Open Access Article
Open Access ArticleDirect synthesis of ultrathin FER zeolite nanosheets via a dual-template approach†
Yuekun Liab,
Duozheng Maab,
Wenhua Fu b,
Chuang Liub,
Yu Wangb,
Zhendong Wang
b,
Chuang Liub,
Yu Wangb,
Zhendong Wang *b and
Weimin Yang*ab
*b and
Weimin Yang*ab
aSchool of Chemical Engineering, East China University of Science and Technology, Shanghai, 200237, PR China. E-mail: yangwm.sshy@sinopec.com
bState Key Laboratory of Green Chemical Engineering and Industrial Catalysis, Sinopec Shanghai Research Institute of Petrochemical Technology, Shanghai, 201208, PR China. E-mail: wangzd.sshy@sinopec.com
First published on 11th May 2022
Abstract
The synthesis of zeolites with nanosheet morphology has been attracting extensive attention. Despite the steady progress, the direct synthesis of ultrathin nanosheets of FER zeolite with thickness of less than 10 nm is still a great challenge. Herein we report a facile synthesis of FER zeolite nanosheets (named SCM-37) by using octyltrimethylammonium chloride (OTMAC) and 4-dimethylaminopyridine (4-DMAP) as dual organic templates. The effects of synthesis parameters, including initial molar ratio of SiO2/Al2O3, crystallization temperature and time were investigated. It turned out that the crystallization field of SCM-37 was narrow. A two-step crystallization method was utilized to obtain pure and completely crystallized SCM-37 zeolite. SCM-37 was characterized by multiple techniques, including X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen physisorption, Fourier transform infrared (FTIR), ammonia-temperature programmed desorption (NH3-TPD) and nuclear magnetic resonance (NMR), and compared with the conventional FER zeolite (C-FER). The two most significant features of SCM-37 are the ultrathin crystal nanosheet and extremely high external surface area. The thickness of SCM-37 along the a-axis is 4∼7 nm, while that of C-FER is over 20 nm. The external surface area reaches 198 m2 g−1, which is over ten times larger than that of C-FER. The catalytic performances of the FER zeolites were evaluated by the cracking of 1,3,5-triisopropylbenzene (TiPB). Although possessing a lower amount of total acid sites, SCM-37 showed higher conversion of TiPB, as well as selectivity to the deep cracking products. The superior performance of SCM-37 was attributed to the higher external surface area arising from the ultrathin nanosheets.
Introduction
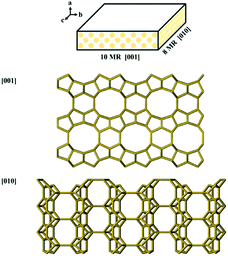
Zeolites are a class of crystalline microporous materials composed of SiO4 and AlO4 tetrahedras,1–4 which are widely used in many fields such as catalysis, ion exchange, and adsorption/separation owing to their tunable chemical composition, strong acidity, excellent thermal/hydrothermal stability and uniform apertures.5–10 The nanopores of zeolites, diameters of which are usually below 1 nm, offer excellent places for shape-selective catalysis.11,12 However, zeolites also suffer from intracrystalline diffusion limitations because of the poor accessibility of confined active sites. To shorten the diffusion path and boost the diffusion efficiency consequently, significant efforts have been made to prepare nanosized zeolites, such as nanoparticles, nanoneedles, nanosheets, single-walled zeolitic nanotubes, and zeolites with hollow structure.13–19FER zeolite is composed of 10-membered ring (10-MR) channels (0.43 × 0.55 nm) in the [001] direction and 8-MR channels (0.35 × 0.48 nm) in the [010] direction to form a two-dimensional (2D) channel system (Scheme 1).20,21 The 6-MR channel parallel to the 10-MR channel intersects with the 8-MR channel to form an ellipsoidal cage of 0.6 to 0.7 nm (fer cage).22,23 It is effective in diverse catalytic processes, such as skeletal isomerization,24–26 benzylation,27 NOx reduction28 and carbonylation of dimethyl ether.29–32 To overcome diffusion limitation, FER zeolites with nanosized crystals are highly desirable. Although FER zeolites can be hydrothermally synthesized using various organic structure-directing agents (OSDAs),33–36 also called templates, researches on the direct synthesis of ultrathin nanosheets of FER zeolite are still rather limited. The general strategy for preparing ultrathin FER zeolites nanosheets is to employ elaborately designed OSDAs or introduce surfactants as additive. Using piperidine (PI) and tetramethylammonium hydroxide (TMAOH) as cooperative OSDAs, FER nanosheets with hierarchical pores were synthesized. The size of crystals was about 10∼15 μm, while the thickness was less than 50 nm.22 Hong and coworkers used choline as a single organic template to synthesize nano-needle like FER zeolite (FER nanoneedles) with a diameter of 10 nm.37 Corma and coworkers prepared FER nanocrystals with bc flane as low as 10 × 10 nm with the presences of both PI and cetylmethylpiperidinium bromide (C16MPip) as OSDAs.38 FER nanosheets with a thickness of about 10 nm were synthesized by using PI as OSDA and cetyltrimethyl ammonium bromide (CTAB) as morphology regulator.39 Xiao and coworkers reported a method to synthesize the ultrathin FER nanosheets using N,N-diethyl-cis-2,6-dimethyl piperidinium (DMP) as OSDA, which not only directed the crystallization of FER zeolite structure, but also inhibited the growth of FER zeolite in [100] direction.40 Wu and coworkers synthesized highly delaminated FER nanosheets using a simple imidazole molecule as the single OSDA.41
Here we report a new dual-template method for the direct synthesis of ultrathin FER zeolite nanosheets, designated as SCM-37 (Sinopec Composite Material No. 37), by using both octyltrimethylammonium chloride (OTMAC) and 4-dimethylaminopyridine (4-DMAP) as templates. Compared to conventional FER zeolites (C-FER), SCM-37 showed significantly better performance in the catalytic cracking of 1,3,5-triisopropylbenzene (1,3,5-TiPB).
Experimental
Synthesis of zeolite
In a typical synthesis of SCM-37, colloidal silica solution (40.0 wt% SiO2, LUDOX HS40, Aldrich), sodium aluminate (≥41% Al2O3, China Medicine (Group) Shanghai Chemical Reagent Co., Ltd), NaOH (99.5% NaOH, China Medicine (Group) Shanghai Chemical Reagent Co., Ltd), octyltrimethylammonium chloride (OTMAC, 99 wt%, Aladdin), 4-dimethylaminopyridine (4-DMAP, 99 wt%, TCI shanghai) and deionized water were mixed in a certain order to obtain the synthesis mixture with molar composition of 1.0 SiO2: 0.04 Al2O3: 0.18 NaOH: 0.30 OTMAC: 0.20 4-DMAP: 20 H2O. After stirring at room temperature for 3 h, the final synthesis mixture was transferred into a Teflon-lined stainless steel autoclave and crystallized dynamically (20 rpm) at 150 °C for 6 days followed by at 170 °C for 3 days. The obtained solid product was recovered by centrifugation, washed repeatedly with deionized water, and dried overnight at 90 °C. The product was calcined at 550 °C for 6 h to remove the organic species. The obtained sample was denoted as SCM-37.The conventional FER zeolite (denoted as C-FER) was prepared according to the previous literature, where cyclohexylamine (CHA) was used as OSDA.36 The molar composition of the initial gel was 1.0 SiO2: 0.04 Al2O3: 0.20 NaOH: 0.20 CHA: 20 H2O.
The H-form zeolite samples were obtained by ion-exchanging the calcined samples in 0.5 M NH4Cl solution at 70 °C for 2 h with solid-to-liquid weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The ion-exchange procedure was repeated twice. After that, the solid was recovered by centrifugation, washed, and then dried overnight at 90 °C and calcined at 550 °C for 6 h.
20. The ion-exchange procedure was repeated twice. After that, the solid was recovered by centrifugation, washed, and then dried overnight at 90 °C and calcined at 550 °C for 6 h.
Physicochemical characterization
X-ray powder diffraction (XRD) patterns were recorded on a PANalytical X'Pert PRO diffractometer (Holland) with Cu Kα radiation (λ = 1.54 Å), operating at 40 kV and 40 mA. The data were collected in 2θ range of 3–50° at a speed of 10° min−1.The morphology and size of zeolite sample were observed by scanning electron microscopy (SEM) on a Hitachi S-4800 microscope under accelerated voltage of 3.0 kV.
The high-resolution transmission electron microscopy (HRTEM) images were collected under a Cs-corrected STEM (FEI Titan Cubed Themis G2 300) operated at 300 kV with a convergence semi-angle of 25 mrad.
Nitrogen adsorption/desorption analysis was carried out at −196 °C on a BELSORP-MAX instrument after the sample was degassed under vacuum at 300 °C for 5 h. The specific surface area was calculated with the BET equation while the microporous volume was distinguished from the mesoporous one by the t-plot method.
Acidity was measured by temperature-programmed desorption of ammonia (NH3-TPD) with an Altamira AMI-3300 chemisorption equipment equipped with a thermal conductivity detector (TCD). Typically, 100 mg of sample was pre-treated in the helium stream (30 mL min−1) at 550 °C for 1 h. The adsorption of NH3 (30 mL min−1) was carried out at 100 °C for 30 min. The sample was flushed with helium at 100 °C for 1 h to remove the physisorbed NH3 from the catalyst. The TPD profile was recorded at a heating rate of 10 °C min−1 from 100 °C to 600 °C.
SiO2/Al2O3 molar ratio (SAR) of the prepared FER zeolites was quantified by inductive coupled plasma atomic emission spectrometer (ICP-AES) on a Varian 725-ES after dissolving the samples in HF solution.
IR spectra of hydroxyl (4000–2800 cm−1) stretching were all recorded on a Nicolet 5700 FTIR spectrometer.
The nature and concentration of acid sites of the samples were characterized by Fourier transformed infrared spectra after adsorption of pyridine molecule (Py-IR), using a Nicolet Nexus spectrometer equipped with an in situ vacuum system containing a secondary vacuum. Wafers with a weight of 15 mg and a diameter of 13 mm were pre-treated under vacuum at 400 °C for 2 h (background spectrum). Then the samples were cooled to 150 °C to adsorb pyridine probe molecules. After that, the samples were thermally desorbed at different temperatures (150 °C and 350 °C) followed by the spectra recording. The concentrations of Brønsted acid sites (B acid) and Lewis acid sites (L acid) were calculated by comparing the integrated areas of the bands at 1545 cm−1 and 1455 cm−1. For calculation, integration distinct molar extinction coefficients of 1.67 and 2.22 cm for pyridine interacting with B acid and L acid centers were used, respectively.
27Al and 13C solid-state magic angle spinning solid nuclear magnetic resonance (MAS NMR) spectra were recorded on a JNM-ECZ500R/S1 (JEOL) spectrometer under one-pulse condition. The 13C MAS NMR spectra were recorded at a 13C frequency of 125.765 MHz and a spinning rate of 18.0 kHz with a π/2 rad pulse length of 5.0 μs, 13C chemical shifts were reported relative to adamantane (ADA). The 27Al MAS NMR spectra were recorded at a 27Al frequency of 130.322 MHz and a spinning rate of 18.0 kHz with a π/2 rad pulse length of 1.4 μs, the 27Al chemical shifts were referenced to an Al(NO3)3 solution (1 M).
Catalytic test
Cracking of 1,3,5-triisopropylbenzene (TiPB) was used as the probe reaction to evaluate the catalytic property of zeolite. The reaction was carried out in a fixed-bed pulse microreactor with an inner diameter of 6 mm equipped with an Agilent automatic liquid sampler (ALS). Before catalytic test, zeolite powder was pressurized into wafers and crushed and sieved to 40–60 meshes. In a typical test, 50 mg catalyst was mixed with 100 mg of quartz sand and loaded in the reactor, 1 μL of TiPB was injected via ALS at certain temperature (250 °C, 275 °C, 300 °C, 325 °C, and 350 °C). The result of the first pules at each temperature was analyzed using Agilent 8840 gas chromatograph (GC) apparatus equipped with a flame ionization detector (FID).The conversion of TiPB and selectivity to isopropylbenzene and benzene (iPB + Bz) were calculated as follows:
 | (1) |
 | (2) |
Results and discussion
The effect of initial molar ratio of silica to alumina (SAR) on the products was studied. Fig. 1 illustrates the XRD patterns of as-synthesized zeolites with different SAR, as well as that of C-FER sample and the simulated pattern of FER zeolite. All the samples show patterns corresponding to FER type structure with characteristic diffraction peaks at 2θ of 9.4°, 22.3° and 25.2° ascribed to (200), (031) and (040) plane, respectively. No visible diffraction peaks assigned to other zeolites were detected, which suggests the purity of all FER zeolite materials. The full width at half maximum (FWHM) of the peak at around 9.4° associated with (200) becomes obviously higher with the increase of SAR, which suggests a remarkable loss of ordering along the [100] direction and a decrease of crystal thickness.21 The broadening of the (200) plane associated peak was also observed in the preparation of other FER zeolites with nanosheet morphology.40 After calcination, SCM-37 samples kept the FER framework without any skeleton collapse or the appearance of impurity phase (Fig. S1(b–d)†), which proves their outstanding thermal stability. The reflection peak corresponding to (200) plane does not shift to higher 2θ region, suggesting that the nanocrystalline nature along a-axis are well kept during the calcination. | ||
| Fig. 1 XRD patterns of simulated FER (a), as-synthesized C-FER (b) and SCM-37 zeolites with different SAR of 15 (c), 20 (d), 25 (e). | ||
It's worth mentioning that FER zeolite could not be obtained in the absence of either OTMAC or 4-DMAP (Fig. S2†), suggesting these two compounds might play a cooperative role in the crystallization of SCM-37 zeolite. 13C NMR spectra of the as-synthesized SCM-37 and corresponding OTMAC and 4-DMAP are shown in Fig. 2. It can be seen that the chemical shifts at 13, 22, 25, 28, 31, 52 and 66 ppm in the 13C MAS NMR spectra are assigned to OTMA+, which means the SCM-37 zeolite contains a large number of OTMA+, but the signals at 37, 106 and ∼145 ppm belong to 4-DMAP is relatively weak. Meantime, some small signals can be seen in the 13C solid-state MAS NMR spectra, the reason is not certain, it may be caused by the decomposition of a part of organic OTMAC and 4-DMAP in the synthesis process under high temperature and high pressure. The result suggests that OTMA+ and 4-DMAP participate in the formation of SCM-37 zeolite as OSDA, while only a small amount of 4-DMAP entered the framework of zeolite. The 8-MR channel is possible to accommodate OTMAC, however, neither of the 8-MR or 10-MR channels can accommodate 4-DMAP. Thus, the ammonium head group of OTMA+ is likely located in 8-MR, 10-MR channels and fer cages, whereas the 4-DMAP enables spacing the stacked crystals in the agglomerates.42,43 Therefore, 4-DMAP might be located between the FER layers, preventing crystal growth in the a-direction.
 | ||
| Fig. 2 13C MAS NMR patterns of the as-synthesized SCM-37 zeolite (a), OTMAC solution (b), and 4-DMAP (c). | ||
The effect of crystallization temperature and time on the synthesis of SCM-37 has also been investigated. XRD patterns of samples collected after different crystallization times are shown in Fig. 3. The samples exhibit no obvious characteristic reflection peaks of FER zeolite when the crystallization time was less than 4 days at 150 °C, indicating the forming of amorphous phase. Weak diffraction peaks corresponding to FER structure appears at 5–30° when the crystallization time prolonged to 6 days. The relative crystallinity gradually improved with the increase of synthesis time.
Nevertheless, the crystallization process did not accomplish after 9 days, while prolonging the heating time to 11 days resulted in the emergence of MOR zeolite as impurity (Fig. S3†). This observation implied a narrow crystallization field of FER zeolite. We then employed a two-step crystallization procedure. The initial gel was heated at 150 °C for 6 days and then at 170 °C for various times. The intensities of diffraction peaks strengthened with the prolonged heating period and SCM-37 zeolite with high crystallinity could be obtained after crystalizing at 150 °C for 6 days followed by at 170 °C for 3 days. MOR zeolite emerged after the gel was heated at 170 °C for 3.5 days. If the crystallization temperature was fixed at 160 °C or 170 °C, only amorphous phases would be obtained, regardless of the crystallization time. It is speculated that the preparation of SCM-37 requires a period of crystallization at low temperature to ensure the formation of sufficient crystal nucleus. Shortening the time of crystallizaion at low temperature would result in a decrease in crystallinity probably because of reduced number of crystal nuclei generated at the initial stage. Taking advantage of the two-step crystallization method, SCM-37 zeolite with FER framework was successfully prepared.
The physicochemical properties of SCM-37 and C-FER zeolite materials were systematically studied and compared. For each SCM-37 sample, the peak at 9.4 ° is broader than that of C-FER, proving a shorter dimension along the [100] direction. This demonstrates that SCM-37 crystals are much thinner than C-FER crystals. The relative crystallinity of SCM-37 seems to be a bit lower than that of C-FER, due to the broadening of the diffraction peaks. The comparison of the crystal thickness of SCM-37 and C-FER samples can be realized by SEM and TEM techniques. As depicted in Fig. 4, C-FER consists of typical rectangular flake-like crystals, while SCM-37 (SiO2/Al2O3 = 25) crystals are characterized by a nanosheet-like morphology. No amorphous phase or other zeolitic crystals could be found in the samples. TEM images (Fig. 4C) show that the thickness of C-FER zeolite along the a-axis exceeds 20 nm. In the contrary, the thickness of SCM-37 zeolite crystals is 4∼7 nm, confirming the ultrathin nature of the nanosheets. This observation is in line with the result inferred from XRD patterns. Crystals with clear lattice fringes could be observed through the HRTEM images, indicating the high crystallinity of both materials (Fig. 4E and F). In addition, sharp and clear diffraction spots in the selected area electron diffraction (SAED) indicated that the FER nanolayers are ordered and packed in the [100] direction.
 | ||
| Fig. 4 SEM (A and B) and HRTEM (C–F) images of C-FER (A, C, E) and SCM-37 (B, D, F). The inserted images in panel E and F are the selected area electron diffraction (SAED) patterns. | ||
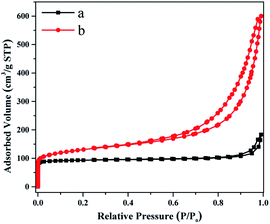
The N2 adsorption–desorption isotherms of C-FER and SCM-37 (SiO2/Al2O3 = 25) are displayed in Fig. 5, and the textural properties are summarized in Table 1. C-FER exhibits a type I isotherm which is characteristic of microporous materials. The total BET surface area (359 m2 g−1), total pore volume (0.28 cm3 g−1) and micropore volume (0.14 cm3 g−1) are all close to those reported in the previous researche.35 The external specific surface area is 18 m2 g−1, which contributes little to the total specific surface area. In contrast, SCM-37 shows a type IV isotherm along with a moderate uptake of N2 in a medium relative pressure range, as well as a remarkable capillary condensation step above a relative pressure of 0.9 due to the inter-crystal voids arising from the stacking of nanosheet crystals. The micropore volume and micropore surface area of SCM-37 are comparable, although slightly lower, to those of C-FER. However, the external surface area of SCM-37 reaches 198 m2 g−1, which is over ten times larger than that of C-FER.
| Sample | SiO2/Al2O3a | SBETb (m2 g−1) | Sextc (m2 g−1) | Smicrod (m2 g−1) | Vtotale (cm3 g−1) | Vmicroc (cm3 g−1) |
|---|---|---|---|---|---|---|
| a Measured by ICP.b Calculated from BET method.c Calculated from t-plot method.d Smicro = SBET − Sext.e Calculated from the N2 volume adsorbed at P/P0 = 0.99. | ||||||
| C-FER | 23.9 | 359 | 18 | 341 | 0.28 | 0.14 |
| SCM-37 | 23.1 | 475 | 198 | 277 | 0.93 | 0.12 |
The NH3-TPD curves of both FER zeolite materials (Fig. S4†) display two obvious desorption peaks at ca. 200 °C and 425 °C, corresponding to the weak and strong acid sites of the zeolites, respectively. Although the two zeolites possessed similar SAR (Table 1), the total acid amount of SCM-37 was lower than that of C-FER sample (955 μmol g−1 vs. 1349 μmol g−1). Fig. S5† shows the Py-IR spectra of the FER zeolites. The calculated amounts of acid sites were listed in Table S1.† The concentrations of both B and L acid sites of C-FER are higher than those of SCM-37. The reason might lie in the slight lower relative crystallinity of SCM-37.44 Furthermore, 27Al MAS NMR spectra (Fig. S6†) prove that almost all Al species in both FER zeolites are isomorphically incorporated into the framework as deduced from the presence of the resonance at 53 ppm and the absence of octahedrally coordinated resonance around 0 ppm. After calcination, less than 5% of the Al species migrated to non-framework sites while the majority of the Al species retained within the framework, indicating excellent thermal stability of both SCM-37 and C-FER materials.
Fig. 6 displays the FTIR spectra of OH-stretching vibrations in the region between 3400 cm−1 and 3800 cm−1. Both zeolites exhibit the characteristic bands at 3745 cm−1 ascribed to the stretching vibration of terminal silanol groups and at 3602 cm−1 contributed by bridging hydroxyl groups (Si–OH–Al). In addition, there is a broad peak at 3640 cm−1 attributed to OH groups connected to non-framework aluminum species.45–47 The intense peak at around 3745 cm−1 for SCM-37 is significantly stronger than that of C-FER, indicating that SCM-37 is partially delaminated and an abundance of terminal silanol groups are exposed on the external surface of crystals.
The catalytic performance of SCM-37 and C-FER were comparatively evaluated by using the catalytic cracking of TiPB carried out at different temperatures. As shown in Fig. 7, the conversion of TiPB increased with the increasing of reaction temperature for both catalysts. The conversion of TiPB over C-FER zeolite was 27% at 250 °C and gradually rose to 50% and 94% as the reaction temperature raised to 300 °C and 350 °C. By contrast, the conversion of TiPB over SCM-37 was 74% at reaction temperature of 250 °C, which was pronouncedly higher than that over C-FER. The conversion kept climbing up to 95% at 300 °C and reached nearly 100% at 350 °C. It is widely accepted that the cracking of TiPB is a diffusion-controlled reaction and promoted accessibility of active sites is significant beneficial to this process. Moreover, TiPB molecular with a critical diameter of 0.95 nm is too bulky to diffuse into the micropore of FER zeolite.48 The reaction occurs at the weak Brønsted acid located on the external surface.49–51 Taking into account the similar SiO2/Al2O3 molar ratio in both FER zeolites and the less acid sites (especially Brønsted acid) of SCM-37 zeolite, the superior catalytic performance of SCM-37 should be related to its remarkably higher external surface area arising from the ultrathin nanosheets. The primary products in the cracking of TiPB are diisopropylbenzene (DiPB) and propylene, while the deep cracking of DiPB gives isopropylbenzene (iPB) and benzene (Bz). The selectivities to iPB and Bz over SCM-37 zeolite were always higher than those over C-FER under the same condition (Fig. 7, Table S2†). It suggests that the increased external surface area of the FER zeolite catalyst not only favors the cracking of TiPB, but also deep cracking of DiPB.
 | ||
| Fig. 7 Conversion of TiPB (asterisk line) and selectivity to Bz + iPB (bars) over C-FER (black) and SCM-37 (red) at different reaction temperatures. | ||
It is broadly believed that the high exposure of external surface of zeolite materials is beneficial to the conversion of organic molecules which are too bulky to be accommodated within the sub-nanopores of zeolites. We believe SCM-37 with ultrathin nanosheets opens up opportunity to make greater contributions to industrial catalysis processes, especially those involving heavy feeds.
Conclusions
In summary, we have developed an effective method for the direct synthesis of ultrathin FER zeolite nanosheets. By using OTMAC and 4-DMAP as dual templates, SCM-37 zeolite with thicknesses along the a-axis of 4∼7 nm was successfully prepared. The synthesis field of SCM-37 was narrow and a two-step crystallization approach offered an opportunity for the successful synthesis of pure FER zeolite with high crystallinity. This novel zeolite material is characterized by remarkably higher external surface area compared to conventional FER zeolites. Thanks to the enhanced accessibility of active sites distributed on the external surface, SCM-37 exhibited a superior performance in the catalytic cracking of TiPB. The new method may also offers a good avenue for the development of other zeolites with ultrathin nanosheets in the future.Author contributions
Yuekun Li performed most experiments, analyzed all the data, and wrote the original draft. Duozheng Ma carried out part of the experiments. Wenhua Fu: data curation, formal analysis, writing – review & editing. Chuang Liu: data curation. Yu Wang: data curation. Zhendong Wang: conceptualization, methodology, funding acquisition, writing – review & editing. Weimin Yang: conceptualization, project administration, funding acquisition, resources, writing – review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Key R&D Program of China (2017YFB0702800), and the National Natural Science Foundation of China (21972168, 21802168).References
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- S. I. Zones, Microporous Mesoporous Mater., 2011, 144, 1–8 CrossRef CAS.
- V. Valtchev and L. Tosheva, Chem. Rev., 2013, 113, 6734–6760 CrossRef CAS PubMed.
- M. Moliner, C. Martínez and A. Corma, Chem. Mater., 2014, 26, 246–258 CrossRef CAS.
- M. Moliner, C. Martínez and A. Corma, Chem. Mater., 2014, 26, 246–258 CrossRef CAS.
- J. Shi, Y. Wang, W. Yang, Y. Tang and Z. Xie, Chem. Soc. Rev., 2015, 44, 8877–8903 RSC.
- M. Dusselier and M. E. Davis, Chem. Rev., 2018, 118, 5265–5329 CrossRef CAS PubMed.
- A. Corma, J. Catal., 2003, 216, 298–312 CrossRef CAS.
- M. Moliner, C. Martínez and A. Corma, Angew. Chem., Int. Ed., 2015, 54, 3560–3579 CrossRef CAS PubMed.
- V. Valtchev, G. Majano, S. Mintova and J. P. Ramírez, Chem. Soc. Rev., 2013, 42, 263–290 RSC.
- T. F. Degnan, J. Catal., 2003, 216, 32–46 CrossRef CAS.
- B. Smit and T. L. M. Maesen, Nature, 2008, 451, 671–678 CrossRef CAS PubMed.
- L. Xu and J. Sun, Adv. Energy Mater., 2016, 6, 1600441 CrossRef.
- S. C. Larsen, J. Phys. Chem. C, 2007, 111, 18464–18474 CrossRef CAS.
- P. Chlubná, W. J. Roth, H. F. Greer, W. Zhou, O. Shvets, A. Zukal, J. Čejka and R. E. Morris, Chem. Mater., 2013, 25, 542–547 CrossRef.
- J. Přech, P. Pizarro, D. P. Serrano and J. Čejka, Chem. Soc. Rev., 2018, 47, 8263–8306 RSC.
- H. Xu, J. Zhu, L. Zhu, E. Zhou and C. Shen, Molecules, 2020, 25, 3722–3739 CrossRef CAS PubMed.
- A. Korde, B. Min, E. Kapaca, O. Knio, I. Nezam, Z. Wang, J. Leisen, X. Yin, X. Zhang, D. S. Sholl, X. Zou, T. Willhammar, C. W. Jones and S. Nair, Science, 2022, 375, 622–666 CrossRef PubMed.
- B. Li, K. M. Kwok and H. C. Zeng, ACS Appl. Mater. Interfaces, 2021, 13, 20524–20538 CrossRef CAS PubMed.
- K. Shen, X. Huang, J. Wang, Z. Chen, Y. Yu, Z. Tang and Y. Liu, Microporous Mesoporous Mater., 2020, 297, 110027 CrossRef CAS.
- H. S. Jung, N. T. Xuan and J. W. Bae, Microporous Mesoporous Mater., 2021, 310, 110669 CrossRef CAS.
- T. Xue, H. Liu and Y. Wang, RSC Adv., 2015, 5, 12131–12138 RSC.
- H. Fjellvag, K. P. Lillerud, P. Norby and K. Sorby, Zeolites, 1989, 9, 152–158 CrossRef.
- R. Byggningsbacka, N. Kumar and L. E. Lindfors, J. Catal., 1998, 178, 611–620 CrossRef CAS.
- Y. Wang, Y. Gao, W. Chu, D. Zhao, F. Chen, X. Zhu, X. Li, S. Liu, S. Xie and L. Xu, J. Mater. Chem. A, 2019, 7, 7573–7580 RSC.
- Y. P. Khitev, I. I. Ivanova, Y. G. Kolyagin and O. A. Ponomareva, Appl. Catal., A, 2012, 441–442, 124–135 CrossRef CAS.
- P. Wuamprakhon, C. Wattanakit, C. Warakulwit, T. Yutthalekha, W. Wannapakdee, S. Ittisanronnachai and J. Limtrakul, Microporous Mesoporous Mater., 2016, 219, 1–9 CrossRef CAS.
- M. Petersson, T. Holma, B. Andersson, E. Jobson and A. Palmqvist, J. Catal., 2005, 235, 114–127 CrossRef CAS.
- P. Cheung, A. Bhan, G. J. Sunley and E. Iglesia, Angew. Chem., Int. Ed., 2006, 118, 1647–1650 CrossRef.
- J. Liu, H. Xue, X. Huang, Y. Li and W. Shen, Catal. Lett., 2010, 139, 33–37 CrossRef CAS.
- X. Li, X. Liu, S. Liu, S. Xie, X. Zhu, F. Chen and L. Xu, RSC Adv., 2013, 3, 16549–16557 RSC.
- X. Feng, P. Zhang, Y. Fang, W. Charusiri, J. Yao, X. Gao, Q. Wei, P. Reubroycharoen, T. Vitidsant, Y. Yoneyama, G. Yang and N. Tsubaki, Catal. Today, 2020, 343, 206–214 CrossRef CAS.
- A. B. Pinar, P. A. Wright, L. Gómez-Hortigüela and J. Pérez-Pariente, Microporous Mesoporous Mater., 2010, 129, 164–172 CrossRef CAS.
- S. J. Xie, J. B. Peng, L. Y. Xu, Z. H. Wu and Q. X. Wang, Chin. J. Catal., 2003, 24, 531–534 CAS.
- W. Chu, F. Chen, C. Guo, X. Li, X. Zhu, Y. Gao, S. Xie, S. Liu, N. Jiang and L. Xu, Chin. J. Catal., 2017, 38, 1880–1887 CrossRef CAS.
- X. X. Chen, T. Todorova, A. Vimont, V. Ruaux, Z. X. Qin, J. P. Gilson and V. Valtchev, Microporous Mesoporous Mater., 2014, 200, 334–342 CrossRef CAS.
- Y. Lee, M. B. Park, P. S. Kim, A. Vicente, C. Fernandez, I.-S. Nam and S. B. Hong, ACS Catal., 2013, 3, 617–621 CrossRef CAS.
- V. J. Margarit, M. R. D. Rey, M. T. Navarro, C. Martínez and A. Corma, Angew. Chem., Int. Ed., 2018, 57, 3459–3464 CrossRef CAS PubMed.
- T. Xue, S. Li and H. Wu, Microporous Mesoporous Mater., 2020, 312, 110748 CrossRef.
- H. Xu, W. Chen, G. Zhang, P. Wei, Q. Wu, L. Zhu, X. Meng, X. Li, J. Fei, S. Han, Q. Zhu, A. Zheng, Y. Ma and F. Xiao, J. Mater. Chem. A, 2019, 7, 16671–16676 RSC.
- M. Peng, Z. Wang, J. Huang, M. Shen, J. Jiang, H. Xu, Y. Ma, B. Hu, X. Gong, H. Wu and P. Wu, Chem. Mater., 2021, 33, 6934–6941 CrossRef CAS.
- R. García, L. Gómez-Hortigüela, I. Díaz, E. Sastre and J. Pérez-Pariente, Chem. Mater., 2008, 20, 1099–1107 CrossRef.
- R. García, L. Gómez-Hortigüela, T. Blasco and J. Pérez-Pariente, Microporous Mesoporous Mater., 2010, 132, 375–383 CrossRef.
- K. Kim, R. Ryoo, H.-D. Jang and M. Choi, J. Catal., 2012, 288, 115–123 CrossRef CAS.
- A. Bolshakov, R. Poll, T. Bergen-Brenkman, S. Wiedemann, N. Kosinov and E. Hensen, Appl. Catal., B, 2020, 263, 118356 CrossRef CAS.
- P. Wu, J. Ruan, L. Wang, L. Wu, Y. Wang, Y. Liu, W. Fan, M. He, O. Terasaki and T. Tatsumi, J. Am. Chem. Soc., 2008, 130, 8178–8187 CrossRef CAS PubMed.
- A. Bonilla, D. Baudouin and J. Pérez-Ramírez, J. Catal., 2009, 265, 170–180 CrossRef CAS.
- S. Mi, T. Wei, J. Sun, P. Liu, X. Li, Q. Zheng, K. Gong, X. Liu, X. Gao, B. Wang, H. Zhao, H. Liu and B. Shen, J. Catal., 2017, 347, 116–126 CrossRef CAS.
- M. S. Aghakhani, A. A. Khodadadi, Sh. Najafi and Y. Mortazavi, J. Ind. Eng. Chem., 2014, 20, 3037–3045 CrossRef CAS.
- S. Zhao, D. Collins, L. Wang and J. Huang, Catal. Today, 2021, 368, 211–216 CrossRef CAS.
- Q. Wang, L. Zhang, Z. Yao, Y. Guo, Z. Gao, J. Zheng, W. Li, B. Fan, Y. Wang, S. Chen and R. Li, Mater. Chem. Phys., 2020, 243, 122610 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01334g |
| This journal is © The Royal Society of Chemistry 2022 |