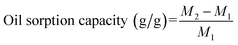Open Access Article
Open Access ArticlePretreatment of coir lignocellulose for preparation of a porous coir–polyurethane composite with high oil adsorption capacity
Phan Huy Hoang *,
Hoang Tien Dat,
Thai Dinh Cuong and
Le Quang Dien
*,
Hoang Tien Dat,
Thai Dinh Cuong and
Le Quang Dien
School of Chemical Engineering, Hanoi University of Science & Technology, No. 1 Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
First published on 17th May 2022
Abstract
In this present work, different treatment methods of coir biomass were investigated to improve the oil sorption capacity. The treated coir material was then used to fabricate an efficient porous coir–polyurethane composite sorbent by incorporating coir into a polyurethane matrix. The new composite possessed an open cell structure with high porosity and high oil sorption efficiency. The suitable technical parameters of the coir treatment process were selected as: hot water treatment at 170 °C for 120 minutes. After treatment under this suitable condition, treated coconut fiber exhibited an oil adsorption capacity of 4.1 g g−1, with an increase of 78.3% compared to that of the original coconut fiber. Furthermore, the application of the as-fabricated porous composite sorbent for oil treatment was examined under various conditions. It was observed that the oil uptake capacity of the new composite sorbent was high, up to 15.2 g g−1 when 20% treated coir material with a particle size of 1 mm was added into the polyurethane matrix. Several advantages of the new porous composite sorbent obtained from coir biomass and polyurethane such as low cost, being eco-friendly, ready availability and high buoyancy make it an efficient sorbent material for oil spill treatment.
Introduction
Along with the development of agriculture and industries, the amount of waste discharged into the environment is increasing. Many issues of environmental pollution, especially the water environment, have been and will be a challenge for human society, including in Vietnam. The problem is to effectively treat wastewater as well as polluted water sources, and to return a clean-living environment for organisms and human life. Many methods for treating pollution from oil, heavy metals, organic substances, colorants, etc. in water have been investigated and applied such as biological, physical, and chemical methods.1–4 In these the adsorption process is the main method with several advantages to separate the organic pollutants including of color substances and oil out of water. Many sorbent materials from agricultural by-products such as rice straw, coconut fiber, shrimp shells, soybean residue, corn cobs, and water hyacinth, etc. has been investigated.2,5–10For oil spill treatment, using adsorbent is economical, efficient, and environmentally friendly method due to the possibility of collection, complete cleanup of the oil and convenient post-treatment of oil-loaded sorbents. The application of natural adsorbents from agricultural wastes with good oil sorption capacity is very suitable and applicable.11,12 For example, modified cellulose fiber,13 rice husk,14 rice straw,15 raw cotton,16 palm leaves and palm endocarp (shell),17 kapok,18 sugarcane bagasse19 sawdust and sponge-gourd20 have been investigated as oil sorbent. These biomass sorbents have several advantages such as biodegradability, economy, technical feasibility, and environmental acceptability for cleanup of oil spill. It can be seen that the amount of agricultural residue or waste after harvesting is very large, and thus there are serious impacts on the environment due to decomposition, illumination and/or burning these wastes in the field. Meanwhile the application of organic natural materials derived from plant sources works as a vital development in sustainable environmental technology.19,21,22 Moreover, utilization of agricultural residue shows many advantages such as readily available raw materials, low cost, environmentally friendly and biodegradable, complete oil removal and simple disposal with minimum environmental hazard as well as do not add secondary pollution sources to the environment.20,23–25 This application at the same time solves both environmental problems: remove of oil out of water and the treatment of agricultural wastes.
Coconut fiber (coir) is one of the biomass-derived adsorbent materials and is quite popular in Vietnam. It can adsorb oil from oil-polluted water thanks to its porous structure with many functional groups. In our country, coir is evaluated as a potential source of biomass material but has not been used effectively because it is considered as agricultural by-product. Therefore, the use of coir to fabricate adsorbent material with high adsorption capacity is a useful solution to reduce water pollution and solve the waste from agriculture. Nevertheless, such lignocellulose material has poor buoyancy characteristics, not high oil sorption capacity.3,13,26 The material thus should be modified to improve the oil uptake capacity for practical application.
There have been some literature reported on treatment of cellulose fiber or lignocellulose material by chemical modification to enhance the oil sorption capacity of biomass material. Sun et al.27 investigated the acetylation of rice straw with acetic anhydride and Wang et al.28 studied on coating Zn layer on cotton fiber to increase hydrophobicity and hence increase the oil sorption capacity. Moreover, many reports have been concentrated on pretreatment of lignocellulose material by hot water, sodium hydroxide and sulfuric acid for improvement of enzymatic hydrolysis and bio-ethanol production.11,23,29 However, there is only one report on hot water treatment of populus fiber to prepare oil sorbent from lignoellulosic material.21 We were, therefore, motivated that the difference treatment method such as hot water, alkaline and acid treatment could be used to enhance oil sorption capacity of natural biomass sorbent.
Furthermore, polyurethane (PU) is a highly porous, lightweight, and commercially produced synthetic organic product which is widely used in wastewater treatment and oil spill adsorption due to their hydrophobic and oleophilic properties.30–32 PU could be also modified or functionalized for improvement the oil uptake ability.30,33,34 Taking all these factors into consideration, it is urgent to find out a new environmentally friendly material with a high oil adsorption capacity by combination of hydrophobic synthetic PU and lignocellulosic waste.
In this current work, we have studied the treatment of coconut fiber by different methods to improve the oil sorption capacity as well as examined the effect of treatment methods on the oil sorption capacity of treated coir. Moreover, in order to further improve the oil adsorption capacity of natural materials, a porous bio-based composite material was fabricated by incorporating treated coir and polyurethane. A combination of these two materials, by taking advantage of agricultural by-products and synthetic PU adsorbent would create a porous coir–polyurethane composite sorbent with high buoyancy characteristics and high porosity for oil spill treatment.
Experimental
Materials
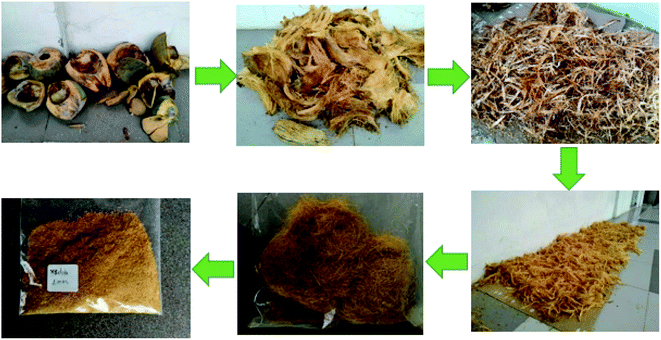
Coconut shell samples were taken from the Middle of Vietnam. The coir was separated from the coconut shell and dried at room temperature for one week (Fig. 1). After that, coir was cut into pieces, grounded, and screened to select samples in different sieve sizes: 1 mm; 2 mm; 4 mm and 6 mm. The grinder used in our work is knife grinder (the grinder with sharp knife) to cut the long coir fibers into short ones. The samples were then stored in a sealed plastic bag for doing further experiment (the moisture of coir sample in plastic bag was about 13 ± 0.5%).Diesel oil (DO, density at 20 °C = 0.85 g cm−3, viscosity = 5.0 mm2 s−1) was purchased from Vietnam Petro Oil Corporation (Vietnam).
Coir treatment to increase adsorption capacity
To increase the oil adsorption efficiency of coir, the treatment processes were carried out by different methods using different agents: hot water, sodium hydroxide and sulfuric acid treatment. In typically, the treatment processes were performed as following:High temperature water treatment: 10 g of coconut fiber with determined components, 400 mL water was put into the 1 L autoclave that was kept at desired temperature (160, 170 and 180 °C) for 60–120 minutes. The vapor pressure inside the autoclave at 160, 170 and 180 °C was around 5.2, 7 and 9.2 bar, respectively. After hot water treatment, the samples were washed carefully, dried in the oven at 50 °C for 2 days and stored in the nylon bag for further use.
Other treatment methods: the treatments of coconut fiber with sodium hydroxide and sulfuric acid agents were carried out similar to the hot water treatment method. In particular, 10 g of coconut fiber with determined components, 400 mL water and NaOH or H2SO4 were put into the 1 L autoclave. The amount of agents (NaOH, H2SO4) was varied from 0.5–1.0 g (correspond to 5–10% w/w of coir biomass), while the treatment temperature was set at 110 °C for 60–120 minutes.
Fabrication of porous coir–polyurethane composite
New sorbent material was fabricated by taking sufficient quantities of components A (polyol part) and B (isocyanate part) of polyurethane matrix (Guangzhou Niubi Special Glass Co., Ltd. Guangdong, China) in certain proportions by mass. First, component A was mixed with treated coir, stirred for several minutes. Treated coir (the coir was treated by above treatment methods) with sizes of 1, 2, 4 and 6 mm were used with amount of 5–25 wt% over mass of PU. Component B was then added to the above mixture and stirred vigorously for about 10–20 seconds. Then the curing process occurred to obtain a porous coir–polyurethane composite sorbent by incorporating the coir filler into PU matrix.Material characterization
Chemical compositions of coconut fiber were characterized using analytical methods according to international standards such as TAPPI – The Technical Association of the Pulp and Paper Industry (American Pulp and Paper Industry Technical Association), GOST (Russian Federation standards) and other commonly used methods in the field of chemical processing of plant biomass. In particular, determination of acid-insoluble lignin in wood and pulp followed the method of T 222 om-02, determination of solvent extractives of wood and pulp followed the method of T 204 cm-97, determination of ash in wood, pulp, paper and paperboard followed the method of T211 om-93, determination of pentosans in wood and pulp followed the method of T 223 cm-10 and determination of cellulose in wood and pulp followed the Kürschner–Hoffer method. Scanning electron microscopy (SEM) was performed using a JSM-7000F machine, JEOL, Japan.To determine the oil uptake capacity of the adsorbent, as-prepared porous coir–PU composite was used to adsorb oil of water containing diesel oil (DO) at room temperature as shown in Fig. 2. The analysis method for measuring the oil adsorption capacity of absorbents is based on ASTM F726 17: standard test method for adsorbent adsorption efficiency for crude oil and related spills. In typically, the procedure is following: 80 mL of water and 20 mL of oil was poured into a 100 mL beaker. 0.5 g of the as-prepared adsorbent material was gently placed onto the oil surface of beaker for different periods of time of 30, 60, 90, 120, 180 min. The sorbent material was then separated and treated under vacuum to remove water on the surface for about 5 minutes before weighing to calculate the adsorption capacity. All experiments were conducted in duplicates.
The sorption capacity was calculated as the ratio of adsorbed amount of oil to initial dry weight of the sorbent as shown in the following equation and given in unit of g oil/g dry sorbent:32
Results and discussion
Modification of coir to increase adsorption capacity
The chemical composition of coir was characterized to be: 41.0 ± 0.5% cellulose, 20.3 ± 0.3% pentosane, 25.9 ± 0.3% lignin, 6.5 ± 0.1% extractives and 4.1 ± 0.1% ash. It is seen that the content of cellulose, pentosane and lignin of coir sample is appropriate to be used as lignocellulose material for bio-refinery process. Density is an important physical property that affects crushing, compaction, diffusion, and chemical penetration into plant cell walls. The density of raw coir was determined approximately 1.12 ± 0.1 g cm−3, a relative low buoyancy characteristic.Different treatment process such as hot water, H2SO4 and NaOH treatment at different conditions were conducted for improvement of the oil sorption capacity of coconut fiber. The decomposition level of lignocellulosic structure of coir biomass depends on the nature of reactive agents and reaction condition resulting in different oil sorption capacity of treated coconut fiber. First attempt was tried by treating coir sample with water at high temperature in order to modify the structure of the coir material, thus increase the surface area and the porosity of treated coir. The results were listed in Table 1.
| Coir sample at treatment conditions | Oil sorption capacity at different sorption time, g g−1 | |||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| 160 °C, 60 min | 1.91 ± 0.10 | 2.14 ± 0.10 | 2.43 ± 0.10 | 2.91 ± 0.10 |
| 160 °C, 90 min | 2.23 ± 0.10 | 2.42 ± 0.10 | 2.72 ± 0.10 | 3.13 ± 0.11 |
| 160 °C, 120 min | 2.72 ± 0.11 | 3.13 ± 0.12 | 3.41 ± 0.12 | 3.61 ± 0.12 |
| 170 °C, 60 min | 2.34 ± 0.11 | 2.61 ± 0.11 | 3.04 ± 0.11 | 3.22 ± 0.11 |
| 170 °C, 90 min | 2.49 ± 0.11 | 2.82 ± 0.11 | 3.22 ± 0.11 | 3.62 ± 0.12 |
| 170 °C, 120 min | 3.1 ± 0.12 | 3.51 ± 0.12 | 3.91 ± 0.12 | 4.12 ± 0.12 |
| 180 °C, 60 min | 2.40 ± 0.11 | 2.81 ± 0.11 | 3.13 ± 0.11 | 3.34 ± 0.11 |
| 180 °C, 90 min | 2.61 ± 0.11 | 2.94 ± 0.11 | 3.32 ± 0.11 | 3.82 ± 0.12 |
| 180 °C, 120 min | 3.20 ± 0.12 | 3.62 ± 0.12 | 4.10 ± 0.12 | 4.34 ± 0.12 |
It can be seen that at harsh treatment condition, the higher oil adsorption capacity was observed. In particular, the adsorption capacity increased when the treatment time and temperature increased. In the process of high temperature water treatment, hemicelluloses were hydrolyzed at elevated temperature, and the hydroxyl groups in the lignocellulose material decreased.21,29 The hydrolysis of the acetyl groups in the hemicellulose component forms acetic acid, which catalyze the dissolution of hemicellulose leading to the deconstruction of lignocellulosic structure.
There was still 88% solid residue retaining after hot water treatment at 160 °C after 60 min. At this condition, the speed of the hydrolysis reaction takes place slowly. Only a small part of polysaccharides, mainly hemicellulose was hydrolyzed. Therefore, at this condition, the deconstruction level of lignin–hemi-cellulose network structure was not high. The porosity of coir material was not significantly increased, leading to a relative low improvement of sorption capacity. When the treatment time and temperature increased, the lignin–hemi-cellulose network structure of the coir was partially deconstructed, leading to the increase of the surface area and pore volume of the material, the adsorption ability thus would improve. As known that the recovery of mass in the solid residue after pretreatment decreased with the increase of pretreatment temperature and time. It means that higher degradation of lignin, hemicellulose even cellulose was observed at higher temperature and longer time.29 It can be attributed to the gradually increase of organic acid catalyst formed when temperatures and times increased.
Besides, treatment at 180 °C for 120 min gave the highest sorption capacity. However, the difference in capacity between treatment at 180 °C for 120 min and 170 °C for 120 min was not high (4.3 g g−1 and 4.1 g g−1, respectively). Hence considering the economic and technical efficiency it is possible to select the appropriate conditions for coir treatment with hot water as: treatment temperature at 170 °C for 120 minutes. After this treatment, the oil uptake ability after 180 minutes was up to 4.1 g g−1, an increase of about 78.3% compared to the oil uptake of the original coir material (2.3 g g−1 after 180 minutes).
Sodium hydroxide treatment of coir was conducted to improve the oil adsorption efficiency. Alkali is an easy-to-find, low-cost chemical that has little impact on the environment when used in small amounts. From the results reported in sodium hydroxide treatment of lignocellulose, it is found that when temperature over 120 °C and longer treatment time than 120 min (at dosage of 5 or 10% of sodium hydroxide) would result in the significant deconstruction of lignocellulose structure and significant degradation of main compound of lignocellulose.23,29,35 It is necessary to lower the temperature because sodium hydroxide is more effective at breaking down lignocellulosic structure of coir biomass than water. Therefore, the concentration of 5 or 10%, time of 60 or 120 min and temperature of 110 °C were chosen to avoid the significant modification and deconstruction of biomass structure as well as ensure the certain modification of biomass. The obtained results were shown in Table 2.
| Coir sample at treatment conditions | Oil sorption capacity at different sorption time, g g−1 | |||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| NaOH 5%, 60 min | 2.22 ± 0.10 | 2.72 ± 0.11 | 3.14 ± 0.12 | 3.53 ± 0.12 |
| NaOH 5%, 120 min | 2.03 ± 0.10 | 2.41 ± 0.10 | 2.73 ± 0.11 | 2.92 ± 0.11 |
| NaOH 10%, 60 min | 1.94 ± 0.10 | 2.33 ± 0.10 | 2.52 ± 0.11 | 2.94 ± 0.11 |
| NaOH 10%, 120 min | 1.81 ± 0.10 | 2.21 ± 0.10 | 2.42 ± 0.10 | 2.73 ± 0.11 |
From the obtained data in Table 2, it can be seen that, treating coir material with sodium hydroxide resulted in a higher oil adsorption efficiency than initial coir. By treating with sodium hydroxide solution, the dissolution of lignin and hemicellulose of lignocellulosic biomass are mainly occurred. This induces the deconstruction of lignin–hemi-cellulose structure network of material leading to the increase the porosity of the material. Consequently, the oil adsorption capacity of the treated material would be enhanced. By treating with NaOH agent at 110 °C, the adsorption capacity of coir treated for 60 minutes is much higher than that of coir treated for 120 minutes.
Moreover, treating with dosage of 5% NaOH resulted in higher oil adsorption capacity than treating with 10% NaOH. In general, the oil sorption capacity of treated coir material after sodium hydroxide treatment was lower than that of treated coir after hot water treatment. It can be explained that in an alkaline environment at harsh condition, lignin and hemicellulose were significantly decomposed, and cellulose was partly depolymerized. The lignocellulosic structure of coir thus was broken quite a lot, leading to achievement of material with low porosity, low pore volume and low surface area, and thus reducing the adsorption capacity of the coir material.11,29 Therefore, the longer the treatment time and higher the dosage of alkali, the higher decomposition level of lignin and hemicellulose, leading to the strongly reduced the sorption capacity of coir.
For sodium hydroxide treatment, the conditions of 60 min treatment at 100 °C with 5% NaOH is suitable treatment to obtain coir material with higher adsorption efficacy. Under this treatment condition, the oil adsorption capacity of the material after 180 minutes was 3.5 g g−1, which is much higher than the adsorption capacity of initial coir materials (with an increase of about 52.2%).
Another treatment was carried out by using sulfuric acid solution and the results were reported in Table 3.
| Coir sample at treatment conditions | Oil sorption capacity at different sorption time, g g−1 | |||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| H2SO4 5%, 60 min | 2.13 ± 0.10 | 2.62 ± 0.12 | 2.91 ± 0.12 | 3.23 ± 0.12 |
| H2SO4 5%, 120 min | 2.02 ± 0.10 | 2.31 ± 0.11 | 2.49 ± 0.11 | 2.82 ± 0.12 |
| H2SO4 10%, 60 min | 1.81 ± 0.10 | 2.19 ± 0.10 | 2.51 ± 0.11 | 2.78 ± 0.11 |
| H2SO4 10%, 120 min | 1.72 ± 0.10 | 1.89 ± 0.10 | 2.01 ± 0.10 | 2.28 ± 0.10 |
Similar to the case of sodium hydroxide treatment, using sulfuric acid agent to treat coir biomass, it was found that the oil adsorption coefficient of coconut fiber was higher than that of initial coir material (except the sample treated at 10% H2SO4 for 120 min). When increasing the treatment time and dosage of H2SO4 the oil sorption capacity of treated material decreased.
This can be attributed to the easily hydrolysis of polysaccharides in the presence of dilute sulfuric acid at elevated temperature. Hemicellulose was almost completely decomposed; a part of lignin may also be dissolved into the solution and cellulose is also slightly depolymerized. Hence the lignocellulosic network of coir biomass was degraded partly leading to the increase porosity and surface area of material. However, the longer the treatment process with the higher the amount of acid used, the higher the solubility level of biomass components. Thus, the lignocellulosic structure would be degraded seriously inducing the reduction of surface area and porosity of biomass, leading to a decrease in adsorption capacity. From the obtained results, the condition of 100 °C for 60 minutes with 5% sulfuric acid was selected as suitable condition that resulted in the adsorption capacity of 3.2 g g−1.
For comparison, the oil adsorption results of coir materials obtained from three treatment methods at suitable condition were summarized and shown in Fig. 3.
As seen from Fig. 3, it was found that the alkaline and acid pretreatment method gave a much lower oil adsorption capacity than the hot water pretreatment method. The sulfuric acid pretreatment method resulted in the almost similar oil adsorption capacity to that of the alkaline pretreatment method. In summary, hot water pretreatment of coir biomass at suitable condition: 170 °C for 120 minutes showed the highest adsorption capacity of 4.1 g g−1. The treated coir materials from this pretreatment method at suitable condition was chosen to use for next experiment of fabrication of porous coir–polyurethane composite.
In the hot water treatment, hemicelluloses are hydrolyzed at elevated temperature and pressure, and the hydroxyl groups in the plant cell walls are reduced.21,29 When the treatment temperature increased above the phase transition temperature of lignin, the lignin droplets would deposit on the residual biomass surfaces.36 These reasons could be attributed to the increase of the hydrophobicity, thus increase the oil sorption capacity of the hot water treated coir fibers.
SEM images of the original coir biomass (Fig. 4A) showed that the original coir has a porous, hollow structure, rough surface with a few pores, ensuring that coir has the certain potential for oil adsorption. However, the oil sorption capacity of original coconut fiber was not high due to the hydrophilic of coir, low buoyancy characteristics and not high porosity. After hot water treatment at 170 °C for 2 hours, treated coir samples was partially degraded and more porous. Surface morphology of treated coir exhibited the extended and interconnected dome-shaped regions with higher number of pore, leading to the increase of oil sorption capacity.19
Fabrication of porous coir–polyurethane composite
As known that coir biomass material has a hydrophilic, low buoyancy characteristics and not high porosity. Even after treatment with hot water, the hydrophobic and porosity was slightly improved but the low buoyancy characteristics was not improved. Therefore, to overcome this disadvantage and to significantly enhance the oil sorption capacity of materials, the research was conducted to fabricate a porous coir–polyurethane composite with high oil adsorption capacity. This combination will take the advantages of two types of natural and synthetic adsorbents to create a new material that has both economic and technical advantages. The treated coir biomass that was treated with hot water was used as filler for replacement of a part of polyurethane (PU) to create porous coir–polyurethane composite.A experiment has been carried out to study the effect of the size of coconut fiber filler on the adsorption capacity of the obtained composite material. Samples of coir with sizes 1, 2, 4 and 6 mm were filled with content of 15 wt% (over mass of PU) for the investigation. The adsorption experiments of as-prepared coir–PU composite sorbent were conducted, and the results were given in the Fig. 5.
 | ||
| Fig. 5 Effect of coir biomass size on oil adsorption capacity of the composite sorbent (15% w/w of coir biomass). | ||
From the obtained results in the Fig. 5, it can be seen that the size of the coir materials was an important factor influencing the oil adsorption capacity of the composite sorbent. When the size of the coir biomass increased, the adsorption capacity of sorbent decreased gradually. Except for the sample using 6 mm coir, all of other samples had a much higher adsorption capacity than that of pristine polyurethane. The adsorption capacity of the porous composite sorbent reached the highest value when using the 1 mm coir sample and slightly decreased when using 2 mm coir sample. When adding 4- and 6 mm coir samples, the adsorption capacity reduced dramatically about 27.1% and 46.6%, compared to that of composite sorbent using 1 mm coir sample, respectively. In particular, when using the 1 mm coir filler, the oil adsorption capacity after adsorption time of 180 min was the highest, about 11.8 g g−1. The adsorption capacity of the composite sorbent after 180 min sorption time reduced to 10.5, 8.6, and 6.3 g g−1 when the coconut fiber sizes of 2, 4 and 6 mm were used, respectively. There was a slight difference in the adsorption capacity between the sorbent material using the coir size of 6 mm and the original polyurethane (but much higher than treated coir without PU, about 4.1 g g−1).
The oil adsorption capacity increased as the fiber size decreased, due to the correlation between the filler size and porosity and pore number of porous coir–PU composite.20 The coir sizes decreased, the surface area of the coir increased, leading to an increase in the surface area and pore volume of the as-prepared porous coir–PU composite. Moreover, at the same amount of filled coir when the size is small, the number of filled coir particles increases, leading to the increase of the number of pore and thus increase in porosity and pore volume of the composite adsorbent material. As known that, the adsorption capacity depends significantly on the surface area and pore volume of the material, therefore using the coir sample of small size, the as-prepared porous adsorbent had a higher oil adsorption capacity. According to the obtained results, 1 mm was chosen as the suitable size of the coir biomass filler for fabrication of the porous coir–PU composite sorbent.
The effect of the filler content on adsorption efficiency was also investigated to optimize the amount of coir lignocellulosic filler used for fabrication of porous coir–PU material. While coir size of 1 mm was used as mentioned earlier, different coir material content of 5–30% were selected to study in the experiment. The results of adsorption were shown in Fig. 6.
 | ||
| Fig. 6 Effect of content of coir biomass on oil adsorption capacity of the composite sorbent (size of coir material of 1 mm). | ||
Similar to the case of coir size, it was found that the content (wt%) of coconut fiber filler in the coir–PU composite greatly affected the oil adsorption capacity of sorbent material. Changing the content of coir filler would result in significant change of oil adsorption capacity of porous materials. The oil adsorption capacity of porous composite material increased as the increase of content of coir filler and reached to the highest value at 20% coir filler (oil sorption coefficient was about 15.2 g g−1 after 180 minutes adsorption time). When the content of coir filler further increased to over 25%, the oil adsorption capacity decreased, even at the content of 30% the adsorption capacity was almost similar to that of original polyurethane. Moreover, when adding with content of coir of 30% by weight, the fracture of the as-prepared porous material started occurring during the experiment, the material was very easy to crack and break due to the low bonding strength of the composite materials.
This can be explained that increase the content of coir would increase the number and size of capillaries or the space volume inside the adsorbent giving the higher oil adsorption capacity of material. The higher content of coir biomass used, the higher open-cell structure of porous coir–PU composite with higher porosity, thus make it more ability to adsorb oil into its porous structure. In addition, lignocellulosic coir itself is also a porous material (and more improvement after hot water treatment), has many capillaries and the ability to absorb oil. While, polyurethane, a synthetic polymer is high porous and oil adsorption capacity that has been extensively studied and applied in adsorption and treatment of oil pollution. Moreover, the buoyancy of new porous composite was increased (with density of about 0.26 g cm−3) and higher than that of original coir thus gave the higher ability to contact with oil for easy adsorption. By combination of lignocellulosic coir and polymer matrix, resulted in a newly fabricated composite material with high oil adsorption capacity and effective oil treatment. At coir fiber content over 25%, the oil adsorption capacity of the composite adsorbent started reducing significantly. It can be explained that using higher amount of coir biomass leading to decrease in amount of polyurethane matrix resulted in a decrease of adsorption capacity of the material. Therefore, the most appropriate content of coconut fiber filler was selected at 20% for the adsorbent fabrication process.
For comparison, an original coir biomass without hot water treatment was used as filler at content of 20% to prepare porous coir–PU composite for oil adsorption. It was observed that coir–PU composite prepared from original coir biomass had a lower adsorption capacity (about 12.5 g g−1) than that of coir–PU composite prepared from hot water treated coir biomass (about 15.2 g g−1). The oil sorption capacity of new coir–PU composite material was comparable or higher than that of Azolla plant,37 several vegetable fibers,20 sugar cane, rice husk and synthetic sorbent.19 Compared with sorption capacity of treated coir fiber without being incorporated in polyurethane matrix, this capacity was improved nearly 4 times. This result confirmed the significantly oil adsorption improvement of coir biomass treatment in fabrication of porous coir–PU composite adsorbent.
To estimate the surface property and porous structure of coir–PU composite, the sorbent material was characterized by SEM analysis and shown in Fig. 7. As seen from Fig. 7A and B that, after adding the coir biomass filler the coir–PU composite showed higher number of capillaries/pores as well as higher pore size than pristine polyurethane. It is consistent and confirmed again from result in SEM image of coir–PU composite (Fig. 7C). SEM image of as-fabricated porous coir–PU composite showed the half-open cell structure, which was composed of many pores, capillaries and fissure. The pore size was increased considerably compared to that of original PU. Pores diameters ranging from a few hundred nanometers to several micrometers composed the hierarchical roughness required for a sorbent surface.30 It is noted that the surface properties as well as porous properties give significant effects on various features of oil adsorption of sorbent materials.
 | ||
| Fig. 7 Optical image of pristine PU foam (A), coir–PU composite (B) and SEM image of porous coir–PU composite (C). | ||
The desorption test was performed by simple squeezing of the coir–PU composite sample. Then sorbent was used for investigating the reusability of the porous coir–PU composite materials. Our experimental effort showed that over 93% of adsorbed oil is extractable. Of course, the collected oil can be sent to a refinery for required processing, making it ready for appropriate applications. The maximum adsorption capacity of the coir–PU composite sample decreased slightly after second recycle run. However, further runs, the adsorption capacity was significantly decreased. It was about 10 g g−1 for third run and 6 g g−1 for fourth run. These results indicated that the porous coir–PU composite sorbent not only had a high adsorption capacity but also had relatively good reusability.
Conclusion
A suitable treatment method was found to improve oil adsorption efficiency of coir biomass. The suitable technical parameters of treatment process were selected as: hot water treatment at 170 °C for 120 minutes. Coconut fiber treated under this suitable condition presented the highest oil adsorption capacity of 4.1 g g−1, with an increase of 78.3% compared to the adsorption capacity of the original coir. Moreover, a porous coir–polyurethane composite sorbent with high adsorption capacity has been fabricated by combination of treated coir biomass and polyurethane. The new designed composite sorbent that was fabricated by mixing and blending polyurethane with coconut fiber originated from Vietnam showed high oil adsorption capacity, up to 15.2 g g−1 when 20% coir material with particle size of 1 mm was added into polyurethane matrix. The porous coir–PU composite sorbent exhibited the high efficiency in oil spill treatment in order to separate contaminated oil from water. Moreover, this sorbent also showed relatively good reusability and adsorbed oil was extractable and reusable.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Vietnam Ministry of Education and Training (MOET) under grant number B2021-BKA-16.References
- R. Asadpour, N. Sapari, Z. Tuan, H. Jusoh, A. Riahi and O. Uka, Application of sorbent materials in oil spill management: a review, Casp. J. Appl. Sci. Res., 2013, 2, 46–58 Search PubMed
.
- M. Hubbe, New Horizons for Use of Cellulose-Based Materials to Adsorb Pollutants from Aqueous Solutions, Lignocellulose, 2014, 2, 386–411 Search PubMed
.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS
.
- A. A. Al-Majed, A. R. Adebayo and M. E. Hossain, A novel technology for sustainable oil spills control, Environ. Eng. Manag. J., 2014, 13, 265–274 Search PubMed
.
- R. Yadav, K. Upadhyay and S. Maru, Colour removal from paper mill effluent using activated Carbon, J. Ind. Pollut. Control, 2012, 28, 45–50 CAS
.
- R. Gong, Y. Jin, J. Sun and K. Zhong, Preparation and utilization of rice straw bearing carboxyl groups for removal of basic dyes from aqueous solution, ( 2008) Search PubMed
.
- D. F. Arsene, C. A. Peptu and C. Teodosiu, Textile Dyes From Aqueous Effluents, Cellul. Chem. Technol., 2017, 51, 175–184 Search PubMed
.
- T. Dizhbite, L. Jashina, A. Andersone, V. Solodovniks and G. Telysheva, Lignocellulosic Wastes As Bio-Sorbents For Removal Of Organic And Inorganic Eco-Toxicants From Water Solutions Lignocelulozes Atkritumi K Ā Biosorbenti Organisko Un Neorganisko Eko- Pies Ā R Ĥ Ot Ā Ju Aizv Ā Kšanai No Ū Dens Š Ėī Dumiem, in International conference Eco-Balt, Riga, Latvia, 2007, p. 7555916 Search PubMed
.
- S. Suni, A. L. Kosunen, M. Hautala, A. Pasila and M. Romantschuk, Use of a by-product of peat excavation, cotton grass fibre, as a sorbent for oil-spills, Mar. Pollut. Bull., 2004, 49, 916–921 CrossRef CAS PubMed
.
- M. Lahieb Faisal, Z. T. Al-Sharify and M. Farah Faisal, Role of rice husk as natural sorbent in paracetamol sorption equilibrium and kinetics, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 870, 012053 CrossRef
.
- L. E. Q. Dien, N. T. M. Phuong, P. H. Hoang and D. T. Hoa, Efficient Pretreatment of Vietnamese Rice Straw by Soda and Sulfate Cooking Methods for Enzymatic Saccharification, Appl. Biochem. Biotechnol., 2015, 175, 1536–1547 CrossRef CAS PubMed
.
- J. R. Vicente, M. G. G. Nillos, H. S. Taberna Jr, I. G. Pahila and N. C. Añasco, Sorbents derived from lignocellulosic waste materials: characterization and potential removal of surfactants, phenolic compounds, and nutrients, Adv. Environ. Sci., 2014, 6, 91–100 Search PubMed
.
- D. M. Suflet, I. Popescu and I. M. Pelin, Preparation and Adsorption Studies of Phosphorylated Cellulose Microspheres, Cellul. Chem. Technol., 2017, 51, 23–34 CAS
.
- D. Angelova, I. Uzunov, S. Uzunova, A. Gigova and L. Minchev, Kinetics of oil and oil products adsorption by carbonized rice husks, Chem. Eng. J., 2011, 172, 306–311 CrossRef CAS
.
- S. H. Taufik, S. A. Ahmad, N. N. Zakaria, N. A. Shaharuddin, A. A. Azmi, F. E. Khalid, F. Merican, P. Convey, A. Zulkharnain and K. A. Khalil, Rice straw as a natural sorbent in a filter system as an approach to bioremediate diesel pollution, Water, 2021, 13, 3317 CrossRef CAS
.
- V. Singh, R. J. Kendall, K. Hake and S. Ramkumar, Crude oil sorption by raw cotton, Ind. Eng. Chem. Res., 2013, 52, 6277–6281 CrossRef CAS
.
- V. Da Silva, J. B. Lopez-Sotelo, A. Correa-Guimaraes, S. Hernandez-Navarro, M. Sanchez-Bascones, L. M. Navas-Gracia, P. Martin-Ramos and J. Martin-Gil, an Example of Lignocellulosic Waste Reuse in Two Consecutive Steps: Sorption of Contaminants and Enzymatic Hydrolysis, Cellul. Chem. Technol., 2017, 51, 127–136 CAS
.
- J. Wang, Y. Zheng, Y. Kang and A. Wang, Investigation of oil sorption capability of PBMA/SiO2 coated kapok fiber, Chem. Eng. J., 2013, 223, 632–637 CrossRef CAS
.
- N. Alia, M. El-Harbawia, A. A. Jabal and C. Y. Yin, Characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: oil removal suitability matrix, Environ. Technol., 2012, 33, 481–486 CrossRef PubMed
.
- T. R. Annunciado, T. H. D. Sydenstricker and S. C. Amico, Experimental investigation of various vegetable fibers as sorbent materials for oil spills, Mar. Pollut. Bull., 2005, 50, 1340–1346 CrossRef CAS PubMed
.
- Y. Zhang, S. Yang, J. Q. Wu, T. Q. Yuan and R. C. Sun, Preparation and characterization of lignocellulosic oil sorbent by hydrothermal treatment of Populus fiber, Materials, 2014, 6, 6733–6747 CrossRef PubMed
.
- P. H. Hoang, N. M. Dat, N. T. Thanh, D. Co, V. Street, V. Trong and P. Street, Preparation of Activated Bio-char from Corn Stalk for Color Treatment of Effluent from Packaging Paper Mill. Water Air, Soil Pollut., 2021, 232, 385–392 CrossRef CAS
.
- N. T. M. Phuong, P. H. Hoang, L. Q. Dien and D. T. Hoa, Optimization of sodium sulfide treatment of rice straw to increase the enzymatic hydrolysis in bioethanol production, Clean Technol. Environ. Policy, 2017, 19, 1313–1322 CrossRef
.
- A. P. Vieira, S. A. A. Santana, C. W. B. Bezerra, H. A. S. Silva, J. A. P. Chaves, J. C. P. Melo, E. C. S. Filho and C. Airoldi, Removal of textile dyes from aqueous solution by babassu coconut epicarp (Orbignya speciosa), Chem. Eng. J., 2011, 173, 334–340 CrossRef CAS
.
- D. Kołodyńska, M. Gęca, I. V. Pylypchuk and Z. Hubicki, Development of New Effective Sorbents Based on Nanomagnetite, Nanoscale Res. Lett., 2016, 11, 152 CrossRef PubMed
.
- R. D. S. Bezerra, R. C. Leal, M. S. Da Silva, A. I. S. Morais, T. H. C. Marques, J. A. Osajima, A. B. Meneguin, H. da S. Barud and E. C. Da Silva Filho, Direct modification of microcrystalline cellulose with ethylenediamine for use as adsorbent for removal amitriptyline drug from environment, Molecules, 2017, 22, 1–23 CrossRef PubMed
.
- X. F. Sun, R. Sun and J. X. Sun, Acetylation of rice straw with or without catalysts and its characterization as a natural sorbent in oil spill cleanup, J. Agric. Food Chem., 2002, 50, 6428–6433 CrossRef CAS PubMed
.
- J. Wang, F. Han, B. Liang and G. Geng, Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions, J. Ind. Eng. Chem., 2017, 54, 174–183 CrossRef CAS
.
- C. Wan, Y. Zhou and Y. Li, Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility, Bioresour. Technol., 2011, 102, 6254–6259 CrossRef CAS PubMed
.
- B. Li, X. Liu, X. Zhang, J. Zou, W. Chai and J. Xu, Oil-absorbent polyurethane sponge coated with KH-570-modified graphene, J. Appl. Polym. Sci., 2015, 132, 1–7 Search PubMed
.
- H. Li, L. Liu and F. Yang, Oleophilic Polyurethane Foams for Oil Spill Cleanup, Procedia Environ. Sci., 2013, 18, 528–533 CrossRef CAS
.
- H. Li, L. Liu and F. Yang, Hydrophobic modification of polyurethane foam for oil spill cleanup, Mar. Pollut. Bull., 2012, 64, 1648–1653 CrossRef CAS PubMed
.
- M. V. G. Zimmermann, A. J. Zattera, B. R. Fenner and R. M. C. Santana, Sorbent system based on organosilane-coated polyurethane foam for oil spill clean up, Polym. Bull., 2021, 78, 1423–1440 CrossRef CAS
.
- Q. Wei, O. Oribayo, X. Feng, G. L. Rempel and Q. Pan, Synthesis of Polyurethane Foams Loaded with TiO2 Nanoparticles and Their Modification for Enhanced Performance in Oil Spill Cleanup, Ind. Eng. Chem. Res., 2018, 57, 8918–8926 CrossRef CAS
.
- E. M. Karp, B. S. Donohoe, M. H. O'Brien, P. N. Ciesielski, A. Mittal, M. J. Biddy and G. T. Beckham, Alkaline pretreatment of corn stover: Bench-scale fractionation and stream characterization, ACS Sustain. Chem. Eng., 2014, 2, 1481–1491 CrossRef CAS
.
- H. Li, Y. Pu, R. Kumar, A. J. Ragauskas and C. E. Wyman, Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms, Biotechnol. Bioeng., 2014, 111, 485–492 CrossRef CAS PubMed
.
- J. Sayyad Amin, M. Vared Abkenar and S. Zendehboudi, Natural Sorbent for Oil Spill Cleanup from Water Surface: Environmental Implication, Ind. Eng. Chem. Res., 2015, 54, 10615–10621 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2022 |