Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polyaniline inside the pores of high surface area mesoporous silicon as composite electrode material for supercapacitors†
Saima Nawazab,
Yaqoob Khan*b,
Shaimaa A. M. Abdelmohsenc,
Sadia Khalidb,
Emma M. Björkd,
Muhammad Asim Rasheede and
M. Siddiq *a
*a
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad 45320, Pakistan. E-mail: m_sidiq12@yahoo.com; Tel: +92 5190642147
bNanoscience and Technology Department, National Centre for Physics, QAU Campus, Shahdra Valley Road, Islamabad 45320, Pakistan. E-mail: yaqoob@ncp.edu.pk; Fax: +92 512077389; Tel: +92 3455235423
cDepartment of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P. O. Box 84428, Riyadh 11681, Saudi Arabia
dNanostructured Materials, Department of Physics, Chemistry and Biology (IFM), Linköping University, SE-581 83 Linköping, Sweden
eDepartment of Physics and Applied Mathematics, Pakistan Institute of Engineering and Applied Sciences (PIEAS), Islamabad, 45650, Pakistan
First published on 10th June 2022
Abstract
Mesoporous silicon (mSi) obtained by the magnesiothermic reduction of mesoporous silica was used to deposit polyaniline (PANI) in its pores, the composite was tested for its charge storage application for high performance supercapacitor electrodes. The mesoporous silica as confirmed by Small Angle X-ray Scattering (SAXS) has a Brunauer–Emmett–Teller (BET) surface area of 724 m2g−1 and mean pore size of 5 nm. After magnesiothermic reduction to mSi, the BET surface area is reduced to 348 m2g−1 but the mesoporousity is retained with a mean pore size of 10 nm. The BET surface area of mesoporous silicon is among the highest for porous silicon prepared/reduced from silica. In situ polymerization of PANI inside the pores of mSi was achieved by controlling the polymerization conditions. As a supercapacitor electrode, the mSi–PANI composite exhibits better charge storage performance as compared to pure PANI and mesoporous silica–PANI composite electrodes. Enhanced electrochemical performance of the mSi–PANI composite is attributed to the high surface mesoporous morphology of mSi with a network structure containing abundant mesopores enwrapped by an electrochemically permeable polyaniline matrix.
Introduction
Supercapacitors are the next generation of energy storage devices designed to traverse the gap between capacitors and batteries. Supercapacitors have an immense scope in the energy sector to replace batteries due to their fast charging–discharging rate for storing energy with high power density, intermediate specific energy and excellent long cycle life. They are a stronger candidate for the working territory where devices demand urgent power to be sustained for a short duration e.g., in electric vehicles. Based on the mechanism of charge storage, supercapacitors are categorized in two types i.e., pseudo capacitors and electric double layer capacitors (EDLCs). Energy is stored in pseudocapacitors via fast reversible redox reactions and this mechanism is seen in electrically conductive polymers and transition metal oxides. EDLCs have a storage mechanism of ion absorption at the interface of the electrolyte and the electrical double layer at the electrode. Carbon-based active materials comprising high surface area fall in the category of EDLCs. Pseudocapacitors exhibit higher specific capacitance as compared to EDLCs.1–9 Typically faradaic pseudocapacitors have further two different types comprising redox pseudocapacitance (surface or near-surface redox reactions) and intercalation pseudocapacitance (electrolyte ions intercalate into the tunnels/layers of the electrode materials accompanied by faradaic charge-transfer with no phase transitions).10,11Transition metal oxides and hydroxides, transition metal dichalcogenides and carbon based materials have been studied as electrode materials for charge storage applications.12 After the discovery of graphene, rapid development of other new emerging layered structures such as MXenes,13 layered nanoclay, phosphorene, bismuthene and 2D graphene analogues have excellent charge storage capabilities.14 Many structural evolutions, chemical changes and hybridizations of previously used traditional materials in industry are being made to utilize the synergistic effect of constituent materials to ultimately achieve better electrochemical performance. These hybrids/nanocomposites integrate the advantageous attributes and compensate for the disadvantages associated with its single components. New advances include porous 2D and 3D graphene materials,15 transition metal oxide–hydroxide heterostructure (e.g. Co3O4/Co(OH)2 heterostructure via interfacial layer control12), spinel-type Co3O4 and its modification in spinel cobaltites MCo2O4 (M = Co, Mn, Zn).16 Recently Huan Pang et al. work suggests that MOFs (metal–organic frameworks) and its various derivatives such as multimetallic MOFs (i.e. bimetallic and trimetallic MOFs)17 and MIL-96-Al18 with controllable shapes and sizes also possess enhanced charge storage (sulfur storage).
For a material to be an efficient supercapacitor electrode, there should be some spaces inside structure to store charge e.g. MnO2 exist in many structural phases (i.e., α, β, γ, λ and δ) each of which differs in its shape, size and dimensions of tunnels. So the specific capacitance (Cs) of β-MnO2 [narrow (1 × 1) tunnels] is lower while δ-MnO2 (interlayer separation ∼ 7 Å) exhibits high Cs values being layered structure.2,19–21 N. Munichandraiah et al. synthesized nanostructured MnO2 samples with different crystal structures, and investigated as electrode materials for electrochemical capacitors in aqueous 0.1 M Na2SO4 solution. The Cs values are 240 F g−1 for α-MnO2 and 236 F g−1 for δ-MnO2. Alternatively, they are as low as 9 F g−1 for β-MnO2 and 21 F g−1 for λ-MnO2.19
Beside materials containing intrastructural spaces, porous structures are becoming suitable candidates for supercapacitors. Recently Vlad and Balducci showed higher normalized capacitance relative to their BET surface areas of Ni3(hexaiminotriphenylene)2 metal–organic framework (MOF) as compared to activated carbons, carbon nanotubes, zeolite-templated carbon, carbide-derived carbon and graphene.22
Porous materials can be categorized on the basis of pore size i.e. macroporous (>50 nm), mesoporous (2–50 nm) and microporous (≤2 nm).2,23–25
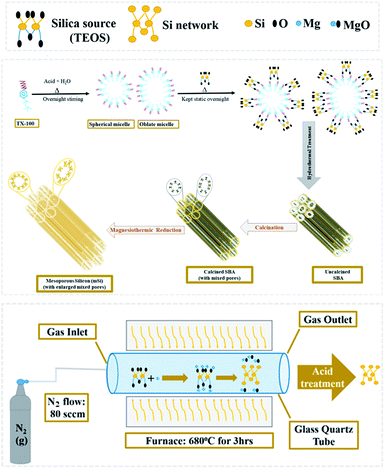
Recently porous silicon has been widely studied for variety of applications, most specifically as anode material in lithium-ion batteries.26–28 Previously established synthetic route of mesoporous silicon from silicon is physical etching which require long duration, various pre-steps and involves many harsh corrosive reagents.29 Three dimensionally structured silicon replicas could be produced from parent silica diatom by magnesiothermic reduction (i.e., vaporized/liquefied magnesium at higher temperatures reduces different metal oxides). Currently, magnesiothermic reduction, being simple and lower temperature method to convert silica to nanostructured silicon is trending which usually results in porous silicon contaminated by unreacted silica.30
We have developed a scheme to synthesize the mesoporous silicon by magnesiothermic reduction of mesoporous silica. The focus of this research was to keep intact the porosity of mesoporous silica in resultant silicon which can be used in the energy areas like supercapacitors and hydrogen generation in the form of composites. Ultimately, we can avail the advantage of this new technique by using it to produce mesoporous silicon having good cycling stability. Our developed synthetic route comprises of just two steps. Firstly, mesoporous silica was synthesized from silica precursor via soft template method. Resultant mesoporous silica was converted into mesoporous silicon by magnesiothermic reduction.
Polyaniline (PANI) due to its easy synthesis, high controllable electrical conductivity (due to its conjugated structure having delocalised p-electrons along its backbone) and environmental stability is considerably attractive choice among several conducting polymers (CPs).31,32 It offers many advantages being pseudo-capacitive electrode material containing high theoretical specific capacitance, as short path lengths for ionic transport allow faster ionic diffusion within the polymer network so energy is delivered relatively at rapid rate.33 Its high surface area in contact with the electrolyte, allows comparatively fast or high charge/discharge rates. However, the shrinkage and swelling occurring due to doping–dedoping phenomena result in poor mechanical characteristics and low cycle life limit (i.e. poor cycling stability) when used as individual electrode material.34,35 The strategies used to overcome the issue with its cyclic life mainly include irradiation, sonication during fabrication or compositing it with fillers which increase volume of polymer, enhances porosity and provide room for its swelling.33 Compositing with other materials such as carbon based materials (carbon nanotubes and graphene), inorganic oxides (such as SnO2, MnO2, TiO2), sulphides and hydroxides and other metal compounds have been proved to enhance cyclic stability as well as maximize the capacitance of resultant composites.6,15,33,36–42
In order to alleviate the limitation associated with PANI-matrix for charge storage application, PANI was added inside synthesized mesoporous silicon (which contains mixed pores within mesoporosity range) to increase its charge storage, cyclic stability and specific capacitance. Besides this, PANI was also added in the mesoporous silica under same conditions and was compared with simple PANI and PANI with mesoporous silicon. This convenient and scalable synthesis procedure developed here indicates a significant potential towards cost-effective electrode materials for practical applications. Moreover the surface area of mesoporous silicon (348 m2g−1) attained by magnesiothermic reaction is amongst the highest published in literature so far.30
Experimental
Materials
All the chemical reagents of analytical grade were purchased from Sigma-Aldrich and were utilized without any further treatment.Synthesis of mesoporous silica (SBA)
2 mL of Triton X-100 (TX-100) was added in 80 mL of 1.3 molar aqueous solution of HCl in a beaker and stirred overnight at room temperature. 5.5 mL tetraethylorthosilicate (TEOS) was then added dropwise to TX-100 and acid solution under vigorous stirring to achieve clear solution. After it, the mixture was kept static at 35 °C overnight, followed by hydrothermal treatment for 24 h at 100 °C in a Teflon-lined autoclave. White solid product was collected by filtration process. Later it was washed with water then dried for 3 h at 60 °C and calcined for 6 h at 550 °C.26 The attained sample was designated as SBA.Magnesiothermic reduction of SBA to mesoporous silicon (mSi)
SBA powder placed in an alumina crucible was evenly covered with flakes of magnesium (Mg). The weight ratio of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SBA was 0.8
SBA was 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The loaded crucible was kept in a tube furnace under N2 flow (80 sccm) for 3 h at 680 °C.
1. The loaded crucible was kept in a tube furnace under N2 flow (80 sccm) for 3 h at 680 °C.| SiO2 + 2Mg → Si + 2MgO | (1) |
When SiO2 (silica) and Mg (magnesium) react during magnesiothermic process, Si (mesoporous silicon) forms and MgO (magnesium oxide) is produced as a by-product (Fig. 1).
The powder thus obtained was washed with 1 M hydrochloric acid (HCl) and then vacuum filtered. A greyish brown fine powder of mesoporous silicon (mSi) was attained as end product.
Synthesis of polymer (P) and polymer composites (mSi–P & SBA–P)
Aniline monomer was first dissolved into a dilute HCl aqueous solution. Aniline/aqueous acid solution was placed in an ice-salt bath to maintain the temperature at 4–5 °C. For synthesis of mesoporous silicon–polyaniline (mSi–P) and mesoporous silica–polyaniline (SBA–P) composites, the fillers (mSi and SBA respectively) were added in aniline/acid solution mixture at this stage. The polymerization of aniline in aqueous medium was done in the presence of an initiator i.e. ammonium persulfate, (NH4)2SO4. Aqueous solution of (NH4)2SO4 in ice-cold water was slowly added to the prepared mixture under moderate stirring condition to get homogeneous mixture. Then stirring was stopped and the whole homogeneous mixture was placed to age at 4–5 °C for 16 h under static condition. Resultant polymer/composite was filtered and washed with distilled water. Same procedure was followed for polyaniline (P) without addition of mSi or SBA.Characterization
XRD and SAXS patterns of the samples were collected using a Bruker D8 Advance diffractometer. SAXS data was collected by keeping the X-ray source fixed at 0.1 degree. XPS data was collected using a Scienta Omicron nanotechnology GmBH equipped with a hemispherical analyser. Nitrogen sorption isotherms were obtained using an ASAP 2020 system (Micromeritics) operated at 77 K. The samples were degassed at 200 °C for 4 h prior to the analysis. The Brunauer–Emmett–Teller (BET) method based on the adsorption data (within the range of P/P0 = 0.05–0.20) was used to calculate the specific surface area. The total pore volume was obtained from the adsorbed amount of N2 at P/P0 = 0.99. Barrett–Joyner–Halenda (BJH) analysis was used on the adsorption branch to evaluate the average pore diameter. Hitachi Su-70 Schottky field emission microscope was used for SEM. TEM analysis was done using a FEI TECNAI G2 microscope operated at 200 kV. The samples were prepared by dispersing the materials in acetone and depositing them on hollow carbon grids. FT-IR data was collected on a Bio-Rad Excalibur FT-IR Spectrometer in the range 400–4000 cm−1 using pellets of KBr at ambient temperature.Electrochemical measurements
The electrochemical properties of the synthesized samples were studied in the three-electrode system with 1 M H2SO4 aqueous solution as electrolyte. Reference electrode was saturated calomel electrode (SCE) while graphite rod was utilized as counter electrode. The working electrodes were prepared by mixing active materials and polytetrafluoroethylene (PTFE) as the binder. A Gamry REF3000-electrochemical workstation was used to collect the cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge (GCCD) data. CV scans were carried out in the potential range from −0.2 V to 1 V at various scan rates. Cyclic stability was checked for 50 cycles at 20 mV s−1. The specific capacitance can be determined by integration of the CV curve to calculate the average area under the curve for a complete cycle using the relation43 given in the eqn (2).
 | (2) |
Specific capacitance of three electrode system can be estimated also by slope of discharge curve of GCCD measurement by following formula44 given in eqn (3).
 | (3) |
Results and discussion
Structural studies
In the XRD patterns of as reduced mSi, peaks from MgO were observed (ICCD#: 01-077-2364, 01-077-2109) (Fig. 3) along with reflections from Si indicating the complete reduction of SBA to mSi. After acid washing, the reflections from MgO disappeared and only the three prominent peaks of Si at 28.4°, 47.3° and 56.1° were present (ICDD#: 00-026-14181). There is no evident peak of mesoporous silica so it shows that magnesiothermic reduction with consequent acid washing left no residues of parent material (Fig. 3).
 | ||
| Fig. 3 X-ray diffractograms of mesoporous silicon reduced from mesoporous silica before (mSi–Mg) and after (mSi) the acid treatment. | ||
PANI exhibits X-ray diffraction peaks at 14.4°, 19.6°, and 25.4° for (121), (113) and (322) reflections respectively.45,46 Intensity of these peaks decreases slightly in XRD diffractograms of composites of mSi–P & SBA–P, which is due to the interaction between PANI and filler (ESI Fig. S1†).
Morphological studies
Surface studies
 | ||
| Fig. 6 BJH pore size & volume analysis – Brunauer–Emmett–Teller (BET) of (a) SBA and (b) mSi by Halsey: Faas correction. | ||
For mSi, the N2 adsorption–desorption isotherm still shows a type H1 hysteresis loop followed by a type H3 hysteresis. This indicates that the metallic Si retains part of the mesopores, even though some pore shrinkage is observed in the pore size distribution in Fig. 6. The strong H3 loop is most probably due to the slit shaped pores formed between Si crystals, see Fig. 4.47–52 The specific surface area of mSi is 348 m2 g−1. This significant decrease in specific area is due to a decreased mesoporosity upon the formation of crystalline Si. The narrow pore size distribution of mSi indicates a homogeneous material reduction.
 | ||
| Fig. 7 X-ray photoelectron spectroscopy (XPS) of mSi–P showing peaks of (a) Si 2p, (b) N 1s, (c) C 1s and (d) O 1s. | ||
In the XPS survey spectrum of mSi–P, peaks from Si 2p were due to mSi while peaks from C 1s and N 1s were observed due to the presence of polyaniline in the composite. N 1s was fitted into three components arising from the –NH2+, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+, and
NH+, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– moieties in PANI. C 1s was fitted into four components consisting of C–C, C
N– moieties in PANI. C 1s was fitted into four components consisting of C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N/C
C, C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and COOH groups. The three components under the O 1s spectra corresponds to adsorbed O, Si–O and, C–O groups.
N, and COOH groups. The three components under the O 1s spectra corresponds to adsorbed O, Si–O and, C–O groups.
Electrochemical studies
Cyclic voltammetry (CV) measurements were performed in 1 M H2SO4 aqueous solution as the electrolyte to investigate the electrochemical behaviour of samples. The CV scans were taken at scan rates of 5 to 250 mV s−1 (i.e., 5, 10, 20, 50, 100, 150, 200, 250 mV s−1) in voltage range of −0.2 to 1 V (versus SCE) for all samples.Cyclic voltammograms of polyaniline (P) (Fig. S4(a)†) at various scan rates exhibit a redox peak which is attributed to the transition of PANI from leucoemeraldine (semiconducting state) to emeraldine (conductive form). This redox process caused pseudo capacitance of PANI. Apparently, the mSiP composite (Fig. 8(a)) has a similar electrochemical response as that of the P, but peak current of mSi–P increases greatly which implies a larger electrode capacitance. High electrochemical utilization of mSi–P is due to its larger specific surface area as compared to P and hence more electroactive sites.
 | ||
| Fig. 8 Cyclic voltammogram (CV) of (a) mSi–P and (b) mSi at different scan rates on GCE in 1 M H2SO4 electrolyte vs. SCE. | ||
The PANI matrix is electrochemically permeable i.e. it does not block the counter-ions to reach the mSi because of the close contact established between the two during in situ polymerization inside the pores of mSi.
Comparison of the shapes of the cyclic voltammograms (Fig. 8(a), S4(b) and (c)†) depicts that the contribution of the mSi and SBA to the total capacitance of mSi–P and SBA–P composite electrodes is of the double-layer type which is also apparent in the form of CV curves for pure mSi (Fig. 8(b)) and SBA (Fig. S4(b)†). The electrochemical behaviour of mSi–P (Fig. 8(a)) is still better than SBA–P (Fig. S4(c)†) which is because of enlarged pores of mSi than SBA which facilitate the charge transfer. P (Fig. S4(a)†) is showing good capacitance response but its efficiency decreases after multiple cyclic charge–discharge phenomena due to changes in its volume and physical properties which is inevitable in its pure polymer matrix.
The rectangular shape and mirror images are observed in the CV curves for all samples (Fig. 8 and S4†), indicating high electrochemical reversibility.36–38,56,57
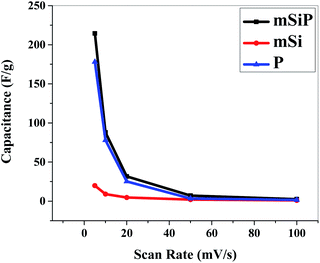
At 5 mV s−1 scan rate and 1.2 V potential, the specific capacitance of mSi is 19.85 F g−1 while for P its value is 178 F g−1. Specific capacitance of mSi–P is 214.45 F g−1.
Fig. 9 and S5† show the cyclic voltammograms recorded at 20 mV s−1 scan rate for 50 cycles in 1 M H2SO4 aqueous electrolyte solution. The observed CV scans are quite stable for 50 cycles and no significant decrease in current is observed for any material which depicts that materials have high cycling stability. Fig. 9(a) is showing the good cyclic stability of the electrode which is due to the cohesive inter-molecular contact between P and mSi which inhibits the dissolution of filler. Fig. 10 and S6† show specific capacitance decreases by increasing scan rate.
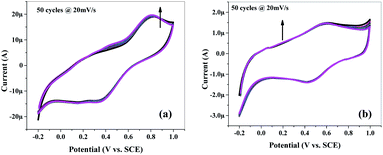 | ||
| Fig. 9 Cyclic voltammetry (CV): cyclic stability of (a) mSi–P and (b) mSi at scan rate of 20 mV s−1 for 50 cycles on GCE in 1 M H2SO4 electrolyte vs. SCE. | ||
Further, relative contributions of the capacitance from the bulk or surface mechanism is derived by analyzing the CV data at various scan rates (5–250 mV s−1) shown in Fig. S10† and using the power law58,59 given in eqn (4).
| i = avb | (4) |
According to power law, slope of the plot log(i) i.e. redox peak current versus log(v) i.e. various scan rates provides the b-value which can be used to estimate the controlling mechanism of the active material in electrode. For ideal capacitor type materials, b-value is close to 1 where pseudocapacitive behavior is dominant. If the value of b is close to 0.5, it depicts the material is battery type and contains diffusion controlled phenomenon as dominant one. Fig. S10† shows a plot between log(i) and log(v) showing the cathodic and anodic peak currents of mSi–P as indicated in Fig. 8(a). The b-values from the anodic and cathodic peaks are 1.009 and 1.022 respectively. It suggests that total contribution to capacitance is mainly due to peudocapacitance phenomenon and mSi–P is showing mainly capacitive behavior. Using the Dunn method of differentiation,60 it is estimated that 99.78% of the total capacitance is from pseudocapacitive contribution and 0.22% is diffusion controlled behavior.
EIS is performed in the frequency range of 106 to 10−1 Hz (at AC voltage = 10 mV rms, open circuit potential amplitude = 0.34 and stabilization time = 10 s) to evaluate the resistance of electrochemical phenomenon.
Fig. 11 and S7† show the Nyquist plots of samples. Fig. 11(a) shows the frequency response of mSi–P/1 M H2SO4 (aq.) system in the plotted form of two impedance components against each other, one of which is real component i.e. Z′ and other one is imaginary component i.e. Z′′. The Nyquist plots are fitted using an equivalent circuit model shown in the inset of Fig. 11, where Rs is solution ohmic resistance, Rct is charge transfer resistance, CPE is constant phase element and W is Warburg impedance. In Fig. 11(a), absence of semicircle in the domain of high frequency shows less Rct. Rct calculated by fitting equivalent circuit model is 232.6 × 10−6 Ω. Rct existing between mSi–P electrode and 1 M H2SO4 (aq.) electrolyte is considerably less due to the highly conductive cross-linked mSi and PANI. It may relate to a high material electrochemical activity (pseudo capacitance), and indicates the surface properties of mSi–P electrode is favouring facile fast charge transfer kinetics within electrodes.61
Large impedance values observed for mSi electrodes in Fig. 11(b) are indicative of high Rct (1.868 × 103 Ω) which is greater than that of mSi–P composite (Fig. 11(a)).
In Fig. 11(a) at low frequency region a steeper curve is evident of decreased Warburg impedance which depicts the accelerated diffusion and adsorption rate of counter-ions of electrolyte in the system (in/on the electrode material). The reason behind it is that the network of PANI enwrapping mSi (having mesoporous configuration) with high surface area provide more space with short and equal diffusion path length for transportation of redox species (i.e. counter-ions) of 1 M H2SO4 electrolyte consequences in excellent capacitive behavior.
 | ||
| Fig. 12 EIS (electrochemical impedance spectroscopy): Bode plots of (a) mSi–P and (b) mSi vs. OC (open circuit) at 10 mV rms AC perturbation (in 1 M H2SO4 electrolyte). | ||
Nyquist plot (Fig. 11(b)) for mSi shows larger Rct and Warburg impedance as compared to mSi–P; Fig. 11(a) which is due to high obstruction in the movement of counter-ions and higher variations in ion diffusion path lengths respectively.
The slope of each electrode (Fig. 11 and S7†) decreases with increasing AC frequency (potential) which demonstrates an enhanced Warburg resistance which is consequently causing reduction in effective charge storage at the electrode. Fig. 12 and S8† show the Bode plots of samples.
The electrochemical stability was examined by galvanostatic charge/discharge (GCCD) measurements in 1 M H2SO4 aqueous solution by consecutive charge–discharge cycles within the potential range of −0.2 to 1 V at discharge current of 1 μA and capacity of 0.0025 A h. GCCD measurements are executed to attain the quantitative information of the electrochemical capacitance for mSi–P, mSi, P, SBA and SBA–P electrodes (Fig. 13 and S9†).
The higher capacitance observed for mSi–P composite is due to low Rct (also discussed in impedance analysis) which is the consequence of its unique morphology (with more electroactive sites due to mesoporosity of mSi for reversible redox reaction) while having continuous conductive network of PANI.
The specific capacitance calculated by discharge curve of GCCD curves for mSi–P is equal to 68.21 F g−1 at 0.187 A g−1 current density.
Capacitance retention and coulombic efficiency of mSi–P for 1000 cycles in 1 M H2SO4 electrolyte is shown in Fig. 14.
 | ||
| Fig. 14 GCCD (galvanostatic cyclic charge–discharge): capacitance retention and coulombic efficiency of mSi–P vs. cycle number for 1000 cycles (in 1 M H2SO4 electrolyte). | ||
Conclusions
We have reported a facile and cost effective method to obtain mesoporous silicon with a remarkably high surface area (348 m2 g−1) by magnesiothermic reduction of mesoporous silicon (SBA). The conducting mesoporous network can be used to host small polymeric molecules such as PANI for most of applications. As supercapacitor electrode, mSi–PANI composite was able to achieve specific capacitance of 214.45 F g−1 at 5 mV s−1. The composite demonstrates improved charge storage performance and excellent cyclic stability (100% capacitance retention and 91.87% coulombic efficiency at 0.18 A g−1 after 1000 cycles). The results demonstrate that the pseudocapacitive contribution in total specific capacitance is dominant. The predominant pseudocapacitive behaviour is attributed to synergistic effect between mesoporous silicon and polymer matrix. This work suggests a simple and scalable route for the development of mesoporous silicon based hybrid composites favourable to supercapacitor applications.Author contributions
MS: supervision, reviewing, and editing, YK: conceptualization, supervision, administration, resources, reviewing, and editing, EB: help in synthesis of mesoporous silica and TEM analysis, AR: BET measurement and analysis, SA: SEM analysis, SK: methodology, investigation, formal analysis, SN: methodology, investigation, formal analysis, writing-original draft preparation, validation, data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their gratitude to Princess Nourah Bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R61), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia. Ms Saima Nawaz acknowledges HEC Pakistan for the six months fellowship (IRSIP) at the University of Manchester. Emma Björk acknowledges the Swedish Research Council (VR) (2015-00624) and the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU no. 2009-00971) for financial support.Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. Wang, J. Guo, T. Wang, J. Shao, D. Wang and Y.-W. Yang, Nanomaterials, 2015, 5, 1667–1689 CrossRef CAS PubMed.
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- M. F. El-Kady, Y. Shao and R. B. Kaner, Nat. Rev. Mater., 2016, 1, 16033 CrossRef CAS.
- A. Borenstein, O. Hanna, R. Attias, S. Luski, T. Brousse and D. Aurbach, J. Mater. Chem. A, 2017, 5, 12653–12672 RSC.
- J. Yang, Y. Liu, S. Liu, L. Li, C. Zhang and T. Liu, Mater. Chem. Front., 2017, 1, 251–268 RSC.
- E. Pomerantseva and Y. Gogotsi, Nat. Energy, 2017, 2, 845 CrossRef.
- M. Schalenbach, Y. E. Durmus, H. Tempel, H. Kungl and R.-A. Eichel, Sci. Rep., 2022, 12, 3375 CrossRef CAS PubMed.
- R. Ramkumar and M. Minakshi Sundaram, New J. Chem., 2016, 40, 2863–2877 RSC.
- P. Gao, R. J. Koch, A. C. Ladonis and S. T. Misture, J. Electrochem. Soc., 2020, 167, 160523 CrossRef CAS.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2018, 5, 1700322 CrossRef PubMed.
- P. Gao, Y. Zeng, P. Tang, Z. Wang, J. Yang, A. Hu and J. Liu, Adv. Funct. Mater., 2022, 32, 2108644 CrossRef CAS.
- Y. Bai, C. Liu, T. Chen, W. Li, S. Zheng, Y. Pi, Y. Luo and H. Pang, Angew. Chem., Int. Ed., 2021, 60, 25318–25322 CrossRef CAS PubMed.
- C. Yang, R. Gao and H. Yang, EnergyChem, 2021, 3, 100062 CrossRef CAS.
- Y. Zhang, Q. Wan and N. Yang, Small, 2019, 15, 1903780 CrossRef CAS PubMed.
- P. Tang, P. Gao, X. Cui, Z. Chen, Q. Fu, Z. Wang, Y. Mo, H. Liu, C. Xu, J. Liu, J. Yan and S. Passerini, Adv. Energy Mater., 2022, 12, 2102053 CrossRef CAS.
- W. Li, X. Guo, P. Geng, M. Du, Q. Jing, X. Chen, G. Zhang, H. Li, Q. Xu, P. Braunstein and H. Pang, Adv. Mater., 2021, 33, 2105163 CrossRef CAS PubMed.
- P. Geng, L. Wang, M. Du, Y. Bai, W. Li, Y. Liu, S. Chen, P. Braunstein, Q. Xu and H. Pang, Adv. Mater., 2022, 34, 2107836 CrossRef CAS PubMed.
- S. Devaraj and N. Munichandraiah, J. Phys. Chem. C, 2008, 112, 4406–4417 CrossRef CAS.
- Y. Khan, S. K. Durrani, M. Mehmood and M. R. Khan, J. Mater. Res., 2011, 26, 2268–2275 CrossRef CAS.
- M. Huang, F. Li, F. Dong, Y. X. Zhang and L. L. Zhang, J. Mater. Chem. A, 2015, 3, 21380–21423 RSC.
- A. Vlad and A. Balducci, Nat. Mater., 2017, 16, 161–162 CrossRef CAS PubMed.
- M.-H. Sun, S.-Z. Huang, L.-H. Chen, Y. Li, X.-Y. Yang, Z.-Y. Yuan and B.-L. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- S. Leyva-García, D. Lozano-Castelló, E. Morallón and D. Cazorla-Amorós, J. Mater. Chem. A, 2016, 4, 4570–4579 RSC.
- Y. Zhang, S. Yu, G. Lou, Y. Shen, H. Chen, Z. Shen, S. Zhao, J. Zhang, S. Chai and Q. Zou, J. Mater. Sci., 2017, 52, 11201–11228 CrossRef CAS.
- A. Sayari, B.-H. Han and Y. Yang, J. Am. Chem. Soc., 2004, 126, 14348–14349 CrossRef CAS PubMed.
- Y. Zhu, H. Li, J. Xu, H. Yuan, J. Wang and X. Li, CrystEngComm, 2011, 13, 402–405 RSC.
- V. F. Vavsari, G. M. Ziarani and A. Badiei, RSC Adv., 2015, 5, 91686–91707 RSC.
- V. Lehmann, Electrochemistry of silicon: instrumentation, science, materials and applications, Wiley Online Library, 2002, vol. 1 Search PubMed.
- J. Entwistle, A. Rennie and S. Patwardhan, J. Mater. Chem. A, 2018, 6, 18344–18356 RSC.
- L. Yu, M. Gan, L. Ma, H. Huang, H. Hu, Y. Li, Y. Tu, C. Ge, F. Yang and J. Yan, Synth. Met., 2014, 198, 167–174 CrossRef CAS.
- Z. Lei, Z. Chen and X. S. Zhao, J. Phys. Chem. C, 2010, 114, 19867–19874 CrossRef CAS.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- P. Naskar, A. Maiti, P. Chakraborty, D. Kundu, B. Biswas and A. Banerjee, J. Mater. Chem. A, 2021, 9, 1970–2017 RSC.
- R. Ramkumar and M. M. Sundaram, New J. Chem., 2016, 40, 7456–7464 RSC.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- K. Wang, J. Huang and Z. Wei, J. Phys. Chem. C, 2010, 114, 8062–8067 CrossRef CAS.
- T. Liu, L. Finn, M. Yu, H. Wang, T. Zhai, X. Lu, Y. Tong and Y. Li, Nano Lett., 2014, 14, 2522–2527 CrossRef CAS PubMed.
- M. E. Abdelhamid, A. P. O'Mullane and G. A. Snook, RSC Adv., 2015, 5, 11611–11626 RSC.
- A. Eftekhari, L. Li and Y. Yang, J. Power Sources, 2017, 347, 86–107 CrossRef CAS.
- Q. B. Le, T.-H. Nguyen, H. Fei, C. Bubulinca, L. Munster, N. Bugarova, M. Micusik, R. Kiefer, T. T. Dao, M. Omastova, N. E. Kazantseva and P. Saha, Sci. Rep., 2022, 12, 664 CrossRef CAS PubMed.
- M. Rapisarda, F. Marken and M. Meo, Commun. Chem., 2021, 4, 169 CrossRef CAS.
- M. R. Biradar, A. V. Salkar, P. P. Morajkar, S. V. Bhosale and S. V. Bhosale, New J. Chem., 2021, 45, 5154–5164 RSC.
- Y. Zhao, Y. Xu, J. Zeng, B. Kong, X. Geng, D. Li, X. Gao, K. Liang, L. Xu, J. Lian, S. Huang, J. Qiu, Y. Huang and H. Li, RSC Adv., 2017, 7, 55513–55522 RSC.
- J. Zhou, L. Yu, W. Liu, X. Zhang, W. Mu, X. Du, Z. Zhang and Y. Deng, Sci. Rep., 2015, 5, 17858 CrossRef CAS PubMed.
- L. Ren, G. Zhang, J. Wang, L. Kang, Z. Lei, Z. Liu, Z. Liu, Z. Hao and Z. h. Liu, Electrochim. Acta, 2014, 145, 99–108 CrossRef CAS.
- E. K. Richman, C. B. Kang, T. Brezesinski and S. H. Tolbert, Nano Lett., 2008, 8, 3075–3079 CrossRef CAS PubMed.
- K. H. Kim, D. J. Lee, K. M. Cho, S. J. Kim, J.-K. Park and H.-T. Jung, Sci. Rep., 2015, 5, 9014 CrossRef CAS PubMed.
- A. Xing, S. Tian, H. Tang, D. Losic and Z. Bao, RSC Adv., 2013, 3, 10145–10149 RSC.
- X. Li, M. Gu, S. Hu, R. Kennard, P. Yan, X. Chen, C. Wang, M. J. Sailor, J.-G. Zhang and J. Liu, Nat. Commun., 2014, 5, 4105 CrossRef CAS PubMed.
- Z. Jiang, C. Li, S. Hao, K. Zhu and P. Zhang, Electrochim. Acta, 2014, 115, 393–398 CrossRef CAS.
- S.-z. Zeng, X. Zeng, L. Huang, H. Wu, Y. Yao, X. Zheng and J. Zou, RSC Adv., 2017, 7, 7990–7995 RSC.
- R. J. Robson and E. A. Dennis, J. Phys. Chem., 1977, 81, 1075–1078 CrossRef CAS.
- H. Yin, P. Zheng, J. Zhao and W. Shen, Soft Matter, 2017, 13, 5888 RSC.
- J. M. Borreguero, P. A. Pincus, B. G. Sumpter and M. Goswami, Macromolecules, 2017, 50, 1193–1205 CrossRef CAS.
- B. K. Kuila, B. Nandan, M. Böhme, A. Janke and M. Stamm, Chem. Commun., 2009, 5749–5751 RSC.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294–1301 RSC.
- Y. Zhang, C. Wang, H. Jiang, Q. Wang, J. Zheng and C. Meng, Chem. Eng. J., 2019, 375, 121938 CrossRef CAS.
- P. Sharma, M. Minakshi Sundaram, T. Watcharatharapong, S. Jungthawan and R. Ahuja, ACS Appl. Mater. Interfaces, 2021, 13, 56116–56130 CrossRef CAS PubMed.
- J. Ning, M. Xia, D. Wang, X. Feng, H. Zhou, J. Zhang and Y. Hao, Nano-Micro Lett., 2020, 13, 2 CrossRef PubMed.
- N. Ogihara, Y. Itou, T. Sasaki and Y. Takeuchi, J. Phys. Chem. C, 2015, 119, 4612–4619 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Polyaniline inside the pores of high surface area mesoporous silicon as composite electrode material for supercapacitors. Fig. S1: X-ray diffraction (XRD) pattern of polyaniline (P), SBA–P and mSi–P composites. Fig. S2(a): scanning electron microscopy (SEM) images of mSi, P and mSi–P composite. Fig. S2(b): energy dispersive X-ray (EDX) pattern of mSi–P composite. Fig. S3: Fourier transform infrared spectroscopy (FT-IR) of SBA (mesoporous silica), polyaniline (P) and composites (mSi–P and SBA–P). Fig. S4: cyclic voltammogram (CV) of (a) P, (b) SBA and (c) SBA–P at different scan rates on GCE in 1 M H2SO4 electrolyte vs. SCE. Fig. S5: cyclic voltammetry (CV): cyclic stability of (a) P, (b) SBA and (c) SBA–P at scan rate of 20 mV s−1 for 50 cycles on GCE in 1 M H2SO4 electrolyte vs. SCE. Fig. S6: scan rate vs. specific capacitance. Fig. S7: EIS (electrochemical impedance spectroscopy): Nyquist plots of (a) P, (b) SBA and (c) SBA–P vs. OC (open circuit) at 10 mV rms AC perturbation (in 1 M H2SO4 electrolyte). Fig. S8: EIS (electrochemical impedance spectroscopy): Bode plots of (a) P, (b) SBA and (c) SBA–P vs. OC (open circuit) at 10 mV rms AC perturbation (in 1 M H2SO4 electrolyte). Fig. S9: galvanostatic cyclic charge–discharge (GCCD): discharge curve; potential vs. real time (a) P (at charge density = 0.143 A g−1), (b) SBA (at charge density = 0.250 A g−1) and (c) SBA–P (at charge density = 0.388 A g−1) and in 1 M H2SO4 electrolyte. Fig. S10: plot of log(i) versus log(v) for the anodic and cathodic current peaks of mSi–P. Fig. S11: proposed mechanism of electrochemical reaction during charging and discharging in 1 M H2SO4 electrolyte. See https://doi.org/10.1039/d2ra01829b |
| This journal is © The Royal Society of Chemistry 2022 |