Open Access Article
Open Access ArticleRh(III)-catalyzed synthesis of dibenzo[b,d]pyran-6-ones from aryl ketone O-acetyl oximes and quinones via C–H activation and C–C bond cleavage†
Wei
Yang
 *ab,
Haonan
Zhang
a,
Yu
Liu
*ab,
Cuiman
Tang
a,
Xiaohui
Xu
a and
Jiaqi
Liu
a
*ab,
Haonan
Zhang
a,
Yu
Liu
*ab,
Cuiman
Tang
a,
Xiaohui
Xu
a and
Jiaqi
Liu
a
aSchool of Chemical Engineering, Northeast Electric Power University, Jilin 132012, China. E-mail: yangw467@163.com; greatliudy123@163.com
bGongqing Institute of Science and Technology, Gongqing 332020, China
First published on 12th May 2022
Abstract
A redox-neutral synthesis of dibenzo[b,d]pyran-6-ones from aryl ketone O-acetyl oximes and quinones has been realized via Rh(III)-catalyzed cascade C–H activation annulation. A possible Rh(III)–Rh(V)–Rh(III) mechanism involving an unprecedented β-C elimination step was proposed.
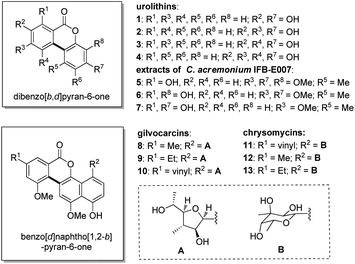
The dibenzo[b,d]pyran-6-one is one of the most important structural motifs widely present in natural products with pharmacological relevance,1 such as gut microbiota metabolites urolithins (1–4) that show anti-inflammatory, antiglycative and neuroprotective effects,2–4 and the extracts of an endophytic fungus Cephalosporium acremonium IFB-E007 (5–7) that have pronounced anticancer activities.5 In addition, the related heterocyclic structure benzo[d]naphtho[1,2-b]pyran-6-one is found in some bactericidal and antitumor natural products including gilvocarcins6,7 (8–10) chrysomycins8,9 (11–13), etc. (Fig. 1). Therefore, a number of approaches to access dibenzo[b,d]pyran-6-ones have been developed via the intra- or inter-molecular biaryl formation as the key step.10 However, many of these methodologies require multi-step reactions, and the development of new efficient synthetic methods, especially those easy one-step reactions that are still of great interest.
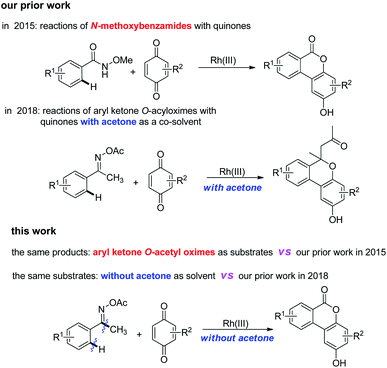
In the past decade, transition-metal-catalyzed C–H bond activation has proven to be a powerful tool in organic syntheses11 and several methods for the synthesis of dibenzo[b,d]pyran-6-ones via C–H activation have been reported.12 Actually, in 2015, our group reported Rh(III)-catalyzed synthesis of dibenzo[b,d]pyran-6-ones from N-methoxybenzamides and quinones through C–H activation annulation.13 Interestingly, we obtained the same products using aryl ketone O-acetyl oximes as substrates to react with quinones under Rh(III)-catalyzed conditions in this work. Rh(III)-catalyzed C–H activation using ketoximes as substrates has been developed for synthesis of various substituted heterocycles.14 Compared to the previous reports, this reaction undergoes a novel mechanism involving an unexpected C–C bond cleavage, which is attractive. Moreover, our study demonstrated that solvent is vital to these reactions. In 2018, we reported Rh(III)-catalyzed annulation of aryl ketone O-acetyl oximes with quinones to synthesize 6H-benzo[c]chromenes with acetone as a co-solvent.15 Herein, we described Rh(III)-catalyzed synthesis of dibenzo[b,d]pyran-6-ones using the same substrates without acetone (Scheme 1).
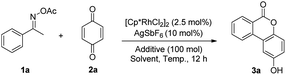
Initially, the reaction of acetophenone O-acetyl oxime 1a with benzoquinone 2a was employed to optimize the reaction conditions (Table 1). When the reaction was conducted in the presence of [Cp*RhCl2]2 (2.5 mol%), AgSbF6 (10 mol%) and PivOH (100 mol%) in MeOH at 50 °C for 12 h, 2-hydroxy-6H-dibenzo[b,d]pyran-6-one 3a was obtained in 12% yield (Table 1, entry 1). Elevating the reaction temperature led to a higher yield of 3a (Table 1, entries 1–4). Solvent screening (Table 1, entries 4–9) revealed that reaction in MeOH gave a higher yield of 3a (Table 1, entry 4). Among the additives tested, benzoic acid was the most favorable with respect to product yield (Table 1, entries 4, 10 and 11). Decreasing the amount of benzoic acid to 75 mol% resulted in the best yield of 3a (Table 1, entry 12).
| Entry | Additive | Solvent | Temp °C | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), [Cp*RhCl2]2 (2.5 mol%), additive (100 mol%), solvent (1 mL) for 12 h. b Isolated yields. c Benzoic acid (75 mol%) was added. d Benzoic acid (50 mol%) was added. | ||||
| 1 | PivOH | MeOH | 50 | 12 |
| 2 | PivOH | MeOH | 70 | 20 |
| 3 | PivOH | MeOH | 90 | 36 |
| 4 | PivOH | MeOH | 110 | 43 |
| 5 | PivOH | EtOH | 110 | 26 |
| 6 | PivOH | DMF | 110 | 37 |
| 7 | PivOH | THF | 110 | 16 |
| 8 | PivOH | HFIP | 110 | 0 |
| 9 | PivOH | Acetone | 110 | Trace |
| 10 | HOAc | MeOH | 110 | Trace |
| 11 | Benzoic acid | MeOH | 110 | 50 |
| 12c | Benzoic acid | MeOH | 110 | 70 |
| 13d | Benzoic acid | MeOH | 110 | 63 |
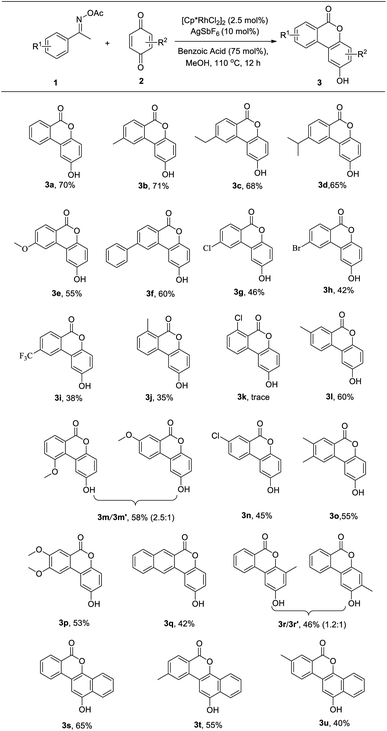
Under the obtained optimum reaction conditions above (Table 1, entry 12), we surveyed the reaction scope (Table 2). First, the reactions of various aryl ketone O-acetyl oximes 1 with 2a were examined. For acetophenone O-acetyl oximes, substrates with electron-donating groups or phenyl at the para-position of aryl groups participated well in this reaction and the corresponding products were obtained in good yields (3a–3f). Substrates with halogens or strong electron-withdrawing group trifluoromethyl gave the products in lower yields (3g–3i). Substrate bearing the methyl or chlorine at the meta-position provided the desired products 3l and 3n with exclusive regioselectivity toward the less-hindered site, whereas the meta-methoxy-substituted derivative gave a mixture of regioisomers (3m/3m′ = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), revealing that the nature of the substituent at the meta-position had an effect on the regioselectivity. 3,4-Disubstituted acetophenone O-acetyl oximes smoothly reacted to result the corresponding dibenzo[b,d]pyran-6-ones 3o and 3p in moderate yields. 2-Acetonaphthone O-acetyl oxime also produced the target product 2-hydroxy-6H-naphtho[2,3-c]chromen-6-one 3q. Next, we examined the reactivity of quinone derivatives with 1a under the established conditions. Methyl benzoquinone afforded the desired molecule in 46% yield with regioisomers (3r/3r′) in a ratio of approximately 1.2
1), revealing that the nature of the substituent at the meta-position had an effect on the regioselectivity. 3,4-Disubstituted acetophenone O-acetyl oximes smoothly reacted to result the corresponding dibenzo[b,d]pyran-6-ones 3o and 3p in moderate yields. 2-Acetonaphthone O-acetyl oxime also produced the target product 2-hydroxy-6H-naphtho[2,3-c]chromen-6-one 3q. Next, we examined the reactivity of quinone derivatives with 1a under the established conditions. Methyl benzoquinone afforded the desired molecule in 46% yield with regioisomers (3r/3r′) in a ratio of approximately 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The naphthoquinone could be also well tolerated, giving benzo[d]naphtho[1,2-b]pyran-6-one 3s in 65% yield. Furthermore, para-methyl-substituted or meta-methyl-substituted acetophenone O-acetyl oximes also smoothly reacted with naphthoquinone to give the corresponding products 3t or 3u. Thus, several tetracyclic benzo[d]naphtho[1,2-b]pyran-6-ones were synthesized successfully.
1. The naphthoquinone could be also well tolerated, giving benzo[d]naphtho[1,2-b]pyran-6-one 3s in 65% yield. Furthermore, para-methyl-substituted or meta-methyl-substituted acetophenone O-acetyl oximes also smoothly reacted with naphthoquinone to give the corresponding products 3t or 3u. Thus, several tetracyclic benzo[d]naphtho[1,2-b]pyran-6-ones were synthesized successfully.
| a Standard conditions. |
|---|
|
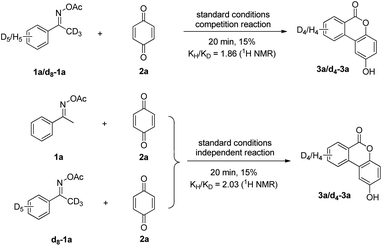
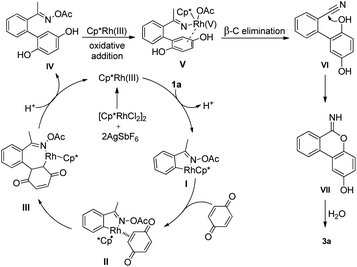
To shed light on the reaction mechanism of this annulation, the reaction of acetophenone O-acetyl oxime 1a with benzoquinone 2a under standard conditions was detected by GC-MS, and benzonitrile was observed (detected by GC-MS; see ESI†). This result suggested this reaction might undergo a β-C elimination. Then, deuterium-labeling experiments were further carried out to gain some insights into the catalytic mechanism. A competition between protio and deutero 1a showed a KIE value of 1.86 at early conversion. The KIE was further measured from two side-by-side reactions using protio and deutero 1a with 2a and a KIE value of 2.03 was observed (Scheme 2). These results demonstrated that the C–H bond cleavage process might be involved in the rate-determining step.
On the basis of our previous work, present observations and literature precedent,11,13,15,16 a mechanistic pathway is proposed (Scheme 3, taking the reaction of substrate 1a with benzoquinone 2a as an example). First, O-acetyl oxime 1a reacts with the active Cp*Rh(III) species through directed C–H cleavage to form a five-membered rhodacycle intermediate I. Next, coordination of the benzoquinone affords intermediate II, which undergoes migratory insertion into the incipient Rh–C bond to form a seven-membered rhodacycle III. Protonolysis and aromatization deliver biaryl intermediate IV. Then, an oxidative addition of Rh(III) into the O–N bond is possible to produce the Rh(V) species V,17 followed by β-C elimination to give the intermediate VI.18 A subsequent intramolecular nucleophilic addition of intermediate VI delivers the intermediate VII, which undergoes hydrolysis to generate the final product 3a.
Conclusions
In summary, we have developed a novel Rh(III)-catalyzed cascade C–H activation annulation with readily available and inexpensive substrates for the convenient and direct synthesis of dibenzo[b,d]pyran-6-ones. In this process, we proposed a possible Rh(III)–Rh(V)–Rh(III) pathway, which might undergo an unprecedented β-C elimination step. This is the first example of β-C elimination via Rh(III)-catalyzed C–H bond functionalization. Further studies into the detailed reaction mechanism is ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from The Education Department of Jilin Province (Grant No. JJKH20190698KJ), Jilin Science and Technology Bureau (Grant No. 20190104142) and Northeast Electric Power University (Grant No. BSZT06202106).Notes and references
- (a) J. M. Sayer, Y. Haruhiko, A. W. Wood, A. H. Conney and D. M. Jerina, J. Am. Chem. Soc., 1982, 104, 5562 CrossRef CAS; (b) K. Koch, J. Podlech, E. Pfeiffer and M. J. Metzler, Org. Chem., 2005, 70, 3275 CrossRef CAS PubMed; (c) W. Sun, L. D. Cama, E. T. Birzin, S. Warrier, L. Locco, R. Mosley, M. L. Hammond and S. P. Rohrer, Bioorg. Med. Chem. Lett., 2006, 16, 1468 CrossRef CAS PubMed; (d) N. Tibrewal, P. Pahari, G. Wang, M. K. Kharel, C. Morris, T. Downey, Y. Hou, T. S. Bugni and J. Rohr, J. Am. Chem. Soc., 2012, 134, 18181 CrossRef CAS PubMed; (e) J. S. Lazo, E. R. Sharlow, M. W. Epperly, A. Lira, S. Leimgruber, E. M. Skoda, P. Wipf and J. S. Greenberger, J. Pharmacol. Exp. Ther., 2013, 347, 669 CrossRef CAS PubMed; (f) Z. Mao, W. Sun, L. Fu, H. Luo, D. Lai and L. Zhou, Molecules, 2014, 19, 5088 CrossRef PubMed; (g) Y. L. Garazd and M. M. Garazd, Chem. Nat. Compd., 2016, 52, 1 CrossRef CAS.
- J. C. Espín, M. Larrosa, M. T. García-Conesa and F. T. Barberan, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 15 Search PubMed.
- T. Yuan, H. Ma, W. Liu, D. B. Niesen, N. Shah, R. Crews, K. N. Rose and D. A. Vattem, ACS Chem. Neurosci., 2016, 7, 26 CrossRef CAS PubMed.
- J. P. Piwowarski, S. Granica, J. Stefanska and A. K. Kiss, J. Nat. Prod., 2016, 79, 3022 CrossRef CAS PubMed.
- H. W. Zhang, W. Y. Huang, Y. C. Song, J. R. Chen and R. X. Tan, Helv. Chim. Acta, 2005, 88, 2861 CrossRef CAS.
- (a) S. Horii, H. Fukase, E. Mizuta, K. Hatano and K. Mizuno, Chem. Pharm. Bull., 1980, 28, 3601 CrossRef CAS; (b) H. Nakano, Y. Matsuda, K. Ito, S. Ohkubo, M. Morimoto and F. Tomita, J. Antibiot., 1981, 34, 266 CrossRef CAS PubMed.
- (a) T. Matsumoto, T. Hosoya and K. Suzuki, J. Am. Chem. Soc., 1992, 114, 3568 CrossRef CAS; (b) I. Takemura, K. Imura, T. Matsumoto and K. Suzuki, Org. Lett., 2004, 6, 2503 CrossRef CAS PubMed; (c) P. R. Nandaluru and G. J. Bodwell, J. Org. Chem., 2012, 77, 8028 CrossRef CAS PubMed.
- (a) F. Strelitz, H. Flon and I. N. Asheshov, J. Bacteriol., 1955, 69, 280 CrossRef CAS PubMed; (b) U. Weiss, K. Yoshihira, R. J. Highet, R. J. White and T. T. Wei, J. Antibiot., 1982, 35, 1194 CrossRef CAS PubMed.
- (a) I. R. Pottie, P. R. Nandaluru, W. L. Benoit, D. O. Miller, L. N. Dawe and G. J. Bodwell, J. Org. Chem., 2011, 76, 9015 CrossRef CAS PubMed; (b) S. K. Jain, A. S. Pathania, R. Parshad, C. Raina, A. Ali, A. P. Gupta, M. Kushwaha, S. Aravinda, S. Bhushan, S. B. Bharate and R. A. Vishwakarma, RSC Adv., 2013, 3, 21046 RSC.
- (a) W. R. Bowman, E. Mann and J. Parr, J. Chem. Soc., Perkin Trans. 1, 2000, 1, 2991 RSC; (b) H. Abe, K. Nishioka, S. Takeda, M. Arai, Y. Takeuchi and T. Harayama, Tetrahedron Lett., 2005, 46, 3197 CrossRef CAS; (c) N. Thasana, R. Worayuthakarn, P. Kradanrat, E. Hohn, L. Young and S. J. Ruchirawat, Org. Chem., 2007, 72, 9379 CrossRef CAS PubMed; (d) M. E. Jung and D. A. Allen, Org. Lett., 2009, 11, 757 CrossRef CAS PubMed; (e) K. Vishnumurthy and A. Makriyannis, J. Comb. Chem., 2010, 12, 664 CrossRef CAS PubMed; (f) Y. Li, Y. J. Ding, J. Y. Wang, Y. M. Su and X. S. Wang, Org. Lett., 2013, 15, 2574 CrossRef CAS PubMed; (g) S. Luo, F. X. Luo, X. S. Zhang and Z. J. Shi, Angew. Chem., Int. Ed., 2013, 52, 10598 CrossRef CAS; (h) H. N. Lv, P. F. Tu and Y. Jiang, Mini-Rev. Med. Chem., 2014, 14, 603 CrossRef CAS PubMed.
- For selected reviews, see: (a) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (b) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (c) F. Wang, S. Yu and X. Li, Chem. Soc. Rev., 2016, 45, 6462 RSC; (d) L. Liu and J. L. Zhang, Youji Huaxue, 2017, 37, 1117 CrossRef CAS; (e) T. H. L. Nguyen, N. Gigant and D. Joseph, ACS Catal., 2018, 8, 1546 CrossRef CAS; (f) Y. Y. Xiang, C. Wang, Q. P. Ding and Y. Y. Peng, Adv. Synth. Catal., 2019, 361, 919 CrossRef CAS; (g) S. Kumar, S. Nunewar, S. Oluguttula, S. Nanduri and V. Kanchupalli, Org. Biomol. Chem., 2021, 19, 1438 RSC.
- (a) K. Inamoto, J. Kadokawa and Y. Kondo, Org. Lett., 2013, 15, 3962 CrossRef CAS; (b) T. H. Lee, J. Jayakumar, C. H. Cheng and S. C. Chuang, Chem. Commun., 2013, 49, 11797 RSC; (c) K. Sasano, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2013, 135, 10954 CrossRef CAS PubMed; (d) F. Ji, X. Li, W. Wu and H. Jiang, J. Org. Chem., 2014, 79, 11246 CrossRef CAS PubMed; (e) Y. Wang, J. Y. Gu and Z. J. Shi, Org. Lett., 2017, 19, 1326 CrossRef CAS PubMed; (f) R. Chen and S. Cui, Org. Lett., 2017, 19, 4002 CrossRef CAS PubMed; (g) L. Pan, J. Dong, D. Xie, Y. Li and Q. Liu, Adv. Synth. Catal., 2018, 360, 958 CrossRef CAS; (h) Y. Dong, J. T. Yu, S. Sun and J. Cheng, Chem. Commun., 2020, 56, 6688 RSC.
- W. Yang, S. Wang, Q. Zhang, Q. Liu and X. Xu, Chem. Commun., 2015, 51, 661 RSC.
- (a) P. C. Too, S. H. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed; (b) H. Jiang, J. Yang, X. Tang and W. Wu, J. Org. Chem., 2016, 81, 2053 CrossRef CAS PubMed; (c) H. Huang, J. Cai and G. J. Deng, Org. Biomol. Chem., 2016, 14, 1519 RSC; (d) V. Vinayagam, A. Mariappan, M. Jana and M. Jeganmohan, J. Org. Chem., 2019, 84, 15590 CrossRef CAS PubMed; (e) N. Aravindan and M. Jeganmohan, J. Org. Chem., 2021, 86, 14826 CrossRef CAS PubMed.
- W. Yang, J. Wang, H. Wang, L. Li, Y. Guan, X. Xu and D. Yu, Org. Biomol. Chem., 2018, 16, 6865 RSC.
- (a) L. Yang, B. Qian and H. Huang, Chem. –Eur. J., 2012, 18, 9511 CrossRef CAS PubMed; (b) W. Yang, J. Sun, X. Xu, Q. Zhang and Q. Liu, Chem. Commun., 2014, 50, 4420 RSC; (c) Z. Qi and X. Li, Chin. J. Catal., 2015, 36, 48 CrossRef CAS; (d) W. Yang, J. Wang, Z. Wei, Q. Zhang and X. Xu, J. Org. Chem., 2016, 81, 1675 CrossRef CAS; (e) W. Yang, J. Dong, J. Wang and X. Xu, Org. Lett., 2017, 19, 616 CrossRef CAS.
- (a) W. Guo and Y. Xia, J. Org. Chem., 2015, 80, 8113 CrossRef CAS PubMed; (b) Z. Zhou, M. Bian, L. Zhao, H. Gao, J. Huang, X. Liu, X. Yu, X. Li and W. Yi, Org. Lett., 2018, 20, 3892 CrossRef CAS PubMed.
- H. Li, M. L. Wang, Y. W. Liu, L. J. Li, H. Xu and H. X. Dai, ACS Catal., 2022, 12, 82 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02074b |
| This journal is © The Royal Society of Chemistry 2022 |