Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of mixed musks via Eschenmoser–Tanabe fragmentation, enyne metathesis and Diels–Alder reaction as key steps†
Sambasivarao Kotha *,
Arpit Agrawal‡
and
Yellaiah Tangella‡
*,
Arpit Agrawal‡
and
Yellaiah Tangella‡
Department of Chemistry, Indian Institute of Technology, Bombay, Powai, Mumbai, 400076, Maharashtra, India. E-mail: srk@chem.iitb.ac.in
First published on 11th May 2022
Abstract
Musk analogues containing different macrocyclic ring systems as well as different annulated ring systems were synthesised by a simple and useful strategy. This strategy includes Eschenmoser–Tanabe fragmentation, enyne metathesis and Diels–Alder reaction as key steps. Starting from easily available (n) macrocyclic ketones, (n + 3) macrocyclic systems were assembled using the basic organic reactions.
Introduction
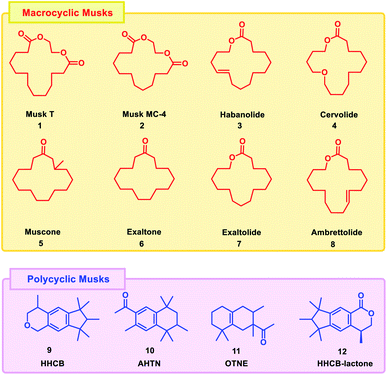
A musk is a compound with unusual fragrance properties.1 The market of the fragrance industry has gained momentum from the 20th century, and large quantities of synthetic musks have been synthesized in the world. In the flavour and fragrance industry, musks are used as perfume additives on a large scale.2 These compounds are also used as personal care products which include insect repellent, UV filters, preservatives and antimicrobial agents. There are a wide range of personal care products (PCPs) that contain synthetic musks, including lotions, shampoos, perfumes, softeners, air fresheners, washing powders etc.3Among various synthetic fragrances available on the market, polycyclic musks (61%) and nitro musks (35%) play a dominant role in production volume.4 Most nitro musks have been withdrawn from the market due to their potential toxicity. Exposure to nitro musks is associated with an increased risk of tumorigenesis in mice. Evidence suggests that nitro musk itself is not genotoxic, but may increase genotoxicity of other chemicals. However, animal models of exposure to nitro musk have proven to be problematic because certain results are species-specific. It is also found that nitro musk compounds were not easily degraded, which makes them very stable and ubiquitous in the environment.5 After a sharp decline in the use and production of nitro musks, polycyclic musk compounds (PMCs) have become the leading commercial synthetic musks that now dominates the global market. Mainly, PMCs sold as Galaxolid® (HHCB) and Tonalide® (AHTN) account for approximately 95% of all fragrances in the perfume industry and are the most frequently detected PMCs in environmental compartments and living tissues at an environmentally suspicious level. Concerns about potential impacts relate not only to the environment, but also to food safety and thus to public health. Nonetheless, studies have been conducted to report potential oestrogenic and anti-oestrogenic effects in several PMCs, due to this reason their use in cosmetics is uncertain.6 Macrocyclic musks occupies a small part (3–4%) of the market and is used almost exclusively for perfumes. In 2017, the flavor and fragrance market was valued at $24.8 billion. Macrocyclic musks supports all consumer categories in the fragrance industry and has good environmental performance, renewable options and other sustainability resulting in high demand. As a result, both scientific and industrial synthetic chemists are continuously developing new strategies for the preparation of macrocycles. Desirable macrocycles include structures with one carbonyl unit and 13–19 carbon atoms.
These structural properties are known to produce the scent of musk, a well-known scent used in perfumes, colognes, and personal care products. Small structural changes to macrocycles, such as the incorporation of olefins, can create a characteristic odor profile. In addition, stereochemistry and the location of alkyl group substitutions or double bonds within the macrocycle skeleton can affect the resulting musk odor. Also due to their stronger odor, less amounts are needed to achieve the same level of performance as other synthetic musk varieties. Structural similarity of macrocyclic musks with that of the natural musks leads to the conclusion that these compounds appear to be more environment friendly and environmentally degradable than their predecessor class.7 Some of the examples of macrocyclic and polycyclic musks are given in Fig. 1.
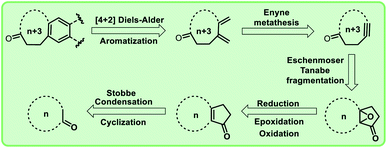
In view of the importance of macrocyclic ketones as well as our long-term interest in olefin metathesis and other strategies to design polycycles,8 we conceived a new synthetic approach (Schemes 1–5) to mixed musks containing fused cyclic ketone derivatives based on cross-enyne metathesis as key step. Retrosynthetic analysis to these macrocyclic compounds is shown in Fig. 2.
Results and discussion
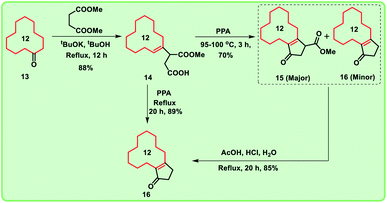
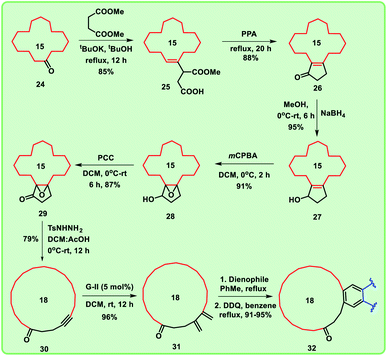
Considering the importance of macrocyclic ring systems in designing musks, we started with commercially available cyclododecanone 13. The compound 13 underwent Stobbe condensation with dimethyl succinate under basic conditions to produce the compound 14 in good yield (88%).Further, compound 14 was treated with polyphosphoric acid (PPA) at 95–100 °C for 3 h, we obtained cyclopentane derivative 15 as major product with the corresponding decarboxylated product 16.9 Under acidic conditions, the mixture 15 and 16 collectively converted into decarboxylated enone 16.
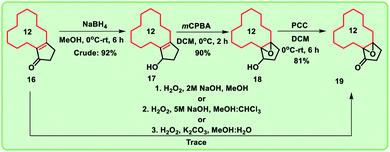
Alternatively, when the compound 14 was heated with PPA for 20 h, enone 16 was formed exclusively (Scheme 1). To obtain the key intermediate 19, compound 16 was treated with hydrogen peroxide (H2O2) under different reaction conditions at room temperature in the presence of bases like NaOH and K2CO3 for 12 h. Unfortunately, trace amount of keto-epoxide 19 was observed. In this regard, to get higher yield of the compound 19 from enone 16, alternate method was adopted in which enone 16 was initially reduced to hydroxy derivative 17 using sodium borohydride (NaBH4). Compound 17 was then treated with mCPBA to give hydroxy-epoxide 18 which further on reaction with pyridinium chlorochromate (PCC) produced the oxidised keto-epoxide 19 (Scheme 2). It is worthy to mention, by using this alternate route, we obtained the desired key intermediate 19 in good yield.
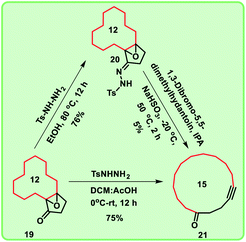
Further, we are intended to synthesize a metathesis precursor i.e. macrocyclic alkyne 21 using compound 19. In view of this aspect, compound 19 was treated with tosyl hydrazide in ethanol under reflux conditions to afford the compound 20, which subsequently on reaction with 1,3-dibromo-5,5-dimethylhydantoin in isopropyl alcohol (IPA) delivered the corresponding rearranged 15-membered macrocyclic derivative 21 in very low yield (5%). This problem was rectified by using the key reaction Eschenmoser–Tanabe fragmentation.10
To access enlarged 15-membered macrocyclic compound 21 in good yield, compound 19 was directly treated with tosyl hydrazide in the presence of DCM:AcOH mixture (Scheme 3).
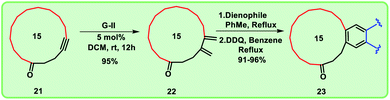
To prepare the diene, cross-enyne metathesis11 was performed on compound 21 using Grubbs II generation catalyst and the diene 22 was formed in excellent yield, which is a useful substrate for the Diels–Alder reaction. To access the targeted fused macrocyclic systems, the macrocyclic diene 22 was subjected to Diels–Alder reaction12 followed by aromatization with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Here, we used different dienophiles to realize [4 + 2] cycloaddition products to generate 15 membered mixed musks (Scheme 4).
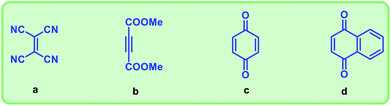
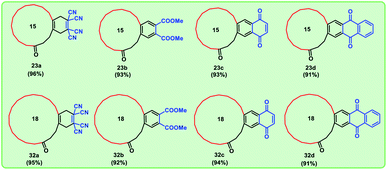
We have used four types of dienophiles here such as tetracyanoethylene (a), dimethyl acetylenedicarboxylate (b), benzoquinone (c) and naphthoquinone (d) to assemble mixed musks having 15 membered macrocycle (Fig. 3).
Using these optimized conditions, we have carried out the sequence with commercially available cyclopentadecanone 24, which depicted in Scheme 5. By using a similar strategy, we successfully synthesized four different mixed musks in good yields with the difference in macrocyclic ring system. Thus, we obtained eight targeted mixed musks (Fig. 4).
Conclusions
We have successfully demonstrated a new synthetic strategy to prepare mixed musks having macrocyclic ring systems fused with polycycles in good yields. This route includes the usage of commercially available materials and facile reaction conditions. In targeted molecules (Fig. 4), 15-membered and 18-membered macrocyclic systems are shown here, as the difference in ring system can lead to a change in the properties of musks. This strategy may be valuable addition to the flavor and fragrance industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank, Rasiklal Hemani Funds for financial support. A. A. thanks Ministry of Education, Government of India for Prime Minister Research Fellowship (PMRF). Y.T. thank to IITB for the award of IPDF position.Notes and references
- (a) L. Ruzicka, Helv. Chim. Acta, 1926, 9, 230 CrossRef CAS; (b) O. R. P. David, Chem.– Eur. J., 2020, 26, 7537–7555 CrossRef CAS PubMed.
- (a) A. Sytniczuk, M. Milewski, A. Kajetanowicz and K. Grela, Russ. Chem. Rev., 2020, 89, 469–490 CrossRef CAS; (b) T. Zhang, W. Jin, S. Yang, Y. Li, M. Zhang, M. Shi, X. Guo, D. Li, B. Zhang, S. Liu and D. Hu, PLoS ONE, 2021, 16, e0245677 CrossRef CAS PubMed.
- (a) V. Homem, J. A. Silva, N. Ratola, L. Santos and A. Alves, J. Environ. Manage., 2015, 149, 168–192 CrossRef PubMed; (b) W. D. Wombacher and K. C. Hornbuckle, J. Environ. Eng., 2009, 135, 1192–1198 CrossRef CAS.
- H. Nakata, M. Hinosaka and H. Yanagimoto, Ecotoxicol. Environ. Saf., 2015, 111, 248–255 CrossRef CAS PubMed.
- K. M. Taylor, M. Weisskopf and J. Shine, Environ. Health, 2014, 13, 14 CrossRef PubMed.
- (a) F. O. Ehiguese, M. J. González-Delgado, C. Garrido-Perez, C. V. M. Araújo and M. L. Martin-Diaz, Processes, 2021, 9, 371 CrossRef CAS; (b) X. Li, Y. Zhao, B. Chen, Z. Zhu, Q. Kang, T. Husain and B. Zhang, Environ. Int., 2022, 158, 106911 CrossRef CAS PubMed; (c) W.-J. Tseng and S.-W. Tsai, Sci. Total Environ., 2019, 669, 160–167 CrossRef CAS PubMed; (d) F. Wong, M. Robson, L. Melymuk, C. Shunthirasingham, N. Alexandrou, M. Shoeib, E. Luk, P. Helm, M. L. Diamond and H. Hung, Environ. Sci.: Processes Impacts, 2019, 21, 74–88 RSC; (e) L. Roosens, A. Covaci and H. Neels, Chemosphere, 2007, 69, 1540–1547 CrossRef CAS PubMed; (f) Y. Lyu, S. Ren, F. Zhong, X. Han, Y. He and Z. Tang, Ecotoxicol. Environ. Saf., 2021, 213, 112074 CrossRef PubMed; (g) S. Patel, A. Homaei and S. Sharifian, Environ. Dev. Sustain., 2021, 23, 4764–4781 CrossRef; (h) J.-H. Hong, J.-Y. Lee, H.-J. Ha, J.-H. Lee, S.-R. Oh, Y.-M. Lee, M.-Y. Lee and K.-D. Zoh, Water, 2021, 13, 392 CrossRef CAS.
- (a) É. Morin, J. Sosoe, M. Raymond, B. Amorelli, R. M. Boden and S. K. Collins, Org. Process Res. Dev., 2019, 23, 283–287 CrossRef; (b) A. S. Williams, Synthesis, 1999, 1999, 1707–1723 CrossRef.
- (a) P. K. Thallapally, K. Chakraborty, H. L. Carrell, S. Kotha and G. R. Desiraju, Tetrahedron, 2000, 56, 6721–6728 CrossRef CAS; (b) S. Kotha and N. Sreenivasachary, Bioorg. Med. Chem. Lett., 2000, 10, 1413–1415 CrossRef CAS PubMed; (c) S. Kotha, K. Mandal, A. C. Deb and S. Banerjee, Tetrahedron Lett., 2004, 45, 9603–9605 CrossRef CAS.
- K. Biemann, G. Büchi and B. H. Walker, J. Am. Chem. Soc., 1957, 79, 5558–5564 CrossRef CAS.
- (a) A. Eschenmoser, D. Felix and G. Ohloff, Helv. Chim. Acta, 1967, 50, 708–713 CrossRef CAS; (b) C. Fehr, G. Ohloff and G. Büchi, Helv. Chim. Acta, 1979, 62, 2655–2660 CrossRef CAS.
- (a) S. Kotha, A. S. Chavan and D. Goyal, ACS Omega, 2019, 4, 22261–22273 CrossRef CAS PubMed; (b) S. Kotha, M. Meshram and A. Tiwari, Chem. Soc. Rev., 2009, 38, 2065 RSC; (c) V. Dragutan, I. Dragutan, A. Demonceau and L. Delaude, Beilstein J. Org. Chem., 2020, 16, 738–755 CrossRef CAS PubMed; (d) A. Sytniczuk, A. Leszczyńska, A. Kajetanowicz and K. Grela, ChemSusChem, 2018, 11, 3157–3166 CrossRef CAS PubMed; (e) S. Kotha and A. K. Ghosh, Tetrahedron, 2004, 60, 10833–10841 CrossRef CAS; (f) K. Kashinath, P. D. Jadhav and D. S. Reddy, Org. Biomol. Chem., 2014, 12, 4098–4103 RSC; (g) C. Fischmeister and C. Bruneau, Beilstein J. Org. Chem., 2011, 7, 156–166 CrossRef CAS PubMed.
- (a) S. Kotha and G. T. Waghule, J. Org. Chem., 2012, 77, 6314–6318 CrossRef CAS PubMed; (b) S. Kotha, A. Agrawal and S. Ansari, ChemistrySelect, 2021, 6, 11178–11181 CrossRef CAS; (c) S. Kotha, E. Brahmachary and N. Sreenivasachary, Tetrahedron Lett., 1998, 39, 4095–4098 CrossRef CAS; (d) S. Kotha and A. S. Chavan, J. Org. Chem., 2010, 75, 4319–4322 CrossRef CAS PubMed; (e) S. Kotha and P. Khedkar, J. Org. Chem., 2009, 74, 5667–5670 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01458k |
| ‡ Both the authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |