically oxidative coupling of thiyl radicals led to the quantitative production of the corresponding disulfides in 6 min. Mechanistic experiments on the mechanochemically oxidative coupling with BaTiO3 as piezocatalyst showed that superoxide radical anion and thiyl radicals were involved in the reaction process. This solid-state mechanoredox oxidative coupling of thiols with air as the sole oxidant avoids the usage of any solvents or catalysts. In most cases, the pure product could be collected via simple operation, while no further purification by gel chromatography on silica gel is needed. The piezocatalyst BaTiO3 could be recycled 20 times with no loss in reactivity or product yields. Compared to solution methods, the operational simplicity, wide substrate tolerance, and short reaction time of this mechanochemical technique offer promising potential in industrial manufacturing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Henan Province (No. 222300420528), and the Key-Area Research and Development Program of Guangdong Province (No. 2020B010188003).Notes and references
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- D. Tan and T. Friščić, Eur. J. Org. Chem., 2018, 2018, 18–33 CrossRef CAS.
- T. Stolar and K. Užarević, CrystEngComm, 2020, 22, 4511–4525 RSC.
- O. V. Lapshin, E. V. Boldyreva and V. V. Boldyrev, Russ. J. Inorg. Chem., 2021, 66, 433–453 CrossRef CAS.
- F. Shen, X. Xiong, J. Fu, J. Yang, M. Qiu, X. Qi and D. C. W. Tsang, Renewable Sustainable Energy Rev., 2020, 130, 109944 CrossRef CAS.
- A. A. L. Michalchuk, E. V. Boldyreva, A. M. Belenguer, F. Emmerling and V. V. Boldyrev, Front. Chem., 2021, 9, 359 Search PubMed.
- D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC.
- F. Chen, H. Huang, L. Guo, Y. Zhang and T. Ma, Angew. Chem., Int. Ed., 2019, 58, 10061–10073 CrossRef CAS PubMed.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rödel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed.
- K.-S. Hong, H. Xu, H. Konishi and X. Li, J. Phys. Chem. Lett., 2010, 1, 997–1002 CrossRef CAS.
- X. Xu, Z. Wu, L. Xiao, Y. Jia, J. Ma, F. Wang, L. Wang, M. Wang and H. Huang, J. Alloys Compd., 2018, 762, 915–921 CrossRef CAS.
- H. Mohapatra, M. Kleiman and A. P. Esser-Kahn, Nat. Chem., 2017, 9, 135–139 CrossRef CAS.
- Z. Wang, X. Pan, J. Yan, S. Dadashi-Silab, G. Xie, J. Zhang, Z. Wang, H. Xia and K. Matyjaszewski, ACS Macro Lett., 2017, 6, 546–549 CrossRef CAS PubMed.
- K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500 CrossRef CAS PubMed.
- Y. Pang, J. W. Lee, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2020, 59, 22570–22576 (Angew. Chem., Int. Ed., 2020, 50, 22759–22765) CrossRef CAS PubMed.
- C. Schumacher, J. G. Hernández and C. Bolm, Angew. Chem., Int. Ed., 2020, 59, 16357–16360 (Angew. Chem., Int. Ed., 2020, 132, 16499–16502) CrossRef CAS PubMed.
- S. Tu, Y. Guo, Y. Zhang, C. Hu, T. Zhang, T. Ma and H. Huang, Adv. Funct. Mater., 2020, 30, 2005158 CrossRef CAS.
- F. Dénès, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef PubMed.
- Y. Dou, X. Huang, H. Wang, L. Yang, H. Li, B. Yuan and G. Yang, Green Chem., 2017, 19, 2491–2495 RSC.
- H. Huang, J. Ash and J. Y. Kang, Org. Biomol. Chem., 2018, 16, 4236–4242 RSC.
- S. Molaei and M. Ghadermazi, Appl. Organomet. Chem., 2020, 34, e5328 CrossRef CAS.
- R. Gaur, M. Yadav, R. Gupta, G. Arora, P. Rana and R. K. Sharma, ChemistrySelect, 2018, 3, 2502–2508 CrossRef CAS.
- M. Oba, K. Tanaka, K. Nishiyama and W. Ando, J. Org. Chem., 2011, 76, 4173–4177 CrossRef CAS PubMed.
- T. J. Trivedi, K. S. Rao, T. Singh, S. K. Mandal, N. Sutradhar, A. B. Panda and A. Kumar, ChemSusChem, 2011, 4, 604–608 CrossRef CAS PubMed.
- L. Yang, S. Li, Y. Dou, S. Zhen, H. Li, P. Zhang, B. Yuan and G. Yang, Asian J. Org. Chem., 2017, 6, 265–268 CrossRef CAS.
- Y. Wang, Z. Zhang, L. Deng, T. Lao, Z. Su, Y. Yu and H. Cao, Org. Lett., 2021, 23, 7171–7176 CrossRef CAS PubMed.
- Z. Wang, J. Ayarza and A. P. Esser-Kahn, Angew. Chem., Int. Ed., 2019, 131, 12151–12154 (Angew. Chem., Int. Ed., 2019, 58, 12023–12026) CrossRef.
- H. Mohapatra, J. Ayarza, E. C. Sanders, A. M. Scheuermann, P. J. Griffin and A. P. Esser-Kahn, Angew. Chem., Int. Ed., 2018, 57, 11208–11212 (Angew. Chem., 2018, 130, 11378–11382) CrossRef CAS PubMed.
- H. Mohapatra, M. Kleiman and A. P. Esser-Kahn, Nat. Chem., 2017, 9, 135–139 CrossRef CAS.
- I. Zaborniak and P. Chmielarz, Materials, 2019, 12, 3600 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, and 1H and 13C NMR spectra. See https://doi.org/10.1039/d2ra02255a |
| This journal is © The Royal Society of Chemistry 2022 |