Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, characterization and application of oxovanadium(IV) complexes with [NNO] donor ligands: X-ray structures of their corresponding dioxovanadium(V) complexes†
Rakhimoni Borah ,
Surabhi Lahkar
,
Surabhi Lahkar ,
Naranarayan Deori
,
Naranarayan Deori and
Sanfaori Brahma
and
Sanfaori Brahma *
*
Department of Chemistry, Gauhati University, Guwahati 781014, Assam, India. E-mail: sanfaoribrahma@gmail.com
First published on 6th May 2022
Abstract
Two oxovanadium(IV) complexes ligated by [NNO] donor ligands have been synthesized and characterized by ESI-HRMS, elemental (CHN) analysis and spectroscopic (UV-Vis, IR and EPR) techniques. Block shaped brown crystals from the methanolic solutions of these oxovanadium(IV) complexes were obtained during the crystallization process. Crystallographic structures of the resulting crystals revealed that the original oxovanadium(IV) complexes have been transformed into new dioxovanadium(V) complexes with concomitant oxidation of VIV to VV. The original oxovanadium(IV) complexes have been identified to be an efficient catalyst for the CO2 cycloaddition reaction with epoxides resulting up to 100% cyclic carbonate products. The geometries of oxovanadium(IV) complexes are optimized by the density functional theory (DFT) calculations at the uB3LYP/6-31G**/LANL2DZ level of theory. The geometry and structural parameters of optimized structures of oxovanadium(IV) complexes are in excellent agreement with the parameters of X-ray structures of their dioxovanadium(V) counterparts. Further, TD-DFT and Spin Density Plots for the oxovanadium(IV) complexes are performed in order to get more insights about their electronic absorption and EPR spectroscopies, respectively.
1 Introduction
Carbon dioxide, a major greenhouse gas, is being released into the Earth's atmosphere in a large scale due to the combustion of fossil fuels leading to global warming, which is at present posing a serious environmental threat.1–5 Nevertheless, fossil fuel burning is still inevitable due to the increasing demand of energy and lack of feasible technologies for sustainable energies.1 Therefore, the strategies that need to be immediately adopted are the reduction in carbon dioxide emission and the attenuation of carbon dioxide concentration in the atmosphere. On the other hand, CO2 is an abundant, renewable, inexpensive and non-toxic C1 source of chemical carbon in organic syntheses.1–15 Although utilization of carbon dioxide as C1 feedstock is challenging in view of its thermodynamic and kinetic inertness,16 research that deals with sustainable use of CO2 in producing value-added chemicals is advancing at a rapid rate.17–21 Of many important CO2 transformation reactions,22 cycloaddition of CO2 with epoxides to form cyclic carbonates stands out to be a very attractive transformation process.23–57 The process is 100% atom economic and the resulting cyclic carbonate products are commercially important chemicals which can be used as electrolytes in lithium-ion battery,58 polar aprotic solvents,59 functionalized building blocks for synthesizing valuable organic products,60 monomers for polycarbonates61 and isocyanate-free polyurethanes,62 intermediates for pharmaceuticals and fine chemicals.63 Interestingly, cyclic carbonate moieties are also seen in some natural products64–66 thereby making the CO2 transformation reaction to cyclic carbonate a very significant and naturally relevant reaction.Catalytic cycloaddition of CO2 with epoxides to yield cyclic carbonates using both homogeneous and heterogeneous catalyst systems that encompass metal complexes,31–39,50–56 ionic liquids,40,41 metal organic frameworks (MOFs),42,57 covalent organic frameworks (COFs),43 porous organic polymers (POPs),44 organo-catalysts,45 bi-functional catalysts,46 alkali metal salts,47,48 and metal oxides49 have been demonstrated by various research groups. Currently, vanadium complexes have gained considerable attention from scientific community to be used as catalyst in the CO2 cycloaddition with epoxides due to the Lewis acidic nature of high-valent VV and VIV centers, suitable for activating epoxides as well as relatively non-toxic nature of naturally abundant vanadium metal.50–57 Lee and co-workers67 were first to report commercial VCl3 catalysed cycloaddition reaction of CO2 with epoxides, however it required high temperature (90–120 °C) and pressure (14.8 bar).
Herein, we report synthesis, characterization and the catalytic study of substituted salicylidin-2-picolylimine ligated vanadium complexes in the CO2 cycloaddition with epoxides yielding cyclic carbonates with very good to excellent conversions under relatively benign conditions.
2 Results and discussion
2.1 Syntheses and structures of the vanadium complexes
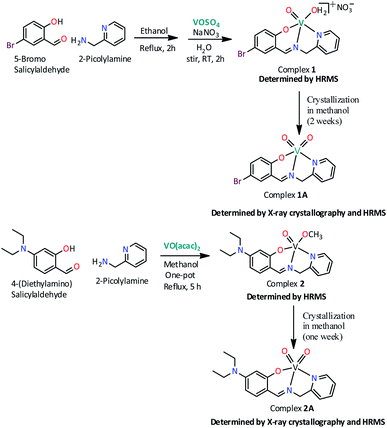
Scheme 1 illustrates the synthetic route for two new oxovanadium complexes 1, [VIVO(H2O)L1]NO3 and 2, [VIVO(OCH3)L2] and their corresponding dioxovanadium complexes 1A, [VVO2L1] and 2A, [VVO2L2] (HL1: 5-bromosalicylidin-2-picolylimine; HL2: 4-diethylaminosalicylidin-2-picolylimine).Complex 1 was prepared by first refluxing an equimolar mixture of 5-bromosalicylaldehyde and 2-picolylamine in ethanol for 2 h, followed by addition of aqueous VOSO4 solution and subsequent refluxing for another 1 h. Green solid complex 1 was obtained when an aqueous solution of sodium nitrate was added to the former solution and stirred for 1 h at room temperature. Dioxovanadium(V) complex, 1A was obtained as brown blocked shaped crystals from the methanolic solution of complex 1 after around two weeks. Similarly, complex 2 has been synthesized in one pot by refluxing equimolar mixture of 4-(diethylamino)salicylaldehyde and 2-picolylamine in methanol for 2 h and subsequently refluxing further for 3 h after addition of 1 equivalent [VO(acac)2]. The solution obtained was filtered and from the filtrate greenish color solid is collected and dried. Dioxovanadium(V) complex, 2A was obtained as brown blocked shaped crystals from the methanolic solution of complex 2 after around one week.
2.2 X-ray crystallography
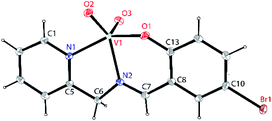
Single crystals suitable for X-ray crystallographic analysis of dioxovanadium(V) complexes 1A, [VVO2L1] and 2A, [VVO2L2] were obtained from their corresponding oxovanadium(IV) complexes 1, [VIVO(H2O)L1]NO3 and 2, [VIVO(OCH3)L2]. However, crystals of complexes 1 and 2 could not be obtained in spite of repeated attempts. Brown block shaped crystals of 1A suitable for X-ray diffraction were obtained via slow evaporation of solution containing complex 1 in methanol for around 2 weeks. Complex 1A crystallises in a monoclinic crystal system with P21/c space group. Perspective view of the asymmetric unit of complex 1A and the crystal packing are illustrated in Fig. 1 and Fig. S1 (ESI†), respectively. Crystal data collection parameters and the selected bond distances and angles are shown in Table S1 and S2 (ESI†), respectively. V(1)–O(2), 1.637(2) Å and V(1)–O(3), 1.620(2) Å bond lengths are in accordance with the usual V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances.68 V(1)–N(1) and V(1)–N(2) bond lengths are comparable which are 2.115(3) and 2.153(3) Å, respectively. However, V(1)–O(1) bond length, 1.900(2) Å is significantly smaller than V–N bond distances. C(6)–N(2) and C(7)–N(2) bond distances, 1.475(4) Å and 1.293(4) Å are consistent with the C–N and C
O bond distances.68 V(1)–N(1) and V(1)–N(2) bond lengths are comparable which are 2.115(3) and 2.153(3) Å, respectively. However, V(1)–O(1) bond length, 1.900(2) Å is significantly smaller than V–N bond distances. C(6)–N(2) and C(7)–N(2) bond distances, 1.475(4) Å and 1.293(4) Å are consistent with the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond distances. Moreover, C(10)–Br(1) bond distance, 1.901(3) Å is consistent with C–Br bond distances. O(2)–V(1)–O(3) bond angle is in line with the previously reported values 109.32(12)°.
N bond distances. Moreover, C(10)–Br(1) bond distance, 1.901(3) Å is consistent with C–Br bond distances. O(2)–V(1)–O(3) bond angle is in line with the previously reported values 109.32(12)°.
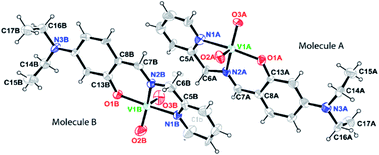
Brown block shaped crystals of 2A were grown via slow evaporation of solution containing complex 2 in methanol for around 7 days. Complex 2A crystallises in a monoclinic crystal system with P21 space group. Perspective view of the asymmetric unit of complex 2A and the crystal packing are illustrated in Fig. 2 and S2 (ESI†), respectively. Asymmetric unit of the crystal contains two symmetry-independent molecules viz A and B. It is observed that molecules are crystallographically non-equivalent as the corresponding bond distances and angles differ slightly in these two molecules (Table S3, ESI†). The V–N bond distances in molecule A is somewhat shorter than those in molecule B [V(1A)–N(1A), 2.136(5) Å and V(1B)–N(1B), 2.140(5) Å; V(1A)–N(2A), 2.097(5) Å and V(1B)–N(2B), 2.127(4) Å in molecule A and B, respectively]. However, the V–O bond distance in molecule A is somewhat longer compared to that in molecule B, V(1A)–O(1A), 1.910(4) Å and V(1B)–O(1B), 1.888(3) Å. It is worthy to mention that the bond distances C(6A)–N(2A), 1.504(7) Å and C(6B)–N(2B), 1.434(8) Å in molecules A and B, respectively significantly differ. Moreover, both the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances in molecule A [V(1A)–O(2A), 1.638(6) Å; V(1A)–O(3A), 1.654(5) Å] is comparatively longer compared to those in molecule B [V(1B)–O(2B), 1.609(6) Å; V(1B)–O(3B), 1.595(6) Å].
O bond distances in molecule A [V(1A)–O(2A), 1.638(6) Å; V(1A)–O(3A), 1.654(5) Å] is comparatively longer compared to those in molecule B [V(1B)–O(2B), 1.609(6) Å; V(1B)–O(3B), 1.595(6) Å].
2.3 Structure optimization of [1 – NO3−]+ (without NO3− counter anion) and 2
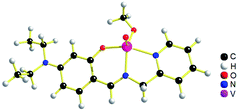
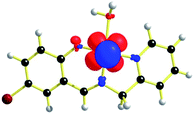
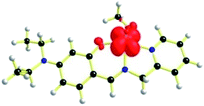
The geometries of [1 – NO3−]+ and 2 are optimized by the density functional theory (DFT) calculations at the uB3LYP/6-31G**/LANL2DZ level of theory. The geometry and structural parameters of complexes [1 – NO3−]+ and 2 determined by DFT calculations are in excellent agreement with the parameters of X-ray structures of their dioxovanadium(V) counterparts. In both the complexes, the coordination geometry around vanadium(IV) center is square pyramidal with oxo ligands in the axial position (Fig. 3 and 4). Excluding the bonds, V–O(H2O) and V–O(OCH3) which are respectively absent in 1A and 2A, the selected bond distances of the optimised structures of both the complexes [1 – NO3−]+ and 2 (Table 1) are similar with the experimentally determined X-ray structures of their dioxovanadium(V) counterparts, 1A and 2A.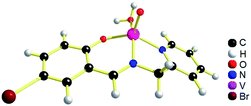 | ||
| Fig. 3 uB3LYP/LANL2DZ/6-31G** optimized structure of complex [1 – NO3−]+ (without NO3− counter anion). | ||
| Bond lengths (Å) | [1 – NO3−]+ | 2 |
|---|---|---|
V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1.582 | 1.601 |
| V–O (H2O) | 2.134 | — |
| V–O (OCH3) | — | 1.854 |
| V–O (phenolate) | 1.879 | 1.946 |
| V–N (Py) | 2.103 | 2.168 |
| V–N (imine) | 2.026 | 2.093 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1.302 | 1.308 |
| C–Br | 1.953 | — |
2.4 NMR spectroscopy
In spite of repeated attempts, no good 1H NMR spectrum of complex 1 was obtained, may be due to the presence of paramagnetic vanadium(IV) center.69 However, 1H NMR spectrum of complex 2 is reasonably good (Fig. S3†) although the signals are broad. The resonance at 8.80 ppm is due to the imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) proton. There appear four signals (8.51, 7.98, 7.61 and 7.44 ppm) for picolyl aromatic protons. The resonance at 5.33 ppm can be attributed to the picolyl methylene proton. Moreover, the phenolate proton signals appear at 7.22, 6.20 and 5.90 ppm. It is worth mentioning that –CH2 protons of ethyl groups and –OCH3 protons resonate in the same region with residual water impurity.
N) proton. There appear four signals (8.51, 7.98, 7.61 and 7.44 ppm) for picolyl aromatic protons. The resonance at 5.33 ppm can be attributed to the picolyl methylene proton. Moreover, the phenolate proton signals appear at 7.22, 6.20 and 5.90 ppm. It is worth mentioning that –CH2 protons of ethyl groups and –OCH3 protons resonate in the same region with residual water impurity.
2.5 EPR spectroscopy
EPR spectrum (Fig. 5) of complex 1, [VIVO(H2O)L1]NO3 in chloroform exhibited eight-lines revealing the oxidation state of vanadium as +4. The hyperfine structure corresponds to the interaction of a single unpaired electron (S = 1/2) with a vanadium nucleus (I = 7/2). The typical eight-line pattern X-band EPR spectrum of the complex 1 (giso = 1.9905, Aiso = 96.695 G) recorded in room temperature reveals the presence of a single vanadium(IV) center in the complex.70 Spin density plot of the complex [1 – NO3−]+ (without NO3− counter anion) shown in Fig. 6 reveals the localisation of single spin (S = 1/2) on vanadium(IV) center with no spin delocalisation over ligand, thus supporting the experimental EPR signal.71,72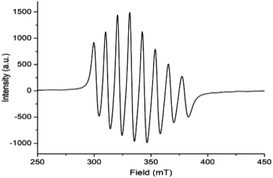 | ||
| Fig. 5 Room temperature X-band EPR spectrum of complex 1, [VIVO(H2O)L1]NO3 (HL1 = 5-bromosalicylidin-2-picolylimine) in chloroform. | ||
Expectedly, similar kind of EPR spectrum (Fig. 7) is also observed for the oxovanadium(IV) complex 2 (giso = 1.9935, Aiso = 96.557 G). Moreover, the spin density plot of the complex 2 (Fig. 8) also indicates the localisation of spin density on oxovanadium(IV) center like that in complex [1 – NO3−]+.
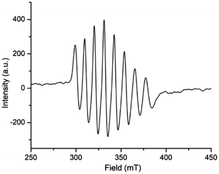 | ||
| Fig. 7 Room temperature X-band EPR spectrum of complex 2, [VIVO(OCH3)L2] (HL2 = 4-diethylaminosalicylidin-2-picolylimine) in methanol. | ||
2.6 IR spectroscopy
The IR spectra of oxovanadium(IV) complex 1, [VIVO(H2O)L1]NO3 and complex 2, [VIVO(OCH3)L2] are shown in Fig. S4 and S5 (ESI†), respectively. The IR spectrum of oxovanadium complex 1 displayed a strong band at 964 cm−1 that can be attributed to ν(V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the vanadyl moiety present in the complex.73 Moreover, the peak at 1627 cm−1 is related to vibration of the azomethine moiety (C
O) of the vanadyl moiety present in the complex.73 Moreover, the peak at 1627 cm−1 is related to vibration of the azomethine moiety (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).74 Further the peaks at 1761, 1383 and 830 cm−1 are attributable to the NO3−.75 In a similar way, the IR spectrum of oxovanadium complex 2 reveals the formation of the complex which shows a peak at 924 cm−1 corresponding to ν(V
N).74 Further the peaks at 1761, 1383 and 830 cm−1 are attributable to the NO3−.75 In a similar way, the IR spectrum of oxovanadium complex 2 reveals the formation of the complex which shows a peak at 924 cm−1 corresponding to ν(V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching of the vanadyl group. Further the strong band observed at 1596 cm−1 corresponds to ν(C
O) stretching of the vanadyl group. Further the strong band observed at 1596 cm−1 corresponds to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of the azomethine group.76
N) of the azomethine group.76
2.7 UV-visible spectroscopy
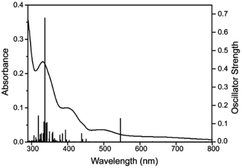
The UV-visible spectrum of oxovanadium(IV) complex 1 (5.0 × 10−5 M) in dichloromethane have been illustrated in Fig. 9. UV-visible spectrum of 1 displays a peak in the higher energy region at 331 nm, which is due to ligand centred transitions.77 Band appearing at 402 nm is attributed to the LMCT transitions originating from the p orbital of phenolate oxygen to the empty d orbital of vanadium(IV) center.78 Moreover, d–d transition in complex 1 is apparent (500 and 660 nm) when the concentration of 1 was increased to 3.0 × 10−3 M.79 Similarly, UV-visible spectrum of 2 (Fig. S6,† left) exhibits a band at 349 nm corresponding to π–π* transition of azomethine chromophore.80,81 The intense band observed at 402 nm can be attributed to the charge transfer transition (LMCT) from the p orbital of the phenolate oxygen to empty d orbital of vanadium(IV).78 However, the bands due to d–d transitions could not be distinguished properly as they are probably under the tail of much stronger LMCT band at ca. 465 nm (shoulder).82 Moreover, the UV-visible spectrum (Fig. S6,† right) recorded in DMF solvent of dioxovanadium(V) complex 2A shows similar absorption pattern showing LMCT bands at 396 and 465 nm (shoulder) as that of oxovanadium(IV) complex 2, suggesting that complex 2 get oxidised to complex 2A.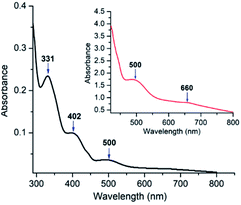 | ||
| Fig. 9 UV-visible spectra in dichloromethane of complex 1 (5 × 10−5 M) at 298 K. Inset represents UV-visible spectrum of 1 (3 × 10−3 M) in dichloromethane. | ||
The time-dependent density functional theory (TD-DFT) calculation of [1 – NO3−]+ shown in Fig. 10 indicates the presence of absorption band at longer wavelength (546 nm; oscillator strength f = 0.1281) that can be attributable to the d–d transition.
2.8 ESI-HRMS spectrometry
The formation of complex 1, [VIVO(H2O)L1]NO3 and 2, [VIVO(OCH3)L2] were further unambiguously confirmed by the electrospray ionization high-resolution mass spectrometry (ESI-HRMS). A peak appearing at m/z 373.9460 in the HRMS (Fig. S7, ESI†) corresponds to [1 – NO3−]+ (calcd, 373.9471) and an additional peak at m/z 396.9604 corresponds to [1 − NO3− + Na]+ (calcd, 396.9369). The other fragment ions observed in the spectrum at m/z 355.9344 and 386.9532 correspond to [1 − NO3− − H2O]+ (calcd, 355.9365) and [1 − NO3− − H2O + CH3OH]+ (calcd, 386.9549), respectively. Formation of complex 2 was also revealed by the ion peak at m/z 380.0787 (Fig. S8, ESI†) that corresponds to the [2]+ (calcd, 380.1179). Further, the transformation of complex 2, [VIVO(OCH3)L2] to 2A, [VVO2L2] during the course of crystallization process was also confirmed when the crystals of complex 2A were subjected to mass spectrometry that showed an ion peak at m/z 366.1039 corresponding to the [2A + H]+ (calcd, 366.1023) (Fig. S9, ESI†). The other peak observed in the mass spectrum of 2A at m/z 284.1775 (Fig. S9, ESI†) corresponds to the detached ligand HL2, 4-diethylaminosalicylidin-2-picolylimine (calcd, 284.1763).2.9 Cycloaddition reaction of CO2 with epoxides
CO2 cycloaddition with epoxides for the formation of cyclic carbonates was performed by loading vanadium(IV) complexes 1 or 2 with tetrabutylammonium bromide (TBAB) co-catalyst in a stainless-steel autoclave equipped with a magnetic stirring bar and dosing appropriate pressure of CO2 (Scheme 2, Table 2). The formation of cyclic carbonates has been confirmed by 1H NMR (Fig. S10–S22, ESI†) showing downfield shifting of the peaks compared to epoxides because of the addition of CO2. The formation of cyclic carbonate products was also supported by the IR spectroscopic technique, which showed characteristic peaks in a range of 1783–1786 cm−1 that correspond to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of carbonate moiety (Fig. S23–S25†). Preliminary investigation of the potentiality of the catalyst system 1-TBAB (1 mol%: 2 mol%) was performed at 60 °C and 5 bar CO2 pressure taking epichlorohydrin (ECH) and the epibromohydrin (EBH) as the substrates. To our delight, quantitative conversions of ECH (entry 1, 100% conv.) and EBH (entry 2, 100% conv.) to their corresponding cyclic organic carbonates (COCs) were achieved. To see the catalytic activity of the oxovanadium(IV) complex 1 alone, we have performed the reaction in the absence of TBAB co-catalyst (entry 3). However, it is revealed that vanadium complex 1 alone does not have any capability to transform the styrene oxide (SO) into its corresponding COC. To see the catalytic efficiency of the complex 2, a similar kind of reaction was performed taking ECH as substrate and found 2-TBAB catalyst system to have the same extent of efficiency (entry 4, 100% conv.) as that of 1-TBAB. To see the substrate scope of the catalyst systems, we have chosen three other substrates viz. styrene oxide (SO), allyl glycidyl ether (AGE) and butyl glycidyl ether (BGE). Both the catalyst systems 1-TBAB and 2-TBAB showed similar kinds of conversions when SO was taken as substrate (compare entry 5 and 6, 95% and 93% conv., respectively). Comparing the entry 5 (95% conv.) and entry 7 (26% conv.), it is observed that 1-TBAB catalyst system is superior to TBAB alone, as more than around 68% conversion was found when the combination of complex 1 and TBAB was used as a catalyst system instead of TBAB alone. Catalyst system 2-TBAB appears to be a bit better than 1-TBAB in case of the substrate AGE (compare entry 8 and 9, 85% and 92% conv., respectively). For the substrate BGE, both the vanadium complexes 1 and 2 showed similar potentiality as catalysts resulting in 85% (entry 10) and 82% (entry 11) conversions for catalyst 1 and 2, respectively. To investigate the effect of CO2 pressure on the catalytic activity of the catalyst system, a reaction was performed with SO as substrate under 1 bar of CO2 pressure keeping other parameters the same. It is seen that 100% conversion of SO (entry 12) to its corresponding COC could be achieved within 6 h of duration in a mere 1 bar of CO2 pressure indicating 1-TBAB combination to be a very efficient catalyst.
O) of carbonate moiety (Fig. S23–S25†). Preliminary investigation of the potentiality of the catalyst system 1-TBAB (1 mol%: 2 mol%) was performed at 60 °C and 5 bar CO2 pressure taking epichlorohydrin (ECH) and the epibromohydrin (EBH) as the substrates. To our delight, quantitative conversions of ECH (entry 1, 100% conv.) and EBH (entry 2, 100% conv.) to their corresponding cyclic organic carbonates (COCs) were achieved. To see the catalytic activity of the oxovanadium(IV) complex 1 alone, we have performed the reaction in the absence of TBAB co-catalyst (entry 3). However, it is revealed that vanadium complex 1 alone does not have any capability to transform the styrene oxide (SO) into its corresponding COC. To see the catalytic efficiency of the complex 2, a similar kind of reaction was performed taking ECH as substrate and found 2-TBAB catalyst system to have the same extent of efficiency (entry 4, 100% conv.) as that of 1-TBAB. To see the substrate scope of the catalyst systems, we have chosen three other substrates viz. styrene oxide (SO), allyl glycidyl ether (AGE) and butyl glycidyl ether (BGE). Both the catalyst systems 1-TBAB and 2-TBAB showed similar kinds of conversions when SO was taken as substrate (compare entry 5 and 6, 95% and 93% conv., respectively). Comparing the entry 5 (95% conv.) and entry 7 (26% conv.), it is observed that 1-TBAB catalyst system is superior to TBAB alone, as more than around 68% conversion was found when the combination of complex 1 and TBAB was used as a catalyst system instead of TBAB alone. Catalyst system 2-TBAB appears to be a bit better than 1-TBAB in case of the substrate AGE (compare entry 8 and 9, 85% and 92% conv., respectively). For the substrate BGE, both the vanadium complexes 1 and 2 showed similar potentiality as catalysts resulting in 85% (entry 10) and 82% (entry 11) conversions for catalyst 1 and 2, respectively. To investigate the effect of CO2 pressure on the catalytic activity of the catalyst system, a reaction was performed with SO as substrate under 1 bar of CO2 pressure keeping other parameters the same. It is seen that 100% conversion of SO (entry 12) to its corresponding COC could be achieved within 6 h of duration in a mere 1 bar of CO2 pressure indicating 1-TBAB combination to be a very efficient catalyst.
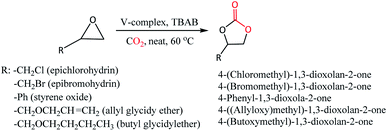 | ||
| Scheme 2 Catalytic cycloaddition reaction of CO2 with epoxides using oxovanadium(IV) complexes 1 and 2 in presence of co-catalyst TBAB. | ||
| Entry | Epoxide | Catalyst | 1 or 2 (mol%) | TBAB (mol%) | Time (h) | % Convb | Ref. |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: ECH = 0.5 mL (6.38 mmol); SO = 0.5 mL (4.38 mmol); AGE = 0.5 mL (4.27 mmol); BGE = 0.5 mL (3.50 mmol); EBH = 0.5 mL (5.88 mmol).b Percentage conversions of the catalytic reactions were evaluated with the help of 1H NMR spectroscopy according to the conversion formula shown in Fig. S26.c 5 bar CO2 pressure.d 1 bar CO2 pressure. | |||||||
| 1 | ECH | 1-TBAB | 1 | 2 | 4 | 100 | Twc |
| 2 | EBH | 1-TBAB | 1 | 2 | 4 | 100 | Twc |
| 3 | SO | 1 | 1 | — | 4 | 0 | Twc |
| 4 | ECH | 2-TBAB | 1 | 2 | 4 | 100 | Twc |
| 5 | SO | 1-TBAB | 1 | 2 | 4 | 95 | Twc |
| 6 | SO | 2-TBAB | 1 | 2 | 4 | 93 | Twc |
| 7 | SO | TBAB | — | 2 | 4 | 26 | Twc |
| 8 | AGE | 1-TBAB | 1 | 2 | 4 | 85 | Twc |
| 9 | AGE | 2-TBAB | 1 | 2 | 4 | 92 | Twc |
| 10 | BGE | 1-TBAB | 1 | 2 | 4 | 85 | Twc |
| 11 | BGE | 2-TBAB | 1 | 2 | 4 | 82 | Twc |
| 12 | SO | 1-TBAB | 1 | 2 | 6 | 100 | Twd |
| 13 | SO | TBAB | — | 2 | 6 | 33 | Twd |
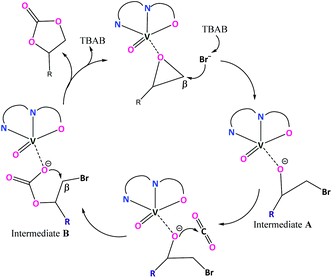
2.10 Catalytic cycle
As suggested from previously reported work52 on metal complex catalysed conversion of carbon dioxide and epoxides into cyclic carbonates, an attempt has been made to sketch a plausible reaction pathway for cycloaddition of carbon dioxide to epoxides catalysed by oxovanadium(IV) catalysts 1 and 2 (Scheme 3). Lewis acidic V(IV) center of oxovanadium(IV) complexes 1 and 2 activates the epoxide by coordinating through the epoxide O-atom which results in the weakening of β C–O bond as well as increase in the electrophilic nature of the β carbon of the epoxide. As a result, bromide anion from TBAB attacks epoxide β-carbon more facilely and cleaves the O–CH2 bond to produce intermediate A. In the next step, oxyanion intermediate A makes a nucleophilic attack at the carbon center of CO2 to generate a carbonate species (intermediate B). In the final step, the ring closure through nucleophilic attack by carbonate species at β-carbon results in the cyclic carbonate products.3 Conclusion
Two new oxovanadium(IV) complexes 1, [VIVO(H2O)L1]NO3 and 2, [VIVO(OCH3)L2] with [NNO] donor ligand have been synthesized and characterized by spectroscopic techniques (UV-Vis, IR and EPR), ESI-HRMS and elemental (CHN) analysis. Block shaped brown crystals obtained from the methanolic solutions of complexes 1 and 2 were crystallographically characterised and revealed to be dioxovanadium complexes 1A, [VVO2L1] and 2A, [VVO2L2] respectively. Crystal structures determined for complexes 1A and 2A indirectly give an evidence for the formation of complexes 1 and 2 where the vanadium(IV) centers were slowly oxidised to vanadium(V) during the crystallization process. However, structures of oxovanadium(IV) complexes [1 − NO3−]+ and 2 have been optimized by the density functional theory (DFT) calculations at the uB3LYP/6-31G**/LANL2DZ level of theory. It is worthy to mention that the structural parameters of the optimized structures are in very good agreement with their dioxovanadium(V) counterparts, 1A and 2A. TD-DFT calculation for the complex 1 (without NO3− anion) could support the presence of electronic absorption bands at longer wavelengths (546 nm, oscillator strength f = 0.1281) attributable to d–d transitions. Moreover, spin density plot of the oxovanadium(IV) complexes [1 − NO3−]+ and 2 show localization of spin density on the V(IV) centers supporting the experimental EPR lines. An investigation on the catalytic activity of the oxovanadium(IV) complexes in the cycloaddition reaction of carbon dioxide with the epoxides to yield cyclic organic carbonates as products revealed that V-complexes 1 and 2 are efficient catalysts. Applicability of dioxovanadium(V) complexes 1A and 2A as catalysts in various reactions are also being investigated.4 Experimental and computational section
4.1 Materials
All chemicals used in this work were purchased from Sigma Aldrich, Spectrochem, and Alfa Aesar, and were used without further purification.4.2 Instrumentation
IR spectra were recorded on a BRUKER infrared spectrometer (model no ALPHA II). Elemental analysis (CHN) measurements were done in Thermo Scientific FlashSmart CHNS/O analyzer. The electronic spectra were recorded using a Shimadzu UV-2600 spectrophotometer. 1H NMR spectra were recorded on Bruker 400 MHz, Bruker 500 MHz, Bruker 600 MHz, JEOL 400 MHz and JEOL 500 MHz spectrometers with tetramethyl silane (TMS) as an internal standard. The solvents used were CDCl3 and [D6] DMSO. Chemical shifts are reported in parts per million (δ) and spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), m (multiplet) and dd (doublet of doublets). A WATERS XEVO-G2XSQTOF was used for taking mass spectra. The X-band electron paramagnetic resonance (EPR) spectra were recorded on a JESFA200 ESR spectrometer at room temperature with the experimental conditions [frequency, 9.439 GHz; power, 0.995 mW; field center, 490.000 mT; width, ± 500.000 mT; sweep time, 30.0 s; modulation frequency, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 kHz; width, 1.0000 mT; amplitude, 100; and time constant, 0.03 s]. The crystals of complex 1 and 2 were coated with light hydrocarbon oil and mounted at 273(2) K and 296(2) K, respectively on a Bruker SMART APEX CCD diffractometer, and the intensity data were collected using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The data integration and reduction were processed with SAINT software.83 An absorption correction was applied.84 The structure was solved by the direct method using SHELXT 2014/5. The structure was refined on F2 by the full-matrix least-squares technique using the SHELXL-2018/3 program package.85 Non-hydrogen atoms were refined anisotropically. In the refinement, hydrogens were treated as riding atoms using SHELXL default parameter.
00 kHz; width, 1.0000 mT; amplitude, 100; and time constant, 0.03 s]. The crystals of complex 1 and 2 were coated with light hydrocarbon oil and mounted at 273(2) K and 296(2) K, respectively on a Bruker SMART APEX CCD diffractometer, and the intensity data were collected using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The data integration and reduction were processed with SAINT software.83 An absorption correction was applied.84 The structure was solved by the direct method using SHELXT 2014/5. The structure was refined on F2 by the full-matrix least-squares technique using the SHELXL-2018/3 program package.85 Non-hydrogen atoms were refined anisotropically. In the refinement, hydrogens were treated as riding atoms using SHELXL default parameter.
4.3 Synthesis of complexes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1521, 1452, 1383 (NO3−), 1310, 1151, 964 (V
N), 1521, 1452, 1383 (NO3−), 1310, 1151, 964 (V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 830 (NO3−) and 710. ESI-HRMS(+) [M − NO3−]+: m/z (calcd) = 373.9471, m/z (found) = 373.9460.
O), 830 (NO3−) and 710. ESI-HRMS(+) [M − NO3−]+: m/z (calcd) = 373.9471, m/z (found) = 373.9460.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1507, 1392, 1346, 1248, 1141, 924 (V
N), 1507, 1392, 1346, 1248, 1141, 924 (V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 772. ESI-HRMS(+) [M]+: m/z (calcd) = 380.1179, m/z (found) = 380.0787. 1H NMR δH (600 MHz, DMSO-d6) 8.80 (–CH
O) and 772. ESI-HRMS(+) [M]+: m/z (calcd) = 380.1179, m/z (found) = 380.0787. 1H NMR δH (600 MHz, DMSO-d6) 8.80 (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1H), 8.51 (Picolyl py-H, 1H), 7.98 (Picolyl py-H, 1H), 7.61 (Picolyl py-H, 1H), 7.44 (Picolyl py-H, 1H), 7.22 (Ph-H, 1H), 6.20 (Ph-H, 1H), 5.90 (Ph-H, 1H), 5.33 (picolyl-CH2, 2H), 3.25 (ethyl-CH2, –OCH3, 7H), and 0.99 (–CH3, 6H).
N, 1H), 8.51 (Picolyl py-H, 1H), 7.98 (Picolyl py-H, 1H), 7.61 (Picolyl py-H, 1H), 7.44 (Picolyl py-H, 1H), 7.22 (Ph-H, 1H), 6.20 (Ph-H, 1H), 5.90 (Ph-H, 1H), 5.33 (picolyl-CH2, 2H), 3.25 (ethyl-CH2, –OCH3, 7H), and 0.99 (–CH3, 6H).4.4 General procedure for catalytic reactions yielding cyclic carbonates
The oxovanadium(IV) complex 1 or 2 (1 mol%), TBAB (2 mol%) and epoxide (0.5 mL) were mixed together in a 100 mL stainless steel autoclave and the existing air in the reaction vessel was evacuated with a flush of CO2 gas for some time. The reaction vessel was then pressurized with appropriate pressure of CO2 and stirred for desired time at 60 °C. Upon completion of the catalytic reaction, the residual CO2 of the autoclave was vented out carefully. After cooling the autoclave to room temperature, the reaction mixture was collected for analysis.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163 (C–O).
O), 1163 (C–O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1161 (C–O).
O), 1161 (C–O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1167 (C–O).
O), 1167 (C–O).Author contributions
Rakhimoni Borah: Data curation, methodology, investigation, validation, visualization and writing - original draft. Surabhi Lahkar: Writing - review & editing. Naranarayan Deori: Software. Sanfaori Brahma: Conceptualization, funding acquisition, project administration, supervision and resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Rakhimoni Borah thanks CSIR, New Delhi for research fellowship. We thank the Department of Chemistry, Gauhati University for various facilities and Sophisticated Analytical Instrumentation Facility (SAIF), Gauhati University for the single crystal X-ray diffraction facility. We also acknowledge Mr Sabyasachi Sarkar for his help in computational analysis.Notes and references
- M. Ding, R. W. Flaig, H. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- A. A. Marciniak, K. J. Lamb, L. P. Ozorio, C. J. A. Mota and M. North, Curr. Opin. Green Sustain. Chem., 2020, 26, 100365 CrossRef.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- A. Rehman, F. Saleem, F. Javed, A. Ikhlaq, S. W. Ahmad and A. Harvey, J. Environ. Chem. Eng., 2021, 9, 105113 CrossRef CAS.
- A. J. Kamphuis, F. Picchioni and P. P. Pescarmona, Green Chem., 2019, 21, 406–448 RSC.
- A. Jangam, S. Das, N. Dewangan, P. Hongmanorom, W. M. Hui and S. Kawi, Catal. Today, 2020, 358, 3–29 CrossRef CAS.
- V. Aomchad, À. Cristòfol, F. Della Monica, B. Limburg, V. D'Elia and A. W. Kleij, Green Chem., 2021, 23, 1077–1113 RSC.
- C. C. Truong and D. K. Mishra, J. CO2 Util., 2020, 41, 101252 CrossRef CAS.
- I. Omae, Coord. Chem. Rev., 2012, 256, 1384–1405 CrossRef CAS.
- T. Fujihara and Y. Tsuji, Front. Chem., 2019, 7, 1–8 CrossRef PubMed.
- G. Yuan, C. Qi, W. Wu and H. Jiang, Curr. Opin. Green Sustain. Chem., 2017, 3, 22–27 CrossRef.
- S. H. Kim, K. H. Kim and S. H. Hong, Angew. Chem., Int. Ed., 2014, 53, 771–774 CrossRef CAS PubMed.
- Y. Tsuji and T. Fujihara, Chem. Commun., 2012, 48, 9956–9964 RSC.
- A. Otto, T. Grube, S. Schiebahn and D. Stolten, Energy Environ. Sci., 2015, 8, 3283–3297 RSC.
- A. Dibenedetto, A. Angelini and P. Stufano, J. Chem. Technol. Biotechnol., 2014, 89, 334–353 CrossRef CAS.
- M. Aresta and A. Dibenedetto, J. Chem. Soc. Dalton Trans., 2007, 2975–2992 RSC.
- A. Banerjee, G. R. Dick, T. Yoshino and M. W. Kanan, Nature, 2016, 531, 215–219 CrossRef CAS PubMed.
- K. Nozaki, Bull. Chem. Soc. Jpn., 2021, 94, 984–988 CrossRef CAS.
- H. Long Ngo, D. Kumar Mishra, V. Mishra and C. Chien Truong, Chem. Eng. Sci., 2021, 229, 116142 CrossRef CAS.
- P. R. Yaashikaa, P. Senthil Kumar, S. J. Varjani and A. Saravanan, J. CO2 Util., 2019, 33, 131–147 CrossRef CAS.
- X. Xiaoding and J. A. Moulijn, Energy Fuel., 1996, 10, 305–325 CrossRef CAS.
- T. Sakakura, J. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- F. Gou, J. Liu, N. Ye, X. Jiang and C. Qi, J. CO2 Util., 2021, 48, 101534 CrossRef CAS.
- P. P. Pescarmona, Curr. Opin. Green Sustain. Chem., 2021, 29, 100457 CrossRef.
- C. Calabrese, F. Giacalone and C. Aprile, Catalysts, 2019, 9, 325 CrossRef.
- F. Zhang, Y. Wang, X. Zhang, X. Zhang, H. Liu and B. Han, Green Chem. Eng., 2020, 1, 82–93 CrossRef.
- K. Kiatkittipong, M. Amirul, A. Mohamad, W. Kiatkittipong, J. W. Lim, P. L. Show and M. K. Lam, Processes, 2020, 8, 548 CrossRef CAS.
- C. Claver, M. Bin Yeamin, M. Reguero and A. M. Masdeu-Bultó, Green Chem., 2020, 22, 7665–7706 RSC.
- M. Liu, X. Wang, Y. Jiang, J. Sun and M. Arai, Catal. Rev. - Sci. Eng., 2019, 61, 214–269 CrossRef CAS.
- J. W. Comerford, I. D. V. Ingram, M. North and X. Wu, Green Chem., 2015, 17, 1966–1987 RSC.
- Á. Mesías-Salazar, Y. R. Yepes, J. Martínez and R. S. Rojas, Catalysts, 2021, 11, 2 CrossRef.
- M. Cavalleri, N. Panza, A. Biase, G. Tseberlidis, S. Rizzato, G. Abbiati and A. Caselli, Eur. J. Org. Chem., 2021, 2021, 2764–2771 CrossRef CAS.
- S. Suleman, H. A. Younus, N. Ahmad, Z. A. K. Khattak, H. Ullah, J. Park, T. Han, B. Yu and F. Verpoort, Appl. Catal. Gen., 2020, 591, 117384 CrossRef.
- F. Della Monica, A. Buonerba and C. Capacchione, Adv. Synth. Catal., 2019, 361, 265–282 CrossRef CAS.
- R. R. Shaikh, S. Pornpraprom and V. D'Elia, ACS Catal., 2018, 8, 419–450 CrossRef CAS.
- C. Martín, G. Fiorani and A. W. Kleij, ACS Catal., 2015, 5, 1353–1370 CrossRef.
- V. D'Elia, J. D. A. Pelletier and J. Basset, ChemCatChem, 2015, 7, 1906–1917 CrossRef.
- A. Decortes, A. M. Castilla and A. W. Kleij, Angew. Chem., Int. Ed., 2010, 49, 9822–9837 CrossRef CAS PubMed.
- Y. M. Shen, W. L. Duan and M. Shi, J. Org. Chem., 2003, 68, 1559–1562 CrossRef CAS PubMed.
- Z. J. Li, J. F. Sun, Q. Q. Xu and J. Z. Yin, ChemCatChem, 2021, 13, 1848–1866 CrossRef CAS.
- B. H. Xu, J. Q. Wang, J. Sun, Y. Huang, J. P. Zhang, X. P. Zhang and S. J. Zhang, Green Chem., 2015, 17, 108–122 RSC.
- T. K. Pal, D. De and P. K. Bharadwaj, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS.
- R. Luo, Y. Yang, K. Chen, X. Liu, M. Chen, W. Xu, B. Liu, H. Ji and Y. Fang, J. Mater. Chem. A., 2021, 9, 20941–20956 RSC.
- R. Luo, M. Chen, X. Liu, W. Xu, J. Li, B. Liu and Y. Fang, J. Mater. Chem. A., 2020, 8, 18408–18424 RSC.
- L. Guo, K. J. Lamb and M. North, Green Chem., 2021, 23, 77–118 RSC.
- F. Zhang, S. Bulut, X. Shen, M. Dong, Y. Wang, X. Cheng, H. Liu and B. Han, Green Chem., 2021, 23, 1147–1153 RSC.
- Y. Hao, T. Tian, Y. Kang, T. Chang, X. Fu, Z. Zhu, X. Meng, B. Panchal and S. Qin, New J. Chem., 2020, 44, 15811–15815 RSC.
- S. Suleman, H. A. Younus, N. Ahmad, Z. A. K. Khattak, H. Ullah, S. Chaemchuen and F. Verpoort, Catal. Sci. Technol., 2019, 9, 3868–3873 RSC.
- N. Kulal, V. Vasista and G. V. Shanbhag, J. CO2 Util., 2019, 33, 434–444 CrossRef CAS.
- R. Borah, N. Deori and S. Brahma, New J. Chem., 2020, 44, 2547–2554 RSC.
- S. Lahkar, R. Borah, N. Deori and S. Brahma, Polyhedron, 2020, 192, 114848 CrossRef CAS.
- S. Ta, M. Ghosh, R. A. Molla, S. Ghosh, M. Islam, P. Brandão, V. Félix and D. Das, ChemistrySelect, 2019, 4, 10254–10259 CrossRef CAS.
- C. Miceli, J. Rintjema, E. Martin, E. C. Escudero-Adán, C. Zonta, G. Licini and A. W. Kleij, ACS Catal., 2017, 7, 2367–2373 CrossRef CAS.
- A. I. Elkurtehi and F. M. Kerton, ChemSusChem, 2017, 10, 1249–1254 CrossRef CAS PubMed.
- D. Bai, Z. Zhang, G. Wang and F. Ma, Appl. Organomet. Chem., 2015, 29, 240–243 CrossRef CAS.
- A. Coletti, C. J. Whiteoak, V. Conte and A. W. Kleij, ChemCatChem, 2012, 4, 1190–1196 CrossRef CAS.
- R. R. Kuruppathparambil, R. Babu, H. Jeong, Y. H. Jang, M. H. Lee and D. W. Park, Korean J. Chem. Eng., 2019, 36, 643–649 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed.
- W. Guo, J. E. Gómez, À. Cristòfol, J. Xie and A. W. Kleij, Angew. Chem., Int. Ed., 2018, 57, 13735–13747 CrossRef CAS PubMed.
- V. Besse, F. Camara, C. Voirin, R. Auvergne, S. Caillol and B. Boutevin, Polym. Chem., 2013, 4, 4545–4561 RSC.
- L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau and H. Cramail, Chem. Rev., 2015, 115, 12407–12439 CrossRef CAS PubMed.
- P. Rollin, L. K. Soares, A. M. Barcellos, D. R. Araujo, E. J. Lenardão, R. G. Jacob and G. Perin, Appl. Sci., 2021, 11, 5024 CrossRef CAS.
- Z. Liu, P. R. Jensen and W. Fenical, Phytochemistry, 2003, 64, 571–574 CrossRef CAS PubMed.
- S. Mizobuchi, J. Mochizuki, H. Soga, H. Tanba and H. Inoue, J. Antibiot., 1986, 39, 1776–1778 CrossRef CAS PubMed.
- S. Chatterjee, G. C. Reddy, C. M. Franco, R. H. Rupp, B. N. Ganguli, H. W. Fehlhaber and H. Kogler, J. Antibiot., 1987, 40, 1368–1374 CrossRef CAS PubMed.
- T. Bok, E. K. Noh and B. Y. Lee, Bull. Korean Chem. Soc., 2006, 27, 1171–1174 CrossRef CAS.
- W. Plass and H. P. Yozgatli, Z. Anorg. Allg. Chem., 2003, 629, 65–70 CrossRef CAS.
- F. H. Kohler, P. Hofmann and W. Prossdorf, J. Am. Chem. Soc., 1981, 103, 6359–6367 CrossRef.
- B. Priya, A. Kumar and N. Sharma, J. Chem. Res., 2020, 44, 460–470 CrossRef CAS.
- D. Dragancea, N. Talmaci, S. Shova, G. Novitchi, D. Darvasiová, P. Rapta, M. Breza, M. Galanski, J. Kožíšek, N. M. R. Martins, L. M. D. R. S. Martins, A. J. L. Pombeiro and V. B. Arion, Inorg. Chem., 2016, 55, 9187–9203 CrossRef CAS PubMed.
- A. E. King, M. Nippe, M. Atanasov, T. Chantarojsiri, C. A. Wray, E. Bill, F. Neese, J. R. Long and C. J. Chang, Inorg. Chem., 2014, 53, 11388–11395 CrossRef CAS PubMed.
- M. R. Maurya, S. Agarwal, C. Bader, M. Ebel and D. Rehder, Dalton Trans., 2005, 537–544 RSC.
- B. Baruah, S. P. Rath and A. Chakravorty, Eur. J. Inorg. Chem., 2004, 2004, 1873–1878 CrossRef.
- F. A. Miller and C. H. Wilkins, Anal. Chem., 1952, 24, 1253–1294 CrossRef CAS.
- O. Tsave, E. Halevas, M. P. Yavropoulou, E. Yovos, A. Hatzidimitriou, V. Psycharis, K. Ypsilantis, P. Stathi and A. Salifoglou, New J. Chem., 2019, 43, 17872–17890 RSC.
- S. P. Dash, A. K. Panda, S. Dhaka, S. Pasayat, A. Biswas, M. R. Maurya, P. K. Majhi, A. Crochet and R. Dinda, Dalton Trans., 2016, 45, 18292–18307 RSC.
- M. R. Maurya, N. Jangra, F. Avecilla, N. Ribeiro and I. Correia, ChemistrySelect, 2019, 4, 12743–12756 CrossRef CAS.
- M. R. Maurya, B. Sarkar, F. Avecilla and I. Correia, Dalton Trans., 2016, 45, 17343–17364 RSC.
- G. Sahu, E. R. T. Tiekink and R. Dinda, Inorganics, 2021, 9, 66 CrossRef CAS.
- A. B. Deilami, M. Salehi, A. Amiri and A. Arab, J. Mol. Struct., 2019, 1181, 190–196 CrossRef CAS.
- N. Ribeiro, I. Bulut, B. Cevatemre, C. Teixeira, Y. Yildizhan, V. André, P. Adão, J. C. Pessoa, C. Acilan and I. Correia, Dalton Trans., 2021, 50, 157–169 RSC.
- G. M. Sheldrick, SHELXL-2018: Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 2018 Search PubMed.
- SAINT+, 6.02, Bruker AXS, Madison, WI, 1999 Search PubMed.
- G. M. Sheldrick, SADABS 2.0, 2000 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, J. A. Vreven, T. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, revision B.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. H. Vosko and L. Wilk, Phys. Rev. B: Condens. Matter Mater. Phys., 1980, 22, 3812 CrossRef CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- https://www.chemcraftprog.com.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2154336 for 1A and 2154347 for 2A. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra01448c |
| This journal is © The Royal Society of Chemistry 2022 |