Open Access Article
Open Access ArticleTherapeutic hydrophobic deep eutectic solvents of menthol and fatty acid for enhancing anti-inflammation effects of curcuminoids and curcumin on RAW264.7 murine macrophage cells†
Kantapich Kongpol ab,
Preenapan Chaihaoa,
Parichat Shuapana,
Ploypailin Kongduka,
Warangkana Chunglok
ab,
Preenapan Chaihaoa,
Parichat Shuapana,
Ploypailin Kongduka,
Warangkana Chunglok a and
Gorawit Yusakul
a and
Gorawit Yusakul *cd
*cd
aSchool of Allied Health Sciences, Walailak University, Nakhon Si Thammarat, Thailand
bResearch Excellence Center for Innovation and Health Product, Walailak University, Nakhon Si Thammarat, Thailand
cSchool of Pharmacy, Walailak University, Nakhon Si Thammarat, Thailand. E-mail: gorawit.yu@mail.wu.ac.th; Tel: +66-75-67-2839
dBiomass and Oil Palm Center of Excellence, Walailak University, Nakhon Si Thammarat, Thailand
First published on 13th June 2022
Abstract
Owing to their water insolubility, low stability, and poor absorption, anti-inflammatory curcuminoids (CUN) are difficult to be extracted and delivered to the action site. As a result, therapeutic hydrophobic deep eutectic solvents (HDESs), containing menthol and fatty acids (capric, caprylic, and oleic acids), are being developed for CUN solubilization and delivery. In this study, the anti-inflammatory effects of various combinations of HDESs with CUN and curcumin (CUR) were investigated on RAW264.7 macrophage cells. The results showed that CUN can be solubilized using the HDESs. The HDESs of oleic acid (OLA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios) exhibited anti-inflammatory effects, and OLA
1 molar ratios) exhibited anti-inflammatory effects, and OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) increased the anti-inflammatory effects of CUR. The cytotoxicity of CUN and CUR was also lowered when combined with some OLA
1 molar ratio) increased the anti-inflammatory effects of CUR. The cytotoxicity of CUN and CUR was also lowered when combined with some OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDESs. The combination of OLA, menthol, and CUR entirely suppressed NO secretion without significant cytotoxicity. These results clearly indicate the potential of HDESs to solubilize CUN and impart anti-inflammatory properties. Furthermore, these solvents could replace organic solvents for CUN extraction, with the added benefit of being therapeutic, biodegradable, and safe for human consumption.
menthol HDESs. The combination of OLA, menthol, and CUR entirely suppressed NO secretion without significant cytotoxicity. These results clearly indicate the potential of HDESs to solubilize CUN and impart anti-inflammatory properties. Furthermore, these solvents could replace organic solvents for CUN extraction, with the added benefit of being therapeutic, biodegradable, and safe for human consumption.
Introduction
Even though Curcuma longa L. (CL) and its chemical ingredients have garnered significant attention for use as therapeutics for inflammatory disorders, the extraction and application of curcuminoids (CUN) from CL rhizomes is limited because of their water insolubility, poor stability, and poor absorption.1,2 This has warranted a search for better solvents, such as therapeutic deep eutectic solvents (THEDES), which also exhibit anti-inflammatory properties. Inflammation is the response by the immune system against harmful stimuli, such as infectious agents and injury, and promotes the healing process.3 However, excessive and prolonged inflammation can contribute to the pathogenesis of several chronic diseases, such as cardiovascular disease, metabolic syndrome, and neurodegenerative diseases, by interfering with the homeostasis of tissue function.4 Reducing inflammation can prevent and treat underlying diseases in many organs. CL has been reported to have benefits against chronic inflammatory diseases, such as osteoarthritis,5 rheumatoid arthritis, ulcerative colitis,6 and diabetes mellitus.7 The pharmacological activities of CL result from CUN, which consists of curcumin (CUR), bisdemethoxycurcumin (BIS), and desmethoxycurcumin (DEM).2 In addition, turmeric oils are bioactive volatiles present in CL.Bioactive turmeric compounds are often extracted using organic solvents due to their low solubility in water and poor stability in aqueous solutions.8 Such processes are time-consuming, and the solvent must be removed after extraction, resulting in chemical waste. Therefore, effective and safe solvents are required to extract and manufacture CL-derived products. In particular, the use of edible solvents for extraction is highly desired.
Deep eutectic solvents (DESs) have been employed in various applications, including bioactive molecule extraction, enzymatic processes, agricultural and pharmaceutical applications, and protein stabilization. DESs have lower melting points than that of their constituents while remaining liquid at ambient temperature. THEDESs are DESs that provide medicinal benefits; in particular, they compensate for several shortcomings of the drug formulation and improve bioavailability.9 In addition, they have several advantages, including biodegradability, low toxicity, low cost, sustainability, and eco-friendliness.10 DESs are viable alternatives to organic solvents as they also exhibit low flammability, variable polarity, and excellent stability. In particular, hydrophobic DESs (HDESs) are regarded as suitable solvents for extracting natural compounds with low water solubility and efficiently delivering those that exhibit poor bioavailability. For example, CUNs have low water solubility; therefore, HDESs can be ideal solvents for effectively solubilizing and delivering them.
Menthol and saturated fatty acids have been previously used to produce HDESs. Among them, menthol and stearic acid are the most promising combination because they show no cytotoxicity, improve wound healing, and display antibacterial activities against Staphylococcus epidermis and Staphylococcus aureus strains.10 HDESs containing capric acid (CA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol exhibited antibacterial activity against Escherichia coli and Staphylococcus aureus.11 DESs containing menthol and short chain fatty acids, including propionic acid, butanoic acid, hexanoic acid, and levulinic acid, also exhibit antimicrobial activities.12 Deep eutectic mixtures of drugs have also been shown to improve pharmacological action. For example, the deep eutectic mixture of limonene and ibuprofen (IBU) inhibited HT29 proliferation and increased the anti-inflammatory action of IBU, both of which are essential in anti-cancer therapy.13 A menthol-based deep eutectic combined with acetylsalicylic acid, benzoic acid, and phenylacetic acid enhanced the dissolution rates while maintaining antibacterial effectiveness.14 Capric acid (CA) and menthol HDES improved the solubility of drugs, such as fluconazole and mometasone furoate.15 Although the antimicrobial effect of HDESs containing menthol and fatty acids is known, their anti-inflammatory activity has not been evaluated. HDESs based on menthol/fatty acids may increase CUN delivery and also provide therapeutic benefits. Menthol exhibits anti-inflammatory and antioxidant properties;16,17 CA has anti-inflammatory characteristics, where its anti-inflammatory action is mediated by the reduction of NF-κB activation and phosphorylation of the MAP kinase.18 Oleic acid (OLA) and caprylic acid (CPA) also have anti-inflammatory effects.19,20 Safe and effective HDESs that provide anti-inflammatory action can be used in the extraction and CUN delivery systems of various CL-based products. Therefore, the aim of this study is to evaluate the anti-inflammatory behavior of menthol–fatty acid HDESs with different combinations of CUNs on LPS-induced RAW264.7 murine macrophage cell lines. The multifunctional behavior of HDESs can facilitate their application in the pharmaceutical and food industries, where this green chemistry process will be utilized.
menthol exhibited antibacterial activity against Escherichia coli and Staphylococcus aureus.11 DESs containing menthol and short chain fatty acids, including propionic acid, butanoic acid, hexanoic acid, and levulinic acid, also exhibit antimicrobial activities.12 Deep eutectic mixtures of drugs have also been shown to improve pharmacological action. For example, the deep eutectic mixture of limonene and ibuprofen (IBU) inhibited HT29 proliferation and increased the anti-inflammatory action of IBU, both of which are essential in anti-cancer therapy.13 A menthol-based deep eutectic combined with acetylsalicylic acid, benzoic acid, and phenylacetic acid enhanced the dissolution rates while maintaining antibacterial effectiveness.14 Capric acid (CA) and menthol HDES improved the solubility of drugs, such as fluconazole and mometasone furoate.15 Although the antimicrobial effect of HDESs containing menthol and fatty acids is known, their anti-inflammatory activity has not been evaluated. HDESs based on menthol/fatty acids may increase CUN delivery and also provide therapeutic benefits. Menthol exhibits anti-inflammatory and antioxidant properties;16,17 CA has anti-inflammatory characteristics, where its anti-inflammatory action is mediated by the reduction of NF-κB activation and phosphorylation of the MAP kinase.18 Oleic acid (OLA) and caprylic acid (CPA) also have anti-inflammatory effects.19,20 Safe and effective HDESs that provide anti-inflammatory action can be used in the extraction and CUN delivery systems of various CL-based products. Therefore, the aim of this study is to evaluate the anti-inflammatory behavior of menthol–fatty acid HDESs with different combinations of CUNs on LPS-induced RAW264.7 murine macrophage cell lines. The multifunctional behavior of HDESs can facilitate their application in the pharmaceutical and food industries, where this green chemistry process will be utilized.
Experimental
Chemicals and reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), curcumin (CUR, ≥99.5%), oleic acid (OLA, ≥98%), Griess reagent (modified), and lipopolysaccharides (LPS) from Escherichia coli O111:B4 (LPS) were purchased from Sigma-Aldrich (MO, USA). Caprylic acid (CPA, ≥98%), capric acid (CA, ≥98%), and L-menthol (>99.0%) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). CUN containing CUR (73.5 ± 0.6%), BIS (0.136 ± 0.001%), and DEM (27.3 ± 0.0%) was obtained from Acros Organics™ (Thermo Fisher Scientific Inc., NJ, USA). Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco BRL (Life Technologies, Inc., NY, USA).Preparation of HDESs and solubility studies
The HDESs were prepared using a mixture of menthol and fatty acids, CPA, CA, and OLA at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. After mixing the components, HDESs were initiated by stirring and heating at 70 °C for 20 min.10 The obtained mixtures were transparent. The solubility of CUN in the established HDES was evaluated in the following manner: CUN (fine crystalline powder, 100 mg) was suspended in each HDES (1 mL) and ultrasonically treated for 1 h at 37 kHz. Thereafter, the mixtures were centrifuged for 10 min at 7155 × g and 25 °C, yielding clear solutions, which were evaluated for CUN solubility. The solubility of CL extracts was also evaluated using the same procedure, where 100 mg extracts replaced the pure CUN. The CL extract used in this study was prepared by macerating the CL rhizome in 95% ethanol for two days at 25 °C. Subsequently, the extract was collected, dried with a vacuum rotary evaporator, and lyophilized.
1, respectively. After mixing the components, HDESs were initiated by stirring and heating at 70 °C for 20 min.10 The obtained mixtures were transparent. The solubility of CUN in the established HDES was evaluated in the following manner: CUN (fine crystalline powder, 100 mg) was suspended in each HDES (1 mL) and ultrasonically treated for 1 h at 37 kHz. Thereafter, the mixtures were centrifuged for 10 min at 7155 × g and 25 °C, yielding clear solutions, which were evaluated for CUN solubility. The solubility of CL extracts was also evaluated using the same procedure, where 100 mg extracts replaced the pure CUN. The CL extract used in this study was prepared by macerating the CL rhizome in 95% ethanol for two days at 25 °C. Subsequently, the extract was collected, dried with a vacuum rotary evaporator, and lyophilized.
For the solubility experiment, the concentration of solubilized CUN was measured using a spectrophotometer. First, serial concentrations of pure CUN solutions were prepared at 0.390, 0.781, 1.56, 3.13, 6.25, 12.5, 25.0, and 50.0 μg mL−1 in 80% ethanol. The sample solutions (soluble CUN in the HDESs) were diluted in 80% ethanol before analysis. A 96-well plate was loaded with 200 μL of the referent CUN and sample solutions, and the absorbance was measured at 430 nm using a microplate spectrophotometer (EonTM, BioTek Instruments, Inc., VT, USA). The analyses were performed in triplicate (n = 3).
Cell culture and treatment
The macrophage cell line (RAW264.7) was obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10% (v/v) heat inactivated FBS, 100 units per mL penicillin/streptomycin, and 25 μg mL−1 amphotericin B. The culture was maintained at 37 °C in a humidified atmosphere containing 95% air and 5% CO2 until cell growth achieved a 70–80% confluence.For cell treatment, the effect of all the HDESs on the inflammation and cell viability of the LPS-induced RAW264.7 cells was determined first. The HDESs that were effective and less cytotoxic were selected for further investigations, where they were combined with CUN and CUR. The fatty acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDESs were investigated at ratios of 1
menthol HDESs were investigated at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–128
1–128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 μM ratios. Effects of CUN and CUR on the LPS-induced RAW264.7 cells were investigated at concentrations of 0.305–19.5 μg L−1 and 1.0–64 μM, respectively. For the various combinations of CUN, CUR, and HDESs, the compounds were dissolved in the selected HDESs and then diluted in DMEM containing 0.1% DMSO for various concentrations (0.305–19.5 μg L−1 CUN and 1.0–64 μM CUR). Working solutions of the samples were diluted in DMEM containing 0.1% DMSO.
64 μM ratios. Effects of CUN and CUR on the LPS-induced RAW264.7 cells were investigated at concentrations of 0.305–19.5 μg L−1 and 1.0–64 μM, respectively. For the various combinations of CUN, CUR, and HDESs, the compounds were dissolved in the selected HDESs and then diluted in DMEM containing 0.1% DMSO for various concentrations (0.305–19.5 μg L−1 CUN and 1.0–64 μM CUR). Working solutions of the samples were diluted in DMEM containing 0.1% DMSO.
Determination of NO production
The RAW264.7 cells were seeded at seeding densities of 1 × 105 cells per well into 96-well cell culture plates. The cells were pretreated with various concentrations of HDESs, CUN, CUR, and their combinations, as mentioned in “Cell culture and treatment”. The pretreatment was performed for 1 h before inflammatory induction by LPS (100 ng mL−1) for 23 h. For cell treatment, solutions of 0.1% (v/v) DMSO in DMEM was used as the untreated group. NO production from the cells was monitored by the Griess assay, as previously described.21 Briefly, 150 μL of the culture medium was mixed with 130 μL deionized water and 20 μL of the Griess reagent. After 30 min, the optical density was measured at 548 nm. The NO level was expressed in μM based on the nitrite calibration curve (r2 > 0.990). Thereafter, the relative NO production (%) was calculated using the following equation and compared to that of the LPS-treated group eqn (1).| NO secretion (%) = (nitrite level in (substance+LPS)-treated cell/nitrite level in LPS-treated cell) × 100 | (1) |
Furthermore, IC50 indicates the concentration of the solutions that inhibit NO secretion by 50%, as determined by the dose–response curve using non-linear regression in GraphPad Prism version 9 (GraphPad Software, CA, USA).
Determination of cytotoxicity
Cell cytotoxicity was determined to ensure that NO inhibition was not due to cell death. The cultured media were removed and replaced with a fresh culture medium with 0.5 mg mL−1 MTT reagent. The cells were then incubated for 2 h, followed by removing the remaining MTT reagent and adding DMSO to solubilize the formazan crystals. The solution was collected and analyzed using a spectrophotometer microplate reader at a wavelength of 595 nm. The results were expressed as the relative cell viability (%) using the following eqn (2):| Cell viability (%) = (OD of treated cells/OD of untreated cells) × 100 | (2) |
The cell viability percentage was plotted against the concentration of the drug. CC50 was determined by the dose–response curve using non-linear regression in GraphPad Prism version 9 (GraphPad Software, CA, USA).
Statistical analysis
GraphPad Prism version 9 (GraphPad Software, CA, USA) and SPSS version 26 were used for the statistical analysis. All data are presented as means with the standard error of the mean (SEM). All comparisons were assessed using the analysis of variance (ANOVA), followed by the Dunnett's multiple comparison and Tukey–Kramer test. The statistical significance level was set at p < 0.05.Results and discussion
Establishment of HDESs and the solubility of the CUN
The HDESs in this study were obtained by combining menthol with OLA, CPA, and CA at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The solubility of CUN in the as-prepared HDESs was tested, which showed that pure CUN was the most soluble (2.16 ± 0.14 mg g−1) in the CPA
1. The solubility of CUN in the as-prepared HDESs was tested, which showed that pure CUN was the most soluble (2.16 ± 0.14 mg g−1) in the CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) HDES. The best solvent for the CL extract was CPA
2 molar ratio) HDES. The best solvent for the CL extract was CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (2
menthol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), which consisted of a CUN concentration of 34.2 ± 1.1 mg g−1. However, the CUN solubility decreased as the chain length of the fatty acids increased (Table 1).
1 ratio), which consisted of a CUN concentration of 34.2 ± 1.1 mg g−1. However, the CUN solubility decreased as the chain length of the fatty acids increased (Table 1).
| Hydrophobic deep eutectic solvents (HDESs)b | Solubility (mg g−1) | ||
|---|---|---|---|
| Mixtures | Molar ratio | Curcuminoidsc | CL extractd |
| a The same and different letters indicate the nonsignificant and significant differences, respectively, in the solubility studies between HDESs. One-way ANOVA was used to determine the statistical significance, followed by the Tukey–Kramer test (p < 0.05).b The HDESs are formulated from menthol and fatty acids, including caprylic acid (CPA), capric acid (CA), and oleic acid (OLA).c Solubility studies were conducted using the fine crystalline powder of curcuminoids.d Solubility studies were conducted using the crude extract of CL rhizome. | |||
CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2.16 ± 0.14a | 24.4 ± 0.7a |
CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.69 ± 0.05b | 27.2 ± 0.8b |
CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.44 ± 0.06c | 34.2 ± 1.1c |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1.45 ± 0.04c | 30.5 ± 0.7c |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.61 ± 0.01bc | 24.7 ± 0.5a |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.04 ± 0.04d | 20.7 ± 0.8d |
OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1.68 ± 0.05b | 15.2 ± 1.0e |
OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.07 ± 0.06d | 16.1 ± 0.8e |
OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol menthol |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.941 ± 0.038d | 11.0 ± 0.4f |
Despite its vast range of therapeutic effects, the health utility of CUR has been limited because of its extremely low solubility, poor gastrointestinal absorption, low oral bioavailability, and variable effects. As previously reported, the solubility of CUR in pure water is only 0.6–7.4 μg mL−1 (ref. 22) and that in the oil of medium-chain triglycerides is 2.90 ± 0.15 mg g−1,23 whereas their solubility in edible oils is 0.41–0.53 mg g−1.24 The solubility of CUN in hydrophilic DES containing choline chloride and glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) is 7.25–8.6 mg g−1 at 20–60 °C.25 In another case, a 1
1 molar ratio) is 7.25–8.6 mg g−1 at 20–60 °C.25 In another case, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of D-(+)-glucose to sucrose led to a CUR solubility of 0.0521 ± 0.0065 mg mL−1.26 In comparison to hydrophilic DESs, the HDESs based on fatty acids and menthol are less viscous.27 CPA
1 molar ratio of D-(+)-glucose to sucrose led to a CUR solubility of 0.0521 ± 0.0065 mg mL−1.26 In comparison to hydrophilic DESs, the HDESs based on fatty acids and menthol are less viscous.27 CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and CA
menthol and CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol exhibited a viscosity of less than 20 mPa s at 25 °C,27 compared to that of a previously reported hydrophilic DES (choline chloride + urea, 1
menthol exhibited a viscosity of less than 20 mPa s at 25 °C,27 compared to that of a previously reported hydrophilic DES (choline chloride + urea, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with a viscosity of 859.45 mPa s.28 The viscosity of a solvent is one of the key factors that determine the extraction yields when applying HDES for CL extraction. The high viscosity of most DESs at room temperature limits their application due to the slow mass transfer.29 Although the viscosity of a hydrophilic DES can be adjusted by the addition of water, this decreases the solubility of CUN. The solubility of CUN is higher in the established HDESs compared to that in water and the edible oils that are usually used as vehicles for CUN delivery.
2) with a viscosity of 859.45 mPa s.28 The viscosity of a solvent is one of the key factors that determine the extraction yields when applying HDES for CL extraction. The high viscosity of most DESs at room temperature limits their application due to the slow mass transfer.29 Although the viscosity of a hydrophilic DES can be adjusted by the addition of water, this decreases the solubility of CUN. The solubility of CUN is higher in the established HDESs compared to that in water and the edible oils that are usually used as vehicles for CUN delivery.
Menthol significantly enhances the percutaneous flux and enhancement ratio of CUR across the rat epidermis.30 Menthol in the HDES also functions as a penetration enhancer of CUN, which may improve bioavailability.
The solubility of pure CUN was lower than that of the CL extract. The pure CUN used in this study was in the form of a fine crystalline powder, in which the crystalline structure of the CUN may render it insoluble in pure water.31 The CL extract contains various constituents, including turmeric oils such as α-zingiberene, ar-turmerone, β-sesquiphellandrene, α-turmerone, β-turmerone, and β-bisabolene, which may contribute to the solubility of CUN in the HDES. The solubility is mediated by bonding between the solute and solvent. Hydrogen bonds and van der Waals forces are formed between the HDES components and the substances in the CL extract. In particular, the components of turmeric oil may interact with the HDES and CUN to increase its solubility. The specific interactions that are fundamental to solubility should be further investigated.
These results indicate that HDESs based on menthol/fatty acids can be CUN solvents. The composition of the HDES and the molar ratios of its constituents influence the solubility of the target CUN, and further optimization of these parameters is necessary to improve the solubility. This type of HDES has low toxicity potential, making it ideal for use in food and pharmaceutical products.
Anti-inflammatory and cytotoxicity evaluation of HDESs
The effects of menthol-based HDESs, namely CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, CA
menthol, CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, and OLA
menthol, and OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
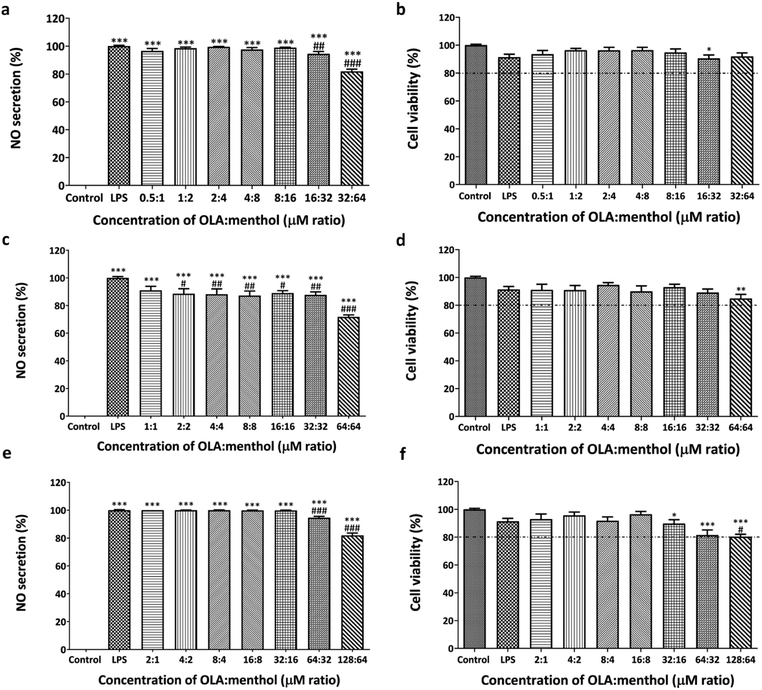
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, on RAW264.7 cells are shown in Fig. S1, S2† and 1, respectively. The NO secretion of LPS-treated RAW264.7 cells was 100%, and statistical analysis indicated that LPS considerably induced NO secretion compared to the untreated group. The LPS-induced inflammation in the cells via NO production and the upregulation of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α mRNAs).32 LPS also induced apoptotic cell death in the RAW264.7 cells.32 However, based on our experiments, the LPS-treated groups showed a cell viability of over 80% compared with that of the untreated group.
menthol, on RAW264.7 cells are shown in Fig. S1, S2† and 1, respectively. The NO secretion of LPS-treated RAW264.7 cells was 100%, and statistical analysis indicated that LPS considerably induced NO secretion compared to the untreated group. The LPS-induced inflammation in the cells via NO production and the upregulation of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α mRNAs).32 LPS also induced apoptotic cell death in the RAW264.7 cells.32 However, based on our experiments, the LPS-treated groups showed a cell viability of over 80% compared with that of the untreated group.
As stated above, the composition of HDES has a significant influence on the cell viability of the RAW264.7 cells. In the case of CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, the cell survival was ≥80% by HDESs at concentration ratios of ≤8
menthol, the cell survival was ≥80% by HDESs at concentration ratios of ≤8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, 1
16, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μM ratios, respectively (Fig. S1†). Accordingly, CPA
1 μM ratios, respectively (Fig. S1†). Accordingly, CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) was quite toxic for the RAW264.7 cells, when compared to the other molar ratios. Furthermore, it did not induce a significant reduction in NO production at any concentration, with ≥80% cell viability. Although CPA did not affect NO production in the LPS-stimulated RAW264.7 cells, CPA has previously been shown to decrease inflammation via other inflammatory molecules, such as the Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), nuclear factor-κB (NF-κB), TNF-α, IKKα, and IKKβ mRNA expression.20 Furthermore, CPA suppresses inflammation via TLR4/NF-kBα signaling and ameliorates atherosclerosis by inhibiting the production of IL-1β, IL-6, NF-κB, and TNF-α.20 Thus, the above-mentioned mechanism of the anti-inflammatory effect, cytotoxicity, and underlining pathway of CPA
1 molar ratio) was quite toxic for the RAW264.7 cells, when compared to the other molar ratios. Furthermore, it did not induce a significant reduction in NO production at any concentration, with ≥80% cell viability. Although CPA did not affect NO production in the LPS-stimulated RAW264.7 cells, CPA has previously been shown to decrease inflammation via other inflammatory molecules, such as the Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), nuclear factor-κB (NF-κB), TNF-α, IKKα, and IKKβ mRNA expression.20 Furthermore, CPA suppresses inflammation via TLR4/NF-kBα signaling and ameliorates atherosclerosis by inhibiting the production of IL-1β, IL-6, NF-κB, and TNF-α.20 Thus, the above-mentioned mechanism of the anti-inflammatory effect, cytotoxicity, and underlining pathway of CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol requires further examination.
menthol requires further examination.
The highest CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol concentration ratios providing ≥80% cell survival were 16
menthol concentration ratios providing ≥80% cell survival were 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32, 8
32, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, and 2
8, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μM ratios (Fig. S2†). Furthermore, the CA
1 μM ratios (Fig. S2†). Furthermore, the CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDESs significantly reduced NO production. When cell viability (≥80%) was considered, CA
menthol HDESs significantly reduced NO production. When cell viability (≥80%) was considered, CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (2
menthol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μM ratio) inhibited NO production the most (25.4%). CA has previously been shown to reduce TNF-α and IL-6 levels in cyclophosphamide-induced intestinal inflammation.33 Furthermore, CA
1 μM ratio) inhibited NO production the most (25.4%). CA has previously been shown to reduce TNF-α and IL-6 levels in cyclophosphamide-induced intestinal inflammation.33 Furthermore, CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol can inhibit inflammation via multiple inflammatory mediators. CA
menthol can inhibit inflammation via multiple inflammatory mediators. CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol is probably safer than CPA
menthol is probably safer than CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol. However, at high concentrations of CA
menthol. However, at high concentrations of CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, especially at a molar ratio of 2
menthol, especially at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, considerable cell death occurred. Significantly, OLA
1, considerable cell death occurred. Significantly, OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol was less toxic to RAW264.7 cells, with cell survival exceeding 80% when compared to that of the untreated cells (Fig. 1). At a 64
menthol was less toxic to RAW264.7 cells, with cell survival exceeding 80% when compared to that of the untreated cells (Fig. 1). At a 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 μM ratio, OLA
64 μM ratio, OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol suppressed NO production the most (28.2%), and inhibition occurred in a concentration-dependent manner. Through the activation of NF-κB, OLA is linked to COX-2 and iNOS downregulation.34,35
menthol suppressed NO production the most (28.2%), and inhibition occurred in a concentration-dependent manner. Through the activation of NF-κB, OLA is linked to COX-2 and iNOS downregulation.34,35
At the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios of fatty acid
1 molar ratios of fatty acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, a decreasing trend of cell toxicity with high menthol proportion was observed. Menthol has immunomodulatory and anti-inflammatory effects in ethanol-induced gastric ulcers, where it reduces the pro-inflammatory cytokines, TNF-α, and IL-6, while increasing the levels of the anti-inflammatory cytokine IL-10.16 This suggests that a higher menthol
menthol, a decreasing trend of cell toxicity with high menthol proportion was observed. Menthol has immunomodulatory and anti-inflammatory effects in ethanol-induced gastric ulcers, where it reduces the pro-inflammatory cytokines, TNF-α, and IL-6, while increasing the levels of the anti-inflammatory cytokine IL-10.16 This suggests that a higher menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fatty acid ratio may protect the cell from over-inflammation.
fatty acid ratio may protect the cell from over-inflammation.
In the LPS-treated group, the murine macrophages experienced cell death at 10–13%. Additional cell death was observed with CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and CA
menthol and CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol. LPS triggers cell death via the autocrine release of TNF-α and NO.36 Furthermore, menthol decreased the LPS-induced cytokine production in the RAW264.7 cells (TNF-α, IL-6, and IL-1β).17 Although CPA and CA have been associated with anti-inflammatory properties, cell death remained high for their respective HDESs. This may be attributed to other mechanisms that CPA
menthol. LPS triggers cell death via the autocrine release of TNF-α and NO.36 Furthermore, menthol decreased the LPS-induced cytokine production in the RAW264.7 cells (TNF-α, IL-6, and IL-1β).17 Although CPA and CA have been associated with anti-inflammatory properties, cell death remained high for their respective HDESs. This may be attributed to other mechanisms that CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and CA
menthol and CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol uses to trigger apoptosis. On the contrary, antioxidant effects of OLA may be correlated with the enhanced cell viability. Pyroptosis occurs when cytosolic LPS binds to the precursor of caspase-4/5/11.37 Because menthol appears to change the cell membranes, it is possible that menthol might also change cell permeability.38 Additionally, cell properties are influenced by several factors, such as HDES ratios, concentration, and the type of fatty acids; therefore, the effect of menthol/fatty acid HDESs on the cells remains unclear.
menthol uses to trigger apoptosis. On the contrary, antioxidant effects of OLA may be correlated with the enhanced cell viability. Pyroptosis occurs when cytosolic LPS binds to the precursor of caspase-4/5/11.37 Because menthol appears to change the cell membranes, it is possible that menthol might also change cell permeability.38 Additionally, cell properties are influenced by several factors, such as HDES ratios, concentration, and the type of fatty acids; therefore, the effect of menthol/fatty acid HDESs on the cells remains unclear.
HDESs containing CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and OLA
menthol and OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol significantly inhibited NO production, while those with OLA
menthol significantly inhibited NO production, while those with OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol protected against cell death. OLA can reduce the LPS-induced expression of iNOS, COX-2, and IL-6 mRNA and significantly decrease COX-2 and iNOS protein expression.35 Therefore, OLA
menthol protected against cell death. OLA can reduce the LPS-induced expression of iNOS, COX-2, and IL-6 mRNA and significantly decrease COX-2 and iNOS protein expression.35 Therefore, OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol can preserve cell viability and reduce inflammation. These results corroborate with several previous studies that demonstrated the low toxicity of DESs.10,39 Notably, compared to our HDESs, the previously reported menthol-based THEDESs, such as menthol
menthol can preserve cell viability and reduce inflammation. These results corroborate with several previous studies that demonstrated the low toxicity of DESs.10,39 Notably, compared to our HDESs, the previously reported menthol-based THEDESs, such as menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid, menthol
lauric acid, menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) myristic acid, and menthol
myristic acid, and menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) stearic acid, showed lower toxicity toward the HaCaT cell line despite their high concentrations (mM concentration).10 Our study is the first to report the non-toxicity of the OLA
stearic acid, showed lower toxicity toward the HaCaT cell line despite their high concentrations (mM concentration).10 Our study is the first to report the non-toxicity of the OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDES in RAW264.7 cells (Fig. 1).
menthol HDES in RAW264.7 cells (Fig. 1).
Overall, the HDESs containing OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol are anti-inflammatory and less cytotoxic than the HDESs of the other fatty acids. Consequently, the OLA
menthol are anti-inflammatory and less cytotoxic than the HDESs of the other fatty acids. Consequently, the OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDESs were used in subsequent studies, where they were combined with CUN and CUR. The HDESs were defined as HDES-OM1, HDES-OM2, and HDES-OM3 with OLA
menthol HDESs were used in subsequent studies, where they were combined with CUN and CUR. The HDESs were defined as HDES-OM1, HDES-OM2, and HDES-OM3 with OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol molar ratios of 1
menthol molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
Anti-inflammatory and cytotoxicity of CUN and CUR
CUN and CUR decrease inflammation by inhibiting inflammatory molecules, such as interleukins, cyclooxygenase, TNF-α, and iNOS.40,41 However, the effect of CUN and CUR in combination with OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
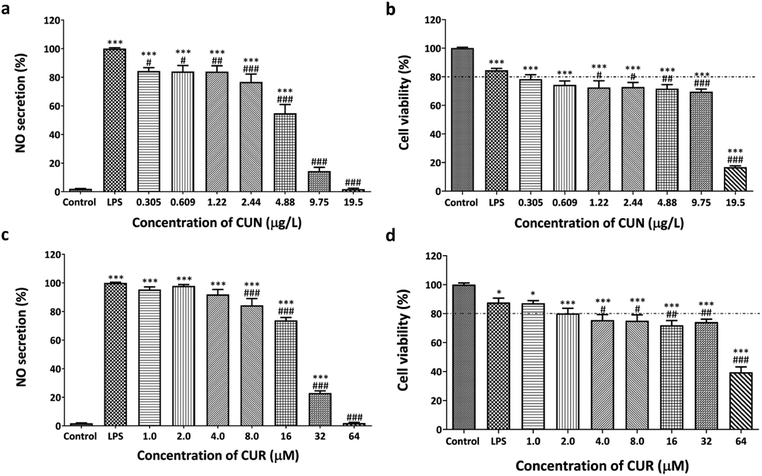
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol HDES has not been explored. Both CUN and CUR decreased NO secretion in a dose-dependent manner (Fig. 2). Furthermore, the IC50 values of CUN and CUR were 5.59 ± 0.56 μg L−1 and 20.7 ± 1.6 μM, respectively. Compared to the control cells, the CUN-treated cells at 0.305–19.5 μg L−1 significantly decreased cell viability by less than 80% in a dose-dependent manner (Fig. 2b), while the same effect was observed at 4.0–64 μM for the CUR-treated cells (Fig. 2d). The results of the CUN and CUR treatments indicated that increasing the concentration decreased the cell viability of the RAW264.7 cells with CC50 values of 11.9 ± 1.1 μg L−1 and 52.9 ± 5.5 μM, respectively. These results corroborate well with previous studies, which report that more than 25 μM concentrations of CUR are toxic to RAW264.7 cells.42 Furthermore, in LPS-stimulated human monocytes, alveolar macrophages, and glucose-induced human monocytes, a modest dose of CUR (1–5 μM) was sufficient to suppress inflammatory cytokines with low cytotoxicity.42–44 There is limited information on the anti-inflammatory effects of the CUN concentration, and CUR is a well-known and principal component of CUN. For the subsequent experiments, the CUR and CUN concentrations were combined with HDES-OM1, HDES-OM2, and HDES-OM3.
menthol HDES has not been explored. Both CUN and CUR decreased NO secretion in a dose-dependent manner (Fig. 2). Furthermore, the IC50 values of CUN and CUR were 5.59 ± 0.56 μg L−1 and 20.7 ± 1.6 μM, respectively. Compared to the control cells, the CUN-treated cells at 0.305–19.5 μg L−1 significantly decreased cell viability by less than 80% in a dose-dependent manner (Fig. 2b), while the same effect was observed at 4.0–64 μM for the CUR-treated cells (Fig. 2d). The results of the CUN and CUR treatments indicated that increasing the concentration decreased the cell viability of the RAW264.7 cells with CC50 values of 11.9 ± 1.1 μg L−1 and 52.9 ± 5.5 μM, respectively. These results corroborate well with previous studies, which report that more than 25 μM concentrations of CUR are toxic to RAW264.7 cells.42 Furthermore, in LPS-stimulated human monocytes, alveolar macrophages, and glucose-induced human monocytes, a modest dose of CUR (1–5 μM) was sufficient to suppress inflammatory cytokines with low cytotoxicity.42–44 There is limited information on the anti-inflammatory effects of the CUN concentration, and CUR is a well-known and principal component of CUN. For the subsequent experiments, the CUR and CUN concentrations were combined with HDES-OM1, HDES-OM2, and HDES-OM3.
Anti-inflammation and cytotoxicity evaluation of the HDES combined with CUN and CUR
In this section, HDES-OM1, 2, and 3 were used to define the OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol molar ratios of 1
menthol molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Various concentrations of CUN and CUR were dissolved in HDES-OM1–3 and then diluted in DMEM containing 0.1% DMSO for cell treatment. Anti-inflammation and cytotoxicity assays were performed in the same manner as previously described. CUN and CUR in HDES-OM1–3 substantially reduced NO secretion in a dose-dependent manner, as detailed below.
1, respectively. Various concentrations of CUN and CUR were dissolved in HDES-OM1–3 and then diluted in DMEM containing 0.1% DMSO for cell treatment. Anti-inflammation and cytotoxicity assays were performed in the same manner as previously described. CUN and CUR in HDES-OM1–3 substantially reduced NO secretion in a dose-dependent manner, as detailed below.
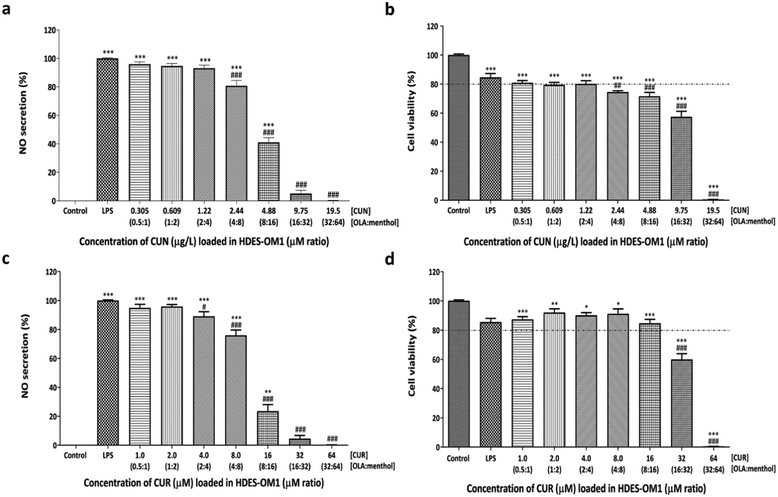
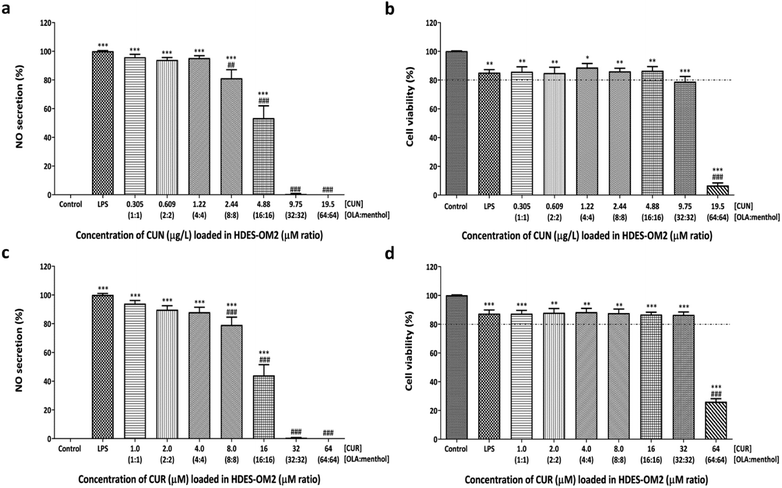
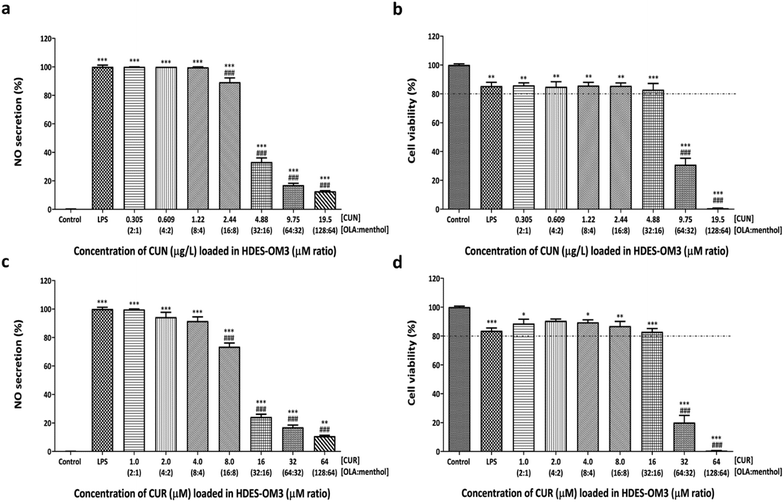
Compared to the LPS-treated cells, CUN in HDES-OM1 at concentrations of 2.44–19.5 μg L−1 considerably reduced NO production in LPS-induced cells; however, all treatments significantly reduced cell viability (Fig. 3). It is speculated that the inhibition of NO production may result in cell death. CUR in HDES-OM1 at concentrations of 4.0–64 μM also significantly decreased NO secretion; however, only 4.0–16 μM CUR demonstrated a cell survival of more than 80% compared to the untreated group. Therefore, at 4.0–16 μM, CUR in HDES-OM1 inhibited NO production with less cell toxicity than that with CUR treatment alone. CUR exhibited less cytotoxicity when combined with HDES-OM1. CUN concentrations of up to 4.88 μg L−1 in HDES-OM2 preserved cell viability over 80% (Fig. 4). Furthermore, CUN concentrations of 2.44 and 4.88 μg L−1 significantly suppressed NO secretion. The CUR concentration in HDES-OM2 of up to 32 μM exhibited a cell viability of over 80%. Therefore, CUN and CUR in HDES-OM2 prevented cytotoxicity, and its efficacy was superior to that of HDES-OM2 without CUN and CUR. Increasing the CUR concentration (8.0–32 μM) decreased the NO secretion, reaching 99.4% inhibition at 32 μM with minimal cytotoxicity.
In HDES-OM3, significant cytotoxicity was not observed for CUN and CUR concentrations of up to 4.88 μg L−1 and 16 μM, respectively, as compared to the LPS-treated group (Fig. 5). Furthermore, CUN (2.44–4.88 μg L−1) and CUR (8.0–16 μM) significantly inhibited NO secretion. HDES-OM2 and 3 both, have a tendency to protect cells from CUN cytotoxicity in a similar manner; however, HDES-OM2 was the most effective in reducing CUR toxicity.
For CUN (4.88 μg L−1) combined with HDES-OM1, HDES-OM2, and HDES-OM3, NO secretion was 41.0, 53.3, and 33.2%, respectively, while cell viability was 71.5%, 86.4%, and 82.8%, respectively. NO secretion was 54.8% and cell survival was 71.6% in cells treated with CUN (4.88 μg L−1) without HDES (Tables 2 and 3). These results suggest that HDES-OM3 is a suitable choice for delivering CUN to the cells. However, cell death was considerably elevated at CUN concentrations of ≥9.75 μg L−1. At 32 μM, CUR combined with HDES-OM1, HDES-OM2, and HDES-OM3 suppressed NO secretion to 4.45, 0.547, and 16.9%, respectively, while cell viability was 59.8%, 86.4%, and 20.0%, respectively. NO secretion was 22.9% and cell survival was 74.1% in cells treated with CUR (32 μM) without HDES. Therefore, it is apparent that CUR in HDES-OM2 outperformed the other solvents, completely blocking NO release while maintaining high cell viability.
| Solvents | NO secretion (%) | |||||
|---|---|---|---|---|---|---|
| CUN (2.44 μg L−1) | CUN (4.88 μg L−1) | CUN (9.75 μg L−1) | CUR (8.0 μM) | CUR (16 μM) | CUR (32 μM) | |
| a The same and different letters represent the nonsignificant and significant differences, respectively, of NO secretion (%) of CUN or CUR in DMSO and CUN or CUR loaded in HDES-OM1-3 at the same concentration. One-way ANOVA was used to determine the statistical significance, followed by the Tukey–Kramer test (p < 0.05). | ||||||
| DMSO | 76.7 ± 5.5a | 54.8 ± 6.2a | 14.4 ± 2.7a | 84.3 ± 4.7a | 73.7 ± 2.1a | 22.9 ± 1.4a |
| HDES-OM1 | 80.7 ± 3.8a | 41.0 ± 3.3ab | 5.11 ± 2.2b | 75.9 ± 3.8a | 23.4 ± 4.6b | 4.45 ± 2.30b |
| HDES-OM2 | 81.1 ± 5.9a | 53.3 ± 8.7ab | 0.432 ± 0.370b | 79.1 ± 5.5a | 43.9 ± 7.6c | 0.547 ± 0.214b |
| HDES-OM3 | 89.2 ± 3.0a | 33.2 ± 2.9b | 16.9 ± 1.3a | 73.5 ± 2.6a | 24.1 ± 2.0b | 16.9 ± 1.5a |
| Solvents | Cell viability (%) | |||||
|---|---|---|---|---|---|---|
| CUN (2.44 μg L−1) | CUN (4.88 μg L−1) | CUN (9.75 μg L−1) | CUR (8.0 μM) | CUR (16 μM) | CUR (32 μM) | |
| a The same and different letters represent the nonsignificant and significant differences, respectively, of cell viability (%) of CUN or CUR in DMSO and CUN or CUR loaded in HDES-OM1-3 at the same concentration. One-way ANOVA was used to determine the statistical significance, followed by the Tukey–Kramer test (p < 0.05). | ||||||
| DMSO | 72.8 ± 3.2a | 71.6 ± 2.9a | 69.5 ± 1.9a | 75.0 ± 4.1a | 71.9 ± 3.4a | 74.1 ± 2.1a |
| HDES-OM1 | 74.5 ± 0.9a | 71.5 ± 2.8a | 57.4 ± 3.8b | 91.1 ± 3.5b | 84.6 ± 2.8b | 59.8 ± 4.1b |
| HDES-OM2 | 86.0 ± 2.2b | 86.4 ± 2.9b | 78.9 ± 3.6a | 87.6 ± 2.9ab | 86.6 ± 1.7b | 86.4 ± 2.0a |
| HDES-OM3 | 85.4 ± 2.1b | 82.8 ± 4.4ab | 30.8 ± 4.5c | 86.8 ± 3.3ab | 82.9 ± 2.2b | 20.0 ± 5.0c |
As previously reported, these findings are most likely related to the structure of the DESs. DES mixtures of menthol terpene molecules, in particular, can improve solubility, permeability, and absorption.45,46 When menthol was coupled with IBU, phenylacetic acid, or benzoic acid, the permeability increased.46
This suggests that our HDESs derived from menthol and OLA unsaturated fatty acids may improve the penetration of CUN in cells. CUR causes apoptosis in cells by dephosphorylating Akt, downregulating the anti-apoptotic Bcl-2, Bcl-X L, and IAP proteins, releasing cytochrome c, and activating caspase 3 in a sequential manner.47,48 The increase in the pro-apoptotic Bak protein levels caused by palmitic acid was considerably reduced by OLA.49 In the palmitic acid-treated cells, OLA restored the low levels of the anti-apoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL, and Mcl-1).49 Therefore, it can be expected that OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol can deliver anti-inflammatory CUN and CUR while also protecting cells from cytotoxicity. When limonene and IBU are combined as a eutectic mixture, the anti-inflammatory effect of IBU is enhanced.13 A DES containing choline and geranate demonstrated a broad-spectrum antibacterial efficacy against a variety of drug-resistant bacteria, fungi, and viruses.50
menthol can deliver anti-inflammatory CUN and CUR while also protecting cells from cytotoxicity. When limonene and IBU are combined as a eutectic mixture, the anti-inflammatory effect of IBU is enhanced.13 A DES containing choline and geranate demonstrated a broad-spectrum antibacterial efficacy against a variety of drug-resistant bacteria, fungi, and viruses.50
Furthermore, the solvent-removal step can be omitted as the solvent is bioactive and improves solubility, enabling its immediate application in formulation manufacturing. Therefore, owing to the bioactivity and biodegradability of the menthol/OLA-based HDESs, CUR and CUN can be chemically extracted in a sustainable manner and used as therapeutics.
Conclusions
This study is the first to demonstrate that HDESs composed of fatty acids and menthol can enhance the therapeutic effect of CUN and CUR as THEDES. Among the fatty acids analyzed in this study, the THEDESs prepared from the HDESs containing CPA and menthol with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, or 2
1, or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were the most successful in dissolving CUN and CUR. However, the anti-inflammatory activities of the OLA
1 were the most successful in dissolving CUN and CUR. However, the anti-inflammatory activities of the OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol-HDESs were superior to those of CPA
menthol-HDESs were superior to those of CPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and CA
menthol and CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, with low cytotoxicity. Notably, OLA
menthol, with low cytotoxicity. Notably, OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol can enhance the anti-inflammatory properties of CUN and CUR and minimize their cytotoxicity. At a 1
menthol can enhance the anti-inflammatory properties of CUN and CUR and minimize their cytotoxicity. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μM ratio of OLA
1 μM ratio of OLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, NO secretion can be entirely suppressed by CUR without causing significant cell death, as compared to the LPS-treated control group. These results provide a foundation for further research and development of safe solvents with pharmacological activity. Furthermore, these findings suggest that HDESs are a potentially viable biodegradable alternative to conventional organic solvents for producing more effective therapeutics and safe CL products for consumption.
menthol, NO secretion can be entirely suppressed by CUR without causing significant cell death, as compared to the LPS-treated control group. These results provide a foundation for further research and development of safe solvents with pharmacological activity. Furthermore, these findings suggest that HDESs are a potentially viable biodegradable alternative to conventional organic solvents for producing more effective therapeutics and safe CL products for consumption.
Author contributions
Kantapich Kongpol: conceptualization, funding acquisition, data curation, formal analysis, investigation, methodology, software, visualization, writing – original draft. Preenapan Chaihao: investigation. Parichat Shuapan: investigation. Ploypailin Kongduk: investigation. Warangkana Chunglok: resources. Gorawit Yusakul: conceptualization, funding acquisition, methodology, resources, supervision, writing – review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.Acknowledgements
This study was supported by a personal research grant [grant number: WU-IRG-64-017], Walailak University, Thailand. The authors would also like to thank the research instrument for health sciences, Walailak University, for providing all the facilities and equipment. This research was financially supported by a new strategic research project (P2P) for fiscal year 2022, Walailak University, Thailand.References
- H.-X. Li, H.-L. Zhang, N. Zhang, N. Wang, Y. Yang and Z.-Z. Zhang, LWT–Food Sci. Technol., 2014, 57, 446–451 CrossRef CAS.
- Y. Liu, J. Li, R. Fu, L. Zhang, D. Wang and S. Wang, Ind. Crops Prod., 2019, 140, 111620 CrossRef CAS.
- L. Chen, H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang and L. Zhao, Oncotarget, 2018, 9, 7204–7218 CrossRef PubMed.
- R. Medzhitov, Cell, 2010, 140, 771–776 CrossRef CAS PubMed.
- L. Zeng, G. Yu, W. Hao, K. Yang and H. Chen, Biosci. Rep., 2021, 41, BSR20210817 CrossRef CAS PubMed.
- A. Ebrahimzadeh, F. Abbasi, A. Ebrahimzadeh, A. T. Jibril and A. Milajerdi, Complement. Ther. Med., 2021, 61, 102773 CrossRef PubMed.
- L. T. Marton, E. S. L. M. Pescinini, M. E. C. Camargo, S. M. Barbalho, J. Haber, R. V. Sinatora, C. R. P. Detregiachi, R. J. S. Girio, D. V. Buchaim and P. Cincotto Dos Santos Bueno, Front. Endocrinol., 2021, 12, 669448 CrossRef PubMed.
- M. I. Landim Neves, M. M. Strieder, R. Vardanega, E. K. Silva and M. A. A. Meireles, RSC Adv., 2019, 10, 112–121 RSC.
- F. Santos and A. R. C. Duarte, in Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances, ed. S. Fourmentin, M. Costa Gomes and E. Lichtfouse, Springer International Publishing, Cham, 2021, ch. 3, pp. 103–129, DOI:10.1007/978-3-030-53069-3_3.
- J. M. Silva, C. V. Pereira, F. Mano, E. Silva, V. I. B. Castro, I. Sa-Nogueira, R. L. Reis, A. Paiva, A. A. Matias and A. R. C. Duarte, ACS Appl. Bio Mater., 2019, 2, 4346–4355 CrossRef CAS PubMed.
- C. Zeng, Y. Liu, Z. Ding, H. Xia and S. Guo, J. Mol. Liq., 2021, 338, 116950 CrossRef CAS.
- N. A. N. Azmi, A. Elgharbawy, H. M. Salleh and A. Hayyan, E3S Web Conf., 2021, 287, 02010 CrossRef CAS.
- C. V. Pereira, J. M. Silva, L. Rodrigues, R. L. Reis, A. Paiva, A. R. C. Duarte and A. Matias, Sci. Rep., 2019, 9, 14926 CrossRef PubMed.
- I. M. Aroso, J. C. Silva, F. Mano, A. S. Ferreira, M. Dionisio, I. Sa-Nogueira, S. Barreiros, R. L. Reis, A. Paiva and A. R. Duarte, Eur. J. Pharm. Biopharm., 2016, 98, 57–66 CrossRef CAS PubMed.
- F. Al-Akayleh, H. H. M. Ali, M. M. Ghareeb and M. Al-Remawi, J. Drug Delivery Sci. Technol., 2019, 53, 101159 CrossRef CAS.
- A. L. Rozza, F. M. de Faria, A. R. S. Brito and C. H. Pellizzon, PLoS One, 2014, 9, e86686 CrossRef PubMed.
- M. Shahid, M. Y. Lee, A. Yeon, E. Cho, V. Sairam, L. Valdiviez, S. You and J. Kim, Sci. Rep., 2018, 8, 10859 CrossRef PubMed.
- W. C. Huang, T. H. Tsai, L. T. Chuang, Y. Y. Li, C. C. Zouboulis and P. J. Tsai, J. Dermatol. Sci., 2014, 73, 232–240 CrossRef CAS PubMed.
- A. B. Santamarina, L. P. Pisani, E. J. Baker, A. D. Marat, C. A. Valenzuela, E. A. Miles and P. C. Calder, Food Funct., 2021, 12, 7909–7922 RSC.
- X. Zhang, C. Xue, Q. Xu, Y. Zhang, H. Li, F. Li, Y. Liu and C. Guo, Nutr. Metab., 2019, 16, 40 CrossRef PubMed.
- S. Mayura, C. Changtam, R. Kimseng, U. Tanyarath, L. Monthon, A. Suksamrarn and W. Chunglok, Asian Pac. J. Cancer Prev., 2014, 15, 1807–1810 CrossRef PubMed.
- B. T. Kurien, A. Singh, H. Matsumoto and R. H. Scofield, Assay Drug Dev. Technol., 2007, 5, 567–576 CrossRef CAS PubMed.
- M. Kharat, Z. Du, G. Zhang and D. J. McClements, J. Agric. Food Chem., 2017, 65, 1525–1532 CrossRef CAS PubMed.
- M. Takenaka, T. Ohkubo, H. Okadome, I. Sotome, T. Itoh and S. Isobe, Food Sci. Technol. Res., 2013, 19, 655–659 CrossRef CAS.
- T. Jelinski, M. Przybylek and P. Cysewski, Pharm. Res., 2019, 36, 116 CrossRef PubMed.
- K. O. Wikene, E. Bruzell and H. H. Tonnesen, Eur. J. Pharm. Sci., 2015, 80, 26–32 CrossRef CAS PubMed.
- C. Florindo, L. C. Branco and I. M. Marrucho, ChemSusChem, 2019, 12, 1549–1559 CrossRef CAS PubMed.
- H. Shekaari, M. T. Zafarani-Moattar and B. Mohammadi, J. Mol. Liq., 2017, 243, 451–461 CrossRef CAS.
- L. Duan, L.-L. Dou, L. Guo, P. Li and E. H. Liu, ACS Sustainable Chem. Eng., 2016, 4, 2405–2411 CrossRef CAS.
- N. A. Patel, N. J. Patel and R. P. Patel, Pharm. Dev. Technol., 2009, 14, 80–89 CAS.
- G. H. Shin, J. Li, J. H. Cho, J. T. Kim and H. J. Park, J. Food Sci., 2016, 81, N494–N501 CrossRef CAS PubMed.
- X. Luo, H. Zhang, X. Wei, M. Shi, P. Fan, W. Xie, Y. Zhang and N. Xu, Molecules, 2018, 23, 517 CrossRef PubMed.
- S. I. Lee and K. S. Kang, Sci. Rep., 2017, 7, 16530 CrossRef PubMed.
- H. Kim, K. Youn, E.-Y. Yun, J.-S. Hwang, W.-S. Jeong, C.-T. Ho and M. Jun, J. Funct. Foods, 2015, 14, 1–11 CrossRef CAS.
- A. K. Muller, F. Albrecht, C. Rohrer, A. Koeberle, O. Werz, W. Schlormann, M. Glei, S. Lorkowski and M. Wallert, Nutrients, 2021, 13, 4437 CrossRef PubMed.
- L. George, T. Ramasamy, K. Sirajudeen and V. Manickam, Immunol. Invest., 2019, 48, 451–465 CrossRef CAS PubMed.
- Y. Y. Wang, X. L. Liu and R. Zhao, Front. Oncol., 2019, 9, 971 CrossRef PubMed.
- H. Wang and F. Meng, J. Mol. Model., 2017, 23, 279 CrossRef PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- S. J. Hewlings and D. S. Kalman, Foods, 2017, 6, 92 CrossRef PubMed.
- A. H. Rahmani, M. A. Alsahli, S. M. Aly, M. A. Khan and Y. H. Aldebasi, Adv. Biomed. Res., 2018, 7, 38 CrossRef PubMed.
- M. R. Guimaraes, F. R. Leite, L. C. Spolidorio, K. L. Kirkwood and C. Rossa Jr, Arch. Oral Biol., 2013, 58, 1309–1317 CrossRef CAS PubMed.
- D. Chen, M. Nie, M. W. Fan and Z. Bian, Pharmacology, 2008, 82, 264–269 CrossRef CAS PubMed.
- Y. Abe, S. Hashimoto and T. Horie, Pharmacol. Res., 1999, 39, 41–47 CrossRef CAS PubMed.
- I. M. Aroso, R. Craveiro, A. Rocha, M. Dionisio, S. Barreiros, R. L. Reis, A. Paiva and A. R. Duarte, Int. J. Pharm., 2015, 492, 73–79 CrossRef CAS PubMed.
- A. R. Duarte, A. S. Ferreira, S. Barreiros, E. Cabrita, R. L. Reis and A. Paiva, Eur. J. Pharm. Biopharm., 2017, 114, 296–304 CrossRef CAS PubMed.
- J. H. Woo, Y. H. Kim, Y. J. Choi, D. G. Kim, K. S. Lee, J. H. Bae, D. S. Min, J. S. Chang, Y. J. Jeong, Y. H. Lee, J. W. Park and T. K. Kwon, Carcinogenesis, 2003, 24, 1199–1208 CrossRef CAS PubMed.
- D. Bandyopadhyay, Front. Chem., 2014, 2, 113 Search PubMed.
- J. H. Ahn, M. H. Kim, H. J. Kwon, S. Y. Choi and H. Y. Kwon, Korean J. Physiol. Pharmacol., 2013, 17, 43–50 CrossRef CAS PubMed.
- M. Zakrewsky, A. Banerjee, S. Apte, T. L. Kern, M. R. Jones, R. E. Sesto, A. T. Koppisch, D. T. Fox and S. Mitragotri, Adv. Healthcare Mater., 2016, 5, 1282–1289 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01782b |
| This journal is © The Royal Society of Chemistry 2022 |