Open Access Article
Open Access ArticleSelenium substituted axitinib reduces axitinib side effects and maintains its anti-renal tumor activity†
Ying Fu‡
 a,
Rengui Saxu‡a,
Kadir Ahmad Ridwana,
Cai Zhaoa,
Xiangshun Konga,
Yao Ronga,
Weida Zhengb,
Peng Yu*a and
Yuou Teng
a,
Rengui Saxu‡a,
Kadir Ahmad Ridwana,
Cai Zhaoa,
Xiangshun Konga,
Yao Ronga,
Weida Zhengb,
Peng Yu*a and
Yuou Teng *a
*a
aChina International Science and Technology Cooperation Base of Food Nutrition/Safety and Medicinal Chemistry, College of Bioengineering, Tianjin University of Science and Technology, Tianjin 300457, China. E-mail: Tyo201485@tust.edu.cn
bMedical College, Yanbian University, No.977 Gongyuan Road, Yanji City, Jilin Province 133002, P. R. China
First published on 8th August 2022
Abstract
Axitinib is a potent vascular endothelial growth factor receptor (VEGFR) inhibitor, which has a strong inhibitory effect on the three isoforms of VEGFR 1–3. Having strong therapeutic efficacy, its broad use is limited by its side effects such as hypertension, proteinuria, cardiovascular damage, and liver and kidney dysfunction. Selenium compounds are broadly reported to have a good protective effect on cardiovascular disease, inflammation, infection, and immune function. In this study, a selenium substitute of axitinib was synthesized, and its anti-renal cell carcinoma activity and side effects were investigated. The results of the study indicated that Se-axitinib had potent antitumor activity on renal cell carcinoma (RCC), alleviated vascular hyperpermeability, and also alleviated axitinib-related side effects including hypertension, liver dysfunction and kidney dysfunction significantly. Therefore, we suggest that Se-axitinib could be a solution to the severe side effects of VEGFR inhibitors and provide evidence to improve the outcome of RCC treatment.
1. Introduction
Renal cell cancer or renal cell adenocarcinoma are the most prevalent types of kidney tumor.1 Medical treatment for renal cell carcinoma (RCC) has transitioned from a nonspecific immune approach such as cytokine and interferon, to targeting vascular endothelial growth factor receptors (VEGFR),2 and now to novel immunotherapy agents like PD-1 and PD-L1 inhibitors. Among all factors in the pathogenesis and progression of RCC, the VEGF-VEGFR signalling pathway plays a key role and is a pivotal mediator of tumor angiogenesis.3 Axitinib is an oral second-generation tyrosine kinase inhibitor (TKI)4 which displays a significant and selective inhibitory effect on VEGF receptors 1, 2, and 3.5 In January 2012, the Food and Drug Administration (FDA) approved axitinib for the treatment of advanced RCC after failure of one prior systemic therapy.6Common side effects of axitinib occur in greater than 30% (ref. 7) of patients and include diarrhea, hypertension, fatigue, loss of appetite,8 nausea, dysphonia (hoarse soft voice),9 decrease in kidney function, and anemia.10 Therefore, it is important to get these side effects under control and improve the life standard of the patients who are taking axitinib.11 Selenium is an essential micronutrient,12 in the human body, it is involved with the regulation of cell metabolism,13 DNA, and RNA,14 as well as protein synthesis,15 and is at the active site of several enzymes of the antioxidant network. A considerable number of studies prove that selenoproteins and some small molecular selenium compounds have antitumor,16 cardioprotective,17 anti-inflammatory,18 anti-infective19 and immunity-boosting effects.20 We synthesized new compound (Se-axitinib) by chemical synthesis, replacing the sulphur in the original compound with selenium to investigate whether Se-axitinib can reduce the side effects caused by axitinib, the blood pressure, the liver and kidney function damage and vascular endothelial injury were measured in Wistar rats. And this research also investigated the vascular permeability and the anti-tumor activity of Se-axitinib in mice. In the current study we tested the hypothesis that selenium substitution of axitinib may possibly maintain the antitumor activity of axitinib and decrease drug related side effect.
2. Materials and methods
2.1 Cell line and animals
Human renal cell carcinoma cell line (786-O) was purchased from Kyowa Cells Bank. Cells were cultured in RPMI Medium 1640 basic (1×, Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37 °C of 5% CO2.10 males Wistar rats, 50 females BALB/c nude mice and 9 females C57BL/6 mice between 6–8 weeks' age were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the ethical committee of Tianjin University of Science and Technology.
2.2 Compounds testing
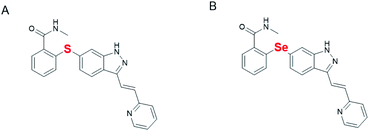
To a mixture of compound 1 (1.0 g, 2.32 mmol) and compound 2 (1.99 g, 4.64 mmol) in DMSO (10 mL), the CuS (4.43 mg, 46.37 μmol), iron powder (77.69 mg, 1.39 mmol), K2CO3 (320.45 mg, 2.32 mmol) were added, then, the reaction mixture was heated to 140 °C under the argon's atmosphere. When the TLC (thin layer chromatography) detection showed that it was completed. The reaction mixture was poured into water then, extracted with ethyl acetate, the organic layer was washed with brine, dried over sodium sulfate. The organic phase was concentrated under vacuum to give crude product. The cured was purified on silica gel column (mobile phase: petroleum ether/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 912 mg of pure compound 3 with 76% of yield. p-Toluenesulfonic acid monohydrate (1.1 g, 5.79 mmol) was added to a solution of compound 3 (0.5 g, 0.96 mmol) in MeOH/H2O (5.0 mL/0.5 mL). And the mixture was stirred for 20 min at room temperature, then, the reaction was completed and filtered it in a dark environment. The filter cake was washed by cooled water 3 times, to give pure compound 4 (0.41 g) in 94% of yield. Compound 4 (200 mg) was added to 4 mL of methylamino in methanol, and then, the reaction mixture was stirred at 70 °C for 36 hours. After the reaction complete, the solvent was evaporated under reduced pressure, 2 mL of methyl tert-butyl ether was added and the products were precipitated, then after filtering, and filter cake drying to obtain 180 mg of Se-axitinib (yield: 90%; 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 8.61–8.58 (m, 2H), 8.24 (d, J = 8.4 Hz, 1H), 7.97 (d, J = 16.4 Hz, 1H), 7.86 (s, 1H), 7.84–7.80 (m, 1H), 7.69–7.67 (m, 2H), 7.60 (d, J = 16.4 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.30–7.22 (m, 3H), 6.90–6.87 (m, 1H), 2.81 (d, J = 4.0 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 167.9, 154.9, 149.6, 142.1, 136.9, 135.6, 133.5, 131.0, 129.5, 129.3, 128.7, 127.9, 125.4, 123.7, 122.7, 122.5, 121.9, 120.7, 118.5, 26.3).
1) to give 912 mg of pure compound 3 with 76% of yield. p-Toluenesulfonic acid monohydrate (1.1 g, 5.79 mmol) was added to a solution of compound 3 (0.5 g, 0.96 mmol) in MeOH/H2O (5.0 mL/0.5 mL). And the mixture was stirred for 20 min at room temperature, then, the reaction was completed and filtered it in a dark environment. The filter cake was washed by cooled water 3 times, to give pure compound 4 (0.41 g) in 94% of yield. Compound 4 (200 mg) was added to 4 mL of methylamino in methanol, and then, the reaction mixture was stirred at 70 °C for 36 hours. After the reaction complete, the solvent was evaporated under reduced pressure, 2 mL of methyl tert-butyl ether was added and the products were precipitated, then after filtering, and filter cake drying to obtain 180 mg of Se-axitinib (yield: 90%; 1H NMR (400 MHz, DMSO-d6) δ 13.43 (s, 1H), 8.61–8.58 (m, 2H), 8.24 (d, J = 8.4 Hz, 1H), 7.97 (d, J = 16.4 Hz, 1H), 7.86 (s, 1H), 7.84–7.80 (m, 1H), 7.69–7.67 (m, 2H), 7.60 (d, J = 16.4 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.30–7.22 (m, 3H), 6.90–6.87 (m, 1H), 2.81 (d, J = 4.0 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 167.9, 154.9, 149.6, 142.1, 136.9, 135.6, 133.5, 131.0, 129.5, 129.3, 128.7, 127.9, 125.4, 123.7, 122.7, 122.5, 121.9, 120.7, 118.5, 26.3).
2.3 Measurements of blood pressure (tail-cuff)
Six-week-old male Wister rats weighing from 180–200 g were adaptively fed for one week, and rats (n = 3–4) were randomly divided into axitinib group, Se-axitinib group and control group.Male rats were used in blood pressure testing that the blood pressure of female rats is more variable in the estrous cycle of 4–5 days than male rats, which makes them unpredictable. And testosterone spikes of male rats make them variable by the hour but comparatively steady in daily and weekly time. Axitinib (25 mg kg−1) and Se-axitinib (25 mg kg−1) were formulated in 0.5% sodium carboxymethylcellulose (CMC-Na). CMC-Na was treated as negative control group, axitinib and Se-axitinib was suspended in CMC-Na for oral administration. Intragastric administration of drugs from the next day was recorded as the first day, and the dosing was performed twice a day with an interval of 8 h for seven consecutive days. Blood pressure was measured after the drug treatment for 4 h each day. For blood pressure testing, rats were placed in tail cuff for 10 min to condition to the procedure, and five consecutive measurements were performed in less than 30 min.
2.4 Determination of biological indicator
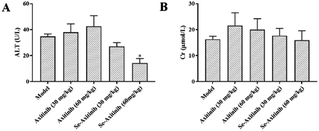
At the sixth day of drug administration, rats were placed in metabolic cage for 24 h to collect the excreta. After treatment process was ended, the rats were sacrificed and blood samples, liver and kidney were used to evaluate the liver function and renal function. The level of and ALT (GPT, glutamic pyruvic transaminase) and LDH (lactic dehydrogenase) in the rat serum was tested to index liver function. The level of Cr and BUN in the rat serum was tested to index renal function. Spectrophotometric commercial kits were used in this procedure and all commercial kits were bought from Nanjing Jiancheng Bioengineer Company (Nanjing, China), and conducted following manufacturer's instruction manual.2.5 Skin vascular permeability assay
In the present study, Miles assay was performed to assess the effect of axitinib and Se-axitinib on VEGF-A induced skin vascular hyperpermeability. Female C57BL/6 mice were used to obtain intact skin samples. Briefly, 8 week age C57BL/6 mice (n = 3) received single oral dose of either axitinib (10 mg kg−1), Se-axitinib (10 mg kg−1) or control CMC-Na followed by an injection of 200 μl histamine inhibitor pyrilamine maleate intraperitoneally. Ten minutes later, 100 μl Evans blue dye was injected through the tail vain and worked for 30 min. Then, the mice were anesthetized with 20% urethane and 20 μl of 1% murine VEGF-A or PBS was intradermally injected into two flanks of the mouse respectively, and made triplicate injections. Four hours after drug treatment, mice were sacrificed and each skin region was dissected and dried, and then immersed in 250 μl formamide in a heating block at 55 °C. The samples were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 20 min and the absorbance at 620 nm was measured.
500 rpm for 20 min and the absorbance at 620 nm was measured.
2.6 Mouse xenograft model
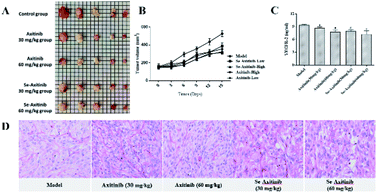
In the mouse xenograft model, 150 μl suspension cells of 786-O (1 × 106 cells) were intradermally injected into the right flank region of BALB/c nude mice. Male mice were aggressive and commonly affected the process of mouse xenograft model establishment and the experimental results. Female BALB/c nude mice were used to establish tumor models. Then, after the tumor volume was reached about 500 mm3, the tumor piece was excised and 1–2 mm3 tumor fragments were implanted into the flank region of other mice. The treatment started when the tumor volume was about 150 mm3, and the mice were separated into five groups (n = 10): model group, axitinib low dose group (30 mg kg−1), axitinib high dose group (60 mg kg−1), Se-axitinib low dose group (30 mg kg−1), Se-axitinib high dose group (60 mg kg−1). Compounds were formulated in 0.5% CMC-Na and dosed orally twice daily for 15 days. Tumor volumes were measured every third day by electronic calipers and calculated according to the equation: tumor growth inhibition (TGI) = (Vmodel − Vtreatment)/Vmodel × 100%. Hematoxylin–eosin staining was conducted by Servicebio Co., Ltd.2.7 Statistical processing
The experimental data was processed using GraphPad Prism7 software, and the experimental result of each group was expressed as mean ± standard deviation (x ± s). The t-test was used to compare the statistical significance between groups. P < 0.05 indicates a statistically significant difference, 0.001 < P < 0.01 indicates a comparatively statistical significance, P < 0.001 indicates a very significant difference.3. Results
3.1 Comparison of drug-related side effects between axitinib and Se-axitinib
Serum creatinine (Cr) and blood urea nitrogen (BUN) are important biochemical indexes to evaluate kidney function. The levels of Cr and BUN after 7 days' drug treatment were shown in Fig. 2G and H. No impact of axitinib and Se-axitinib on kidney function was observed in the present study.
Therefore, it can be concluded that Se-axitinib had lower serum ALT, LDH and higher proportion of liver index which indicated selenium substitution could be a solution for axitinib-related liver dysfunction.
3.2 Effect of different compounds on skin vascular permeability
In Miles assay, each group was divided into VEGF-A group that mediates high blood vessel permeability and PBS control group. As shown in Fig. 3B, in the control group, VEGF-A significantly increased vascular permeability compare to PBS group (170% vs. 100%), which confirmed the successful establishment of the experimental model.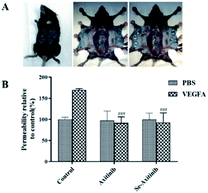 | ||
| Fig. 3 Effect of axitinib and Se-axitinib on skin vascular permeability. (A) Experimental diagram (B) effect of different compounds on skin vascular permeability, ###P < 0.001 vs. PBS group. | ||
Compared with the control group, the PBS group in axitinib group had no significant difference, but the vascular permeability of the VEGF-A group in axitinib group was significantly reduced. Therefore, it can be concluded that axitinib can reduce VEGF-A-related skin vascular hyperpermeability. Similar results were also observed in the Se-axitinib group that PBS group was not significantly different, but the VEGF-A group showed the same effect of alleviating vascular permeability as axitinib. From this result, we suspected that Se-axitinib may be able to be an anti-tumor drug.
3.3 In vivo therapeutic efficacy studies
Fig. 4B showed the changes of tumor volume during 15 days' treatment. It was clear that the tumor volume of the model group increased remarkably while treatment of different doses of axitinib and Se-axitinib significantly reduced the growth of xenograft tumor. The tumor volume of the model group was relatively bigger than other group and the tumors of the high dose group were relatively smaller than the low dose group (Fig. 4A). Tumor tissue from tumor-bearing mice was stained with hematoxylin and eosin to detect histopathological changes after drug administration. As shown in Fig. 4D, tumor atypia and mitotic regions were clearly observed, with a delicate cytoplasm and small nucleus in the model group. In the treatment groups, the nucleus was larger and fewer mitotic regions were detected. A tendency towards highly antitumor activity of axitinib and Se-axitinib were observed in HE staining.
4. Conclusions and discussion
In this study, we synthesized selenium substitute of axitinib and assessed whether selenium substitution of sulphur in axitinib display milder side effects compared with the original drug axitinib and maintain its anti-tumor activity. As expected, VEGFR inhibitor axitinib significantly increased the systolic blood pressure of Wistar rats in 7 days' treatment while selenium substitute of axitinib showed lower BP increasing effect. We hypothesize that the protective effect of selenium substitution against BP increase was mediated by antioxidant and anti-inflammatory activity of selenium,21 as selenoprotein enzyme like glutathione peroxidase (GPX) can reduce hydrogen peroxide and phospholipid hydroperoxides, and therefore reduce the production of free radicals and reactive oxygen species (ROS).22 On the other hand, the mechanisms of TKI induced hypertension were believed to be related with the decreases of nitric oxide and increases in circulating endothelin-1 which causes endothelial dysfunction,23 and whether the blood pressure stabilizing effect of Se-axitinib is mediated by the effect of nitric oxide and endothelin-1 on vascular endothelium remains to be further investigated. One epidemiological survey in China between 2003 and 2018 found that the dietary selenium intake had a negative and non-linear correlation with the risk of stroke in adults, which also supports the beneficial effect of selenium compound on cardiovascular disease.24 Weight increasing effect of Se-axitinib compared to axitinib was also related to the antioxidant activity of selenium compounds, as well as immune regulating effect. In immune response, selenium and selenoprotein work through selenoprotein enzymes like GPXs, and the proper functioning of the immune system relies on adequate dietary selenium intake and exerts its biological effects through its incorporation into selenoproteins.25 Research shows that dietary selenium supplementation can increase body weight gain and feed intake, and regulates immune response of poultry in stress.26 Then, the effects of Se-axitinib and axitinib on liver and kidney function were compared, and Se-axitinib had lower serum ALT, LDH and higher liver index after treatment for 7 days. But no significant difference between treatment group and control group in the levels of BUN and Cr were observed. Thus, Se-axitinib reduced the liver toxicity caused by axitinib, but both had no significant effect on the kidney function indices. Satoshi Imai etc. claimed that27 there might be limited impact of axitinib on renal function, even if accompanied by proteinuria, which was consistent with the results of our research. We hypothesis that the differences of selenium substitute of axitinib on liver function was also related to the antioxidant activity mediated by selenium compounds.Anticancer activity of Se-axitinib is an important field that needs to be illustrated closely in this research. A considerable number of research revealed that organic and inorganic selenium compounds possess strong anticancer activity in vivo and in vitro.28 Inorganic selenium compounds such as selenate, selenite and sodium selenite exhibit excellent chemopreventive and anti-tumor activity by affecting apoptosis and cell proliferation of various types of tumors.29 It is highly believed that the beneficial effect of selenium compound was mediated by selenoproteins, however, selenium substitution did not increase the anticancer activity of the original drug axitinib even if it showed similar anti-renal cancer activity and decreased VEGFA-induced vascular hyperpermeability. The inner mechanism needs to be investigated more closely in future research. Selective inhibition on VEGFR-2 is an important anticancer mechanism of VEGFR inhibitors,30 which was tested in the present study to compare the potency of axitinib and its selenium substitute. Surprisingly, Se-axitinib maintained the inhibitory effect of axitinib on VEGFR-2, which suggested that Se-axitinib might be another potent anticancer drug for RCC treatment.
In conclusion, selenium substituted axitinib reduced the side effect of axitinib, especially blood pressure increasing effect possibly owing to its antioxidant and cardiovascular protective effect. And Se-axitinib displayed weight increasing effect and protective effect on hepatorenal dysfunctions. More importantly, selenium substitute of axitinib maintained the potent anticancer activity of the original drug axitinib, which means Se-axitinib is a novel anticancer agent with lower side effect. On the whole, male animals were used to test side effects and female animals were used to test anticancer activities, the study is preliminary and needs certain improvements, and involving both sexes is one of them, for which male and female show different physiological and behavioral features. In addition, more well designed study is needed to confirm the present result and further investigate the exact mechanism of the action of this new compound.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Thanks for the resource support of Tianjin University of Science and Technology and the valuable comments of the reviewers.References
- S. Vaggers, P. Rice, B. K. Somani, R. Veeratterapillay and B. P. Rai, Turkish Journal of Urology, 2021, 47, S9–S18 CrossRef PubMed.
- P. C. Barata and B. I. Rini, Ca-Cancer J. Clin., 2017, 67, 507–524 CrossRef PubMed.
- R. J. Motzer, K. Penkov, J. Haanen, B. Rini, L. Albiges, M. T. Campbell, B. Venugopal, C. Kollmannsberger, S. Negrier, M. Uemura, J. L. Lee, A. Vasiliev, W. H. Miller Jr, H. Gurney, M. Schmidinger, J. Larkin, M. B. Atkins, J. Bedke, B. Alekseev, J. Wang, M. Mariani, P. B. Robbins, A. Chudnovsky, C. Fowst, S. Hariharan, B. Huang, A. di Pietro and T. K. Choueiri, N. Engl. J. Med., 2019, 380, 1103–1115 CrossRef CAS PubMed.
- L. Chen, D. Oh, M. Ryu, K. Yeh, W. Yeo, R. Carlesi, R. Cheng, J. Kim, M. Orlando and Y. Kang, Cancer Res. Treat., 2017, 49, 851–868 CrossRef CAS PubMed.
- J. C. Bendell, M. Joseph, K. Barnes, M. Mainwaring, D. Shipley, C. Reddy, L. Blakely, R. Blachly, C. M. Lane, C. Earwood and M. Kuzur, Cancer Invest., 2017, 35, 386–392 CrossRef CAS PubMed.
- K. Nagyivanyi and L. Geczi, Orv. Hetil., 2017, 158, 1488–1502 CrossRef PubMed.
- B. Williams, G. Mancia, W. Spiering, E. A. Rosei, M. Azizi, M. Burnier, D. L. Clement, A. Coca, G. de Simone, A. Dominiczak, T. Kahan, F. Mahfoud, J. Redon, L. Ruilope, A. Zanchetti, M. Kerins, S. E. Kjeldsen, R. Kreutz, S. Laurent, G. Y. H. Lip, R. McManus, K. Narkiewicz, F. Ruschitzka, R. E. Schmieder, E. Shlyakhto, C. Tsioufis, V. Aboyans and I. Desormais, Eur. Heart J., 2019, 40, 475 CrossRef PubMed.
- K. J. Henriksen and A. r. Chang, Semin. Nephrol., 2020, 40, 69–75 CrossRef CAS PubMed.
- Z. Y. Li, X. X. Fan, Y. J. Wang, K. Yao, Z. W. Liu, W. T. Pan, Y. L. Ye, P. Yang, Y. C. Huang, Z. M. Wu and F. J. Zhou, Oncotarget, 2016, 7, 35181–35187 CrossRef PubMed.
- S. L. Chan, W. Yeo, F. Mo, A. W. H. Chan, J. Koh, L. Li, E. P. Hui, C. C. N. Chong, P. B. S. Lai, T. S. K. Mok and S. C. H. Yu, Cancer-am Cancer Soc, 2017, 123, 3977–3985 CAS.
- M. O. Grimm, K. Leucht, S. Foller and V. Gruenwald, Urologe, 2020, 59, 155–161 CrossRef PubMed.
- J. L. Benelli, V. R. Poester, L. S. Munhoz, A. M. Melo, M. R. Trapaga, D. A. Stevens and M. O. Xavier, Med. Mycol., 2021, 59, 409–421 CrossRef CAS PubMed.
- G. Liu, X. Yang, J. Zhang, L. Liang, F. Miao, T. Ji, Z. Ye, M. Chu, J. Ren and X. Xu, Int. J. Biol. Macromol., 2021, 179, 418–428 CrossRef CAS PubMed.
- A. B. A. Mahmoud, G. Kirsch and E. Peagle, Curr. Org. Synth., 2017, 14, 1091–1101 Search PubMed.
- Z. Zhou, T. Sun, Y. Qin, Y. Zhu, Y. Jiang, Y. Zhang, J. Liu, J. Wu, S. He and F. Chen, Biomater. Sci., 2019, 7, 5044–5053 RSC.
- V. Gandin, P. Khalkar, J. Braude and A. P. Fernandes, Free Radical Biol. Med., 2018, 127, 80–97 CrossRef CAS PubMed.
- W. Sun, J. Zhu, S. Li, C. Tang, Q. Zhao and J. Zhang, Metallomics, 2020, 12, 1965–1978 CrossRef CAS PubMed.
- B. Wu, Q. Chen, D. Pan, B. Chang and L. Sang, Int. Immunopharmacol., 2021, 96, 107790 CrossRef CAS PubMed.
- B. Afzal, D. Yasin, H. Naaz, N. Sami, A. Zaki, M. A. Rizvi, R. Kumar, P. Srivastava and T. Fatma, Sci. Rep., 2021, 11, 13507 CrossRef CAS PubMed.
- A. Rakib, Z. Nain, S. A. Sami, S. Mahmud, A. Islam, S. Ahmed, A. B. F. Siddiqui, S. M. O. F. Babu, P. Hossain, A. Shahriar, F. Nainu, T. Bin Emran and J. Simal-Gandara, Briefings Bioinf., 2021, 22, 1476–1498 CrossRef CAS PubMed.
- L. V. Papp, J. Lu, A. Holmgren and K. K. Khanna, Antioxid. Redox Signaling, 2007, 9, 775–806 CrossRef CAS PubMed.
- M. P. Rayman, Lancet, 2000, 356, 233–241 CrossRef CAS.
- M. N. Baek, C. Budolfsen, D. Grimm, M. Kruger, M. Infanger, M. Wehland and M. N. E, Int. J. Mol. Sci., 2019, 20, 48–71 Search PubMed.
- W. Shi, L. Su, J. Wang, F. Wang, X. Liu and J. Dou, Ann. Med., 2022, 54, 1395–1402 CrossRef CAS PubMed.
- J. C. Avery and P. R. Hoffmann, Selenium, Selenoproteins, and Immunity, Nutrients, 2018, 10, 1203 CrossRef PubMed.
- A. Calik, N. K. Emami, M. B. White, M. C. Walsh, L. F. Romero and R. A. Dalloul, Poult. Sci., 2022, 101, 101857 CrossRef CAS PubMed.
- S. Imai, H. Miyake and M. Fujisawa, Targeted Oncol., 2016, 11, 309–315 CrossRef PubMed.
- D. Radomska, R. Czarnomysy, D. Radomski and K. Bielawski, Selenium Compounds as Novel Potential Anticancer Agents, Int. J. Mol. Sci., 2021, 22, 1009 CrossRef CAS PubMed.
- H. W. Tan, H.-Y. Mo, A. T. Y. Lau and Y.-M. Xu, Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy, Int. J. Mol. Sci., 2019, 20, 75 CrossRef PubMed.
- B. L. Falcon, S. Chintharlapalli, M. T. Uhlik and B. Pytowski, Pharmacol. Ther., 2016, 164, 204–225 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01882a |
| ‡ Ying Fu and Rengui Saxu contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2022 |