Open Access Article
Open Access ArticleUnravelling the highly efficient synthesis of individual carbon nanodots from casein micelles and the origin of their competitive constant-blue-red wavelength shift luminescence mechanism for versatile applications
R. Blessy Pricilla ,
David Skoda
,
David Skoda ,
Pavel Urbanek
,
Pavel Urbanek ,
Michal Urbanek
,
Michal Urbanek ,
Pavol Suly
,
Pavol Suly ,
Eva Domincova Bergerova
,
Eva Domincova Bergerova and
Ivo Kuritka
and
Ivo Kuritka *
*
Centre of Polymer Systems, Tomas Bata University in Zlin, Tr. T. Bati 5678, Zlin 76001, Czech Republic. E-mail: kuritka@utb.cz
First published on 1st June 2022
Abstract
Synthesis of casein-derived carbon nanodots (CND) using a microwave-assisted approach, giving a high product yield (25%), is reported. Casein was used as a sustainable carbon source, and polyvinylpyrrolidone was used as a stabilizer for the nanodots. The size of the prepared amorphous CND corresponds to individual casein coils, which were only partially carbonized. They were obtained due to the disintegration of casein micelles and submicelles within the microwave-assisted solvothermal process. The resulting nanodots had bright photoluminescence, and their electronic structure and optical properties were investigated. A novel competitive model of their luminescence mechanism was introduced to explain a phenomenon beyond the standard models. The synthesized carbon nanodots were used as luminescent ink for anticounterfeit applications. A polymer matrix nanocomposite was prepared by dispersing the nanodots in a flexible and robust poly(styrene-ethylene-butylene-styrene) tri-block copolymer (SEBS) using the solution cast method. For the first time, the effect of CND on the luminescence and mechanical properties of the SEBS/CND self-supporting films was studied. The film was also studied as a phosphor for light-emitting diodes, with a unique experimental setup to avoid self-absorption, which results in low efficiency and eliminates the excess UV transmitted. Because of their high luminescence, photostability, and mechanical properties, these CND could be used as luminescent labels in the packaging and optoelectronics industries.
1. Introduction
Carbon nanodots (CND) are nanomaterials invading the current industrial era because of their exceptional features. As the name suggests, they belong to the carbon family, having a size of less than 10 nm. The nanodots typically consist of carbon, hydrogen, nitrogen, and oxygen.1 They are expressed in terms of their carbogenic core and their functional groups present on the surface. Their core is disorganized, and a high amount of sp3 carbons are present.2 Furthermore, their properties can be modified by the level of oxidation present on their surface.3 Since their discovery in 2004, they have been widely studied due to their remarkable properties like biocompatibility, good solubility, photostability, low toxicity, and less cost of preparation.4–7 Due to their exceptional characteristics, different methods have been used for their synthesis like hydrothermal, solvothermal, microwave etc.8–10 Depending on the synthesis method used hydrophilic, hydrophobic, or amphiphilic CND are formed.11,12 Their outstanding physicochemical properties attract their operations in different fields. Amongst all their exciting properties, their optical characteristics are widely studied because of their wonderful tuning quality. Their strong absorption and emission properties improve their effectiveness in hiding and storing essential information.13Recently, cost-efficient and environment-friendly polymer/CND nanocomposites have been given much attention because of their marvelous attributes. These polymer/CND nanocomposites have enormous applications like luminescent solar concentrators, photocatalysis and metal ion detection and light-emitting diodes.14–17 CND exhibit different luminescent colours in transparent polymers. Among the various polymers available, poly(styrene-ethylene-butylene-styrene) (SEBS) is an extremely strong and flexible tri-block copolymer. It consists of a hard segment called polystyrene and a soft segment called ethylene co-butylene, providing excellent thermo-mechanical properties. Additionally, it has excellent UV, heat and shatter resistance, toughness, and high transparency.18 This suggests it to be an appreciable candidate for security and packaging applications.
In recent years, counterfeiting of authentic documents, brands, and products has been emerging as a serious problem. However, while advanced manufacturing has its benefits, the number of counterfeit goods is also rising due to the latest technological advances. Hence, various anticounterfeit materials and techniques are being explored to overcome this challenge. The anticounterfeit techniques make the original items difficult to duplicate and simple to validate, providing safety to official and valuable items.19 Different types of technologies developed to reduce the fake transfer of data include holograms, latent fingerprint, security inks, and luminescent tags.19–22 These technologies play an important role in the safety of the country and its people. While various kinds of techniques and materials have been used to regulate the counterfeit of goods, there has always been a search to obtain better materials that are cost-effective and easily produced. Currently, there is a growing trend to use luminescent photoresponsive materials as anticounterfeit materials.23 Among the various luminescent materials available, specific attention has been given to CND, because of their excellent optical properties in the UV and visible light.24,25 These nanodots, because of their high photostability, illumination rate and low toxicity, they have been effectively used to replace the conventional phosphors in LEDs.26,27 Therefore, significant attempts are being made to understand and explore them, which is the necessity of the time.
In our paper, we have reported the synthesis of CND from the disintegration of casein micelles and submicelles using microwave-assisted approach. Compared to the traditional microwaves, the microwave reactor provides uniform heating, less by products and faster rate of synthesis. Casein is an easily available milk protein that has both hydrophobic and hydrophilic domains. It is a sustainable green source compound containing amino acids. It is a natural source of carbon. Polyvinylpyrrolidone (PVP) is used as a stabilizer to prevent the agglomeration of the nanodots. This high quantitative yield obtained through this method is suitable for large-scale production. Besides standard characterization, the electronic structure of CND and its photoluminescence mechanism are studied in detail. A new competitive model has been introduced to explain the luminescence mechanism of the nanodots. Flexible and robust SEBS/CND nanocomposite self-supporting film is obtained using the simple solution cast method. The luminescence and mechanical properties of the film were studied exclusively. According to the best of our knowledge, this is the first time to report the study on SEBS/CND nanocomposite film. We propose the application of the casein-based CND as anticounterfeit inks because of their good emission characteristics. The UV-sensitive luminescent nanocomposite films, because of their good transparency and photostability, can be efficiently used as anticounterfeit films for the packaging of products. Moreover, a peculiar experimental setup is developed to use SEBS/CND nanocomposite film as an efficient phosphor material for light-emitting diodes. Even though carbon dots have been utilized for these applications before, their product yield, synthesis with less time and appropriate temperature has always been challenging.28,29 Sometimes the product yield is not reported or reported with no appreciable yield. In our work the microwave – reactor assisted method has proved to be an efficient method as it gives a high product yield (25%) in comparison with previous reports varying typically from 0.1% up to 19%.30–32 Moreover, the product is obtained in less time and appropriate temperature with significant and exceptional characteristics.
2. Experimental section
2.1. Materials required
Polyvinylpyrrolidone (PVP), styrene–ethylene–butylene–styrene (SEBS) are obtained from Sigma Aldrich. Among many variable grades of milk protein concentrates (MPC) and milk casein concentrates (MCC),33 a product “MPC 85 Micellar Casein” was acquired from a local retailer shop in the Czech Republic as a source of casein. The brand is a natural nutrition and unflavoured. MPC 85 is known for its content of ca. 71% casein, 11% of residual whey, 5% of residual lactose and minerals, and 1% of residual fat.34 The powder was used before the best before date. The size of casein micelles was characterized experimentally in our laboratory which confirmed the suitability of the material for further studies. The distilled water was used for the preparation of various solutions. The polyethylene terephthalate (PET) foil (Novele™) used as substrates for printing was bought from Novacentrix, Texas, USA. A UV protection reflective window film “HooTown Mirror Window Privacy Film Self-Adhesive One-Way Silver Reflective Window Film UV Protection, Sun Protection and Heat Insulation Tinted Film, Silver” was brought commercially from amazon market. This film has 97% reflectance of UVA. The solvents, including ethylene glycol and isopropanol, are purchased from PENTA, Czech Republic. All the chemicals and reagents were used without any further purification.2.2. Instrumental characterization
The CND particle size measurement was carried out using Transmission Electron Microscope (TEM). The TEM model, JEOL JEM 2100 microscope was operated at 300 kV (LaB6 cathode, point resolution 2.3 Å equipped with OLYMPUS SYS TENGRA camera (2048 × 2048 pixels)). The size analysis of the particles was analysed by ImageJ software. The particle size distribution of the casein micelles dispersed in ethylene glycol before the synthesis was determined using Dynamic Light Scattering method (Zetasizer Nano ZS, model 3600, Malvern Instruments Ltd., Worcestershire, UK). The X-ray diffraction pattern was obtained from an X-ray powder diffractometer (Rigaku Miniflex 600) using Co Kα radiation (λ = 1.7903 Å), operated at a beam voltage of 40 kV and a beam current of 100 mA. A UV-Vis spectrophotometer, PerkinElmer Lambda 1050, was used to obtain the absorption studies. The emission studies were attained on photoluminescence (PL) spectrophotometer FLS920, Edinburgh Instruments (Xe lamp with a double monochromator used for excitation in continuous wave regime) at room temperature. Time resolved studies were performed using the same PL spectrophotometer with two different excitation pulse diodes. One diode had an excitation wavelength 275 nm with a pulse width of 860 ps and another had an excitation wavelength of 332 nm with a pulse width of 840 ps. Both were operated at a frequency of 10 MHz. For both UV and PL experiments, 5 mg of the freeze-dried CND sample was dissolved in 5 ml of water. From this solution, 1 ml was taken and made up to 3 ml to perform the studies. Obtained spectra was corrected for both detector sensitivity and excitation source intensity. The Fourier Transform Infrared (FTIR) spectra analysis was carried out on Thermo Scientific Nicolet 6700 spectrometer utilizing the ATR method with the diamond crystal (4000–400 cm−1, resolution 2 cm−1, 64 scans). X-ray photoelectron spectroscopy (XPS) analysis was carried out using a Kratos Axis Supra spectrometer equipped with a monochromatic X-ray source with Al Kα excitation. The binding energy for C 1s at 284.8 eV was used as standard. The printing was achieved on the FUJIFILM DMP-2800 series Dimatix Materials Printer. The viscosity and density measurements of the ink were resolved on a Microviscometer Lovis 2000 ME (1.59 mm capillary) and Density meter DMA 5000 M (Anton Paar s.r.o., Prague, Czech Republic). The surface tension was measured by Tensiometer K100MK3 (Krüss GmbH, Hamburg, Germany) based on the Wilhelmy plate method. The compositional analysis was obtained on Flash 2000 CHNS/O+MAS200R, Thermo Scientific. The thermogravimetric analysis was executed on a Setaram LabSys Evo with TG/DSC sensor in an air atmosphere (heating ramp 5 °C min−1, up to 1000 °C). The mechanical properties of the synthesized films were measured on a Testometric universal-testing machine of type M 350-5CT (Testometric Co. Ltd., Rochdale, UK). The contact angle studies were resolved on SEE System (Advex Instruments, Czech Republic). The total optical transmission of the samples was measured using a spectrometer Lovibond RT850i (Tintometer Limited) along with the ASTM D 1003.2.3. Preparation of carbon dots derived from casein
CND were prepared using casein as a carbon source and PVP as a stabilizer. Various casein to PVP ratios were attempted to find a proper synthesis condition for CND within a preliminary heuristic phase of the research. While the ratio of components was free, the total volume of the Teflon-lined container in the monomodal reactor ERTEC Magnum II (600 W; 2.45 GHz) was given by its construction and used microwave wavelength. The initial amount of casein was always dispersed and mixed with ethylene glycol and PVP to obtain a sufficiently stable yellowish dispersion for the microwave processing. If the concentration of PVP was too low, the final product was in form of large agglomerates obviously unsuitable for production of CND. Increasing the concentration of PVP resulted into a well dispersed and clear final product brown solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 casein to PVP w/w ratio. If the PVP concentration was further increased, irregular structures were obtained again.
2 casein to PVP w/w ratio. If the PVP concentration was further increased, irregular structures were obtained again.
Hence after the initial optimization, 0.1 g of casein was taken in a beaker, and 20 ml of ethylene glycol was added. This solution was kept for ultrasonication for 60 minutes to obtain a good dispersion. Ethylene glycol serves the dual purpose of a solvent and a reducing agent. In another beaker, 0.2 g of PVP was taken and dissolved in 20 ml of ethylene glycol using ultrasonication for 10 minutes. Both the solutions were mixed and poured into a Teflon-lined container and kept in the microwave reactor. The reaction was carried out at 200 °C for 30 minutes with 100% (600 W) power. After cooling, the brown colour solution obtained was filtered using a 0.22 μm membrane filter. The filtered solution was dialyzed using a dialysis bag (3.5 kDa) for two days to remove the undesired low molecular side products or source compound residues. The solution was again centrifuged at 6000 rpm corresponding to a range from RCFmin 1489 g to RCFmax 5514 g all the undesired particles still remaining after dialysis. A centrifuge THERMO Multifuge X3R (Thermo Fisher Scientific, Germany) was used. Finally, the solution was freeze-dried for another two days to obtain the desired product. The product yield (PY) achieved through this method was calculated using the following formula.35
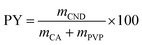 | (1) |
2.4. Preparation of SEBS/CND nanocomposite film
For the preparation of SEBS/CND nanocomposite film, 20 wt% of SEBS solution and 0.25 wt% (C1), 0.5 wt% (C2), and 1 wt% (C3) CND solution were prepared in chloroform. Both the solutions were mixed in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio and stirred for 60 minutes. After this period, the solution was cast on a Petri dish and covered to allow the film to dry slowly overnight. The next day the film could be easily peeled off from the Petri dish, and the desired characterizations were achieved.
1 volume ratio and stirred for 60 minutes. After this period, the solution was cast on a Petri dish and covered to allow the film to dry slowly overnight. The next day the film could be easily peeled off from the Petri dish, and the desired characterizations were achieved.
2.5. Preparation of CND ink
The fluorescent CND ink was prepared by mixing the CND solution and ethylene glycol. Here, 1 wt% of the CND solution was prepared in water and mixed with ethylene glycol in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. Ethylene glycol is added to increase the viscosity of the ink. The prepared ink is mixed well using a sonicator for 10 minutes to obtain proper dispersion. The CND ink was loaded into the cartridge and kept in the cartridge holder. The temperature of the printer head nozzles was maintained at 30 °C. Similarly, the temperature of the PET foil, which was used as a substrate, was controlled at 40 °C.
1 volume ratio. Ethylene glycol is added to increase the viscosity of the ink. The prepared ink is mixed well using a sonicator for 10 minutes to obtain proper dispersion. The CND ink was loaded into the cartridge and kept in the cartridge holder. The temperature of the printer head nozzles was maintained at 30 °C. Similarly, the temperature of the PET foil, which was used as a substrate, was controlled at 40 °C.
2.6. Preparation of CND-LEDs
For the effective distribution of CND into the SEBS polymer solution, 2 ml of 2.5 wt% of CND dispersion in chloroform was added dropwise for 1 hour in 4 ml of 22 wt% SEBS solution of chloroform. After appropriate mixing, the mixture was allowed to be stirred for another hour. This mixture was drop cast on a Petri dish and dried overnight to obtain a uniformly distributed CND/SEBS polymer film. The film was mounted as a flat layer on the LED device a wavelength of about 365 nm. To obtain maximum visible light a cut off filter of 395 nm and a UV protection reflective window film was placed on top of the CND/SEBS polymer film.2.7. Sample preparation for water contact angle studies
To determine the water contact angle (WCA), five measurements were carried out on the same PET substrate, using 5 μl of CND ink for each droplet. After placing a drop on the substrate the angle it formed with the film substrate was analysed using the system. Generally, if the contact angle is less than 90°, the substrate is hydrophilic; if the contact angle is more than 90°, the substrate is hydrophobic.3. Results and discussions
3.1. Characterization of the carbon nanodots
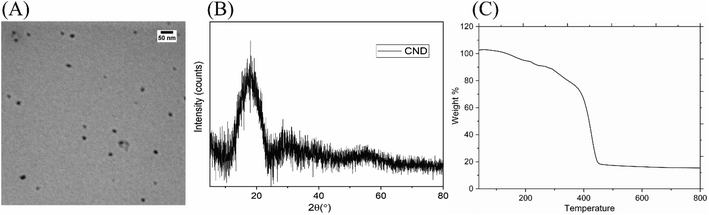
The morphology of the synthesized CND is shown in Fig. 1(A). It is clear from the image that the nanodots are of different sizes. The TEM image shown helps in understanding the spherical nature of the particles. The average size of the particles as calculated by the ImageJ software was found to be 4.3 ± 1.1 nm.Fig. 1(B) shows the XRD pattern of the CND. The broad peak at 18° of two theta corresponds to 0.57 nm interlayer spacing, thus indicating the amorphous nature of the CND,36 rather than graphitization.37,38 The thermal stability of the CND was analyzed using thermal gravimetric analysis in the helium atmosphere as shown in Fig. 1(C). The weight loss at around 100 °C can be attributed to the loss of small molecular weight compounds or adsorbed water. From around 260 °C to 450 °C, the decline observed is due to the decomposition of surface functional groups, the capping polymer, and likely some core components as well.39,40 The residual mass at 800 °C is found to be 15.3%, which also testifies for low carbonization of the prepared CND and partial preservation of the structure of the precursor.
Table 1 shows the elemental analysis of casein and CND. The analysis was carried out to observe the change in the elemental composition between the precursor casein and the CND formed. The experiment was performed in three batches and their average was calculated. The elemental composition of carbon, hydrogen and nitrogen content in the CND was found to be 56, 8, and 10 wt%, respectively. The remaining wt% is assumed to be oxygen which is about 26 wt% in CND, while 36 wt% is in casein. The increase in the carbon wt% is evident due to the carbonization process.
| Element | Casein (wt%) | Carbon nanodots (wt%) |
|---|---|---|
| C | 46 | 56 |
| H | 6 | 8 |
| N | 12 | 10 |
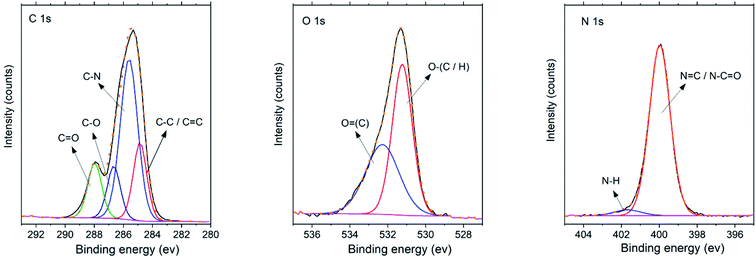
An X-ray photoelectron spectroscopy (XPS) was employed to deeply investigate the bonding relations between elements in the synthesized CND sample as demonstrated in Fig. 2. The overview spectrum is not shown for the sake of brevity. The elemental ratio C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O was 76
O was 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 in at% while the ratio among these elements from Table 1 is 65
13 in at% while the ratio among these elements from Table 1 is 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23. The insignificant difference can be attributed to the experimental errors and differences between the two techniques due to different manifestations of carbon and moisture contamination in a vacuum apparatus and weighing in the ambient atmosphere for elemental analysis. In the C 1s XPS spectrum, four contributions were detected. These components with binding energies 284.9, 285.6, 286.7, and 287.9 eV are assigned to C–C/C
23. The insignificant difference can be attributed to the experimental errors and differences between the two techniques due to different manifestations of carbon and moisture contamination in a vacuum apparatus and weighing in the ambient atmosphere for elemental analysis. In the C 1s XPS spectrum, four contributions were detected. These components with binding energies 284.9, 285.6, 286.7, and 287.9 eV are assigned to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, C–O, and C
C, C–N, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively. It should be noted, that no component is manifested at binding energy ∼288.7 eV corresponding to the absence of carboxyl groups (O–C
O species, respectively. It should be noted, that no component is manifested at binding energy ∼288.7 eV corresponding to the absence of carboxyl groups (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), excluding thus the presence of –COOH or ester groups. The O 1s XPS spectrum revealed the signals at binding energy 531.2 and 532.3 eV corresponding to O
O), excluding thus the presence of –COOH or ester groups. The O 1s XPS spectrum revealed the signals at binding energy 531.2 and 532.3 eV corresponding to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and O–C bonds, respectively. The N 1s XPS spectrum shows the main contribution at 400.0 eV, characteristic of the nitrogen atoms bound to carbon. Concerning the FTIR spectra and composition of the sample, the peak at 400.0 eV is most likely attributed to N–C
C and O–C bonds, respectively. The N 1s XPS spectrum shows the main contribution at 400.0 eV, characteristic of the nitrogen atoms bound to carbon. Concerning the FTIR spectra and composition of the sample, the peak at 400.0 eV is most likely attributed to N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide bonds of the peptide backbone preserved from casein precursor after CND synthesis.41,42 The same group in PVP may contribute to the intensity of the signal as well. The component at the higher binding energy side (401.6 eV) is attributed to NH or NH2 species.43 These findings also match the FTIR spectroscopy (Fig. 3(A)).
O amide bonds of the peptide backbone preserved from casein precursor after CND synthesis.41,42 The same group in PVP may contribute to the intensity of the signal as well. The component at the higher binding energy side (401.6 eV) is attributed to NH or NH2 species.43 These findings also match the FTIR spectroscopy (Fig. 3(A)).
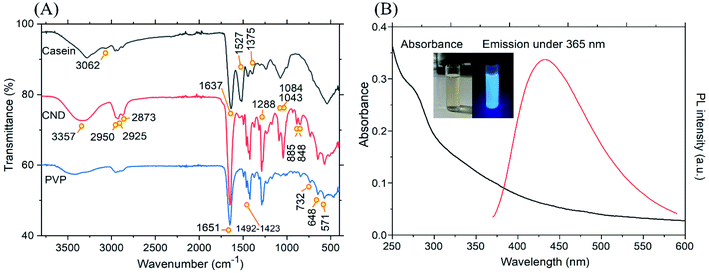 | ||
| Fig. 3 (A) FTIR spectra of casein, CND and PVP and (B) absorption and an example of emission spectra of the CND at 365 nm. | ||
The FTIR spectroscopy helps in the identification of the functional groups present in the CND. The FTIR spectrum of casein, CND and PVP is shown in Fig. 3(A). The FTIR spectra of CND is partially similar to that of PVP and casein. Moreover, it can be expected that the surface of the CND is covered with rich PVP chains.44 The absence of certain bands from PVP and casein, as well as a shift in the position of the bands in the CND, indicate that a chemical reaction, rather than a physical interaction has occurred.
The presence of O–H and N–H vibrations in CND was confirmed by the broad vibrational band from 3600 cm−1 to 3100 cm−1 (CND-3357 cm−1). Due to interactions with amide groups, the shoulder of this band expands extensively to lower wavenumbers in casein, whereas it is considerably inhibited in CND and absent in PVP. It may imply that the polymeric structure of the protein is degraded during the synthesis of CND. The band centered at 3062 cm−1 in casein corresponds to the valence vibration of CH bond in the aromatic system. Symmetric and asymmetric stretching of aliphatic CH at 2950 cm−1 and 2925 cm−1 (CH3 νas and CH3 νsym) and 2873 cm−1 with a shoulder at 2855 cm−1 (CH2 νas and CH2 νsym) are present in all the three spectra. The sharp vibrational band at 1637 cm−1 in casein and 1651 cm−1 in both PVP and CND is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations (Amide I). Increased intensity of this band indicates the presence of large no. of amide (N–C
O stretching vibrations (Amide I). Increased intensity of this band indicates the presence of large no. of amide (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups on the surface. Contrarily, the second typical absorption band classic for polyamides (including proteins) involving both C–N stretching and C–N–H in-plane bending in the stretch-bend mode (Amide II) is centred at 1527 cm−1 in casein and is largely suppressed in CND, though a distinguishable band of low intensity remains. This again testifies for a significant change of backbone structure during synthesis. A group of absorption bands at 1492, 1464, 1438, and 1423 cm−1 correspond to C–N and CH2 scissor vibration and deformations of the cyclic side groups in PVP, which are observable in the spectrum of CND. A week absorption band at 1375 cm−1 is present in all three spectra yet slightly shifted to higher wavenumbers in casein. This band can be assigned to –CH3 βsym (umbrella) vibration, testifying to the presence of terminal CH3 groups in the sample. The strong band at 1288 cm−1 in CND reveals the presence of C–N–H (Amide III) in the stretch-open mode of vibration. The other bands situated in the region of 1300 cm−1 and lower are related to C–H bending. The week bands above 1200 cm−1 (i.e. 1275, 1225 and 1214 cm−1) indicate CH2 wagging, C–N stretching and CH2 twisting, which is confirmed by the week peak at 1169 cm−1. The moderate and strong peaks at 1084 cm−1 and 1043 cm−1, reveal the presence C–O stretching in C–O–C and C–OH groups. The weak band at 885 cm−1 can be associated with CNC groups in amides or with oxygen-containing groups. The weak band located at 848 cm−1 is associated with the vibration modes for C–C bond in the pyrrolidone ring. Finally, the set of weak absorption bands at 732, 648, and 571 cm−1 are associated with CH2 rocking vibrations, thereby confirming the manifestation of long aliphatic chains in both the CND and PVP spectra.45–47 Altogether, the observed features testify for carbonization of the casein due to the partial destruction of peptide bonds in the original protein backbone, partial condensation of the aromatic groups and formation of ether and alcohol groups. On the other hand, no free carboxylic or isolated carbonyl groups (ketones, aldehydes, esters) were formed in the synthesis of CND as can be deduced from the absence of the carbonyl absorption band above 1700 cm−1. Surface stabilization of the CND by PVP is also in line with features observed in FTIR absorption spectra.
O) groups on the surface. Contrarily, the second typical absorption band classic for polyamides (including proteins) involving both C–N stretching and C–N–H in-plane bending in the stretch-bend mode (Amide II) is centred at 1527 cm−1 in casein and is largely suppressed in CND, though a distinguishable band of low intensity remains. This again testifies for a significant change of backbone structure during synthesis. A group of absorption bands at 1492, 1464, 1438, and 1423 cm−1 correspond to C–N and CH2 scissor vibration and deformations of the cyclic side groups in PVP, which are observable in the spectrum of CND. A week absorption band at 1375 cm−1 is present in all three spectra yet slightly shifted to higher wavenumbers in casein. This band can be assigned to –CH3 βsym (umbrella) vibration, testifying to the presence of terminal CH3 groups in the sample. The strong band at 1288 cm−1 in CND reveals the presence of C–N–H (Amide III) in the stretch-open mode of vibration. The other bands situated in the region of 1300 cm−1 and lower are related to C–H bending. The week bands above 1200 cm−1 (i.e. 1275, 1225 and 1214 cm−1) indicate CH2 wagging, C–N stretching and CH2 twisting, which is confirmed by the week peak at 1169 cm−1. The moderate and strong peaks at 1084 cm−1 and 1043 cm−1, reveal the presence C–O stretching in C–O–C and C–OH groups. The weak band at 885 cm−1 can be associated with CNC groups in amides or with oxygen-containing groups. The weak band located at 848 cm−1 is associated with the vibration modes for C–C bond in the pyrrolidone ring. Finally, the set of weak absorption bands at 732, 648, and 571 cm−1 are associated with CH2 rocking vibrations, thereby confirming the manifestation of long aliphatic chains in both the CND and PVP spectra.45–47 Altogether, the observed features testify for carbonization of the casein due to the partial destruction of peptide bonds in the original protein backbone, partial condensation of the aromatic groups and formation of ether and alcohol groups. On the other hand, no free carboxylic or isolated carbonyl groups (ketones, aldehydes, esters) were formed in the synthesis of CND as can be deduced from the absence of the carbonyl absorption band above 1700 cm−1. Surface stabilization of the CND by PVP is also in line with features observed in FTIR absorption spectra.
The optical properties of the CND were studied using a set of characterizations. The UV-Vis absorption spectrum and emission spectra of the synthesized CND at 365 nm are manifested in Fig. 3(B). As can be observed, a small peak can be seen at 278 nm in the otherwise broad absorption spectra tailing over the visible range. The occurrence of the peak is accounted to the presence of π–π* transition of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond or n–π* transition of the C
C bond or n–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,
O, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N,
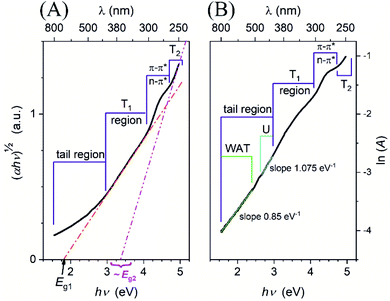
C–N, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O group as well.26,48–50 This suggests the presence of isolated molecular-like states. However, these chromophores or fluorophores are rather a small molecular part or fragment than an edge or molecular state with an extended conjugated π-bond system. Indeed, there is no absorption peak between 300 nm and 400 nm in the spectrum (Fig. 3(B)) otherwise reported for CND with developed core, edge and surface states structure.51 Generally, the spectrum resembles the typical spectral feature of a disordered semiconductor which may be likely due to both structural irregularity and the size distribution of particles.52 The UV-Vis absorption spectrum is deeper analyzed in Fig. 4(A) and (B). Understanding the optical properties of CND remains difficult, as they usually lack a well-defined electronic band structure and have fluctuating properties that vary between sources (e.g. source compound, synthesis method, degree of carbonization, crystallinity or amorphousness, polydispersity). Despite such complexity, Tauc band gap can be considered as a phenomenological parameter for comparison.53,54 Hence, the Tauc plot was constructed.55 In Fig. 4(A), a best linear fit for allowed indirect transition between the valence band (VB) and the conduction band (CB), yielding Eg1 = 1.8 eV was attained. The linear part of the spectrum in the Tauc plot (T1 region) spans approximately from 3 eV to 4 eV (420–310 nm). Above 4 eV, a peak may be observed due to the transitions associated with isolated chromophores. The absorption edge of the CND is not abrupt, and the absorption tail extends deep into the forbidden gap. Thus, at lower energies (i.e. <3 eV) a manifestation of structural disorder is observed.56,57 Two separate strictly linear parts of this tailing region were identified at lower wavelengths (2.95–2.65 eV and 420–466 nm) ascribed as Urbach tail (U region) and at higher wavelengths (1.55–2.40 eV and 800–516 nm) assigned as weak absorption tail (WAT).54,56,58–60
C–O group as well.26,48–50 This suggests the presence of isolated molecular-like states. However, these chromophores or fluorophores are rather a small molecular part or fragment than an edge or molecular state with an extended conjugated π-bond system. Indeed, there is no absorption peak between 300 nm and 400 nm in the spectrum (Fig. 3(B)) otherwise reported for CND with developed core, edge and surface states structure.51 Generally, the spectrum resembles the typical spectral feature of a disordered semiconductor which may be likely due to both structural irregularity and the size distribution of particles.52 The UV-Vis absorption spectrum is deeper analyzed in Fig. 4(A) and (B). Understanding the optical properties of CND remains difficult, as they usually lack a well-defined electronic band structure and have fluctuating properties that vary between sources (e.g. source compound, synthesis method, degree of carbonization, crystallinity or amorphousness, polydispersity). Despite such complexity, Tauc band gap can be considered as a phenomenological parameter for comparison.53,54 Hence, the Tauc plot was constructed.55 In Fig. 4(A), a best linear fit for allowed indirect transition between the valence band (VB) and the conduction band (CB), yielding Eg1 = 1.8 eV was attained. The linear part of the spectrum in the Tauc plot (T1 region) spans approximately from 3 eV to 4 eV (420–310 nm). Above 4 eV, a peak may be observed due to the transitions associated with isolated chromophores. The absorption edge of the CND is not abrupt, and the absorption tail extends deep into the forbidden gap. Thus, at lower energies (i.e. <3 eV) a manifestation of structural disorder is observed.56,57 Two separate strictly linear parts of this tailing region were identified at lower wavelengths (2.95–2.65 eV and 420–466 nm) ascribed as Urbach tail (U region) and at higher wavelengths (1.55–2.40 eV and 800–516 nm) assigned as weak absorption tail (WAT).54,56,58–60
The Urbach tail is related to the disorder, defects, and thermal vibrations in the lattice of a material. They are associated with the transitions from localized electronic centers to extended states or vice versa, but the latter is considered less probable. This region is analyzed when plotted in the ln scale in Fig. 4(B). The following equation can be used to determine Urbach energy, which expresses how absorbance (α) scales with photon energy (hν).58
| α(hν) ∼ ehν/EU | (2) |
Thus, the Urbach energy (EU) may be calculated from the reciprocal slope of the linear portion in Fig. 4(B). The high value of the Urbach energy 0.93 eV obtained for the CND indicates a large structural disorder and broad distribution of localized states. Although these states are usually associated with surface defects,51,57 we expect many defects in the whole structure of our CND as it is amorphous without any sign of a crystalline core structure.
The lowest part of the absorption edge is the WAT region and is related to the purity and perfection of the material. It involves transitions between localized defect states. It is calculated using the following equation.58
| α(hν) ∼ ehν/EWAT | (3) |
The reciprocal slope obtained from the linear portion of the curve in Fig. 4(B) yields weak absorption tail energy (EWAT). The EWAT is always larger than EU. The high value 1.18 eV of EWAT suggests the presence of significant populations of localized tail states and deep band gap states in the electronic structure of the CND. Such rich and wide distribution of defects was inbuilt, most likely due to partial carbonization of casein macromolecules.
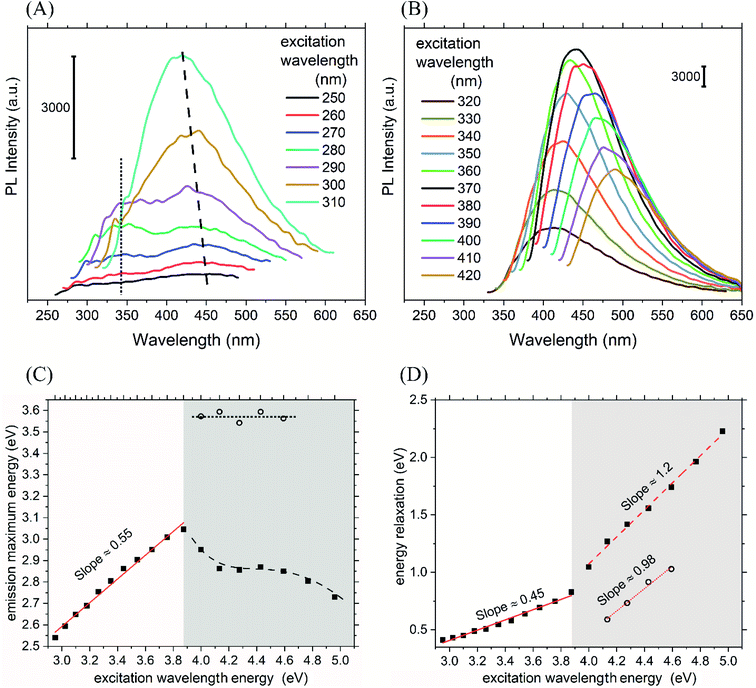
When the solution of CND was illuminated with a UV lamp of 365 nm, blue fluorescence was obtained as illustrated in Fig. 3(B). The absolute quantum yield obtained using the integrating sphere was found to be 4%. The observed excitation-dependent property of the CND is one of their distinguishing emission characteristics.61 A PL emission spectra was recorded for excitation wavelengths 250 nm to 310 nm (Fig. 5(A)) and for excitation wavelengths 320 nm to 420 nm (Fig. 5(B)). A common scale bar of the 3000 arbitrary unit size is shown in both figures to enable intensity comparison. Several trends can be observed in the PL spectra in Fig. 5(B). Firstly, a red-shift in the PL emission maxima (λem,max) is observed with an increase in excitation wavelength (λex) above 320 nm, which implies a relatively simple band structure of CND.54,62 Secondly, the intensity of PL emission increases up to a maximum of 442 nm when excited at 370 nm and then decreases with a further increase of λex. The initial increase in PL intensity may be due to the diminishing self-absorption effect or intersystem conversion. According to the literature, the bathochromic shift observed with decreasing intensity might be due to low optical absorption in the visible range or due to surface defects because of the high level of surface oxidation.51,63 The latter possibility seems to be inappropriate in our case, according to the FTIR and XPS analysis. These PL findings correlate with the PL mechanisms like quantum confinement theory and the surface state model of CND.54 However, more than one radiative relaxation channel is observed in Fig. 5(A) (λex ≤ 320) as indicated by the dot and dash lines connecting corresponding PL emission maxima (λem,max). It is noteworthy that the maximum at lower wavelengths (at ∼347 nm) does not change its position with the change of λex while the maximum at higher wavelengths blue-shifts. This mechanism is analyzed in terms of energy bands and indirect transitions below.
The PL emission with fixed λem,max in the UV range was already observed for carbon dots in carbon nitride nanoflakes and can be attributed to isolated fluorophores in our case as well.57 Although molecular state mechanism is expected for bottom-up synthesized CND, it mostly works well when the PL emission is not excitation-dependent.54,64 There are three common amino acids in casein, namely phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp), exhibiting intrinsic fluorescence. It is expected that their residues are present in the synthesized CND. The observed transition at ∼347 nm fits most likely to the luminescence of Trp. Phe and Tyr transit at shorter wavelengths and may be involved in the UV emissions in 260–310 nm. Their luminescence may be lowered due to the energy transfer between them due to their spectral match.
A more elaborate analysis of the observed phenomenon in terms of energy can be seen in Fig. 5(C) and (D). The full squares in Fig. 5(C) represent the energy (hνem,max) corresponding to λem,max observed at the higher λex, and the empty circles represent hνem,max corresponding to the λem,max observed at the lower λex. The difference between hνem,max and hνex in Fig. 5(D) is interpreted as the vibrational relaxation energy or even intersystem crossing relaxation energy. The two regions below and above 320 nm in Fig. 5(C) and (D) are distinguished by light grey colour. As shown in Fig. 5(C), for λex above 320 nm, the hνem,max obeys a single linear dependency on the hνex with the slope value ∼0.55. This is comparable to the decreasing relaxation energy in Fig. 5(D) with a slope of 0.45, resulting in zero relaxation energy for hνex ≈ 2.0. This value correlates well with the first band gap energy (Eg1) 1.8 eV. Hence, it is expected that the luminescence will vanish at about 620 nm.
Interestingly, two features were revealed at the higher excitation energy region below 320 nm. Firstly, the open circle symbols with constant energy (horizontal) dot line represent the radiative transition channel that is independent of λex. It can be associated with the isolated fluorophores most likely Trp residues as discussed above. A similarly simple pattern is seen (for open circles) in Fig. 5(D), where the energy difference decreases with unity slope, and zero relaxation energy is observed at 3.6 eV. This corresponds perfectly with the fixed emission wavelength ∼347 nm. The point at hνex = 4.0 eV (310 nm) shows the almost vanishing emission intensity, which is in agreement with the absorption edge of Trp. Secondly, the emission maxima (full squares) blue-shifts with increasing excitation wavelength in Fig. 5(C), which is manifested as diminishing progression of the relaxation energy with the slope 1.23 yielding zero relaxation energy for 3.15 eV in Fig. 5(D). This could indicate a splitting of the CB into two (CB1 and CB2), which is also consistent with the PL quantum confinement theory of CND (size of the CND ∼ 4 nm). This is the size range for eventual moderate quantum confinement effects in CND.46,65
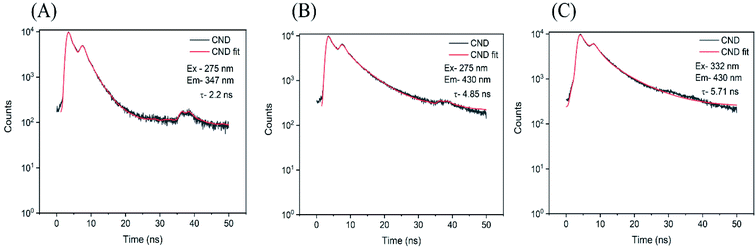
To verify the presence of three possible radiative deexcitation mechanisms and investigate their nature, time resolved PL experiment was performed. A double exponential decay was used for fitting the data and an average lifetime ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) was calculated according to the eqn (4)
was calculated according to the eqn (4)
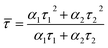 | (4) |
| Excitation wavelength (nm) | Emission wavelength (nm) | Lifetime analysis results | ||||
|---|---|---|---|---|---|---|
| α1 (—) | τ1 (ns) | α2 (—) | τ2 (ns) | ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) (ns) (ns) |
||
| 275 | 347 | 0.055 | 0.94 | 0.017 | 3.35 | 2.20 |
| 275 | 430 | 0.044 | 1.25 | 0.017 | 6.62 | 4.85 |
| 332 | 430 | 0.036 | 1.85 | 0.012 | 8.30 | 5.71 |
A revision of the Tauc plot enables hypothetical linear extrapolation of the absorbance curve at highest energies showing the second gap (Eg2) between 3 and 3.7 eV with the value ∼3.3 eV as the best guess (Fig. 4(A)). It is expected that the LUMO of the isolated fluorophore (possibly Trp) is partially located in the second conduction band (molecular state). For corresponding transitions emitting constantly at ∼347 nm, the lifetime analysis confirmed fluorescence lifetimes. These were similar to those of tryptophan residues in proteins existing in two conformations (with corresponding τ1 = 0.94 ns and τ2 = 3.35 ns) with the average lifetime about 2.2 ns (Fig. 6(A)).67 It is anticipated that the electrons excited to the higher extended states have two different ways of radiative relaxation. Either the excited electron relaxes until trapped at the localized fluorophore and the energy is released at the fixed wavelength (λem,max ∼ 347 nm), or the excited electron may relax within the CB2, and then radiative recombination at a higher wavelength may occur. The closer the hνex energy is to the hνem,max (∼3.6 eV), the less energy relaxation can be observed. This suggests some extinguishing of extended vibrational states in proximity to the isolated fluorophore energy level which works as a trap. Therefore, the lifetime was measured for emission at 430 nm excited by the 275 nm wavelength light, exactly the same as for the Trp fluorophore. A long average lifetime of 4.85 ns, typical for carbon dots was obtained (Fig. 6(B)).68,69 The short living component τ1 = 1.25 ns is quite close to the one of Trp while the long living component τ1 = 6.6 ns suggest a slow energy relaxation in an extended energy level system. It can be hypothesized that electrons excited to higher energies may live longer and relax to lower energies since there is less probability for them to be trapped at the fluorophore. In contrast, for excited electrons closer to the fluorophore lowest vibrational level, less time is available for the energy relaxation of the electron because of its high probability of being trapped. The third lifetime experiment was performed to verify the nature of typical CND luminescence dependent on excitation wavelength as observed for excitations below 3.9 eV in our case. The average lifetime of emission at 430 nm pumped by 332 nm pulses showed slow relaxations typical for extended electronic states for both components τ1 = 1.85 ns and τ2 = 8.30 ns with an average lifetime of about 5.7 ns (Fig. 6(C)).39,68
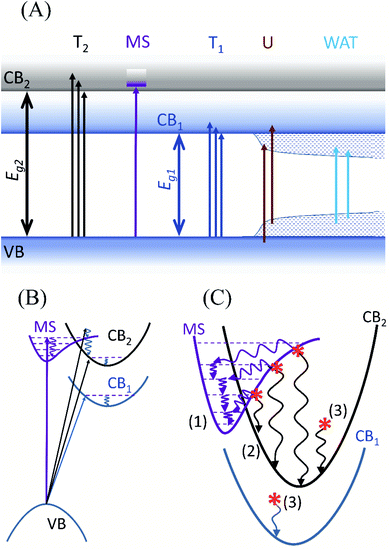
The different types of electronic transitions observed in the CND are summarized in Fig. 7 for improved understanding. The energy level diagram in Fig. 7(A) shows the two identified bands and their possible absorption transitions. It is noteworthy that the Urbach and WAT transitions cannot contribute to the observed UV and visible luminescence features due to their small energy which discriminates the standard surface state PL model from consideration. Although the simple band diagram corresponds to the absorption spectrum in Fig. 3(B) and its analysis in Fig. 4(A) and (B), it cannot fully explain the blue shift of PL emission concurrent and the fixed wavelength emission in the UV region. Hence, a new model is given to reflect the indirect allowed transition between the extended ground states in VB and extended states in CB1 or CB2. Such transitions are likely to occur in a defect-rich semiconducting material. In the band diagram of Fig. 7(B) the vertical arrow indicates the direct transition to the molecular state (MS), while the indirect transitions are depicted by inclined arrows. The vibrational relaxations are indicated by curved arrows in Fig. 7(B) and (C). The electrons (red asterisks) excited by the energies higher than 3.9 eV have such energy that they may follow either of the two possible relaxation pathways. The first channel (1) towards the left in Fig. 7(C) involves the trapping of the electron at the fluorophore followed by a fast relaxation to its lowest excited state and fast emission. The second channel (2) towards the right goes through relaxation in the CB2. As the indirect radiative recombination involves phonon or defect interaction, it takes a longer time than a direct deexcitation from the fluorophore. Therefore, the excited electron has a chance to pass through the indirect band channel only before it is caught by the trap, which results in its limited relaxation manifested as the observed blue shift. To complete the picture for excitations below 3.9 eV, the third radiative channel (3) is the standard one between CB1 and VB or CB2 and VB with vibrational relaxations. This results in the red shift of slower PL emission maximum with the diminishing progression of the difference between λex and λem,max. For the sake of simplicity, the arrows indicating radiative and other deexcitations are not shown. As none of the three standard models known for carbon nanodots can explain sufficiently the observed luminescence phenomena,46,49,58,59 we introduced the above-described model under the name “competitive model”. The name is suggested due to the competition between the first and second radiative deexcitation channels present in the electronic structure of the prepared CND.
3.2. Mechanism for the synthesis of CND
Based on the spectroscopic and microscopic characterization, a synthesis mechanism of CND has been described. For most common proteins, the standard mechanism of carbon dots synthesis from macromolecular sources follows a process of denaturation, aromatization and nucleation.8,70 However, in our case, another mechanism has been introduced to explain the nature of the reaction. Ethylene glycol is a solvent highly absorbing microwave radiation, enabling rapid heating of the reaction mixture. Casein obtained from bovine milk is polydisperse, having spherical colloidal particles of 50–600 nm. These are called “casein micelles” with an average diameter of ∼150 nm.71 In our case, casein was dispersed in ethylene glycol, and its micellar nature was restored before microwave processing. The average diameter of particles obtained after the ultrasonication of the dispersion was 497 nm (with DLS PDI 0.51) according to the DLS measurements. This indicates that the ultrasonication did not destroy the structural integrity of the casein micelles before microwave processing. The resulting CND are of two orders of magnitude smaller, about 4 nm. The diameter of the submicelles consisting of 20 to 25 casein molecules is 12 to 15 nm.72 The number of the casein coils (25) in the submicelle (15 nm) indicates a diameter of about 5 nm for a single casein coil. It is reasonable to expect that the casein micelles were disintegrated into submicelles which were further fragmented into individual casein coils by the fast microwave heating. Further, the denatured casein coils are partially carbonized into CND, retaining still some moieties and structural features of the casein source. The solvent molecules neither take part in transesterification reactions nor allow oxidation. The presence of PVP forms most likely a capping layer around the CND and prevents them from forming agglomerations.3.3. SEBS/CND nanocomposite film
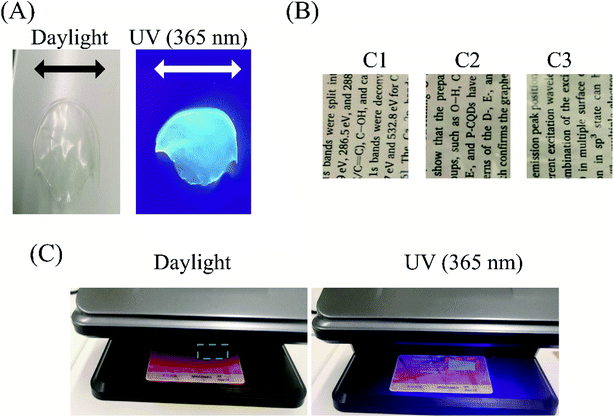
To verify the use of the nanocomposite films for anticounterfeit application, the films were tested under visible and UV light as shown in Fig. 8(A). When the film was irradiated with a UV lamp (365 nm), the film emitted a blue fluorescence. However, under visible light conditions, the film appears to be colourless. These results show that the prepared SEBS/CND nanocomposite film is UV sensitive and can be used for anticounterfeit applications.The light transmittance through these nanocomposite films was studied along ASTM D 1003. It was observed that the composite film (0.5% CND) had optical transparency of 45%. To visually observe the transparency, we kept the synthesized films with different compositions of CND over some text. As can be seen in Fig. 8(B), the letters in the text are visible even up to 1% CND concentration. Fig. 8(C) shows SEBS/CND polymer films on a guest card of Tomas Bata University. The figure beautifully depicts the transparency of the film in the daylight; however, when the films were exposed to a UV banknote detector of wavelength 365 nm, the bright luminescence of the films is observed. Hence, these films can be effectively used in the packaging industries and for anticounterfeit applications.
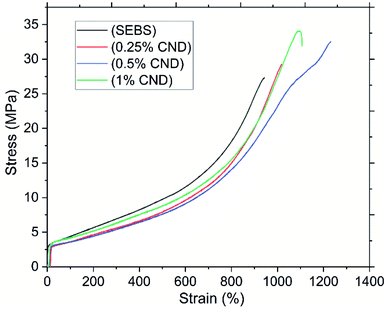 | ||
| Fig. 10 Stress–strain curves of SEBS and SEBS/CND nanocomposite film – one curve selected as an example for each sample. | ||
The elastomer characteristics were observed in the strain, with the increase in the concentration of the CND filler, the strain at break increases from ca. 900% to ca. 1200%. This shows that the addition of filler increases the resistance of the nanocomposite film. However as can be seen, with the increase in the concentration of the filler to 1%, a decrease in the strain is observed. This can be attributed to the fact that with the increase in concentration, the particles tend to agglomerate leading to the break of the nanocomposite film.73 The Young's modulus of the pristine SEBS and the nanocomposite film was found to be (97 ± 8) MPa and (77 ± 9) MPa (C3), respectively. This decrease in the Young's modulus can be accounted to the presence of cracks and pores in the nanocomposite film or to poor adhesion of the filler and matrix. It may also be due to the very different nature of SEBS and PVP, which are normally immiscible and only copolymer grafting results in compatibilisation.74
3.4. Carbon nanodots as anticounterfeit ink
To demonstrate the application of CND as anticounterfeit ink, we created some patterns on a PET substrate. Inkjet printing was used to obtain the desired patterns of the CND ink. The dynamic viscosity, surface tension, and density of the CND ink are measured to be 2.263 mPa s, 41 N m−1, and 1.04 g cm−3.Fig. 11(A) depicts an image of the printed patterns from CND ink with and without the presence of UV (254 nm). The motif is practically invisible in daylight. However, when the patterns were excited at 254 nm using a UV lamp, the patterns emitted an intensive blue fluorescence. Excitation of the patterns by 365 nm wavelength resulted in a less prominent fluorescence on the substrate. These results indicate the effectiveness of the CND to be used as anticounterfeit ink for printing. As seen in Fig. 11(B), the compatibility of the CND ink with the PET substrate was analyzed using water contact angle studies. Five measurements were carried out on the substrate at different places. Their average was calculated to be 18.24°. This indicates the hydrophilic nature of the ink towards the substrate.
 | ||
| Fig. 11 (A) Printed pattern from the CND ink on PET substrate with (right) and without (left) UV (254 nm). (B) Water contact angle on PET substrate. | ||
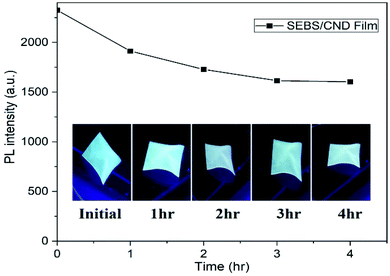
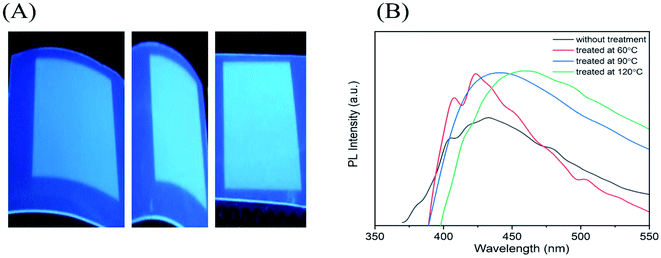
Fig. 12(A) shows the thermal stability of the ink, which was analyzed by treating the printed patterns at three different temperatures 60 °C, 90 °C, 120 °C. The patterns were kept at each temperature for 60 minutes. As observed in Fig. 12(B), the PL intensity of the untreated PET substrate with the ink has less luminescence as compared to the treated PET substrates with the ink. As apparent from the spectra, the luminescence of the ink substrates treated at different temperatures remains almost the same with their maximum red shifted due to prevalent agglomerations with increase in temperature. This indicates a good thermal and photostability of the CND ink.
3.5. Application of CND as phosphors in LEDs
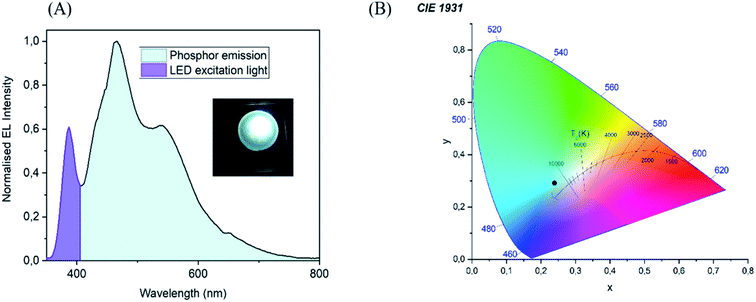
To demonstrate the ability of CND as phosphors, a CND-based phosphor LED was created by layering SEBS/CND nanocomposite film on top of a UV chip with an excitation wavelength of about 365 nm. The luminescence spectra with the operating device and the chromaticity diagram with the final colour of the device is displayed in Fig. 13(A) and (B).Phosphors based on materials with maximum emission wavelength dependent on the excitation wavelength may suffer from problems like self absorption and long wavelength emission. As a result of these factors, the device's efficiency is reduced. These problems were eliminated to a great extent by a proper selection of the film thickness and concentration of CND. Moreover, a commercial reflective UV protection window film was used to enhance the efficiency of CND luminescence. As this was just a preliminary optimization, a cutoff filter at 395 nm was used to suppress the excessive excitation UV light coming through the phosphor film. Further development is necessary to fully optimize the device, however, this is beyond the scope of this article.
4. Conclusions
In summary, the synthesis of casein-based CND has been successfully achieved using a microwave-assisted approach. The advantage of this method is its high product yield of about 25% with respect to the initial weight of casein precursor and PVP stabilizer. A synthesis mechanism has been deduced. Casein micelles were quickly disintegrated into submicelles, which were then separated into individual casein coils of the size that corresponded to the CND. The casein coils were only partially carbonized, and the resulting amorphous CND still included certain structural motifs and chemical moieties from the original source. Regarding the electronic properties, CND showed blue fluorescence when irradiated with a UV lamp (365 nm), and the emission properties manifested excitation-dependent PL characteristics. The unique blue shift followed by red shift in the PL spectra and the portion of UV luminescence independent on the excitation wavelength stands out to be an exciting property of the casein derived CND, which has a scope to be explored more. The mechanism underlying these luminous characteristics can be explained by a new competitive model which is beyond the standard models in CND. The observation of Urbach and WAT region tailing deep into the band gap in the absorption spectrum gave a new perspective to understand the defects and disorders in the electronic structure of CND. The solution cast method was utilized to obtain a flexible SEBS/CND nanocomposite film with good photostability, optical transmittance, and mechanical properties. The synthesized CND has found its application in anticounterfeit market for polymer ink with appreciable thermal stability. The free-standing luminescent nanocomposite film can be effectively employed as anticounterfeit films for packaging and security applications. The potential of CND as phosphor material for LEDs as has been suggested in the work using a unique experimental setup which eliminates excess of UV transmission and avoids self absorption resulting in lower efficiency. Moreover, the transparency attained by this polymeric nanocomposite makes it to be a promising candidate for possible applications in information technology, defense system, transportation, and environmental protection. The promising results obtained and the characteristics like nontoxicity, easy preparation, cost effectiveness of CND makes it a good phosphor material to be used for multipurpose applications.Author contributions
R. Blessy Pricilla: conceptualization, methodology, investigation, formal analysis, visualization, writing – original draft. David Skoda: conceptualization, methodology, investigation, supervision. Pavel Urbanek: investigation. Michal Urbanek: investigation. Pavol Suly: investigation. Eva Domincova Bergerova: investigation. Ivo Kuritka: conceptualization, methodology, formal analysis, writing – review & editing, project administration, funding acquisition, supervision.Conflicts of interest
The authors declare no competing interest.Acknowledgements
The authors appreciate the financial assistance with gratitude from the Ministry of Education, Youth and Sports of the Czech Republic Program – DKRVO (RP/CPS/2022/007). Authors thank P. Machac (Masaryk University) for XPS measurement. CzechNanoLab project LM2018110 funded by MEYS CR is gratefully acknowledged for the financial support of the measurements at CEITEC Nano Research Infrastructure.References
- A. Sciortino, A. Cannizzo and F. Messina, C, 2018, 4, 67 CAS.
- A. Cayuela, M. L. Soriano, C. Carrillo-Carrion and M. Valcarcel, Chem. Commun., 2016, 52, 1311–1326 RSC.
- Y. Park, J. Yoo, B. Lim, W. Kwon and S. W. Rhee, J. Mater. Chem. A, 2016, 4, 11582–11603 RSC.
- J. Zhang and S. H. Yu, Mater. Today, 2016, 19, 382–393 CrossRef CAS.
- W. Lu, X. Gong, Z. Yang, Y. Zhang, Q. Hu, S. Shuang, C. Dong and M. M. F. Choi, RSC Adv., 2015, 5, 16972–16979 RSC.
- A. V. Longo, A. Sciortino, M. Cannas and F. Messina, Phys. Chem. Chem. Phys., 2020, 22, 13398–13407 RSC.
- A. Sharma and J. Das, J. Nanobiotechnol., 2019, 17, 92 CrossRef PubMed.
- L. Sai, J. Chen, Q. Chang, W. Shi, Q. Chen and L. Huang, RSC Adv., 2017, 7, 16608–16615 RSC.
- W. Wang, C. Damm, J. Walter, T. J. Nacken and W. Peukert, Phys. Chem. Chem. Phys., 2016, 18, 466–475 RSC.
- Q. Ren, L. Ga and J. Ai, ACS Omega, 2019, 4, 15842–15848 CrossRef CAS PubMed.
- M. Varisco, D. Zufferey, A. Ruggi, Y. Zhang, R. Erni and O. Mamula, R. Soc. Open Sci., 2017, 4, 170900 CrossRef PubMed.
- K. M. Omer, S. A. Idrees, A. Q. Hassan and L. A. Jamil, New J. Chem., 2020, 44, 5120–5126 RSC.
- M. Li, Y. Feng, Q. Tian, W. Yao, L. Liu, X. Li, H. Wang and W. Wu, Dalton Trans., 2018, 47, 11264–11271 RSC.
- Z. J. Wang, X. J. Zhao, Z. Z. Guo, P. Miao and X. Gong, Org. Electron., 2018, 62, 284–289 CrossRef CAS.
- A. G. El-Shamy and H. S. S. Zayied, Synth. Met., 2020, 259, 116218 CrossRef CAS.
- M. A. Issa and Z. Z. Abidin, Molecules, 2020, 25, 3541 CrossRef CAS PubMed.
- S. Lu, L. Sui, J. Liu, S. Zhu, A. Chen, M. Jin and B. Yang, Adv. Mater., 2017, 29, 1603443 CrossRef PubMed.
- P. Gupta, M. Bera and P. K. Maji, Polym. Adv. Technol., 2017, 28, 1428–1437 CrossRef CAS.
- J. Andres, R. D. Hersch, J.-E. Moser and A. S. Chauvin, Adv. Funct. Mater., 2014, 24, 5029–5036 CrossRef CAS.
- K. T. P. Lim, H. Liu, Y. Liu and J. K. W. Yang, Nat. Commun., 2019, 10, 25 CrossRef CAS PubMed.
- J. Y. Park, J. W. Chung and H. K. Yang, Ceram. Int., 2019, 45, 11591–11599 CrossRef CAS.
- F. Liu, Z. Li, Y. Li, Y. Feng and W. Feng, Carbon, 2021, 181, 9–15 CrossRef CAS.
- P. Kumar, S. Singh and B. K. Gupta, Nanoscale, 2016, 8, 14297–14340 RSC.
- Z. Zhang, W. Sun and P. Wu, ACS Sustainable Chem. Eng., 2015, 3, 1412–1418 CrossRef CAS.
- J. Guo, H. Li, L. Ling, G. Li, R. Cheng, X. Lu, A.-Q. Xie, Q. Li, C.-F. Wang and S. Chen, ACS Sustainable Chem. Eng., 2020, 8, 1566–1572 CrossRef CAS.
- J.-x. Zheng, X.-h. Liu, Y.-z. Yang, X.-g. Liu and B.-s. Xu, New Carbon Mater., 2018, 33, 276–288 CrossRef CAS.
- Y. Ma, X. Zhang, J. Bai, K. Huang and L. Ren, Chem. Eng. J., 2019, 374, 787–792 CrossRef CAS.
- S. S. Jones, P. Sahatiya and S. Badhulika, New J. Chem., 2017, 41, 13130–13139 RSC.
- W. T. Hong and H. K. Yang, Optik, 2021, 241, 166449 CrossRef CAS.
- M. He, J. Zhang, H. Wang, Y. Kong, Y. Xiao and W. Xu, Nanoscale Res. Lett., 2018, 13, 175 CrossRef PubMed.
- H. Li, Y. Xu, L. Zhao, J. Ding, M. Chen, G. Chen, Y. Li and L. Dang, Carbon, 2019, 143, 391–401 CrossRef CAS.
- N. K. Sahoo, G. C. Jana, M. N. Aktara, S. Das, S. Nayim, A. Patra, P. Bhatttacharjee, K. Bhadra and M. Hossain, Mater. Sci. Eng., C, 2020, 108, 110429 CrossRef CAS PubMed.
- L. Cadesky, M. W. Ribeiro, K. T. Kriner, M. V. Karwe and C. I. Moraru, J. Dairy Sci., 2017, 100, 7055–7070 CrossRef CAS PubMed.
- M. Warncke and U. Kulozik, Foods, 2021, 10, 1361 CrossRef CAS PubMed.
- Y. T. Xie, J. X. Zheng, Y. L. Wang, J. L. Wan, Y. Z. Yang, X. G. Liu and Y. K. Chen, Nanotechnology, 2019, 30, 085406 CrossRef CAS PubMed.
- A. Pal, M. P. Sk and A. Chattopadhyay, Mater. Adv., 2020, 1, 525–553 RSC.
- B. Zhang, Y. Liu, M. Ren, W. Li, X. Zhang, R. Vajtai, P. M. Ajayan, J. M. Tour and L. Wang, ChemSusChem, 2019, 12, 4202–4210 CrossRef CAS PubMed.
- K. J. Mintz, M. Bartoli, M. Rovere, Y. Zhou, S. D. Hettiarachchi, S. Paudyal, J. Chen, J. B. Domena, P. Y. Liyanage, R. Sampson, D. Khadka, R. R. Pandey, S. Huang, C. C. Chusuei, A. Tagliaferro and R. M. Leblanc, Carbon, 2021, 173, 433–447 CrossRef CAS.
- R. Bandi, B. R. Gangapuram, R. Dadigala, R. Eslavath, S. S. Singh and V. Guttena, RSC Adv., 2016, 6, 28633–28639 RSC.
- F. Victoria, J. Manioudakis, L. Zaroubi, B. Findlay and R. Naccache, RSC Adv., 2020, 10, 32202–32210 RSC.
- J. Ederer, P. Janoš, P. Ecorchard, J. Tolasz, V. Štengl, H. Beneš, M. Perchacz and O. Pop-Georgievski, RSC Adv., 2017, 7, 12464–12473 RSC.
- D. M. Eby, K. Artyushkova, A. K. Paravastu and G. R. Johnson, J. Mater. Chem., 2012, 22, 9875–9883 RSC.
- C. Wang, C. Wang, P. Xu, A. Li, Y. Chen and K. Zhuo, J. Mater. Sci., 2016, 51, 861–867 CrossRef CAS.
- D. Xu, F. Lei, H. Chen, L. Yin, Y. Shi and J. Xie, RSC Adv., 2019, 9, 8290–8299 RSC.
- A. P. Sirocic, L. K. Krehula, Z. Katancic and Z. H. Murgj, Chem. Biochem. Eng. Q., 2017, 30, 501–509 CrossRef.
- P. K. Sarswat and M. L. Free, Phys. Chem. Chem. Phys., 2015, 17, 27642–27652 RSC.
- D. L. Vien, N. Colthup, W. Fateley and J. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Academic Press, Boston, 1991 Search PubMed.
- C. Zhu, J. Zhai and S. Dong, Chem. Commun., 2012, 48, 9367–9369 RSC.
- B. Manoj, A. M. Raj and G. T. Chirayil, Sci. Rep., 2017, 7, 18012 CrossRef PubMed.
- I. J. Gomez, M. V. Sullerio, A. Doleckova, N. Pizurova, J. Medalova, R. Roy, D. Necas and L. Zajickova, J. Phys. Chem. C, 2021, 125, 21044–21054 CrossRef CAS.
- C. M. Carbonaro, R. Corpino, M. Salis, F. Mocci, S. V. Thakkar, C. Olla and P. C. Ricci, C, 2019, 5, 60 CAS.
- J. T. Margraf, V. Strauss, D. M. Guldi and T. Clark, J. Phys. Chem. B, 2015, 119, 7258–7265 CrossRef CAS PubMed.
- G. D. Gesesse, A. G. Berenguer, M.-F. Barthe and C. O. Ania, J. Photochem. Photobiol., A, 2020, 398, 112622 CrossRef CAS.
- K. J. Mintz, Y. Zhou and R. M. Leblanc, Nanoscale, 2019, 11, 4634–4652 RSC.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627–637 CrossRef CAS.
- C. Murru, R. Badia-Laino and M. E. D. Garcia, Antioxidants, 2020, 9, 1147 CrossRef CAS PubMed.
- D. D. Ferreyra, D. R. Sartori, S. D. E. Riega, H. B. Rodriguez and M. C. Gonzalez, Carbon, 2020, 167, 230–243 CrossRef CAS.
- D. L. Wood and J. Tauc, Weak Absorption Tails in Amorphous Semiconductors, Phys. Rev. B: Solid State, 1972, 5, 3144–3151 CrossRef.
- I. Studenyak, M. Kranjec and M. Kurik, Int. J. Opt. Appl., 2014, 4, 76–83 Search PubMed.
- K. P. Neha Sharma, S. Ilango, S. Dash and A. K. Tyagi, Advanced Materials Proceedings, 2017, 2, 342–346 Search PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. J. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- B. Manoj, A. M. Raj and G. C. Thomas, Sci. Rep., 2018, 8, 13891 CrossRef PubMed.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- B. Zhi, X. X. Yao, Y. Cui, G. Orr and C. L. Haynes, Nanoscale, 2019, 11, 20411–20428 RSC.
- Y. H. Liu, H. Huang, W. J. Cao, B. D. Mao, Y. Liu and Z. H. Kang, Mater. Chem. Front., 2020, 4, 1586–1613 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, USA, 2006 Search PubMed.
- J. R. Alcala, E. Gratton and F. G. Prendergast, Biophys. J., 1987, 51, 597–604 CrossRef CAS.
- S. Kalytchuk, Y. Wang, K. Polakova and R. Zboril, ACS Appl. Mater. Interfaces, 2018, 10, 29902–29908 CrossRef CAS PubMed.
- T. Yu, H. Wang, C. Guo, Y. Zhai, J. Yang and J. Yuan, R. Soc. Open Sci., 2018, 5, 180245 CrossRef PubMed.
- X. Liu, T. Z. Li, Y. Hou, Q. H. Wu, J. Yi and G. L. Zhang, RSC Adv., 2016, 6, 11711–11718 RSC.
- P. F. Fox and A. Brodkorb, Int. Dairy J., 2008, 18, 677–684 CrossRef CAS.
- C. Phadungath, Songklanakarin J. Sci. Technol., 2004, 27, 201–212 Search PubMed.
- J. R. Dios, C. G. Astrain, P. Costa, J. C. Viana and S. L. Mendez, Materials, 2019, 12, 1405 CrossRef CAS PubMed.
- L. Jun, L. Qiaochu, Q. Rongrong and S. Yanhan, China Pat., CN100564416C, 2009 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |