Open Access Article
Open Access ArticleThermostable bacterial laccase for sustainable dyeing using plant phenols
Varsha
Panwar
 a,
Bipasa
Dey
a,
Javed Nabibaksha
Sheikh
b and
Tanmay
Dutta
a,
Bipasa
Dey
a,
Javed Nabibaksha
Sheikh
b and
Tanmay
Dutta
 *a
*a
aEnzyme Technology Laboratory, Department of Chemistry, Indian Institute of Technology Delhi, MS 731, Hauz Khas, New Delhi 110016, India. E-mail: dtanmay@chemistry.iitd.ac.in; Fax: +91 11 2658 1102; Tel: +91 11 2659 1508
bDepartment of Textile Technology, Indian Institute of Technology Delhi, Hauz Khas, New Delhi 110016, India
First published on 21st June 2022
Abstract
Laccase is regarded as an efficacious eco-friendly enzyme in various industries. Thus, various laccases have been explored to mitigate the environmental effects of conventional industrial processing; however, the prospects of laccase in hair dyeing have not been thoroughly explored to date. On account of the adverse environmental and health-related issues posed by chemical hair dyeing, laccase as a natural alternative in dyeing hair has recently gained attention. In this study, we executed hair dyeing with different colours and shades of hair dyes developed from natural plant phenols, including ferulic acid, gallic acid, catechol, and syringaldehyde, catalysed by a novel thermostable bacterial laccase (LacT) from Brevibacillus agri. The dyed hair was characterised in terms of its colourimetric parameters (L*, a*, and b*), colour strength (K/S), reflectance (R) and colour durability. L* means luminosity and is defined by L* values from 0 (black) to 100 (white). A positive value of a* means red shades and a negative value indicates green shades. A positive value of b* shows yellow shades and a negative value indicates blue shades. Optical microscopy of circular and longitudinal sections of the dyed hair revealed that the laccase-catalysed dyes did not merely stick to the surface; instead, they well-penetrated the hair. Furthermore, the dyeing process did not affect the surface morphology of the dyed hair. The dyed hair also exhibited a desirable range of colour diversity in terms of market-driven demands and showed considerable resistance to fading during shampooing and pH alterations. Post-dyeing, the texture and tensile strength of the dyed hair remained nearly unchanged. Overall, the outcomes suggest that LacT holds high potential to be exploited extensively in the hair dyeing industry as an alternative to chemical hair dyes.
1. Introduction
Laccase has become a focal point in recent decades as a ‘green’ catalyst owing to its potential to act as an oxidoreductase.1 A wide array of reaction types such as crosslinking of monomers, degradation of polymers, ring cleavage of aromatic compounds are catalysed by this multi-copper oxidase. The single-electron oxidation of a plethora of phenolic and non-phenolic substrates by laccase-driven catalysis is typically coupled to the four-electron reduction of oxygen to water.2 Nonphenolic substrates require a small molecule mediator as an electron shuttle to promote their oxidation catalysed by laccase. Unlike lignin and Mn2+-dependent peroxidases, which utilise hydrogen peroxides as substrates, laccases use molecular oxygen as a terminal electron acceptor, and thus have attracted significant attention for eco-friendly applications in various industries. Laccases can proficiently catalyse the polymerisation of phenolic structures, which is initiated by abstracting an electron from phenolic moieties, generating a radical cation, and subsequent intermolecular attachment produces a dimer. Laccase-driven catalysis for an extended time generates polymers. Laccases have been reported to carry out numerous biosynthetic polymerization processes in microorganisms and plants.1,2Laccases are widely distributed in fungi, actinomycetes, bacteria, plants, and insects. Due to their broad substrate specificity, laccases have been extensively explored in diverse biotechnological applications such as delignification in kraft pulping; colouration, degradation and decolourization of textile dyes; bioremediation of xenobiotics and organic pollutants; bio-bleaching; biofuel cells; biosensors and organic synthesis.3,4 Among the various types of laccases, fungal laccases are mainly studied because of their high redox potential. However, fungal laccases have many shortcomings at the industrial scale in terms of growth rate, yield, extremotolerant properties, cost-effectiveness, ease of cloning and expression in the host. Thus, to overcome the limitations of fungal laccases, scientists are examining other sources of laccase for industrial deployment. Recently, bacterial laccases have been proven to be feasible for industrial use owing to their extremotolerant properties. Bacterial laccases show high thermal and pH stability, fast growth rate and maintain the optimum conditions of environment and nutrients via bio-stimulation in contrast to fungal laccases.5
One of the emerging areas of laccase applications is hair dyeing. Currently, in commercial hair dyeing, aromatic amines, particularly, phenylenediamines, p-toluylenediamine, substituted p-diamines, o- or p-aminophenols are used as dye precursors.6,7 These precursors are designated in the category of severe allergens by The American Contact Dermatitis Society and their prolonged exposure may lead to hypersensitivity in animals due to the sudden activation of the immune response.8,9 Numerous findings confirmed that p-phenylenediamine (PPD) is a potent carcinogen and mutagen and it generates intense oxidative stress. Reactive oxygen species showed enhanced intracellular concentration upon PPD oxidation, which consequently triggered DNA damage and cytotoxicity to HaCaT cells.10–12 Moreover, the salts of peroxides and persulphates used as bleaching agents are highly reactive, cause harsh effects in form of the eczema, hypersensitivity, occupational asthma, contact allergy, anaphylaxis, contact urticaria, and rhinitis, and their prolonged exposure leads to dermatitis and hair loss.13–15 They can break the disulphide bonds in hair, causing increased porosity in the cuticle, swelling of the hair shaft and enhanced brittleness.15 H2O2 is the most commonly used oxidant in chemical dyes, which induces oxidative stress and suppresses hair growth.16 Therefore, to circumvent the side-effects of chemical dyeing, laccases, by virtue of their ability for the cross-coupling polymerization of phenolic monomers and high oxidation potential, have gained tremendous attention in hair dyeing.
Unlike chemical processes, enzyme-based reactions are environmentally friendly, energy efficient, and generate less waste. These processes are also shorter. Laccase-based dyeing has several advantages over chemical dyeing such as no requirement of precarious organic solvents and H2O2; mild pH, temperature, and pressure reaction conditions; easy handling; fast results and generation of broad colour diversity in dyes.17–20 In the present study, we examined the effectiveness of a thermostable bacterial laccase from Brevibacillus agri (LacT) in hair dyeing. Herein, we show that LacT is a potential enzyme for generating diverse shades of hair colours through the coupling reactions of natural plant phenols without using H2O2. The LacT-mediated dyed hairs were characterised for colour parameters, reflectance, colour strength, resistance to shampooing and pH variations. Furthermore, studies related to the surface morphology, anatomy, hair breakage and energy dispersive X-ray (EDX) analysis of the dyed hair were also conducted for detailed analysis.
2. Experimental methodology
2.1 Materials
Phenolic substrates including ferulic acid, gallic acid, syringaldehyde (SGA) and catechol were purchased from Tokyo Chemical Industry Co., Ltd. 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) and Coomassie Brilliant Blue G-250 were obtained from Sigma-Aldrich. DEAE cellulose and Sephacryl (S-100) were acquired from Bio-Rad and Pharmacia, respectively. Basic chemicals, namely malt extract, ferrous sulphate, acrylamide, bis-acrylamide, ammonium persulphate, TEMED (N,N,N′,N′-tetramethyl ethylenediamine), sodium dodecyl sulphate (SDS), Tris buffer, ammonium sulphate, glutamic acid, monopotassium phosphate, copper and zinc sulphate, were obtained from Himedia. The centrifugal concentrators were obtained from Amicon Millipore, while the dialysis tubing was from Thermo Fischer Scientific. The bundle of human hair was picked up from a local salon. The collected hairs belonged to the same person. All other chemicals used were of analytical grade.2.2 Microorganism, medium optimization and laccase production
Thermostable extracellular laccase-producing bacteria, Brevibacillus agri (NCBI GenBank accession number SUB7371747 VP-1 MT422060), were previously isolated from hot spring water in our laboratory.21 The stock culture of the bacterial strain was maintained on malt extract agar slants (stored at 4 °C) and glycerol stock (stored at −80 °C) with periodic transfer.Optimization of the medium is the main way to improve the enzyme yield. The composition of nutrients plays a pivotal role in the enzyme production. In this study, we optimized the laccase production considering parameters such as incubation time for enzyme production, source of carbon, nitrogen (organic and inorganic), metal ions, salts and inducers. The effect of various carbon sources [2% (w/v) of glucose, fructose, sucrose, malt extract, and starch] on laccase activity was studied. Similarly, laccase production was investigated under several nitrogen sources [0.5% (w/v) of ammonium sulphate, sodium nitrate, soya peptone, glutamic acid, yeast extract and beef extract]. The sample showing the highest laccase activity was taken as 100%. All experiments were performed in triplicate and the results expressed as an independent variable.
After optimization, B. agri cells were grown in the standardized growth medium [composition: malt extract, 2% (w/v) supplemented with glutamic acid, 0.5% (w/v); ammonium sulphate, 0.1% (w/v); monopotassium phosphate, 0.1% (w/v); yeast extract, 0.1% (w/v); magnesium sulphate, 1 mM; sodium chloride, 0.02% (w/v); manganese sulphate tetrahydrate, 0.001% (w/v); ferric chloride hexahydrate, 0.002% (w/v); zinc sulphate monohydrate, 0.001% (w/v) and CuSO4, 200 µM] at 30 °C for 48 h with shaking at 180 rpm for laccase production. The culture medium was centrifuged at 5000×g for 20 min at 4 °C to eliminate cellular debris. The as-obtained supernatant contained the extracellular laccase.
2.3 Laccase purification and assay
In the first step of purification, a finely powered (NH4)2SO4 precipitation was added to the supernatant to 40% saturation on ice. The precipitated proteins were pelleted by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 20 min at 4 °C. To the obtained centrifugate, additional (NH4)2SO4 powder was added to 80% saturation (40–80% cut) and the solution was left on ice for 2 h for the complete precipitation of proteins. The precipitated proteins were collected as a pellet by centrifugation at 10
000×g for 20 min at 4 °C. To the obtained centrifugate, additional (NH4)2SO4 powder was added to 80% saturation (40–80% cut) and the solution was left on ice for 2 h for the complete precipitation of proteins. The precipitated proteins were collected as a pellet by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 20 min at 4 °C. Both protein pellets were resuspended in 20 mM Tris–HCl buffer (pH 7.0) and dialyzed (10 kDa cut-off) overnight at 4 °C against the same buffer. Laccase activity was examined in both protein fractions and found to be associated with that obtained from the 40–80% (NH4)2SO4 cut. Thus, this fraction was utilised in the subsequent steps for laccase purification. The dialyzed proteins were loaded on a DEAE-cellulose column pre-equilibrated with the same buffer (20 mM Tris–HCl buffer; pH 7.0). The unbound proteins were washed with 20 mM Tris–HCl buffer (pH 7.0) and elution of the bound proteins was accomplished by a gradual increase in NaCl concentration (0–1 M in equilibrating buffer). The fractions containing laccase were pooled, desalted, concentrated, and consecutively applied to a Sephacryl (S-100) column pre-equilibrated with 20 mM Tris–HCl buffer (pH 7.0). Sequentially, the fractions were eluted with the same equilibration buffer and the aliquots exhibiting laccase activity were concentrated, filtered (0.22 µm) and stored at −20 °C for future applications. The protein concentration was quantitated by the Bradford method.22
000×g for 20 min at 4 °C. Both protein pellets were resuspended in 20 mM Tris–HCl buffer (pH 7.0) and dialyzed (10 kDa cut-off) overnight at 4 °C against the same buffer. Laccase activity was examined in both protein fractions and found to be associated with that obtained from the 40–80% (NH4)2SO4 cut. Thus, this fraction was utilised in the subsequent steps for laccase purification. The dialyzed proteins were loaded on a DEAE-cellulose column pre-equilibrated with the same buffer (20 mM Tris–HCl buffer; pH 7.0). The unbound proteins were washed with 20 mM Tris–HCl buffer (pH 7.0) and elution of the bound proteins was accomplished by a gradual increase in NaCl concentration (0–1 M in equilibrating buffer). The fractions containing laccase were pooled, desalted, concentrated, and consecutively applied to a Sephacryl (S-100) column pre-equilibrated with 20 mM Tris–HCl buffer (pH 7.0). Sequentially, the fractions were eluted with the same equilibration buffer and the aliquots exhibiting laccase activity were concentrated, filtered (0.22 µm) and stored at −20 °C for future applications. The protein concentration was quantitated by the Bradford method.22
Laccase activity was calculated according to Baltierra-Trejo et al.23 The laccase assay was spectrophotometrically evaluated by measuring the oxidation of ABTS at 420 nm (ε = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) using a UV-Visible spectrophotometer (JASCO V-730). Mathematically, laccase activity is elucidated as the amount of laccase required to oxidise 1 µmol of substrate (ABTS) per minute. The assay mixture consisted of 500 µM ABTS, 50 mM acetate buffer (pH 3.0) and 10 µM of purified laccase. All the assays were repeated in triplicate with appropriate consideration of the standard deviation and standard error.
000 M−1 cm−1) using a UV-Visible spectrophotometer (JASCO V-730). Mathematically, laccase activity is elucidated as the amount of laccase required to oxidise 1 µmol of substrate (ABTS) per minute. The assay mixture consisted of 500 µM ABTS, 50 mM acetate buffer (pH 3.0) and 10 µM of purified laccase. All the assays were repeated in triplicate with appropriate consideration of the standard deviation and standard error.
2.4 SDS-PAGE and zymogram analysis
To ascertain the purity of laccase, SDS-PAGE (10%)24 was carried out with the appropriate size standards. The protein bands were stained with Coomassie Brilliant Blue G-250 and the molecular weight of laccase was calculated by comparing the migration of the standard proteins on the gel. In the zymogram analysis, the native protein was loaded on SDS-PAGE together with markers. After electrophoresis, to eliminate SDS, the gel was repeatedly washed with distilled water. Immediately after, the gel was incubated in a dark box at room temperature for 2–3 min completely immersed in freshly prepared 2 mM ABTS dissolved in 100 mM glycine (pH 2.0) to visualise the band on the gel.2.5 Laccase-mediated dye synthesis
Purified laccase-catalysed dye synthesis was performed with an array of natural plant phenolic monomers including ferulic acid, gallic acid, catechol, and syringaldehyde.25,26 The oxidation of these plant phenols (ferulic acid, gallic acid, catechol and syringaldehyde) by LacT was monitored at three pH (5.5, 7 and 9.5). At the optimum pH, the LacT activity was considered 100%. A typical dye formation reaction was comprised of 5 mM of each phenolic precursor prepared in 0.1 M sodium-acetate buffer (pH 5.5) with 15% ethanol as a dissolving agent and 3.1 µM or 6.2 µM of LacT (depending on the shade). Then, the mixture was incubated for 2 h with continuous shaking at 150 rpm in the dark. The control contained all the constituents excluding the active enzyme with similar physical conditions. All chromogenic reactions were performed at room temperature in the dark to preclude any possible phenol oxidation by light. Lastly, the resulting colourants were filtered and concentrated.2.6 Hair dyeing
After substantiating the formation of dyes in aqueous solution (Section 2.5), the laccase-catalysed colour formation was replicated directly on hair, thus mimicking actual dyeing on the human scalp. The in situ methodology was adopted to dye hair because this approach turned out to be the most practical in comparison with ex situ systems. Firstly, natural human hair was bleached to remove its original pigmentation and the bleached hair was treated as the control. Subsequently, each bundle of bleached hair (1 g) was wetted in the aforementioned reaction mixtures, with the minimum quantity of swelling agent27 at room temperature for 2 h in the dark under static state conditions. Post-incubation, the dyed hair was rinsed under running tap water for 5 min to remove the loosely held dye and blow-dried for 5 min. In the conventional chemical hair dyeing process, highly reactive chemicals such as ammonia, guanidine, thiourea, urea peroxide, polyoxymethylene urea, polyoxymethylene cyanoguanidine urea, hydroxyethyl urea and dimethyl urea are used as swelling agents, which can cause irritation and burning sensation in the scalp, resulting in the loss of moisture and protein from the hair. Moreover, they disturb the pH balance of the hair. Therefore, we used arginine-based swelling agent in the current study, which is a natural and non-toxic compound.2.7 Characterization of dyed hair
By using the CIELAB colour space defined by the International Commission on Illumination, the L*, a* and b* colour values of the dyed hair were examined using a reflectance spectrophotometer (Premier Colourscan model no. SS5100H, India). A white porcelain plate was used as a reference for calibration. All experiments were performed in triplicate with the appropriate control. Luminosity is illustrated by L* values from 0 (black) to 100 (white). A positive value of a* indicates red, while a negative value indicates green shades. Similarly, a positive value of b* indicates yellow and a negative value indicates blue shades (Fig. 2C). In addition, the reflectance profile and colour strength (K/S) in the visible range (400–700 nm) were also determined. The colour strength delineates the potential of a dye to impart colour on materials and is inferred by the absorption property of any dyed sample, which was evaluated using the following Kubelka–Munk equation:| K/S = [(1 − R)2/2R] |
2.8 Dye retention on hair upon shampooing
This test is considered as a litmus test for all types of dyes because it governs the cost of any dye in the market depending on its resistance towards detergents and consequent fading. To demonstrate this appraisal, a hair tress of 50 mg each was washed thrice with 10% shampoo solution (Head & Shoulders, Procter & Gamble) in a rotatory shaker at 200 rpm (25 °C) for 10 min, subsequently rinsed with running tap water, and finally dried in an oven. After each wash, the K/S values of the hair samples were determined.2.9 Effect of pH on retainability of hair dyes
Post-dyeing, various hair styling treatments are conducted in several pH ranges from acidic to alkaline. Thus, it is imperative to ascertain the leaching tendency of dyes under pH variations. 50 mg of each dyed hair was submerged in two sets of pH solution, i.e., acidic (pH 4–6) and alkaline (pH 8 and 9) for 10 min with constant vortexing, and afterwards thoroughly washed with tap water and dried in an oven. Neutral condition (pH 7.0) was taken as the control. The change in colour parameters before and after pH treatment was expressed in terms of ΔE* value.2.10 Surface morphology study of dyed hair
Hair dyes are classified into three categories as temporary, semi-permanent, and permanent depending on the extent of the penetration of the dye molecules in the interior of the hair. Temporary dyes are only confined to the peripheral surface of hair and weakly held to the hair shaft cuticle by van der Waals forces.7,28 These dyes are highly prone to shampooing and in just a few washes, all the pigments are leached out. In contrast, permanent dyes diffuse deep inside and get trapped in the hair cortex. In the present work, to investigate the nature of the dyes formed as being temporary or permanent, longitudinal sections of the hair before dyeing (bleached hair) and after dyeing (dyed hair) were visualised using an optical microscope (Zeiss) on a scale of 50 µm. Cross-sections of the dyed hairs were prepared by embedding them in rubber cork, cutting very thin sections, and then viewing on a scale of 50–80 µm.29 These sections were mounted on glass slides and observed under an optical microscope. Additionally, cross-sections of the bleached and dyed hair were scrutinised using a table-top scanning electron microscope (SEM) with gold coating at 2.5KX magnification on a 10 µm scale.In the surface morphological analysis, the effect of the dyes on the keratin protein of hair was exhaustively probed by inspecting longitudinal sections of the dyed hair using a table-top SEM (Hitachi High Technology) at 1.2k magnification for any possible damage to the cuticle of the hair.30 Images were captured at high vacuum with 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 V accelerating voltage on a scale of 50 µm.
000 V accelerating voltage on a scale of 50 µm.
2.11 Hair breakage studies and energy dispersive X-ray (EDX) analysis
We examined the effect of the application of various dyes on the tensile strength of the hair fibril using an Instron Micro tensile tester (Model 5848, Singapore). The hair samples were completely dried and loaded between two vertically aligned clamps, separated by 25 mm for 50 s with a strain rate of 1 mm s−1. All tests were conducted in ∼60% relative humidity at 27 °C. The breakage tests were repeated fifteen times for each type of hair sample. The bleached hair (without dyeing) was taken as the control. The tensile strength of the hair was expressed in kilogram-force (kgf) and plotted with respect to the corresponding extension (mm) observed. To examine the subsequent dye application on the elemental composition of the hair, energy dispersive X-ray (EDX) analysis of the hair prior and post dyeing was performed at an accelerating voltage of 5.0 kV with an acquisition time and process time of 60 s and 5 s, respectively.3. Results
3.1 Medium optimization and laccase purification
Initially, laccase (LacT) from B. agri was identified as a thermostable enzyme and has been utilized successfully for denim bleaching.21 Owing to the high temperature stability of LacT, it was amenable to use/exploit its phenol oxidase activity. Thus, LacT was purified from the B. agri culture filtrate as an extracellular laccase.In any enzymatic production, the incubation time and type of carbon and nitrogen sources play an important role.31 Thus, in the present study, the optimization of the medium resulted in enhanced laccase activity. After the analysis of the incubation time required for LacT production, LacT showed maximum activity at 48 h. However, the LacT activity continued to decrease afterwards (Fig. 1A). A similar incubation time for laccase production was reported for Bacillus sp. MSK-01.32 The higher incubation time of 72 h was also found for Bacillus sp. A4.33 On the contrary, a shorter incubation time of 24 h was also reported for Bacillus cereus B5.34
The carbon source supplies energy, which is essential for the survival of microbial cells. Thus, the effect of various carbon sources on LacT activity was investigated, as shown in Fig. 1B. LacT showed the maximum activity for malt extract and the minimum activity for starch. In the reported studies, glucose (monosaccharide) was the most favourable carbon source in Bacillus sp. PK4 for laccase production,35 while sucrose (disaccharide) was the best carbon source in Bacillus subtilis MTCC 2414.36 Nitrogen is also necessary for microbes because it is used to produce proteins, amino acids, nucleic acids and cell wall components.35 Among the tested nitrogen sources, the maximum activity of LacT was observed for glutamic acid followed by ammonium sulphate and yeast extract. LacT exhibited 60% activity for soya peptone, 50% activity for beef extract and the minimum activity of 30% for sodium nitrate (Fig. 1C). In other reported studies, a combination of yeast extract and tryptone was used for laccase production in Bacillus sp. MSK-01.32 In some Bacillus species, yeast extract alone was the best source for laccase production.35 In Alcaligenes faecalis XF1, a higher yield of laccase was reported when a combination of three nitrogen sources, viz., yeast extract, beef extract and peptone, was used.37
For the induction of laccase production, copper sulphate is considered the predominant inducer. In a previous study, we observed a ∼1.5-fold increase in LacT activity at 2 mM concentration of Cu2+ and ∼1.8-fold increase in LacT activity at 100 mM concentration of Cu2+.21 Sondhi et al. reported that 100 µM is the optimum concentration of Cu2+ for laccase production in Bacillus tequilensis SN4.38 Ravikumar et al. observed that the laccase activity in mushroom Hypsizygus ulmarius was enhanced to ∼16 U mL−1 by adding Cu2+.39
B. agri was cultured in the optimised growth medium, as described in the “Experimental methodology” for 48 h at 30 °C under shaking. The culture was decanted to collect the spent media as the supernatant and subjected to ammonium sulphate saturation. Proteins were collected from the fraction of 40–80% (NH4)2SO4 saturation. Accordingly, 1.7-fold purification of LacT was achieved. The purified LacT/proteins were passed through a pre-equilibrated DEAE-cellulose column, and finally LacT was purified through size exclusion chromatography. An outline of the entire purification procedure is summarised in Table 1. After completion of each step of the purification process, the total activity, protein concentration, specific activity, yield, and purification fold were calculated to examine the efficacy and relevance of each step. The purification of LacT represents an ∼3-fold purification profile. The purification process was repeated to check the reproducibility of the purification protocol. To examine the purity of LacT, the activities of other similar enzymes such as tyrosinase and peroxidase were calculated in the purified fraction of LacT. No activity of either tyrosinase or peroxidase was detected, further proving that the purified LacT was a pure enzyme.
| Purification step | Total activity (U mL−1) U = µmol min−1 | Protein (mg) | Specific activity (U mg−1) U = µmol min−1 | Yield (%) | Purification fold |
|---|---|---|---|---|---|
| a Values are mean of triplicate ± S.E. | |||||
| Culture supernatant | 97.2 ± 0.2 | 291 ± 0.1 | 334.1 ± 0.5 | 100 | 1 |
| (NH4)2SO4 precipitation | 69.4 ± 0.5 | 118.1 ± 0.2 | 588 ± 0.2 | 71.4 ± 0.4 | 1.7 ± 0.3 |
| DEAE cellulose | 41.7 ± 0.2 | 60.5 ± 0.5 | 688.7 ± 0.5 | 42.9 ± 0.5 | 2.1 ± 0.5 |
| Sephacryl (S-100) | 37.4 ± 0.4 | 35.5 ± 0.1 | 1056.3 ± 0.2 | 38.5 ± 0.2 | 3.2 ± 0.2 |
The purity of LacT was examined by SDS-PAGE and zymogram analysis (Fig. 1D). The purified laccase showed a single band of ∼65 kDa on 10% SDS-PAGE gel, which exactly resembled the green-coloured band on the zymogram gel. The occurrence of a green band (with ABTS as the substrate) in the zymogram study confirmed the presence of laccase. Moreover, the single band revealed that the purified LacT was a monomeric protein.
3.2 Dye formation and hair dyeing
In the present study, we used novel plant-derived phenolic compounds obtained from LacT-catalysed colouration reactions. The LacT-mediated reaction on phenolic moieties results in the formation of coloured compounds for the textile dyeing process. Four natural phenols, i.e., ferulic acid, gallic acid, SGA and catechol, were selected for the LacT-driven dye-formation reaction. Most of these phenols are ubiquitous in plant lignin and exhibit negligible toxicity. Here, we utilized monomeric phenols in the LacT-driven reactions. In these reactions, each monomeric phenol gave rise to a distinct dye colour. The basic scheme of LacT-mediated hair dyeing using the plant phenolic monomers is presented in Fig. 2A.The oxidation activity of LacT for plant phenolic monomers under three different pH (5.5, 7 and 9.5) is listed in Table 2. LacT showed the maximum activity at pH 5.5 for all four tested phenolic monomers, namely, ferulic acid, gallic acid, catechol and syringaldehyde. Otsuka et al. suggested that ΔE°′ (difference in Gibbs free energy) is the main factor regulating the optimum pH of multi-copper oxidases.40
| Substrate | LacT activity (%) | ||
|---|---|---|---|
| pH 5.5 | pH 7 | pH 9.5 | |
| a Values are mean of triplicate ± S.E. | |||
| Ferulic acid | 100 | 85 ± 0.2 | 52 ± 0.1 |
| Gallic acid | 100 | 75 ± 0.5 | 45 ± 0.6 |
| Catechol | 100 | 78 ± 0.3 | 48 ± 0.2 |
| Syringaldehyde | 100 | 82 ± 0.1 | 55 ± 0.5 |
A wide range of colourants in terms of shades, tints and colour parameters (L*, a*, and b*) was derived from the various chromogenic reactions using the monophenolic precursors (Fig. 2B). Black dye colour, which is in trend among the population by virtue of its resemblance to naturally occurring human hair, was derived from the LacT-catalysed reaction of catechol into poly(catechol).41 Commercial black hair dye is usually developed from pernicious synthetic precursors such as PPD, which is a well-proven allergen and carcinogen.8,10 Interestingly, the intensity of the hair dye generated increased with an increase in the amount of enzyme added to the reaction mixture.
Similarly, LacT-driven catalysis of ferulic acid developed a light brown colour with a yellowish tint, whereas gallic acid gave rise to a darker brown colour and the intensity of the colour was increased by enhancing the laccase concentration. Fascinatingly, a distinctive brown dye with a light greenish touch was generated by incubating syringaldehyde with LacT.
The phenolic dye solutions obtained through laccase action on the derivatives of natural phenols yielded their respective shades in the treated hair, confirming that the resulting colourants were effective for hair dyeing. Notably, in situ dyeing was the strategy of choice in the current study to accomplish permanent dyeing of the hair, which is subsequently described. A schematic representation of the profusely coloured dyed hair samples is depicted in Fig. 2A. To reinforce our present findings, specific qualitative and quantitative investigations to prove the retainability of the dyes on the hair were executed, as elaborated below.
3.3 Quantification of colour parameters and characterization of dyed hair
The diagram plotting L* (luminosity) and a*/b* (ratio of green-red to blue-yellow chromatic factors) represents the colour diversity of the resultant dyes (Fig. 2D). The analysis of the colour parameters from the various toned dyed hair suggests that the L* (luminosity) values were uniformly dispersed, ranging from 46.31 (FA) to 14.88 (CL2), which delineates the heterogeneity of the colour shades. Quantitatively, the spectrum of the a*/b* ratio was extended from the lowest side of ∼0.57 (FA) to the highest end of ∼1.37 (CL1). Briefly, LacT has emerged as a highly effective enzyme in dyeing sectors with prospects to take over the hazard-spilling dyeing industries.All the values of a* and b* were positive, indicating that the colour span of the dyed hair samples was primarily concentrated on reddish and yellowish shades, which are highly appealing among the population. The value of a* represents a broad range with an astonishing difference of ∼14 units, with the lowest at 2.24 (CL2) to the highest at 16.52 (FA). These findings clearly demonstrate the credibility of LacT in forming polychromatic dyes with a distinct spectrum of shades. The values of b* were also evenly spaced, ranging from ∼29.13 (FA) to ∼2.6 (CL1) (Table 3). The synthesis of market-driven shades of hair dyes indicates the potential of LacT in the salon industry.
| Polymer of phenols on dyed hair | L* | a* | b* | a*/b* |
|---|---|---|---|---|
| a Values are mean of triplicate ± S.E. L* means luminosity. L* values vary from 0 (black) to 100 (white). Positive values of a* indicate red and negative values indicate green shades. Positive values of b* indicate yellow and negative values indicate blue shades. FA, ferulic acid; GA1, gallic acid (LacT-3.1 µM); GA2, gallic acid (LacT-6.2 µM); SGA, syringaldehyde; CL1, catechol (LacT-3.1 µM); and CL2, catechol (LacT-6.2 µM). | ||||
| Control | 62.15 ± 0.2 | 12.82 ± 0.1 | 27.5 ± 0.2 | 0.47 ± 0.4 |
| FA | 46.31 ± 0.1 | 16.52 ± 0.5 | 29.13 ± 0.2 | 0.57 ± 0.2 |
| GA1 | 26.77 ± 0.5 | 12.27 ± 0.5 | 16.45 ± 0.7 | 0.74 ± 0.1 |
| GA2 | 21.03 ± 0.4 | 8.14 ± 0.1 | 8.86 ± 0.4 | 0.92 ± 0.1 |
| SGA | 26.37 ± 0.2 | 6.76 ± 0.1 | 10.14 ± 0.1 | 0.67 ± 0.4 |
| CL1 | 20.29 ± 0.1 | 4.42 ± 0.3 | 3.23 ± 0.3 | 1.37 ± 0.2 |
| CL2 | 14.88 ± 0.1 | 2.24 ± 0.5 | 2.60 ± 0.2 | 0.86 ± 0.3 |
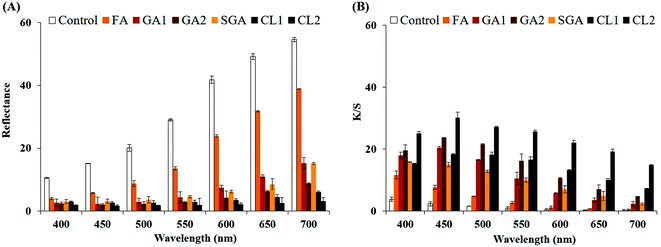
The extent of colour adhered to the hair was investigated by measuring the decrease in the reflectance of the dyed hair compared to the bleached hair, given that the colour formation is inversely proportional to the reflectance. The reflectance value of each dyed hair plotted against wavelength (400–700 nm) (Fig. 3A) reveals that the lighter shades (GA1 and CL1) have higher reflectance units than the corresponding darker shades (GA2 and CL2), respectively. Conversely, in terms of colour strength, the values of colour strength (K/S) of each dyed hair sample increased concomitantly with an increment in the darkness of the shade (Fig. 3B). The lightest brown tint, which developed from ferulic acid, had the highest reflectance of ∼38.9 and lowest K/S of ∼0.5 at 700 nm. On the contrary, the darkest black (CL2) had the lowest reflectance unit of ∼1.6 and highest K/S of ∼30.2 at 450 nm. Additionally, the wide ranges of reflectance and K/S substantiate the potential of the diverse dyes synthesized by LacT.
3.4 Permanence of dyes to shampooing
The capability of dyes to stay on hair against shampooing directly determines their colouring as temporary or permanent. Thus, to investigate the nature of hair dyeing, bleached hair was dyed with the colourants produced by all the phenolic polymers, as described earlier. Subsequently, the dyed hair was washed with excess detergent and dried. The K/S values of the dyed hair after each wash were determined. Fig. 4A shows the plot of K/S vs. number of shampooing cycles. It shows that multiple washings of the dyed hair did not alter the hair colour significantly even after repeated washing with shampoo. This confirms that hair dyes were permanent in nature. Furthermore, the washings/wash water after shampooing was colour free following three consecutive washes, which proves the strong colour fastness of these dyes. The decrease in K/S was the lowest for FA (∼4.8), whereas the highest for CL2 of (∼19) after three consecutive shampooing cycles. Overall, the hair dyes exhibited appreciable resistance to shampooing.3.5 Retainability of hair dyes under pH variations
The rate and degree of leaching of dyes from fabric/hair depends on the pH of the solution. Thus, the stability of dyes under a broad pH range is important from an industrial point of view. To examine the effect of different pH on the retention of dye on the hair, each dyed hair sample was incubated in buffers with different pH under shaking condition for 10 min. Then, each hair sample was dried and the ΔE* value was calculated. It is evident from Fig. 4B that the majority of dyes manifested a good level of colour fastness in both extremes of pH. In acidic conditions, ΔE* fluctuated from ∼0.2 to 3.0, while in alkaline suspensions, it varied from ∼0.1 to 2.7, which implies the strong retention and colouration of the hair cortex. In addition, colour bleeding from the hair was not continuous, rather it stopped after the first wash, proving the pH resistance. The most compelling result arose from the dark shades because they are prone to bleeding even in plain tap water and the majority of dye leaches out quickly, but the LacT-catalysed dyeing displayed encouraging results. As indicated for CL2 (darkest tone), it showed the minimum ΔE* of 0.8 in acidic solvent and 0.2 in the extreme alkaline solvent. Thus far, this is one of the few studies on laccase-formulated dyeing, which exhibited excellent colour fastness in the pH extremes. The property of resistivity of these dyes against harsh pH makes them promising compared to other reported dyes to date for industrial commercialisation.3.6 Surface morphology and anatomy studies of dyed keratin
In situ dyeing of hair through LacT-mediated catalysis of natural phenols was elucidated to be permanent on keratin hair, which prompted us to examine what changes may have happened in keratin hair upon dyeing. To investigate the morphology and anatomy of the hair, we performed optical and scanning electron microscopy of the bleached and dyed hair samples. The longitudinal sections of the undyed and dyed hair viewed under an optical microscope [Fig. 5A–C] clearly revealed that the proposed dyes are permanent because they effectively penetrated and deposited deep in the interior of the hair cortex. The undyed hair (bleached) was completely devoid of any colour inside its cortex, most prominently seen at 40× magnification (Fig. 5C). In contrast, the dyed hair illustrated a uniform dispersion of pigments inside its cortex, unlike the temporary dye, which just stuck on to the surface (hair cuticle) only. The dyes were tightly trapped in the core of the hair cortex, which may be the reason for the exemplary resistance to fading upon shampooing (Section 3.5) and exposure to extreme pH (Section 3.6).For the detailed analysis, thin cross-sections of the dyed hair (Fig. 5D) were observed under the optical microscope to discover the extent of dye invasion inside the hair cortex. The images were totally self-explanatory and specified that the applied colourants on the hair cuticle immediately went deep into the hair cortex and were trapped. This can be explained by understanding the mechanism of dye formation. In the case of temporary dyeing, dyes are directly applied on keratin hair, and thus no chemical reactions are needed to develop the colour on hair. Consequently, they are confined only on the hair cuticle and are loosely trapped on the hair, allowing them to be wiped off in just a few washes. On the contrary, permanent dyes, like in the present study, consist of colourless precursors and colour is generated by a developer. Industrially, H2O2 is the most commonly used developer, which oxidises the colourless precursors and converts them to large-sized coloured compounds. Consequently, these giant coloured molecules get trapped in hair cortex and do not wash away even after repeated shampooing.7,28 Here, LacT acted as a natural developer, replacing H2O2 to impart a wide array of colours. Our dyes also behaved similarly to permanent dyeing, where LacT-mediated catalysis converted the colourless natural plant monomers into large-sized coloured polymers, which were locked firmly inside the hair cortex, making them leach-proof.
In surface morphological studies, the SEM images of the hair cuticle before and after colouration (Fig. 6A) were identical, suggesting that the dyes had no harsh effect on hair morphology. Moreover, neither peeling off nor fracturing of the hair cuticle was observed in the dyed hair. The hair cuticle showed no signs of breakage or lifting up from the surface and the cells of the cuticle maintained their original shape and position. These positive outcomes verify the safety of the LacT-mediated dyes and show their advantage over the damaging H2O2 and other oxidising agents in human hair dyeing.13,16 Moreover, the cross-sectional SEM images of the undyed and dyed hairs also displayed an identical dyeing pattern (Fig. 6B).
3.7 Hair breakage studies and energy dispersive X-ray (EDX) analysis
The specific strength of any fibre is measured in terms of tenacity. The higher tenacity of a fibre denotes its high resistance to breakage, and consequently good tensile strength. The measurement of the tensile strength of the bleached and dyed hair revealed that the tenacity of the coloured hair slightly decreased by 2–4 gf per den in comparison to the control (Fig. 7A). The pattern obtained between load vs. extension of the dyed hair undoubtedly indicates that the LacT-dyes had a negligible effect on the tensile strength of the hair. The dyed hair showed an insignificant decrease in breakage force in comparison with the control, indicating that the hair samples even after dyeing retained their mechanical properties. The detailed assessment of the tensile testing is tabulated in Table 4. Similarly, a minor decline of only 5–15% in strain percentage at the maximum load was recorded in the dyed hair with reference to the control (∼55%). The load-extension curve of the hair is comprised of three regions, i.e., the Hookean region, the first linear stretch, in which the hair showed elasticity; the second region called the transformation region because the uncoiling of the α-helix may occur here, leading to its transformation into a sheet-structure; and third region known as the post-transformation region, which determines the breakage point of the hair.30 The CL1 and SGA hair samples exhibited moderate resistance to breakage given that a trivial decline in load was observed with respect to the control in the entire range of extension. Thus, it can be concluded that the present methodology of hair colouring via natural plant phenols and LacT has no adverse consequences on the mechanical properties of hair.| Polymer of phenols on dyed hair | Tenacity at maximum load [gf per den] | Tenacity at break [gf per den] | Strain at maximum load [%] |
|---|---|---|---|
| a Values are mean of fifteen values ± S.E. gf per den denoted Gram-force per Denier. FA, ferulic acid; GA1, gallic acid (LacT-3.1 µM); GA2, gallic acid (LacT-6.2 µM); SGA, syringaldehyde; CL1, catechol (LacT-3.1 µM); and CL2, catechol (LacT-6.2 µM). | |||
| Control | 11.43 ± 0.1 | 11.428 ± 0.2 | 54.799 ± 0.1 |
| FA | 9.94 ± 0.4 | 9.936 ± 0.4 | 51.783 ± 0.7 |
| GA1 | 10.76 ± 0.7 | 10.760 ± 0.1 | 49.223 ± 0.5 |
| GA2 | 10.33 ± 0.1 | 10.333 ± 0.1 | 48.407 ± 0.8 |
| SGA | 10.25 ± 0.1 | 10.248 ± 0.7 | 48.807 ± 0.1 |
| CL1 | 9.19 ± 0.5 | 9.186 ± 0.4 | 46.607 ± 0.4 |
| CL2 | 7.56 ± 0.5 | 7.557 ± 0.1 | 39.408 ± 0.2 |
The EDX spectrum of the bleached hair showed a substantial similarity to that of the dyed hair, indicating that the elemental composition of the dyed hair remained intact even after dyeing (Fig. 7B). This confirmed that the formulated dyes did not change the natural elemental composition of the hair. Furthermore, the sulphur content in the dyed hair did not increase, proving that our dyes did not contain any sulphur compounds.
4. Discussion
The enzymatic approach of hair dyeing has several major advantages over chemical dyeing. Enzymes-based reactions are less polluting, biodegradable, user friendly, easy to handle and generate non-toxic waste. Therefore, enzymatic methodologies are preferred for industrial applications to reduce the havoc created by industrial waste on the ecosystem. Laccase is a multi-faceted enzyme, which has been utilised in many industrial processing. It is an effective enzyme for dyeing human hair. Due to the low substrate specificity of laccase, it can generate more diversity in the colour of dyes than chemically synthesised dyes. In the current study, a thermostable extracellular bacterial laccase, LacT, was exploited for hair dyeing using plant phenols. The production and purification of LacT were more economical than the production of fungal laccases, which are currently widely used for industrial purposes. Thus, the low-cost LacT production and purification enabled low-cost dye production. In addition, given that the purified enzyme was used to synthesise dyes, there was a very low chance of contamination by metal ions and mediators, which could damage the hair structure or scalp.To date, the majority of reports published on hair dyeing are confined to conventional fungal laccases, which have numerous disadvantages. Although a few studies employed bacterial laccases, they still used noxious aromatic amines as precursors, H2O2 and other chemical oxidizing agents for developing the dyes.25,26,42 To holistically eliminate both aromatic amines and H2O2, we used natural plant phenols in the current study in place of synthetic aromatic amines as substrates without adding H2O2. Accordingly, it was observed that the intrinsic hair structure was not disrupted upon dyeing. The comparison of the SEM images of the hair cuticles before and after dyeing confirmed that the dyes did not damage the natural morphology of the hair surface. Moreover, the comparison of the tensile strength of the dyed/undyed hair depicted that the laccase-driven dyes, unlike commercial oxidizing agents, did not enhance the brittleness of the hair fibres. This fact extends the applicability of LacT-catalysed dyes as user-friendly hair dyes.
A few recent reports demonstrated the development of colourants in Petri dishes. Sun et al. studied in-depth the oxidative polymerization capability of laccase by using phenolic substrates such as resorcinol, hydroquinone, and catechol. Wang et al. prepared a biocolourant for the eco-dyeing of wool-fabrics via the laccase-driven oxidative polymerization of polyphenols.41,43,44 In general, dyeing hair with colourants remains a big task, given that the colourants developed in aqueous solutions do not always impart the same intensity of colour on hair. Thus, although the laccase-catalysed reaction can generate a wide range of colour, only a few dyes can actually show effective hair colouring. In this study, through repeated screening experiments, we ensured that the developed hair dyes could impart effective colouration on hair.
Colour diversity is an important factor in the hair dyeing industry. Therefore, researchers focus on expanding the diversity of colour in the formation of dyes. Among the reported studies, Jeon et al. tested 15 plant-derived phenols for the generation of coloured products via laccase-catalysed polymerization. Thereafter, for further characterisation, they selected three combinations of monomers, viz., ferulic acid and syringic acid (red colour); gallic acid and syringic acid (brown colour); and catechin and catechol (black colour).25 Laccase Denilite II from Aspergillus showed a set of unique colours during the laccase-catalysed oxidative polymerisation of phenolic compounds. In the case of resorcinol, a distinct dark-orange colour was developed. Catechol gave rise to a dark-brown colour, whereas hydroquinone produced a dark wine-red colour.43 Kumar et al. developed natural hair colours through the Bacillus subtilis DS (Lac DS)-based polymerization of natural dye precursors (phenolic and non-phenolic). They generated a golden-yellow colour from gallic acid and ferulic acid; black colour from catechol and pyrogallol; and reddish-brown colour from syringic acid and syringaldehyde.45 Wang et al. developed a pigment from the laccase-catalysed oxidative polymerization of tea polyphenols. This pigment was successfully used to dye silk and wool fabrics.44 In the present study, we generated black colour from catechol, light-brown colour from ferulic acid and dark-brown colour from gallic acid through LacT-driven catalysis.
Saito et al. reported that the colour differences in dyed hair occurred due to the variation in the amount of laccase added to the reaction mixture.46 The colour difference in terms of ΔE*ab of the dyed hair was calculated using 0–0.5 mg of laccase. In the present study, we also observed diverse shades of colour by altering the concentration of LacT. Under two different concentrations of LacT (3.1 µM and 6.2 µM), gallic acid generated two shades of brown colour and catechol produced two shades of black colour.
From a commercial perspective, the market demands for permanent hair and textile dyes are always higher than temporary and semi-permanent dyes. However, the development of permanent colour on hair is quite challenging. In the current study, an in situ approach was implemented to colour hair via the LacT-driven oxidative catalysis of phenols. “In situ” hair dyeing entails the formation of dyes on the hair itself. Small-sized colourless monomeric phenols were mixed with LacT and the resultant mixture was directly applied on the hair. After a certain time, the small-sized colourless monomers were catalysed by LacT into large-sized coloured polymers, which were trapped inside the hair cortex. Herein, the outcome of in situ hair dyeing by LacT emerged to be similar to permanent hair dyeing. Saitta et al. and Jia et al. clearly depicted the difference between temporary and permanent hair dyes through microscopic images.7,47 Accordingly, the microscopic images of the hair cuticles in the current study appeared to be similar to the nature of the hair dyed permanently. This fact was additionally supported by the dye retainability on the hair upon various treatment such as shampooing and alteration in pH.
In our previous study, we used partially purified LacT from B. agri for the decolourization of textile dyes and sustainable denim bleaching.21 The rationale behind using partially purified LacT was to make the processes of decolourization and bleaching economical. Tannic acid was used as a substrate for the production of LacT because it is considered as an inhibitor of tyrosinase.48 This further proved that LacT was a true laccase, which showed no activity in the presence of tyrosine. However, due to the high tendency of tannic acid to form complexes with proteins, we were unable to further purify LacT by anion exchange and size exclusion chromatography.49 Kim et al. also reported the same problem in the purification of laccase3 in their study.50 Due to the formation of a tannic acid-LacT complex, the partially purified LacT showed a molecular weight of 88 kDa on 10% SDS-PAGE gel. Therefore, in this study, we altered the growth medium with malt extract instead of tannic acid for the production and purification of LacT. Hence, the purified LacT in the present study showed the molecular weight of ∼65 kDa on 10% SDS-PAGE gel.
In enzymology, researchers commonly encounter a key impediment in upscaling the catalytic efficiency of enzymes from the laboratory to industrial level. Laccases, which are abundantly utilised presently for industrial processing, lack extremotolerant property to withstand industrial conditions. Thus, to address this problem, we used a novel thermostable LacT in the present study, which can survive high temperature, wide pH range and other extreme conditions generally encountered in industrial reactions. In the current scenario of dye synthesis and its application in colouring hair, LacT possesses immense potential to be extensively exploited in the dyeing industry owing to its distinctive extremophilic character.
For the industrial applications of LacT, enzyme production on large scale is essential at a low cost. However, the classical method for enzyme production and purification is a lengthy and slow process. Thus, the cloning, purification and overexpression of LacT using an appropriate expression system can solve this problem. Clearly much work is needed in forthcoming studies on LacT to successfully exploit it on an industrial scale.
Conflicts of interest
No potential conflicts of interest were disclosed.Author contributions
TD, JNS and VP designed the experiments. VP and BD performed the experiments. TD and VP wrote the paper.Acknowledgements
We acknowledge the Council of Scientific and Industrial Research, Government of India for funding the research grant [No. 37(1728)/19/EMR-II] to TD. VP acknowledges the University Grants Commission (UGC), Govt. of India, for the fellowship [UGC reference no- 3501/(NET-JULY 2016)]. We also sincerely thank Prof. Rabisankar Chattopadhyay, Department of Textile and Fibre Engineering, Indian Institute of Technology Delhi for granting permission to use Tensile Tester. The authors acknowledge Central Research Facility (CRF), IIT Delhi for SEM and Nanoscale Research Facility (NRF), IIT Delhi for EDX.References
- J. R. Jeon and Y. S. Chang, Trends Biotechnol., 2013, 31, 335–341 CrossRef CAS PubMed.
- S. Riva, Trends Biotechnol., 2006, 24, 219–226 CrossRef CAS PubMed.
- Y. Gu, L. Yuan, L. Jia, P. Xue and H. Yao, RSC Adv., 2021, 11, 29498–29506 RSC.
- L. Gioia, C. Manta, K. Ovsejevi, J. Burgueño, P. Menéndez and S. Rodriguez-Couto, RSC Adv., 2014, 4, 34096–34103 RSC.
- D. C. Kalyani, L. Munk, J. D. Mikkelsen and A. S. Meyer, RSC Adv., 2016, 6, 3910–3918 RSC.
- K. H. Kim, E. Kabir and S. A. Jahan, Environ. Int., 2016, 89, 222–227 CrossRef PubMed.
- P. Saitta, C. E. Cook, J. L. Messina, R. Brancaccio, B. C. Wu, S. K. Grekin and J. Holland, J. Clin. Aesthet. Dermatol., 2013, 6, 39 Search PubMed.
- D. Marcoux, P. M. Couture‐Trudel, G. Riboulet‐Delmas and D. Sasseville, Pediatr. Dermatol., 2002, 19, 498–502 CrossRef PubMed.
- I. M. C. Rubin, S. Dabelsteen, M. M. Nielsen, I. R. White, J. D. Johansen, C. Geisler and C. M. Bonefeld, Br. J. Dermatol., 2010, 163, 992–998 CrossRef CAS PubMed.
- Y. C. Huang, W. C. Hung, W. Y. Kang, W. T. Chen and C. Y. Chai, Toxicol. Lett., 2007, 170, 116–123 CrossRef CAS PubMed.
- T. B. Zanoni, F. Hudari, A. Munnia, M. Peluso, R. W. Godschalk, M. V. B. Zanoni and D. P. de Oliveira, Toxicol. Lett., 2015, 239, 194–204 CrossRef CAS PubMed.
- Y. Zhang, S. D. Sanjose, P. M. Bracci, L. M. Morton, R. Wang, P. Brennan, P. Hartge, P. Boffetta, N. Becker, M. Maynadie, L. Foretova, P. Cocco, A. Staines, T. Holford, E. A. Holly, A. Nieters, Y. Benavente, L. Bernstein, S. H. Zahm and T. Zheng, Am. J. Epidemiol., 2008, 167, 1321–1331 CrossRef PubMed.
- J. A. Seo, I. H. Bae, W. H. Jang, J. H. Kim, S. Y. Bak, S. H. Han and K. M. Lim, J. Dermatol. Sci., 2012, 66, 12–19 CrossRef CAS PubMed.
- M. G. Hougaard, T. Menné and H. Søsted, Dermatitis, 2012, 23, 284–287 CrossRef CAS PubMed.
- A. Guerra-Tapia and E. Gonzalez-Guerra, Actas Dermo-Sifiliogr., 2014, 105, 833–839 CrossRef CAS PubMed.
- J. Ohn, S. J. Kim, S. J. Choi, Y. S. Choe, O. Kwon and K. H. Kim, J. Dermatol. Sci., 2018, 89, 91–94 CrossRef CAS PubMed.
- W. Jia, Q. Wang, X. Fan, A. Dong, Y. Yu and P. Wang, RSC Adv., 2017, 7, 12977–12983 RSC.
- K. Wlizło, J. Polak, A. Jarosz-Wilkołazka, R. Pogni and E. Petricci, Enzyme Microb. Technol., 2020, 132, 109398 CrossRef PubMed.
- J. Polak and A. Jarosz-Wilkolazka, Process Biochem., 2012, 47, 1295–1307 CrossRef CAS.
- J. R. Jeon, P. Baldrian, K. Murugesan and Y. S. Chang, Microb. Biotechnol., 2012, 5, 318–332 CrossRef PubMed.
- V. Panwar, J. N. Sheikh and T. Dutta, Appl. Biochem. Biotechnol., 2020, 192, 1238–1254 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- E. Baltierra-Trejo, L. Márquez-Benavides and J. M. Sánchez-Yáñez, J. Microbiol. Methods, 2015, 119, 126–131 CrossRef CAS PubMed.
- U. K. Laemmli, Nature, 1970, 227, 680–685 CrossRef CAS PubMed.
- J. R. Jeon, E. J. Kim, K. Murugesan, H. K. Park, Y. M. Kim, J. H. Kwon, W. G. Kim, J. Y. Lee and Y. S. Chang, Microb. Biotechnol., 2010, 3, 324–335 CrossRef CAS PubMed.
- K. M. Im and J. R. Jeon, J. Visualized Exp., 2016, 118, e54772 Search PubMed.
- S. M. Burkinshaw, P. E. Froehling and M. Mignanelli, Dyes Pigm., 2002, 53, 229–235 CrossRef CAS.
- S. A. Da França, M. F Dario, V. B. Esteves, A. R. Baby and M. V. R. Velasco, Cosmetics, 2015, 2, 110–126 CrossRef.
- C. Battistella, N. C. McCallum, K. Gnanasekaran, X. Zhou, V. Caponetti, M. Montalti and N. C. Gianneschi, ACS Cent. Sci., 2020, 6, 1179–1188 CrossRef CAS PubMed.
- L. Navone and R. Speight, PLoS One, 2018, 13, e0202608 CrossRef PubMed.
- A. Pandey and S. Radhakrishnan, Enzyme Microb. Technol., 1992, 14, 486–488 CrossRef CAS.
- S. Sondhi and K. Saini, Heliyon, 2019, 5, e01718 CrossRef PubMed.
- H. Guo, C. Lin, S. Wang, D. Jiang, B. Zheng, Y. Liu and W. Qin, Bioresources, 2017, 12, 4776–4794 CAS.
- M. M. Allos and A. A. Hussein, Al-Nahrain Journal of Science, 2015, 18, 133–140 Search PubMed.
- M. Rajeswari and V. Bhuvaneswari, Afr. J. Biotechnol., 2016, 15, 1813–1826 CrossRef CAS.
- N. P. Muthukumarasamy, B. Jackson, A. Joseph Raj and M. Sevanan, Biochem. Res. Int., 2015, 1–9 Search PubMed.
- S. Mehandia, S. C. Sharma and S. K. Arya, Biotechnol. Rep., 2020, 25, e00413 CrossRef PubMed.
- S. Sondhi, P. Sharma, N. George, P. S. Chauhan, N. Puri and N. Gupta, 3 Biotech, 2015, 5, 175–185 CrossRef PubMed.
- G. Ravikumar, D. Gomathi, M. Kalaiselvi and C. Uma, Int. J. Pharma Bio Sci., 2012, 3, 355–365 CAS.
- K. Otsuka, T. Sugihara, Y. Tsujino, T. Osakai and E. Tamiya, Anal. Biochem., 2007, 370, 98–106 CrossRef CAS PubMed.
- N. Aktaş, N. Şahiner, Ö. Kantoğlu, B. Salih and A. Tanyolaç, J. Polym. Environ., 2003, 11, 123–128 CrossRef.
- J. Fu, G. S. Nyanhongo, G. M. Gübitz, A. Cavaco-Paulo and S. Kim, Biocatal. Biotransform., 2012, 30, 125–140 CrossRef CAS.
- X. Sun, R. Bai, Y. Zhang, Q. Wang, X. Fan, J. Yuan and P. Wang, Appl. Biochem. Biotechnol., 2013, 171, 1673–1680 CrossRef CAS PubMed.
- F. Wang, J. Gong, X. Zhang, Y. Ren and J. Zhang, Polymers, 2018, 10, 196 CrossRef PubMed.
- D. Kumar, A. Kumar, S. Sondhi, P. Sharma and N. Gupta, 3 Biotech, 2018, 8, 1–10 CAS.
- K. O. Saito, R. Ikeda, K. Endo, Y. Tsujino, M. Takagi and E. Tamiya, J. Biosci. Bioeng., 2012, 113, 575–579 CrossRef CAS PubMed.
- W. Jia, S. Li, Z. Luo, H. Yu, W. Zhu, Q. Mao and A. Dong, Fibers Polym., 2021, 22, 141–148 CrossRef CAS.
- S. Patil, S. Sistla and J. Jadhav, J. Agric. Food Chem., 2014, 62, 11594–11601 CrossRef CAS PubMed.
- K. Aoki, R. Shinke and H. Nishira, Agric. Biol. Chem., 1981, 45, 121–127 CAS.
- J. M. Kim, S. M. Park and D. H. Kim, BMC Biotechnol., 2010, 10, 1–9 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |