Open Access Article
Open Access ArticleFacile hydrothermal synthesis of cobaltosic sulfide nanorods for high performance supercapacitors†
Yin Songa,
Yuanhao Dinga,
Chenghua Yanga,
Xiaokang Peia,
Guangxia Wanga,
Dezhou Zhenga,
Wei Xua,
Fuxin Wang *a and
Xihong Lu
*a and
Xihong Lu *ab
*ab
aSchool of Applied Physics and Materials, Wuyi University, Jiangmen 529020, PR China. E-mail: wangfux91@126.com
bMOE of the Key Laboratory of Bioinorganic and Synthetic Chemistry, The Key Lab of Low-carbon Chem & Energy Conservation of Guangdong Province, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, PR China. E-mail: luxh6@mail.sysu.edu.cn
First published on 14th April 2022
Abstract
With high reactivity, electrical conductivity, theoretical specific capacitance and well redox reversibility, transition metal sulfides are considered as a promising anode material for supercapacitors. Hence, we designed a simple two-step hydrothermal process to grow Co4S3 nanorod arrays in situ on flexible carbon cloth substrates. Benefited from the larger specific surface area of nanoarrays, the binder-free Co4S3 electrode demonstrates a higher specific capacity of 1.97 F cm−2 at a current density of 2 mA cm−2, while the Co3O4 electrode has a capacity of only 0.07 F cm−2 at the same current density. Surprisingly, at a high scan rate of 200 mV s−1, the synthesized Co4S3 electrode still maintains almost 100% of its initial capacitance after 5000 cycles. Moreover, when using the prepared Co4S3 and MnO2 electrode as the anode and cathode, the fabricated flexible supercapacitor obtains a high volumetric energy density of 0.87 mW h cm−3 (power density of 0.78 W cm−3) and a peak power density of 0.89 W cm−3 (energy density of 0.50 mW h cm−3). The excellent electrochemical properties imply that there is a large market for the prepared materials in flexible energy storage devices.
Introduction
With the continuous development of technology, especially the development of mobile electronic devices, and intelligent equipment, the demand for highly efficient energy storage equipment such as lithium-ion batteries, sodium-ion batteries, and supercapacitors has risen sharply.1–4 Among these devices, the supercapacitors have attracted attention and study because of their high energy conversion efficiency, long cycle time (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), and fast charge/discharge performance (10 s to 10 min). Compared with conventional capacitors, supercapacitors possess a relatively high energy density (1–30 W h kg−1).5–7 Based on these advantages, supercapacitors are considered to fill the gap of the batteries and conventional capacitors. Nevertheless, the energy density of supercapacitors is remain poor compared to lithium-ion batteries (about 150 W h kg−1), limiting its large-scale application.8,9 According to the equation E = 1/2CV2, there are two major methods to enhance the energy storage performance of supercapacitors: (i) increasing the specific capacity of the electrode; (ii) expanding the cell voltage.10–12 To scale up the cell voltage, the general research strategy is to replace the aqueous solution-based electrolytes with organic electrolytes or ionic liquids. However, organic electrolytes are flammable and can pose safety hazards, and the traces of water adsorbed in them still limit the further expansion of the voltage window.13–17 Therefore, increasing the specific capacity of electrode materials becomes another key factor. The energy storage performance of supercapacitor devices can be boosted by adjusting the intrinsic properties of the electrode materials.
000 cycles), and fast charge/discharge performance (10 s to 10 min). Compared with conventional capacitors, supercapacitors possess a relatively high energy density (1–30 W h kg−1).5–7 Based on these advantages, supercapacitors are considered to fill the gap of the batteries and conventional capacitors. Nevertheless, the energy density of supercapacitors is remain poor compared to lithium-ion batteries (about 150 W h kg−1), limiting its large-scale application.8,9 According to the equation E = 1/2CV2, there are two major methods to enhance the energy storage performance of supercapacitors: (i) increasing the specific capacity of the electrode; (ii) expanding the cell voltage.10–12 To scale up the cell voltage, the general research strategy is to replace the aqueous solution-based electrolytes with organic electrolytes or ionic liquids. However, organic electrolytes are flammable and can pose safety hazards, and the traces of water adsorbed in them still limit the further expansion of the voltage window.13–17 Therefore, increasing the specific capacity of electrode materials becomes another key factor. The energy storage performance of supercapacitor devices can be boosted by adjusting the intrinsic properties of the electrode materials.
The electrode as a crucial component of the supercapacitors directly influences their performance. In general, electrode materials are primarily comprised of carbon materials (such as activated carbon, graphene, porous carbon), conductive polymers (such as polythiophene, polyaniline), and transition metal compounds (such as MnO2, Co3O4, NiO, CoS2 and etc.).18–23 Materials based on transition metal compounds have been extensively reported because their energy densities are higher than those of electrical double-layer capacitors (carbon-based materials) and conducting polymers.24,25 In recent years, Co-based compounds have exhibited prominent property, and have been a popular topic in the field of supercapacitors. The intrinsically high redox activity of cobalt sulfide, relative to its metal oxide analogs, and the higher electrical conductivity due to the lower optical band gap energy, are of particular interest.26–28 Hence, a series of cobalt sulfide nanomaterials with various morphologies have been designed as electrode materials for supercapacitors, and good electrochemical properties have been obtained. For instance, Peng et al. synthesized CoS2 hollow spheres with high surface area by hydrothermal method. As the anode of the supercapacitor, the CoS2 hollow sphere electrode obtains specific capacitance of 1301 F g−1 at a current densities of 1 A g−1.29 Wang reported a silver fungus-like CoS obtained by mixed solvent thermal method. The electrode material exhibits a high specific capacity due to the three-dimensional pore structure. The electrode material displays a high specific capacity of 350.4 F g−1 at a current density of 1 A g−1.30 Moreover, a series of composite materials, such as NiCo2O4@CoS (1902.5 F g−1 at a current density of 1 A g−1),31 ZnO NFAs/CoS NFs (1416 F g−1 at a current density of 1 A g−1),32 have been designed and obtained good electrochemical properties, demonstrating the greater potential of cobalt sulfide for supercapacitor. However, there is still room for improvement in the rate performance and capacity of cobalt sulfide, and the manufacturing process is also complicated and take a long time. It is not only important but also challenging to explore facile manner so as to further improve the energy storage capacity and rate performance of cobalt sulfide materials.
In this research, Co4S3 nanorod arrays were prepared on flexible carbon cloth substrate by a simple two-step hydrothermal method. Benefiting from in situ sulfurate method, the prepared binder-free Co4S3 electrode possessed the high specific area, good electronic and ionic conductivity, and then obtained a significant specific capacity of 1.97 F cm−2 at a current density of 2 mA cm−2. Additionally, the Co4S3 electrode also exhibited a satisfactory rate performance of 0.78 F cm−2 at a current density of 10 mA cm−2 and cycling life (almost 100% of the initial capacitance after 5000 cycles at a high scan rate of 200 mV s−1). As expected, the fabricated flexible supercapacitors, composed of the as-prepared Co4S3 electrode and MnO2 electrode, achieved a satisfactory volumetric energy density of 0.87 mW h cm−3 and power density of 0.89 W cm−3.
Experimental section
Synthesis of Co3O4 electrode
All chemical reagents in this experiment were of analytical grade, and no further purification. Co3O4 nanorods arrays were prepared by hydrothermal method using flexible conductive carbon cloth as the substrate. Firstly, the carbon cloth was cut into pieces, each 2 cm × 3 cm large, and then sonicated in deionized water, ethanol solution and acetone solution for 10 min, respectively. 10 mmol of Co(NO3)2·6H2O was dissolved in 40 mL of ethanol solution, with the cleaned carbon cloth immersed in the solution for 10 min, and then heated on a heating plate at 400 °C for 10 min, and repeated four times. Then, 5 mmol of Co(NO3)2·6H2O, 10 mmol of NH4F and 25 mmol of CH4N2O were added to a beaker with 50 mL of deionized water, and stirred to entirely dissolution. The prepared solution was moved to a 25 mL Teflon-lined autoclave with the pretreated carbon cloth. Lastly, the Teflon-lined autoclave was placed in an oven and kept at 120 °C for 5 h. After natural cooling, the pink Co3O4 powder was evenly grown on the surface of carbon fiber, before it was washed with deionized water.Synthesis of Co4S3 electrode
The above prepared Co3O4 material was put in an autoclave with Teflon-lined together with 0.3 M Na2S solution. Then it was placed in an oven at 80, 120, 150, and 180 °C for 6 h. After naturally cooling to room temperature, Co4S3-X (X is the 80, 120, 150, 180 °C) electrodes were obtained.Synthesis of MnO2 electrode
MnO2 electrodes were prepared based on the previous literature.33 In brief, MnO2 nanomaterial was electrodeposited on a flexible carbon cloth substrate through a CHI660E electrochemical work-station with a three-electrode system, which was composed of clean carbon cloth as working electrode, carbon rod as auxiliary electrode, and saturated calomel electrode as reference electrode. The electrodeposition solution was a mixture of 0.1 mol L−1 (CH3COO)2Mn and 0.1 mol L−1 Na2SO4. The experiment to make the MnO2 electrode was performed at 1.0 V for 30, 60, 90, 120, and 240 s, respectively.Fabrication of asymmetric supercapacitors (ASCs)
The solid-state ASCs were made up of a NKK separator, PVA/LiCl gel, and the prepared MnO2 and Co4S3 electrodes. Firstly, LiCl (11.48 g), PVA (6 g), and DI water (50 mL) were mixed with magnetic stirring at 80 °C for 30 min. Then the prepared electrodes and separator were dipped in the above solution. After packing, the prepared ASCs was kept at 40 °C for 12 h to eliminate extra water from the electrolyte.Materials characterization
The morphology, crystal type, and microstructure of the prepared materials were characterized by X-ray diffraction (XRD, X'Pert Pro MPD, PANalytical) with Cu Kα radiation, transmission electron microscopy (TEM, JEM-F200, JEOL), and field-emission scanning electron microscopy (SEM, Sigma500, ZEISS). X-ray photoelectron spectroscopy (XPS, NEXSA, Thermo VG) and Raman Spectroscopy (LabRAM HR Evolution, HORIBA) were used to research the element composition and functional group distribution of the synthesized materials. Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) curves, electrochemical impedance spectra (EIS) of the electrodes were performed on an electrochemical workstation with a three-electrode system, while the prepared electrodes, carbon rod electrode, SCE electrode served as the work electrode, counter electrode, and reference electrode, respectively. The electrochemical performance of the prepared asymmetric supercapacitors was also tested by an electrochemical workstation (CHI660E).Results and discussion
The Co3O4 electrodes were manufactured by a simple hydrothermal method. Briefly, the Co3O4 nanomaterials were synthesized hydrothermally with the use of Co(NO3)2·6H2O, NH4F, and CH4N2O on a flexible carbon cloth substrate (details can be seen in the Experimental section). As seen in Fig. 1a and b, the Co3O4 nanorods were evenly coated on the carbon fiber. Then, they were vulcanized to Co4S3 by Na2S through secondary hydrothermal treatment. Fig. 1c and d show that the nanorod arrays remained coated on the surface of the carbon fiber. However, the surface of the Co4S3 nanorods is rougher compared to that of the Co3O4. As shown in Fig. S1,† Co4S3 obtained a larger specific surface area (9.6 m2 g−1) than Co3O4 (6.8 m2 g−1), indicating that it has more active sites. The length of the nanorods was about 4 μm and the average diameter reached 0.2 μm. To further study the microstructure of the electrode materials, a TEM study was performed. Fig. 1e and S2† show that both Co3O4 and Co4S3 electrodes possess the nanorods, corresponding to the SEM results. Selected area electron diffraction (SAED) pattern proves the well crystallinity of Co4S3 nanomaterial (inset in Fig. 1e). Two clear diffraction rings well correspond to the hexagonal crystal system of Co4S3 (101), (110) crystal planes (JCPDF#02-1458). From the high resolution transmission electron microscopy (HRTEM) in Fig. 1f, it can be seen that the nanorods are crystalline with a crystal plane spacing of 0.196 nm, corresponding to the (102) plane of Co4S3 (JCPDS#02-1458), which is consistent with the SAED results. Then, EDS energy spectrum analysis was performed on the nanorods. From Fig. 1g–j, it can be seen that the Co, S, and O elements are uniformly distributed on the nanorods.To further characterize the crystal types, XRD, Raman, and XPS were utilized to study the functional group, and valence composition of the synthesized materials. As shown in Fig. 2a, the characteristic diffraction peaks of the synthesized materials are in good accordance with the Co3O4 (JCPDS#42-1467) and Co4S3 (JCPDS#02-1458), respectively. The comparison of the characteristic peaks indicates that the Co3O4 has been completely converted to Co4S3 by the secondary hydrothermal of sulfidation reaction. The Raman spectra of Co3O4 and Co4S3 are presented in Fig. 2b. It can be clearly seen that there are four characteristic peaks at 187, 466, 512, 609, and 674 cm−1 in the Co3O4 sample, which correspond to the molecular vibration of F2g, Eg, F2g, F2g, and A1g, respectively.33,34 Meanwhile, the peak at 473, 517, 683 cm−1 are attributed to the Eg, F2g, A1g modes of cobalt sulfide,35 which further confirm that the complete sulfidation of Co3O4 to Co4S3. It is worth noting that both samples show two characteristic peaks at about 1350 and 1580 cm−1, originating from carbon cloth substrate, representing the D band and G band of carbon, respectively.36 To further investigate the surface compositions of Co4S3, XPS tests were performed and the results shown in Fig. 2c. In the survey spectrum, both Co4S3 and Co3O4 samples contain the Co and O elements, and only Co4S3 contains sulfur element, which is in line with the EDS results. As indicated in Fig. 2d, there are four peaks of satellite peak, Co 2p1/2 peak, satellite peak, and Co 2p1/2 peak evident in the graph. Further analysis the core level of Co 2p peaks shows that the two peaks located at 780.7 eV and 796.5 eV attributed to the 2p3/2 and 2p1/2 of Co2+, while the peaks at 778.4 eV and 793.3 eV are originate from the Co3+ 2p3/2 and Co3+ 2p1/2.33,37 The core level spectrum of Co 2p XPS results further prove that Co4S3 have been completely synthesized. The core level S 2p spectrum (Fig. S3a†) shows two peaks with binding energy values of 162.0 eV and 169.0 eV, consistent with the S 2p and oxidized sulfur. In addition, as shown in Fig. S3b,† the fitting peaks at 161.6 eV and 163.0 eV can be ascribed to the 2p3/2 and 2p1/2 of sulfur in Co4S3.37,38
 | ||
| Fig. 2 (a) XRD spectra of the Co3O4 and Co4S3 sample. (b) Raman spectra of the Co3O4 and Co4S3 sample. (c) The XPS survey spectra of the Co3O4 and Co4S3 sample. (d) Co 2p spectra of the Co4S3 sample. | ||
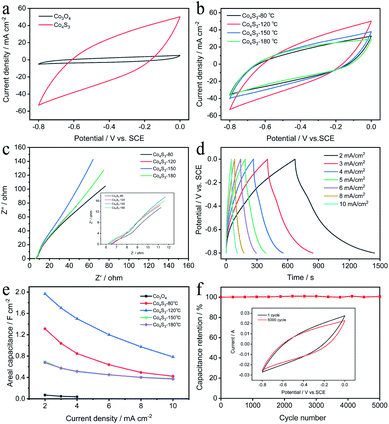
To investigate the electrochemical performance of the Co4S3 electrode, a three-electrode system was constructed with 5 M LiCl as the electrolyte, Co4S3 electrode as the working electrode, SCE as the reference electrode, and carbon rod as the counter electrode with a potential window between −0.8 and 0 V. Firstly, the cyclic voltammetry (CV) curves of the synthesized Co3O4 and Co4S3 electrodes were collected at a scan of 100 mV s−1 in Fig. 3a. It is clear that the electrochemical properties of the materials have been greatly improved by sulphuration treatment, while the specific capacitance was proportional to the average area of a CV curve. Then the sulphuration temperature was optimized (Fig. 3b). It could be clearly seen that the Co4S3 electrode obtained at 120 °C displays the largest area of the CV curves than that of the other reaction temperatures (80, 150, and 180 °C). Fig. 3c shows the electrochemical impedance spectra (EIS) of Co4S3 electrodes at different reaction temperature, where Rs and Rct represented the internal resistance, and charge transfer resistance respectively. They mainly consist of semicircle in high frequency region and lines in the low frequency region. The semicircles are mainly caused by the chemical reaction between the electrode material and the electrolyte, and the lines represent the ion diffusion resistor. The charge-transfer resistance for the Co4S3 electrode is only 0.9 Ω, which is smaller compared with that for other electrodes at different temperatures, demonstrating the superiority of the Co4S3 electrode in conductivity. Fig. 3d and S4† show the GCD and CV curves of Co4S3 electrode at 120 °C at different current densities and scan rates. And the specific capacity of Co4S3 and the different reaction temperature are calculated based on GCD curves of the fabricated electrodes. As shown in Fig. 3e, the specific capacity of Co4S3 is 1.97 F cm−2 at 2 mA cm−2 and 0.78 F cm−2 at 10 mA cm−2, which indicates its excellent rate capacity (a 5-fold increase in the scan rate and the Co4S3 electrode still maintains 39.6% of its initial capacitance). Fig. 3f shows the cycling performance of the Co4S3 electrode at a high scan rate of 200 mV s−1. After 5000 cycles, 96.6% of the specific capacity is retained, which shows that the synthesized electrode is rather stable. The inset in Fig. 3f shows the CV curves before and after 5000 cycles.
The assembly of asymmetric supercapacitor devices is a proven method in increasing the energy density of supercapacitors, allowing for a wide range of operating potentials using different positive and negative electrodes. Hence, a simple ASCs device was assembled from a MnO2 electrode (electroplating for 120 s) as the cathode and a Co4S3 electrode as the anode (denoted as MnO2//Co4S3-ASCs) (see Experimental section for details). For the best capacity of the ASCs device, the balance of the charges of the cathode and anode materials should be achieved before assembly. As shown in Fig. 4a, the CV curves of the cathode and anode were first compared, and it can be seen that the area ratio of about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Fig. 4b shows the CV curves of the assembled ASCs devices gathered at different potential windows. As a result, the potential window of MnO2//Co4S3-ASCs can be stably extended to 1.8 V, indicating that the device has a good energy storage capacity. All these CV curves at different scan rates exhibit regular rectangle, even at high scan rate of 100 mV s−1 (Fig. S5†). In addition, the GCD curves of MnO2//Co4S3-ASCs at different current densities were also collected (Fig. 4c). It can be clearly seen that these GCD curves show good symmetry, indicating that MnO2//Co4S3-ASCs has a satisfactory capacitive behavior and fast charge/discharge characteristics. The volumetric specific capacitance of the assembled MnO2//Co4S3-ASCs can be measured by calculating the discharge time of the GCD curves. As displayed in Fig. 4d, MnO2//Co4S3-ASCs obtained a high volumetric capacitance of 1.9 F cm−3 at a current of 1 mA cm−2. As the current density increases, its specific capacitance gradually decreases. While the current density raised to 5 mA cm−2, it still has a specific capacity of 1.1 F cm−3, which is 57.9% of that at the current of 1 mA cm−2. This result indicates that the assembled devices have a good rate capacity. In addition, all the coulombic efficiencies remain above 84%, indicating its prominent capacitive behavior. The cycling stability of the assembled MnO2//Co4S3-ASCs was conducted at a scan rate of 100 mV s−1. Fig. 4e shows the cycling performance of the device, and it can be seen that the capacity retention is 80% after 5000 cycles, indicating that the device possesses a relatively good cycling stability. In addition, energy density and power density are two critical factors to assess the property of device. Therefore, calculations based on the GCD curves can be performed to obtain a maximum volumetric energy density of 0.87 mW h cm−3 and a power density of 0.89 W cm−3 (Fig. 4f), which is superior to other recently reported devices, such as NiCoS/NF//r-GO-based ASCs (2.04 mW h cm−3 and 42.64 mW cm−3),39 L-VN@AC//α-MnO2-based ASCs (8.5 mW h cm−3 and 34.3 mW cm−3),40 CF/PPY//CNT/MnO2-based ASCs (0.27 mW h cm−3 and 24.1 mW cm−3),41 CFF@V2O5//CFF@AC-based ASCs (0.928 mW h cm−3 and 17.5 mW cm−3),42 MnO2/ZnO//rGO-based ASCs (0.234 mW h cm−3 and 133 mW cm−3),43 CPCC@CuO@Mn(OH)2//CC@AC-based ASCs (0.18 mW h cm−3 and 18 mW cm−3).44 All the results prove the high performance and practicality of the synthesized electrodes.
6. Fig. 4b shows the CV curves of the assembled ASCs devices gathered at different potential windows. As a result, the potential window of MnO2//Co4S3-ASCs can be stably extended to 1.8 V, indicating that the device has a good energy storage capacity. All these CV curves at different scan rates exhibit regular rectangle, even at high scan rate of 100 mV s−1 (Fig. S5†). In addition, the GCD curves of MnO2//Co4S3-ASCs at different current densities were also collected (Fig. 4c). It can be clearly seen that these GCD curves show good symmetry, indicating that MnO2//Co4S3-ASCs has a satisfactory capacitive behavior and fast charge/discharge characteristics. The volumetric specific capacitance of the assembled MnO2//Co4S3-ASCs can be measured by calculating the discharge time of the GCD curves. As displayed in Fig. 4d, MnO2//Co4S3-ASCs obtained a high volumetric capacitance of 1.9 F cm−3 at a current of 1 mA cm−2. As the current density increases, its specific capacitance gradually decreases. While the current density raised to 5 mA cm−2, it still has a specific capacity of 1.1 F cm−3, which is 57.9% of that at the current of 1 mA cm−2. This result indicates that the assembled devices have a good rate capacity. In addition, all the coulombic efficiencies remain above 84%, indicating its prominent capacitive behavior. The cycling stability of the assembled MnO2//Co4S3-ASCs was conducted at a scan rate of 100 mV s−1. Fig. 4e shows the cycling performance of the device, and it can be seen that the capacity retention is 80% after 5000 cycles, indicating that the device possesses a relatively good cycling stability. In addition, energy density and power density are two critical factors to assess the property of device. Therefore, calculations based on the GCD curves can be performed to obtain a maximum volumetric energy density of 0.87 mW h cm−3 and a power density of 0.89 W cm−3 (Fig. 4f), which is superior to other recently reported devices, such as NiCoS/NF//r-GO-based ASCs (2.04 mW h cm−3 and 42.64 mW cm−3),39 L-VN@AC//α-MnO2-based ASCs (8.5 mW h cm−3 and 34.3 mW cm−3),40 CF/PPY//CNT/MnO2-based ASCs (0.27 mW h cm−3 and 24.1 mW cm−3),41 CFF@V2O5//CFF@AC-based ASCs (0.928 mW h cm−3 and 17.5 mW cm−3),42 MnO2/ZnO//rGO-based ASCs (0.234 mW h cm−3 and 133 mW cm−3),43 CPCC@CuO@Mn(OH)2//CC@AC-based ASCs (0.18 mW h cm−3 and 18 mW cm−3).44 All the results prove the high performance and practicality of the synthesized electrodes.
Conclusions
In conclusion, the Co4S3 nanorod arrays were successfully grown on flexible carbon cloth substrate by a two-step hydrothermal method. Benefiting from the larger specific surface area of the nanoarray, the binder-free Co4S3 electrode obtained more active sites with a high specific capacity of 1.97 F cm−2 at a current density of 2 mA cm−2, which is much higher than that of the Co3O4 electrode (0.07 F cm−2). Satisfactorily, the Co4S3 electrode exhibited a long cycling stability, with its initial capacitance remaining almost at 100% after 5000 cycles at a high scan rate of 200 mV s−1. Moreover, the assembled flexible MnO2//Co4S3-ASCs obtained a maximum volumetric energy density of 0.87 mW h cm−3 and power density of 0.89 W cm−3.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22005222), Guangdong province innovation and strong school project (2020ZDZX2004 and 2020KQNCX087), Joint Science Foundation of Wuyi University and HK and Macao (2019WGALH14), the Science Foundation for High-Level Talents of Wuyi University (2019AL022, 5041700133), Jiangmen basic and theoretical scientific research science and technology plan project (2020JC01033).References
- Y. Zhou, H. Qi, J. Yang, Z. Bo, F. Huang, M. S. Islam, X. Lu, L. Dai, R. Amal and C. H. Wang, Energy Environ. Sci., 2021, 14, 1854–1896 RSC.
- Y. Zheng, Y. Yao, J. Ou, M. Li, D. Luo, H. Z. Dou, Z. Q. Li, K. Amine, A. P. Yu and Z. Chen, Chem. Soc. Rev., 2020, 49, 8790–8839 RSC.
- Y. Wan, K. Song, W. Chen, C. Qin, X. Zhang, J. Zhang, H. Dai, Z. Hu, P. Yan, C. Liu, S. Sun, S. Chou and C. Shen, Angew. Chem., Int. Ed., 2021, 60, 11481–11486 CrossRef CAS PubMed.
- W. Cheng, J. Fu, H. Hu and D. Ho, Adv. Sci., 2021, 8, 2100775 CrossRef CAS PubMed.
- J. Zhao and A. F. Burke, J. Energy Chem., 2021, 59, 276–291 CrossRef.
- M. Mansuer, L. Miao, D. Zhu, H. Duan, Y. Lv, L. Li, M. Liu and L. Gan, Mater. Chem. Front., 2021, 5, 3061–3072 RSC.
- P. Xie, W. Yuan, X. Liu, Y. Peng, Y. Yin, Y. Li and Z. Wu, Energy Storage Mater., 2021, 36, 56–76 CrossRef.
- S. Zhao, K. Yan, J. Zhang, B. Sun and G. Wang, Angew. Chem., Int. Ed., 2021, 60, 2208–2220 CrossRef CAS PubMed.
- Z. Li, J. Lin, B. Li, C. Yu, H. Wang and Q. Li, J. Energy Storage, 2021, 44, 103437 CrossRef.
- J. Qin, S. Wang, F. Zhou, P. Das, S. Zheng, C. Sun, X. Bao and Z. Wu, Energy Storage Mater., 2019, 18, 397–404 CrossRef.
- Y. Shao, M. F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn and R. B. Kaner, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- Z. She, D. Ghosh and M. A. Pope, ACS Nano, 2017, 11, 10077–10087 CrossRef CAS PubMed.
- M. Yu, D. Lin, H. Feng, Y. Zeng, Y. Tong and X. Lu, Angew. Chem., Int. Ed., 2017, 56, 5454–5459 CrossRef CAS PubMed.
- W. Zuo, C. Xie, P. Xu, Y. Li and J. Liu, Adv. Mater., 2017, 29, 1703463 CrossRef PubMed.
- L. Liu, J. Niu, Z. Zhang and F. Wang, ACS Appl. Energy Mater., 2021, 4, 7751–7758 CrossRef CAS.
- L. Han, H. Huang, X. Fu, J. Li, Z. Yang, X. Liu, L. Pan and M. Xu, Chem. Eng. J., 2020, 392, 123733 CrossRef CAS.
- X. Li, Y. Tao, J. Song, W. Yang, M. Wang, C. Zhu, W. Zhao, J. Zheng and Y. Lin, Carbon, 2018, 129, 236–244 CrossRef CAS.
- S. Y. Lu, M. Jin, Y. Zhang, Y. B. Niu, J. C. Gao and C. M. Li, Adv. Energy Mater., 2018, 8, 1702545 CrossRef.
- X. Gang, M. Krishnamoorthy, W. Jiang, J. Pan and X. Liu, Carbon, 2021, 171, 62–71 CrossRef CAS.
- M. Y. Zhang, Y. Song, D. Yang, Z. Qin, D. Guo, L. J. Bian, X. G. Sang, X. Sun and X. X. Liu, Adv. Funct. Mater., 2021, 31, 2006203 CrossRef CAS.
- X. Jin, L. Song, H. Yang, C. Dai, Y. Xiao, X. Zhang, Y. Han, C. Bai, B. Lu, Q. Liu, Y. Zhao, J. Zhang, Z. Zhang and L. Qu, Energy Environ. Sci., 2021, 14, 3075–3085 RSC.
- C. Tang, K. Zhao, Y. Tang, F. Li and Q. Meng, Electrochim. Acta, 2021, 375, 137960 CrossRef CAS.
- R. Ahmed and G. Nabi, J. Energy Storage, 2021, 33, 102115 CrossRef.
- R. Liu, A. Zhou, X. Zhang, J. Mu, H. Che, Y. Wang, T. Wang, Z. Zhang and Z. Kou, Chem. Eng. J., 2021, 412, 128611 CrossRef CAS.
- H. Yang, H. Guo, K. Pang, P. Fan, X. Li, W. Ren and R. Song, Nanoscale, 2020, 12, 7024–7034 RSC.
- Y. Zhao, Z. Shi, T. Lin, L. Suo, C. Wang, J. Luo, Z. Ruan, C. A. Wang and J. Li, J. Power Sources, 2019, 412, 321–330 CrossRef CAS.
- M. Wang, J. Yang, S. Liu, C. Hu and J. Qiu, ACS Appl. Energy Mater., 2020, 3, 6977–6984 CrossRef CAS.
- L. Lin, Y. Ding, H. Huang, D. Yu, S. Zhang, H. Chen, S. Ramarkrishna and S. Peng, J. Colloid Interface Sci., 2019, 540, 389–397 CrossRef PubMed.
- S. Peng, L. Li, H. Tan, R. Cai, W. Shi, C. Li, S. G. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Yan, Adv. Funct. Mater., 2014, 24, 2155–2162 CrossRef CAS.
- H. Wang, J. Energy Storage, 2021, 40, 102764 CrossRef.
- W. D. Wang, X. F. Li, P. P. Zhang, B. Q. Wang, S. H. Gong, X. C. Wang, F. Liu and J. P. Cheng, J. Electroanal. Chem., 2021, 891, 115257 CrossRef CAS.
- H. Chen, S. Xiao, Y. Li, X. Ma, Y. Huang, Y. Wang, J. S. Chen and Z. Feng, Chem. Commun., 2021, 57, 10520–10523 RSC.
- W. Xu, J. Chen, M. Yu, Y. Zeng, Y. Long, X. Lu and Y. Tong, J. Mater. Chem. A, 2016, 4, 10779–10785 RSC.
- S. Yang, Y. Liu, Y. Hao, X. Yang, W. Goddard, X. Zhang and B. Cao, Adv. Sci., 2018, 5, 1700659 CrossRef PubMed.
- M. Shi, Q. Wang, J. Hao, H. Min, H. You, X. Liu and H. Yang, Dalton Trans., 2020, 49, 14115–14122 RSC.
- Q. Li, Y. Jiang, Z. Jiang, J. Zhu, X. Gan, F. Qin, T. Tang, W. Luo, N. Guo, Z. Liu, L. Wang, S. Zhang, D. Jia and Z. Fan, Carbon, 2022, 191, 19–27 CrossRef CAS.
- G. Liu, B. Wang, L. Wang, T. Liu, T. Gao and D. Wang, RSC Adv., 2016, 6, 54076–54086 RSC.
- Z. Gao, C. Chen, J. Chang, L. Chen, P. Wang, D. Wu, F. Xu and K. Jiang, Chem. Eng. J., 2018, 343, 572–582 CrossRef CAS.
- P. Prabakaran, S. Prabhu, M. Selvaraj, M. Navaneethan, P. Ramu and R. Ramesh, J. Mater. Sci.: Mater. Electron., 2021, 21, 07251 Search PubMed.
- G. Qu, Z. Wang, X. Zhang, S. Zhao, C. Wang, G. Zhao, P. Hou and X. Xu, Chem. Eng. J., 2022, 429, 132406 CrossRef CAS.
- Y. Xu, Y. Yan, W. Lu, S. Yarlagadda and G. Xu, ACS Appl. Energy Mater., 2021, 4, 10639–10645 CrossRef CAS.
- M. You, W. Zhang, X. Yan, H. Jiang, J. Miao, Y. Li, W. Zhou, Y. Zhu and X. Cheng, Ceram. Int., 2021, 47, 3337–3345 CrossRef CAS.
- Z. Wang, Z. Zhu, J. Qiu and S. Yang, J. Mater. Chem. C, 2014, 2, 1331–1336 RSC.
- Y. Wang, Y. Zhang, D. Dubbink and J. E. Elshof, Nano Energy, 2018, 49, 481–488 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01648f |
| This journal is © The Royal Society of Chemistry 2022 |