Open Access Article
Open Access ArticleWater-soluble optical sensors: keys to detect aluminium in biological environment
Ajmal Roshan Unniram Parambil
 *abc,
Kavyashree P.†
a,
Akshay Silswal†a and
Apurba Lal Koner
*abc,
Kavyashree P.†
a,
Akshay Silswal†a and
Apurba Lal Koner *a
*a
aBionanotechnology Lab, Department of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhopal Bypass Road, Bhauri, 462066 Bhopal, Madhya Pradesh, India. E-mail: akoner@iiserb.ac.in
bDepartment of Chemistry, University of Basel, 4058 Basel, Switzerland. E-mail: ajmalrup@gmail.com
cInstitute of Chemistry and Bioanalytics, School of Life Sciences, University of Applied Sciences and Arts Northwestern Switzerland, 4132 Muttenz, Switzerland
First published on 10th May 2022
Abstract
Metal ion plays a critical role from enzyme catalysis to cellular health and functions. The concentration of metal ions in a living system is highly regulated. Among the biologically relevant metal ions, the role and toxicity of aluminium in specific biological functions have been getting significant attention in recent years. The interaction of aluminium and the living system is unavoidable due to its high earth crust abundance, and the long-term exposure to aluminium can be fatal for life. The adverse Al3+ toxicity effects in humans result in various diseases ranging from cancers to neurogenetic disorders. Several Al3+ ions sensors have been developed over the past decades using the optical responses of synthesized molecules. However, only limited numbers of water-soluble optical sensors have been reported so far. In this review, we have confined our discussion to water-soluble Al3+ ions detection using optical methods and their utility for live-cell imaging and real-life application.
1. Introduction
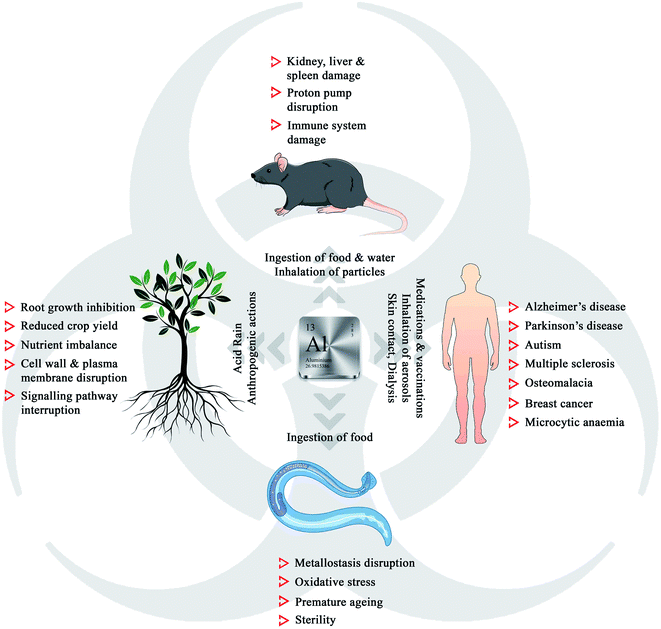
The history of humans and metals goes back to centuries, both physiologically and economically, as most of metals have played a significant role in the progress of civilization.1 Aluminium, being the most abundant metal and the third most element on Earth (8.1% of Earth's mass), is widely used in our daily life, starting from medicines to aircraft. The presence of non-essential aluminium in life forms leaves the question of toxicity in biological systems.2,3 Rigorous research in this field has revealed that aluminium exposure and intake is detrimental to plants, model organisms, and humans (Fig. 1).4,5Aluminium exists in different forms, such as minerals (aluminium oxides and aluminosilicates), as well as organic and inorganic precipitates, making it an essential component of mineral soil. Though naturally occurring silicates and oxides are harmless, soil acidification due to acid rain and anthropogenic actions can trigger the leaching of aluminium, leading to damage to the growth of plants.6 The phytotoxicity of Al3+ includes root growth inhibition, reduced crop yield, cell wall damage, plasma membrane disruption, signalling pathway interruption, and nutrient imbalance.7–16 Aluminium is easily accessible to both animal and human populations through the inhalation of particles and aerosols, ingestion of food and water, medications, vaccinations, skin contact, dialysis, and infusions.17 Recent research in neurobiology has revealed that aluminium could act as a neurotoxin, damaging the nerve tissues, which is associated with several neurogenetic disorders such as Alzheimer's disease, Parkinson's disease, autism, and multiple sclerosis.18 Chronic exposure to aluminium to the model organism Caenorhabditis elegans led to the disruption of metallostasis, oxidative stress, premature aging, and sterility.19 Kamalov et al. reported that an environmentally relevant concentration of aluminium could damage the thymocytes and lymphocytes and gradually the immune system in mice.20 The adverse effects of aluminium urge the detailed investigation on the detection, selective removal, or conversion of Al3+ to non-toxic species, which are highly necessary to control its concentration below the ecotoxic levels in the biosphere.
To date, a variety of techniques such as inductively coupled plasma emission spectroscopy (ICPES), atomic fluorescence spectroscopy (AFS), and X-ray photoelectron spectrometry (XPS) have been developed for Al3+ sensing. Despite their high sensitivity and selectivity, complicated instrumentation, high cost, and tricky operation have hindered their practical utility. In this scenario, the development of fluorescent probe-based chemosensors is an active research field that has garnered tremendous momentum over the past few years.21–26 The use of optical probes is considered as the most convenient, inexpensive, effective, and rapid method for the detection of Al3+. Several probes based on rhodamine,27,28 naphthol,29 fluorescein,30 and spirobenzopyran31 have been synthesized to detect Al3+ cations exploiting their color and fluorescence properties. Recently, Prof. Roy has comprehensively reviewed aluminium sensors derived from 2-hydroxy-1-naphthaldehyde, salicylaldehyde, rhodamine, coumarin, and different metal based-metal–organic frameworks (MOFs).32 However, most of the reported chemosensors require mixed aqueous–organic solvent systems due to their poor solubility in polar buffered/aqueous medium. Besides their poor solubility in water, many fluorescent sensors are being used to detect Al3+ in live cells, Drosophila melanogaster, Escherichia coli, zebrafish, rice seedlings, etc.33–39 However, the utilization of organic solvents for cellular imaging can destroy the cells, causing inaccurate sensing of intracellular Al3+. Therefore, the development of water-soluble fluorescent Al3+ sensors would effectively enhance the efficiency of detection to the next level.
In this review, we will attempt to provide a clear picture on different classes of water-soluble aluminium sensing probes based on colorimetric and fluorometric detection (Fig. 2 and Table 1). We limit our discussion to optical sensors that can act in aqueous solutions with organic solvents less than 2%. Rather than focusing on the synthetic ways, we have discussed the efficiency, spectroscopic properties, and practical applications, deeply examined the sensing mechanisms, compared the selectivity, and detection levels of several probes, and highlight the characteristic requirements of a bio-applicable aluminium detecting fluorescent probe.
| Sensor | Detection method | Solvent | Wavelength/nm | LOD | K/M−1 | Stoichiometrya | Applications | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| λex | λem | |||||||||
| a Receptor: analyte binding ratio.b Initial emission wavelength (blue-shifted emission wavelength). | ||||||||||
| Naphthyl based sensors | 1 | Fluorometric | Water (pH 4.0) | 360 | 387 | 0.65 μg L−1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Water samples | 40,45 |
| 2 | Fluorometric & colorimetric | HEPES buffer (pH 7.4) (water/DMSO 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
355 | 470 | 0.603 μM | 0.35 × 105 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live-cell imaging (HeLa cells) | 46 | |
| 3 | Fluorometric & colorimetric | Water | 450 | 490/495 | 1 μM | 3.148 × 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 47 | |
| 4 | Fluorometric | Bis–tris buffer (pH 7.0) | 450 | 490/515 | 0.13 μM | 2.5 × 103 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live-cell imaging (HeLa cells) | 48 | |
| 5 | Fluorometric & colorimetric | HEPES buffer (pH 7.0) | 320 | 380 | 0.1 μM | 1.19 × 108 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 49 | |
| 6 | Fluorometric | Water | 420 | 575 | 0.056 μM | 0.21 × 102 M−2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 50 | |
| Julolidine & quinoline based sensors | 7 | Fluorometric | Tris buffer (10 mM, pH 7.0) | 450 | 482 | 0.13 μM | 1 × 107 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live-cell imaging (HeLa cells) | 51 |
| 8 | Fluorometric | Bis–tris buffer (10 mM, pH 7.0) | 440 | 487 | 1.12 μM | 2.1 × 105 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live-cell imaging (human dermal fibroblast) | 52 | |
| 9 | Fluorometric & colorimetric | 99% Water/DMSO | 403 | 487 | 54 nM | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Aqueous solution and test paper | 53 | |
| Coumarin & chromone based sensors | 10 | Fluorometric | Tris–HCl (0.1 mM, pH 7.2) | 334 | 445 | 336 nM | 4.2 × 103 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 54 |
| 11 | Fluorometric | Tris–HCl (10 mM, pH 7.2) | 335 | 450 | 66.5 nM | 9.5 × 105 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 58 | |
| 12 | Fluorometric | H2O/DMSO (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
387 | ∼490 | 5.1 μM | 7.6 × 103 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 59 | |
| 13 | Fluorometric | H2O/DMSO (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
310 | ∼455 | 3 μM | 2.9 × 103 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 59 | |
| 2.1 × 109 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||||||
| Peptide-based sensors | 14 | Fluorometric & colorimetric | Hexamine buffer (10 mM, pH 5.5) | 380 | 536 (516)b | 230 nM | 1.84 × 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 66 |
| 15 | Fluorometric | Hexamine buffer (10 mM, pH 5.5) | 315 | 475 (455)b | 195 nM | 1.30 × 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 67 | |
| 16 | Fluorometric | Hexamine buffer (10 mM, pH 5.5) | 315 | 475 (452)b | 155 nM | 1.76 × 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 67 | |
| Polymer-based sensors | 17 | Fluorometric | Water | 345 | 435 | 9.67 nM | 1.30 × 105 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Real water samples | 70 |
| 18 | Fluorometric | Water | 399 | 469 | 6.15 nM | 6.39 × 104 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 68 | |
| 19 | Fluorometric | Water | 367 | 447 | 4.05 nM | 1.18 × 105 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Real water samples | 69 | |
| Nanomaterial based | 20 | Colorimetric | Water | 530/392 | — | 2.0 nM | — | — | Live cell (mouse myeloma cells) and real water samples | 71 |
| 21 | Fluorometric | H2O/DMF (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
∼290 | 390/500 | 677 nM | — | — | Soil samples | 72 | |
| 22 | Fluorometric & colorimetric | Water | 510 | 650 | 20 μM | — | — | Live cell (normal lung AT II cells) | 73 | |
| AIE based | 23 | Fluorometric | HEPES buffer (pH 7.4, 10 mM) | 330 | 455 | 14 nM | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell imaging (A549 cells) and real water samples | 74 |
| 24 | Fluorometric | HEPES buffer (pH 7, 10 mM) | 363 | 511 | 153 nM | 1.99 × 104 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Microfluidic setup for real water samples | 75 | |
2. Results and discussions
2.1 Naphthyl-based sensors
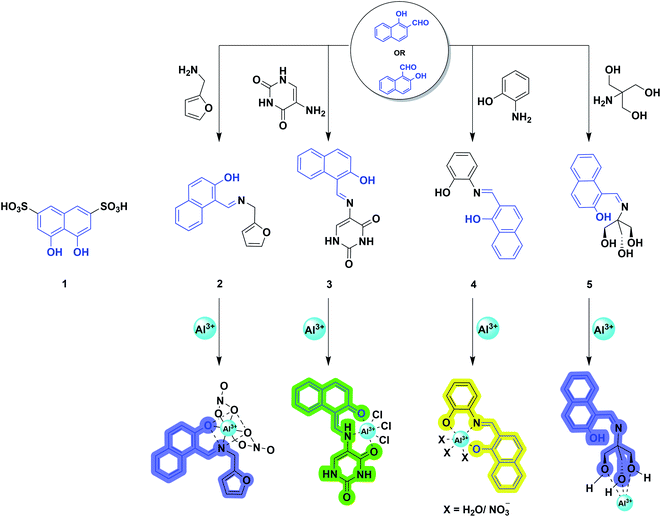
From the historical viewpoint, the ultra-sensitive determination of Al3+ through naphthalene derivative dates to 1994.40 Over the years, the endeavor of organic synthesis judiciously tailored naphthalene into numerous fluorogenic molecular architectures for metal sensing. 2-Hydroxy-1-naphthaldehyde or 1-hydroxy-2-naphthaldehyde (HN) is a vastly explored derivative of naphthalene, and has poor quantum yield, shorter fluorescence lifetime,41 and lower cost.42 In addition, successful condensation with appropriate amines afforded Schiff-bases, where the imine nitrogen and naphthol hydroxy behave as hard donors to efficiently coordinate the hard acid Al3+.43,44 Also, the rational choice of amine counterparts can alter the binding sites to furnish strong interaction with the aluminium ion. The distinguished selective chelation with Al3+ augmented the fluorescence intensity of weakly emissive imine chemosensors. Mechanistically, it involves arresting the photoinduced electron transfer (PET) process and isomerization around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, exhibiting a chelation enhanced fluorescence (CHEF) effect. Therefore, elegantly developed NH-derived Schiff-bases emerged as promising sensors for Al3+ recognition. Herein, we are dealing with some pertinent examples of this category.
N bond, exhibiting a chelation enhanced fluorescence (CHEF) effect. Therefore, elegantly developed NH-derived Schiff-bases emerged as promising sensors for Al3+ recognition. Herein, we are dealing with some pertinent examples of this category.
In 1994, Baksi and coworkers employed chromotropic acid (4,5-dihydroxynaphthalene-2,7-disulfonic acid; 1, Scheme 1) as an ultra-sensitive fluorometric reagent for the selective recognition of Al3+ in nano-trace levels.40 The presence of donor–acceptor moieties at 1,6-position made the probe fluorescent. However, discrepancies related to the absence of wide-range studies on the increase in emission intensity with Al3+ ion, binding stoichiometry, and use of acetic acid/acetate buffer (pH = 4.0) made Bardez and coworkers revisit the sensing properties of 1 in 2004.45 An increase in absorption at 360 nm (Fig. 3a) and a fluorescence enhancement at 387 nm (Fig. 3b) was observed upon Al3+ addition. A detailed study by the authors complemented the previous reports on 1 by forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with a limit of detection of 0.65 μg L−1 at pH = 4.0. They have also demonstrated the application of the probe in determining the Al3+ in different tap water samples. The obtained results were compared with the outcomes attained from the atomic absorption spectroscopy (AAS). However, the methodology is not selective to Al3+, when the interfering ion is Ga3+.
1 complex with a limit of detection of 0.65 μg L−1 at pH = 4.0. They have also demonstrated the application of the probe in determining the Al3+ in different tap water samples. The obtained results were compared with the outcomes attained from the atomic absorption spectroscopy (AAS). However, the methodology is not selective to Al3+, when the interfering ion is Ga3+.
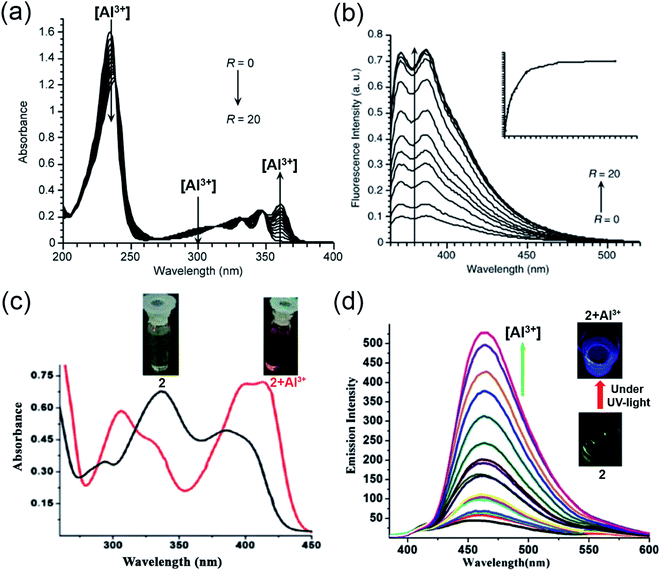 | ||
| Fig. 3 (a and b) represent the absorption and emission spectra for compound 1 with Al3+, R = [Al3+]/[1] in the range R = 0 to 20 at pH = 3.9 ± 0.2, λex = 360 nm. (c) Absorption spectra of 2 with Al3+; the inset displays the visible color change from yellow to reddish-yellow, (d) emission spectra of 2 (12.5 μM) with different concentrations of Al3+ in HEPES buffer (DMSO/water: 1/100) of pH = 7.4 at 25 °C; the inset exhibits the blue color of the complex under UV-light illumination (a and b) were reprinted with permission from ref. 45 copyright 2004. Springer Nature, and (c and d) were reprinted with permission from ref. 46 copyright 2012. Royal Society of Chemistry. | ||
Later, in 2012, Chattopadhyay and coworkers illustrated an efficient dual-mode sensing of Al3+ through colorimetric and fluorescence turn-on behavior of chemosensor 2 (Scheme 1) in HEPES buffer (DMSO/water: 1/100) of pH 7.4.46 The synthesized Schiff-base probe 2 comprises a naphthalene unit behaving as a signalling moiety, whereas the functional groups – hydroxyl and imine provide the chelating site for the selective binding of Al3+. The complexation of the probe with Al3+ rendered a decrease in the absorbance at 335 and 392 nm with concomitant enhanced absorption at 412 nm (Fig. 3c). The remarkable changes in the absorption spectra facilitated a visible color change of the solution from yellow to reddish-yellow, providing naked-eye detection (Fig. 3c; inset image). Also, the probe exhibited a remarkable increase in the emission intensity at 470 nm (Fig. 3d), with the lowest detection limit of 6.03 × 10−7 M and a strong binding constant of 0.35 × 105 M−1. The bidentate and mono-basic nature of the ligand in the rigid chelated product was supported by 1H NMR titration of the probe with Al3+ in DMSO-d6 and DFT calculations. Thus, mechanistically, a reduction in the internal charge transfer (ICT) and improved CHEF probably contributes to the selective detection of Al3+ via 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation with 2. The authors also demonstrated the potential capability of the probe for the intracellular imaging of Al3+ in HeLa cells.
1 complexation with 2. The authors also demonstrated the potential capability of the probe for the intracellular imaging of Al3+ in HeLa cells.
In the very next year, Singh and coworkers redesigned the probe 2 to construct a biologically interesting product 3 (Scheme 1)47 where the furan-derivative amine counterpart was replaced by 5-aminouracil to form the imine moiety. The authors claimed that this is the first report on the Schiff-base of 5-aminouracil with NH for the sensing of Al3+ through the phenomenon of CHEF. In aqueous media, the keto tautomeric form of the probe selectively chelates with Al3+, thus exhibiting an enhancement in the emission intensity at 495 nm (Fig. 4a). Nevertheless, the presence of other cationic species did not alter the fluorescence intensity of the probe. The formation of the stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was established by Job's plot and electrospray ionization (ESI) mass analysis. Ligand 3 behaves as a tridentate Schiff-base by coordinating Al3+ via the carbonyl of uracil, imine nitrogen, and keto group on the naphthalene ring, as is evident from the 1H NMR studies. Further, the binding constant and detection limit were determined to be (1.5 ± 0.009) × 105 M−1 and 1.0 μM, respectively.
1 complex was established by Job's plot and electrospray ionization (ESI) mass analysis. Ligand 3 behaves as a tridentate Schiff-base by coordinating Al3+ via the carbonyl of uracil, imine nitrogen, and keto group on the naphthalene ring, as is evident from the 1H NMR studies. Further, the binding constant and detection limit were determined to be (1.5 ± 0.009) × 105 M−1 and 1.0 μM, respectively.
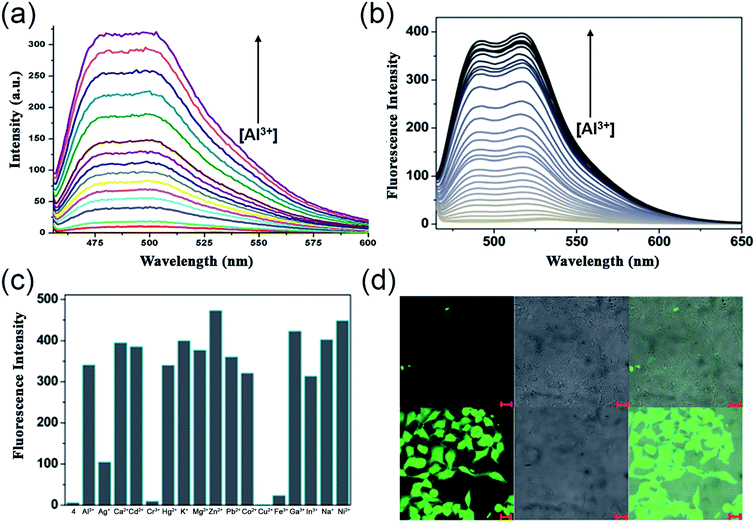 | ||
| Fig. 4 (a) Fluorescence spectra of 3 (20 μM) with 2 equiv. of Al3+, λex = 450 nm, λem = 490 nm, (b) variation in emission intensity of 4 (10 μM) upon Al3+ addition in 50 mM bis–tris buffer [Al3+] = 0–0.6 mM, λex = 450 nm, (c) emission response of 4 (10 μM) toward Al3+ ion (34 μM) addition in the absence and presence of other metal ions (34 μM), (d) fluorescent images of HeLa cells; the above three images were obtained by only incubating with 4, the bottom images were obtained by incubating with 1 mM Al(NO3)3 and 10 μM of 4. Left panel shows fluorescent images (λex = 480 nm, λem = 520 nm), middle panel DIC images and right panel merge images (a) was reprinted with permission from ref. 47 copyright 2013. Elsevier, and (b–d) were reprinted with permission from ref. 48 copyright 2014. Royal Society of Chemistry. | ||
Exploiting the similar Schiff-base approach, a novel phenol-naphthol hybrid chemosensor 4 (Scheme 1) was reported by Kim and coworkers in 2014.48 The developed chemosensor distinguishes Al3+ and CN− from other metal ions through fluorescence enhancement and quenching effect in bis–tris buffer and methanolic medium, respectively. A 100-fold increase in the emission intensity at 490 and 515 nm was observed with the gradual addition of 34 equiv. Al3+ (Fig. 4b). However, turn-on fluorescence feedback got significantly subdued in the presence of competing metal ions such as Ag+, Cu2+, Cr3+, and Fe3+ (Fig. 4c). Mechanistically, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chelation with 4 inhibits the rotation of the aromatic rings around the imine moiety. This brings both the naphthol and phenol rings to the same plane and furnishes a conjugated system that enhances the emission intensity upon Al3+ binding. The stability constant of the complex and the lowest detection limit for aluminium ions were found to be 2.5 × 103 M−1 and 0.13 μM, respectively. In addition, the authors also illustrated the successful tracking of intracellular Al3+ in HeLa cells using 4 (Fig. 4d).
1 chelation with 4 inhibits the rotation of the aromatic rings around the imine moiety. This brings both the naphthol and phenol rings to the same plane and furnishes a conjugated system that enhances the emission intensity upon Al3+ binding. The stability constant of the complex and the lowest detection limit for aluminium ions were found to be 2.5 × 103 M−1 and 0.13 μM, respectively. In addition, the authors also illustrated the successful tracking of intracellular Al3+ in HeLa cells using 4 (Fig. 4d).
Utilizing the excellent sensing properties of HN, in the same year, Liu and coworkers illustrated the design and synthesis of another naphthol aldehyde-tris derivate molecular probe 5 (Scheme 1) for Al3+ detection.49 The UV-Vis absorption spectra revealed a reduction in the O.D. value between 400–420 nm and 310 nm (Fig. 5a) with the concomitant color change of the solution from light yellow to colorless. Concentration-dependent fluorometric titration of aluminium ion (0–2 equiv.) with 5 (10 μM) in HEPES buffer (pH = 7.0) converted the non-fluorescent probe 5 into a blue fluorescent probe. Fig. 5b demonstrates that the developed chemosensor is highly selective to Al3+ over other metal ions with a detection limit of 0.1 μM. The large association constant 1.19 × 108 M−1 indicated the strong metal–ligand interaction. Further, 1H NMR experiments were carried out to investigate the coordinating site of Al3+ in the ligand. Interestingly, chemical shifts revealed the ability of three hydroxyl groups of tris to ligate with the aluminium ion forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. Unlike the previous reports, imine nitrogen and hydroxyl moiety on the naphthalene ring did not participate in forming the Al3+ chelated product. The switch-on mechanism of fluorescence emission was attributed to the blockage of the PET mechanism in the 5-Al3+ complex.
1 complex. Unlike the previous reports, imine nitrogen and hydroxyl moiety on the naphthalene ring did not participate in forming the Al3+ chelated product. The switch-on mechanism of fluorescence emission was attributed to the blockage of the PET mechanism in the 5-Al3+ complex.
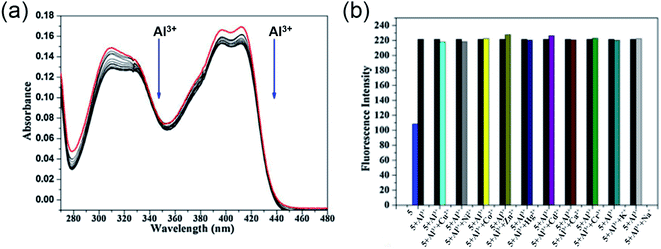 | ||
| Fig. 5 (a) Absorption spectra of 5 with 0–2 equiv. of Al3+ in HEPES buffer, (b) the selectivity assay for 5 with other metal ions (Na+, K+, Ca2+, Cu2+, Zn2+, Cr3+, Cd2+, Co2+, Hg2+, Ni2+, Al3+) in HEPES buffer (pH = 7.0), λex = 320 nm. Reprinted with permission from ref. 49 copyright 2014. Elsevier. | ||
Inspired from the hard acid–hard base concept in Al3+ sensing, in 2017, Yeap and coworkers designed a promising chemosensor 6 that can solely be used in pure water (Fig. 6a).50 The probe 6 was incorporated with the –COOH acid moiety, which acts as a hard base and provides good water solubility. Fig. 6b exhibits the UV-Vis spectral changes of 6 with Al3+. The gradual addition of Al3+ to the aqueous solution of the probe enhances the emission intensity at 575 nm through the CHEF mechanism (Fig. 6c). Synthesized probe 6 was highly selective to Al3+ even in the presence of other metal ions (Fig. 6d). The association constant obtained by the fluorescence titration for the 6-Al3+ complex is 0.21 × 102 M−2. Further, Job's plot analysis and 1H NMR titration experiments were performed to understand the biding stoichiometry and mode of interaction (Fig. 6e). It was noticed that the coordination of two oxygen atoms of the carboxylic group, two phenolic oxygen atoms, and two oxygen atoms of the oxymethylene group of the ligand with Al3+ resulted in the formation of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (6
1 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+) complex (Fig. 6a).
Al3+) complex (Fig. 6a).
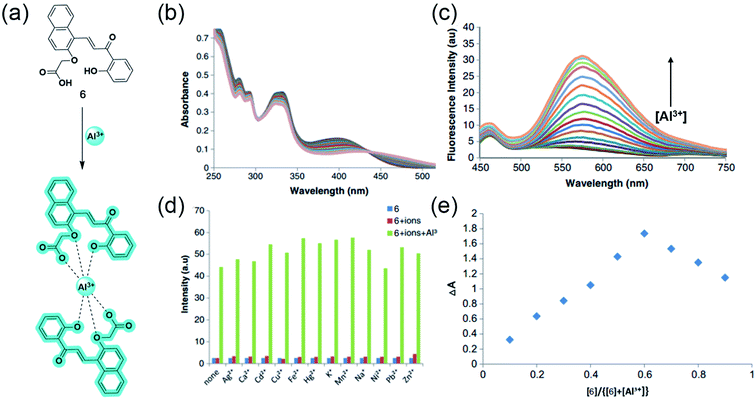 | ||
Fig. 6 (a) The structure of chemosensor 6 and the Al3+ sensing mechanism, (b) and (c) demonstrate the changes in absorption and emission spectra of 6 (100 μM) with Al3+, respectively, (d) Selectivity assay for 6 in the presence of other metal ions, (e) Job's plot showing the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of 6 1 complex of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+. Reprinted with permission from ref. 50 copyright 2017. Springer Nature. Al3+. Reprinted with permission from ref. 50 copyright 2017. Springer Nature. | ||
2.2 Julolidine and quinoline-based sensors
The water-soluble julolidine-based imine probes have grabbed immense attention from the scientific community owing to their selective determination of aluminium ions in the biological system. The imine group attached to julolidine is very fluorescent in the rigid form but C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization causes quenching due to its flexibility (Fig. 7a). This phenomenon has been exploited by researchers for the selective sensing of aluminium ions. The binding of the probe with the metal provides rigidity to the molecule, which in turn produces a fluorescence response.
N isomerization causes quenching due to its flexibility (Fig. 7a). This phenomenon has been exploited by researchers for the selective sensing of aluminium ions. The binding of the probe with the metal provides rigidity to the molecule, which in turn produces a fluorescence response.
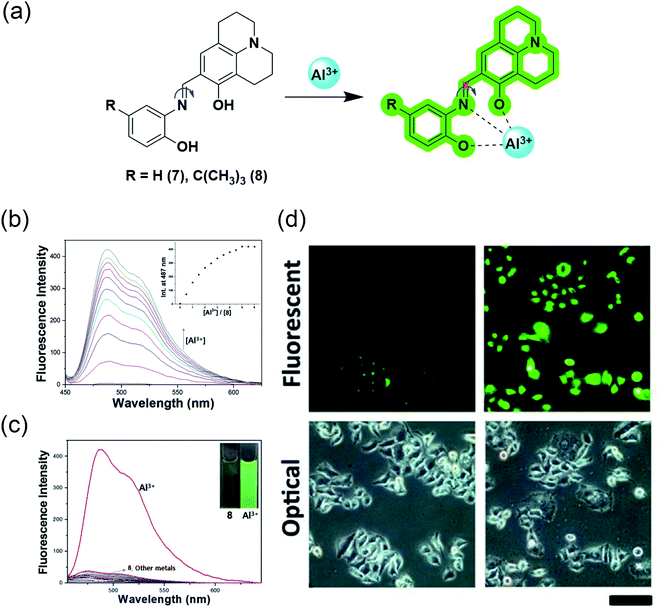 | ||
| Fig. 7 (a) Sensing mechanism of the julolidine-based probes; (b) fluorescence response of probe 8 to Al3+ in bis–tris buffer solution; inset: intensity at 487 nm vs. concentration of Al3+; (c) Fluorescence spectra representing the selectivity of probe 8 for Al3+ over other metal ions in bis–tris buffer solution; (d) observation of changes in the fluorescence intensity in HeLa cells incubated with 0 μM and 100 μM of Al3+ respectively for 5 h using probe 7 (d) was reprinted with permission from ref. 51 copyright 2013. Elsevier, and (b and c) were reprinted with permission from ref. 52 copyright 2016. Elsevier. | ||
J. Y. Noh et al. demonstrated the utilization of a Julolidine-based chemosensor for the selective detection of Al3+ in live-cell imaging.51 They synthesized the chemosensor ortho-phenyljulolidineimine (7) containing the π-conjugated Schiff base using a julolidine-based imine probe (Fig. 7a). The probe showed an excellent turn-on fluorescence response with a 100-fold increase in the fluorescence intensity upon the addition of Al3+ in an aqueous solution. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between 7 and Al3+ was responsible for fluorescence response as it inhibits C
1 complexation between 7 and Al3+ was responsible for fluorescence response as it inhibits C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and excited-state intramolecular proton transfer (ESIPT) processes. The spectroscopic investigation confirmed the high selectivity and sensitivity for Al3+ and possessed a detection limit of 0.13 μM. They also demonstrated the application in live-cell imaging, where HeLa cells exposed to 0.1 μM Al3+ were discernible from unexposed cells (Fig. 7d).
N isomerization and excited-state intramolecular proton transfer (ESIPT) processes. The spectroscopic investigation confirmed the high selectivity and sensitivity for Al3+ and possessed a detection limit of 0.13 μM. They also demonstrated the application in live-cell imaging, where HeLa cells exposed to 0.1 μM Al3+ were discernible from unexposed cells (Fig. 7d).
In 2016, Y. S. Kim et al. further modified the chemosensor and developed 8 by introducing an electron-donating tert-butyl group to enhance the solubility in aqueous solution.52 However, after introducing the tert-butyl group, the authors measured a slight increase in the LOD value, but it is still less than 7.41 μM, as recommended by World Health Organization (WHO) in drinking water. Receptor 8 alone has weak fluorescence proposed due to ESIPT involving the phenolic proton. This probe showed a 90-fold enhancement of the emission intensity at 487 nm with Al3+ treatment (Fig. 7b). This enhancement was proposed due to the chelation-enhanced fluorescence effect, in which the metal ion induces rigidity after chelation and tends to produce fluorescence. Also, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and ESIPT inhibited after chelation leads to fluorescence enhancement. However, the presence of interfering metal ions such as Cu2+, Fe2+, Fe3+, and Cr3+ causes the quenching of the intensity (Fig. 7c). Probe 8 is a suitable and biocompatible sensor for imaging living cells.
N isomerization and ESIPT inhibited after chelation leads to fluorescence enhancement. However, the presence of interfering metal ions such as Cu2+, Fe2+, Fe3+, and Cr3+ causes the quenching of the intensity (Fig. 7c). Probe 8 is a suitable and biocompatible sensor for imaging living cells.
As most of the fluorophores reported do not show high selectivity for only the Al3+ ion, there has always been some interfering metal ion affecting the fluorescence. Achieving high selectivity and specificity for Al3+ ion remains a concern for the scientific community. Quinoline-based fluorophores fascinate scientists to overcome this low selectivity of the Al3+ sensor over the past decade. The ESIPT process in 8-hydroxyquinoline inhibits the fluorescence. Upon binding with the metal ion, the possibility of ESIPT vanishes, which causes turn-on fluorescence. Therefore, researchers well-exploited this phenomenon for aluminium sensing.
In 2017, J. Wang et al. reported hydroxyquinoline-based highly selective Al3+ detection based on a fluorescent chemodosimeter (a system based on analyte-induced irreversible chemical reactions).53 Compound 9 synthesized by them shows high selectivity with a turn-on fluorescence response to Al3+ in aqueous solution of 99% water/DMSO (v/v) with a fluorescent enhancement of 582-fold (Fig. 8a and b). The ESIPT process due to intramolecular hydrogen bonding between the hydroxy and the nitrogen of quinoline moiety causes fluorescence quenching in 8-hydroxyquinoline derivatives. After binding with Al3+, the intramolecular hydrogen bond broke and gave rise to an enhancement in the fluorescence (Fig. 8e). On the other hand, two reasons for the high selectivity were proposed. First, the binding abilities of other metal ions are weak and cannot form the complex. Second, the paramagnetic nature of metal ions such as Cu2+, Ni2+, Co2+, and Fe3+ will cause fluorescence quenching after binding with the fluorophore. Fluorophore 9 had excellent sensitivity and could detect down to 54 nmol L−1 of Al3+ (Fig. 8c and f). Another advantage of this fluorophore was the rapid detection of Al3+ by the naked eye using a fluorescent test paper (Fig. 8d).
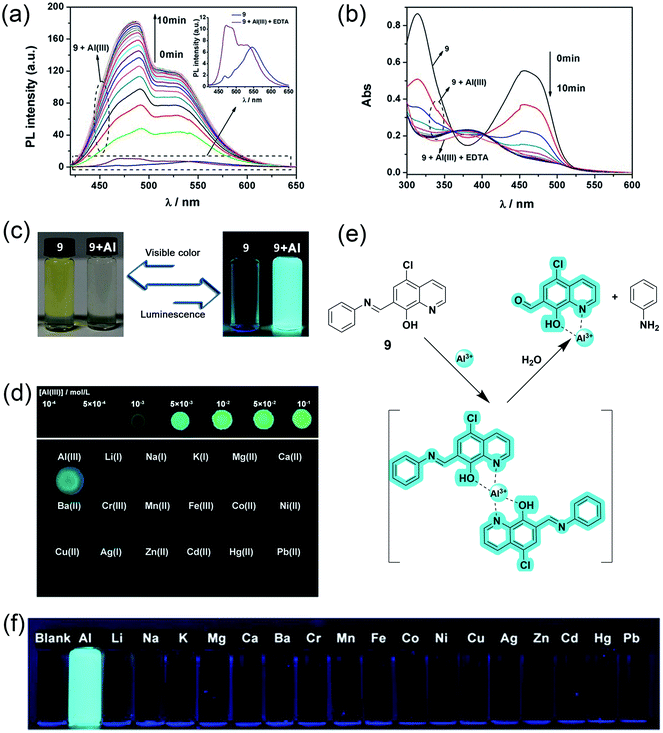 | ||
| Fig. 8 (a) Fluorescence spectra and (b) absorption spectra of compound 9 upon the addition Al3+. Conditions: 99% water/DMSO (v/v) buffered by 50 mmol L−1 NaAc-HAc at pH 5.0, the concentrations of 9 and EDTA were 50 μM, and the concentration of Al3+ was 25 μM, λex = 403 nm, (c) luminescence and visible color change before and after Al3+ addition, (d) images of test papers based on 9 for the detection of Al3+, (e) proposed mechanism for the interaction between 9 and Al3+ and, (f) photos of 9 with 1 equivalent of different metal ions. Reprinted with permission from ref. 53 copyright 2017. Wiley-VCH. | ||
2.3 Coumarin and chromone-based Al3+ sensors
Though fluorescent aluminium receptors are reported with various scaffolds, Schiff base sensors hold a strong position because of their flexibility and tunability. Their N and O comprising hard base donor sites provide a favorable coordination sphere for the hard acid Al3+, thereby changing the fluorescent properties of probes upon metal binding.54 Schiff base-based receptors mostly follow the photoinduced electron transfer (PET) mechanism, where the electron transfer from the fluorophore to another part of the probe gets inhibited by the coordination of the metal ion, resulting in an increase in the fluorescence signal by the CHEF effect. Among the Schiff bases, oxygen-containing heterocyclic coumarin and chromone-based fluorophores have received considerable attention for the naked-eye detection of trivalent aluminium cations over the past decades.Coumarin, an extensively studied fluorophore with high fluorescence quantum yield, visible light emission, and large Stokes' shift stands out as an excellent skeleton for sensing applications.55,56 Moreover, comparably less toxicity and ease of modification enable scientists to use coumarin-based aluminium receptors in biological systems. Similarly, chromones are naturally occurring organic molecules with broad pharmacological applications, ranging from anti-inflammatory to anticancer activities. Ideal photophysical and spectroscopic properties of chromones, including strong blue emission, presence of chromophoric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C moieties, and small singlet-triplet energy gap have attracted a lot of attention in recent times.57 Though many coumarin and chromone based aluminium sensors have been reported, their limited bioavailability prevents their use in the detection of aluminium in living systems. Recently, researchers are interested in developing probes by wisely grafting water-soluble moieties such as amino acids and polyalcohols to the fluorophores to exploit them in vivo.
C moieties, and small singlet-triplet energy gap have attracted a lot of attention in recent times.57 Though many coumarin and chromone based aluminium sensors have been reported, their limited bioavailability prevents their use in the detection of aluminium in living systems. Recently, researchers are interested in developing probes by wisely grafting water-soluble moieties such as amino acids and polyalcohols to the fluorophores to exploit them in vivo.
In 2015, Qi and coworkers developed a coumarin-based fluorescent ‘off–on’ aluminium sensor 10 with great water solubility attained by attaching an L-histidine moiety.54 Probe 10 having an emission maximum of 445 nm showed excellent selectivity and sensitivity toward aluminium cation even in the presence of other biologically relevant metal ions, except Cu2+ in physiological conditions (Fig. 9b and c). UV-Vis titration studies, indicating the deprotonation of hydroxyl functionality upon complexation, Job's plot, and ESI-MS studies, suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between the quadridentate ligand and Al3+. With the aid of 1H NMR, authors reasonably propose a sensing mechanism based on the PET phenomenon, where the electron transfer from the Schiff base nitrogen gets inhibited upon metal binding as the lone pairs are no longer available, leading to an increased fluorescence signal (Fig. 9a). Apart from enhancing the bioavailability, the acid group of L-histidine acts as a hard base donor site for metal coordination. Interestingly, 10 also has a greater sensitivity toward Cu2+ but no fluorescence emission since the complexation does not involve the Schiff base nitrogen and thereby the PET process and the CHEF effect.
1 stoichiometry between the quadridentate ligand and Al3+. With the aid of 1H NMR, authors reasonably propose a sensing mechanism based on the PET phenomenon, where the electron transfer from the Schiff base nitrogen gets inhibited upon metal binding as the lone pairs are no longer available, leading to an increased fluorescence signal (Fig. 9a). Apart from enhancing the bioavailability, the acid group of L-histidine acts as a hard base donor site for metal coordination. Interestingly, 10 also has a greater sensitivity toward Cu2+ but no fluorescence emission since the complexation does not involve the Schiff base nitrogen and thereby the PET process and the CHEF effect.
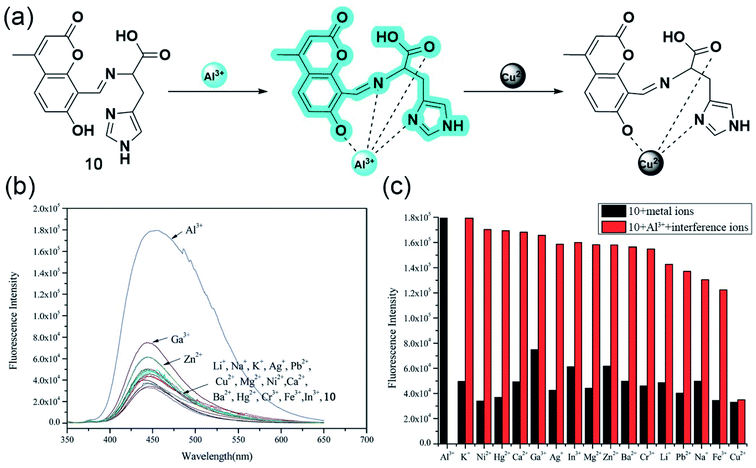 | ||
| Fig. 9 (a) The proposed mechanism for emission changes of 10 with Al3+, and in the presence of Cu2+. (b) Fluorescence spectra (10.0 mM, λex = 334 nm) with the addition of various metal ions (10.0 equiv.) in aqueous solution (Tris–HCl, 0.1 mM, pH 7.2). (c) Fluorescence intensity of 10 and its complexation with Al3+ in the presence of various metal ions. Black bar: 10 (10.0 mM) and 10 with 10.0 equiv. of other ions stated. Red bar: 10.0 mM of 10 and 10.0 equiv. of Al3+ with 10.0 equiv. of metal ions stated (λex = 334 nm). Reprinted with permission from ref. 54 copyright 2015. Royal Society of Chemistry. | ||
Following the line, the same group developed yet another aluminium sensor 11 based on the coumarin-L-serine hybrid moiety.58 The amine part of the Schiff base was sensibly chosen to enhance the water solubility and to provide coordination sites. As Fig. 10b and c suggests, the highly quantum efficient probe exhibited great selectivity toward Al3+ and Zn2+. However, the interference of other metal ions quenched the fluorescence significantly for the latter in aqueous media (Fig. 10d). The blue emitting ‘turn-on’ sensor binds with aluminium having a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry following the well-explored PET process. Electron transfer from the Schiff base nitrogen and oxygen of L-serine gets repressed upon the coordination of Al3+, relapsing the CHEF signal (Fig. 10a).
1 stoichiometry following the well-explored PET process. Electron transfer from the Schiff base nitrogen and oxygen of L-serine gets repressed upon the coordination of Al3+, relapsing the CHEF signal (Fig. 10a).
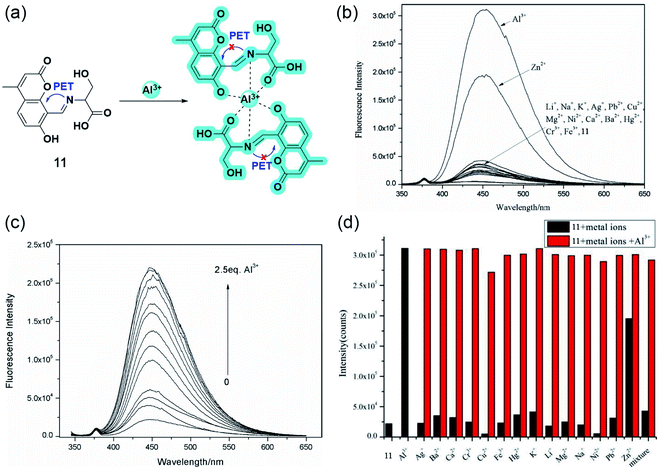 | ||
| Fig. 10 (a) The plausible binding mode of 11 with Al3+ and fluorescence mechanism of the PET processes. (b) Fluorescence spectra (10.0 μM, λex = 335 nm) with the addition of various metal ions (10.0 equiv.) in aqueous solution (Tris–HCl, 10 mM, pH 7.2). (c) Fluorescence spectra (10.0 μM, λex = 335 nm) upon the titration of Al3+ (0–2.5 equiv.) in aqueous solution (Tris–HCl, 10.0 mM, pH 7.2). (d) Fluorescence responses (10.0 μM, λex = 335 nm) to various metal ions (10.0 μM) (black column) (10 equiv.) and upon the subsequent addition of Al3+ (red column) in aqueous solution (Tris–HCl, 10 mM, pH 7.2). Reprinted with permission from ref. 58 copyright 2015. Japan Society for Analytical Chemistry. | ||
In 2016, Vladimír Král and coworkers synthesized two novel water-soluble blue-emitting chromone based-Schiff bases having high affinity toward Al3+ (Fig. 11).59 The grafting of the polyhydroxylated glucitol moiety improved the bioavailability to a greater extent. The computational studies suggested that 12 can bind to Al3+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, whereas 13 can bind either as 1
1 ratio, whereas 13 can bind either as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the latter being one of the strongest coordination having log(K) = 9.32.
1, the latter being one of the strongest coordination having log(K) = 9.32.
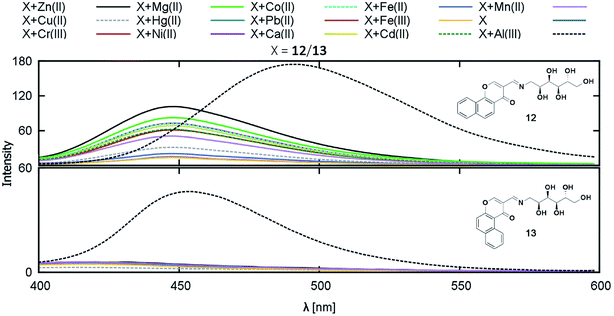 | ||
| Fig. 11 Fluorescence response of compounds 12 and 13 on addition of the selected cations. Reprinted with permission from ref. 59 copyright 2017. Taylor & Francis Academic Journals. | ||
Though several coumarin and chromone-based water-soluble aluminium sensors with desirable association constants and detection limits have been developed, their potential bioanalytical applications are seldom investigated.
2.4 Peptide and polymer-based Al3+ sensors
Besides single amino acids, the combination of conjugated fluorophores with short peptides opens up a new arena of ion-selective probes with immense scope in biological systems. The high water-solubility, biocompatibility, structural diversity, and excellent metal ion binding ability of peptides make them a potential candidate for sensing applications.60,61 Since hard Al3+ binds easily with negatively charged oxygen donor groups, amino acids such as Glu, Ser, Asp, or Thr are preferred in peptide-based aluminium receptors.62,63 On the other hand, the immobilization of conventional small molecular probes onto hydrophilic polymer matrix is also an emerging strategy to develop biocompatible sensors. Similar to peptides, polymers possess desirable characteristic features such as better water solubility, simplicity of use, signal amplification, and ease of fabrication into devices.64,65 Peptide and polymer-based sensors show exceptional bioavailability and ultrasensitive detection regardless of their bulky structures.Lee and coworkers reported the first-ever peptide-based water-soluble reversible aluminium sensor 14 in 2016 by grafting a tripeptide (Ser–Glu–Glu) with dansyl fluorophore bearing a sulfonamide group, which can act as a ligand for hard metal ions (Fig. 12a).66 Emission studies revealed that the ‘turn-on’ probe has excellent sensitivity toward Al3+ in acidic aqueous buffered solution with a detection limit in the nanomolar range. The sensing was associated with an eight-fold emission enhancement and a 20 nm blue shift (536 nm to 516 nm), resulting in a color change from yellow to bright green under UV light (Fig. 12b and c). However, the detection ability was pH dependent within the range of 4–7 and a maximum at pH = 5.5. Job's plot and ESI-MS confirm the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry along with the indication of carboxyl group deprotonation upon complexation. The downfield shifts of protons of α-carbons in 1H NMR suggests a quadra-coordinating binding mode where the hydroxyl and carboxyl group of tripeptide and sulfonamide group of dansyl moiety contribute in Al3+ chelation.
1 stoichiometry along with the indication of carboxyl group deprotonation upon complexation. The downfield shifts of protons of α-carbons in 1H NMR suggests a quadra-coordinating binding mode where the hydroxyl and carboxyl group of tripeptide and sulfonamide group of dansyl moiety contribute in Al3+ chelation.
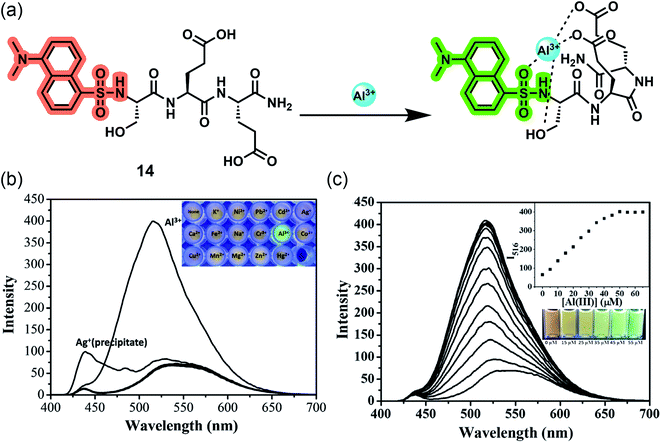 | ||
| Fig. 12 (a) The proposed binding mode of 14 with Al3+. (b) Fluorescence emission spectra of 14 (10 μM) in the presence of 5 equiv. of various metal ions in aqueous buffered solution (10 mM hexamine, pH 5.5) (λex = 380 nm, slit = 12/8 nm, 430 nm cut off). (c) Fluorescence emission spectra and visible emission color change (inset) of 14 (10 μM) in aqueous buffered solution (10 mM hexamine, pH 5.5) with increasing concentration of Al3+ (λex = 380 nm, slit = 12/8 nm, 430 nm cut off). Reprinted with permission from ref. 66 copyright 2016. Elsevier. | ||
Following the same line, Ramezanpour and coworkers improved the detection ability by replacing the dansyl probe with more water-soluble and cheaper 2-aminobenzoyl moiety, keeping the tripeptide the same to give 15.67 The authors designed another chemosensor 16 with the same fluorophore based on the heptapeptide sequence Ala–Glu–Pro–Glu–Ala–Glu–Pro, a naturally occurring Apidaecin 1 gene peptide with three glutamic acids, which can potentially act as ligating sites for Al3+. Both turn-on fluorescent sensors are highly specific and selective to Al3+ in water with emission maxima at 475 nm after complexation (Fig. 13c and d). Similar to 14, these sensors are also sensitive to pH, working efficiently between pH 4–7, and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion binding brings a prominent blue shift of 20–23 nm (Fig. 13e and f). A deeper investigation with the aid of spectroscopic techniques deciphered the ‘off–on’ mechanism based on ICT. 2-Aminobenzoyl moiety act as the electron donor, whereas the amide group of the first amino acid attached to the donor, acts as the electron-withdrawing moiety and binds to the guest cation, lowering the HOMO–LUMO energy gap and facilitating ICT, thereby giving fluorescence enhancement (Fig. 13a and b).
1 ion binding brings a prominent blue shift of 20–23 nm (Fig. 13e and f). A deeper investigation with the aid of spectroscopic techniques deciphered the ‘off–on’ mechanism based on ICT. 2-Aminobenzoyl moiety act as the electron donor, whereas the amide group of the first amino acid attached to the donor, acts as the electron-withdrawing moiety and binds to the guest cation, lowering the HOMO–LUMO energy gap and facilitating ICT, thereby giving fluorescence enhancement (Fig. 13a and b).
 | ||
| Fig. 13 Structures and proposed binding modes of 15 (a) and 16 (b). Fluorescence responses of 10 μM of 15 (c) and 16 (d) to metal ions in the presence of 5 equiv. of various metal ions by an excitation wavelength at 315 nm in 10 mM hexamine buffer at pH 5.5. Fluorescence emission spectra of 10 μM of 15 (e) and 16 (f) in 10 mM hexamine buffer with increasing concentrations of Al3+. Reprinted with permission from ref. 67 copyright 2021. Springer Nature. | ||
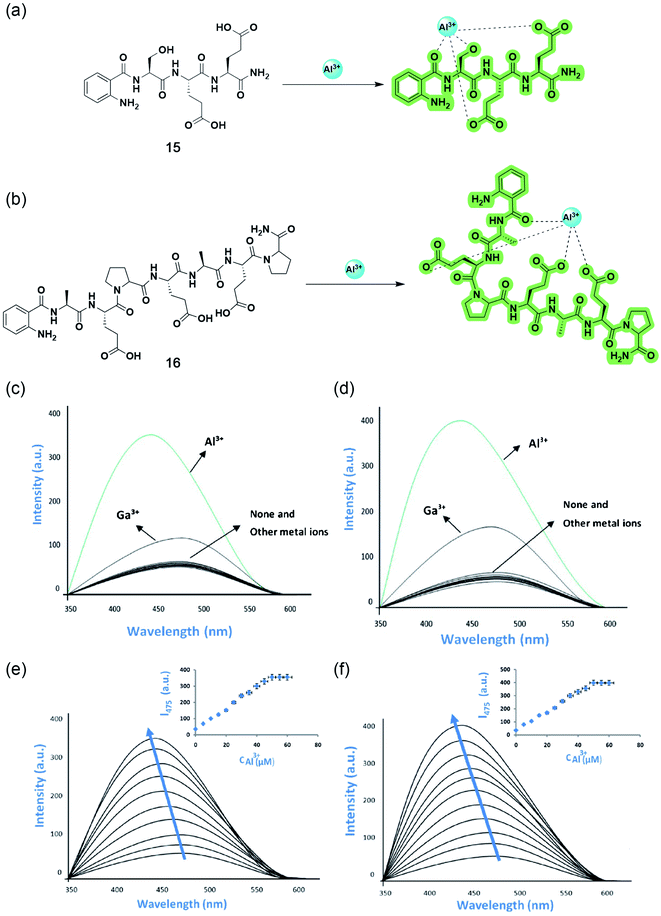
In 2019, Wang and coworkers reported three polymer-based highly selective and sensitive ‘turn-on’ Al3+ sensors by incorporating conventional Schiff base fluorophores onto the non-toxic, biocompatible, water-soluble polyethylene glycol (PEG) matrix.68–70 The one-step esterification of benzoylhydrazine Schiff base derivative with carboxylated PEG yielded 17 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and an excited state intramolecular proton transfer (ESIPT)-based sensing mechanism upon complexation. The chelation of Al3+ stops the C
1 stoichiometry and an excited state intramolecular proton transfer (ESIPT)-based sensing mechanism upon complexation. The chelation of Al3+ stops the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and restricts the ESIPT process, making the complex rigid and thereby enhancing fluorescent intensity to a greater extent (Fig. 14a). While the grafting of PEG with naphthol- and salicylidene-based Schiff bases resulted in 18 and 19, respectively, which binds with Al3+, having a 2
N isomerization and restricts the ESIPT process, making the complex rigid and thereby enhancing fluorescent intensity to a greater extent (Fig. 14a). While the grafting of PEG with naphthol- and salicylidene-based Schiff bases resulted in 18 and 19, respectively, which binds with Al3+, having a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Both sensors were non-fluorescent in their free state due to the photoinduced electron transfer (PET) from imine to naphthalene or benzene rings, but the rigidity arising from complexation prevents the process, resulting in a bright blue/cyan emission (Fig. 14b and c). All three sensors could detect Al3+ even in the presence of other highly concentrated analytes, with a quick response time below 20 seconds over a wide range of pH from 5 to 10 (Fig. 14d–i). The reversible recognition property makes them monomolecular circuits, exhibiting an INHIBIT type logic gate with Al3+ and EDTA (displaces Al3+, thereby halting the fluorescence) as the two chemical inputs and the emission intensity at the maxima as the output (Fig. 14k). Sensors 17 and 19 could also give promising results in the naked-eye detection of Al3+ in real water samples using test strips (Fig. 14j and l).
1 ratio. Both sensors were non-fluorescent in their free state due to the photoinduced electron transfer (PET) from imine to naphthalene or benzene rings, but the rigidity arising from complexation prevents the process, resulting in a bright blue/cyan emission (Fig. 14b and c). All three sensors could detect Al3+ even in the presence of other highly concentrated analytes, with a quick response time below 20 seconds over a wide range of pH from 5 to 10 (Fig. 14d–i). The reversible recognition property makes them monomolecular circuits, exhibiting an INHIBIT type logic gate with Al3+ and EDTA (displaces Al3+, thereby halting the fluorescence) as the two chemical inputs and the emission intensity at the maxima as the output (Fig. 14k). Sensors 17 and 19 could also give promising results in the naked-eye detection of Al3+ in real water samples using test strips (Fig. 14j and l).
 | ||
| Fig. 14 Structures and proposed binding modes of 17 (a), 18 (b), and 19 (c). Fluorescence spectra of 10 μM 17 (d), 18 (e), and 19 (f) in the presence of various metal ions (2 equiv.) in aqueous solution. Inset: The photograph of 17, 18, and 19 with various metal ions (2 equiv.) in aqueous solution under a 365 nm UV lamp. Competition analysis of 10 μM 17 (g), 18 (h), and 19 (i) to various metal ions in aqueous solutions. The black bars represent the intensity when mixed with 2 equiv. of other metal ions; the red bars represent the intensity when mixed with 2 equiv. of Al3+ and 2 equiv. of other metal ions. (j) Fluorescent detection of Al3+ using 17 by test paper under a 365 nm UV lamp after being immersed in different aqueous solutions (10−4 M) (from left to right, up: blank, Al3+, Co2+, Cr3+, Cu2+, and Fe3+; down: Hg2+, Mn2+, Na+, Ni2+, Pb2+, and Zn2+). (k) The monomolecular circuit for the INHIBIT logic gate. (l) Photographs of 19 test strips under a 365 nm UV lamp after being dipped into aqueous solutions of different metal ions (100 μM) (from left to right, up: Ba2+, Ce3+, Cd2+, Co2+, Al3+, Cr3+, Cu2+, and Fe3+; down: Hg2+, In3+, K+, Mn2+, Na+, Ni2+, Pb2+, and Zn2+). Reprinted with permission from ref. 68 and 70 copyright 2019. Elsevier. | ||
2.5 Nanomaterials-based Al3+ sensors
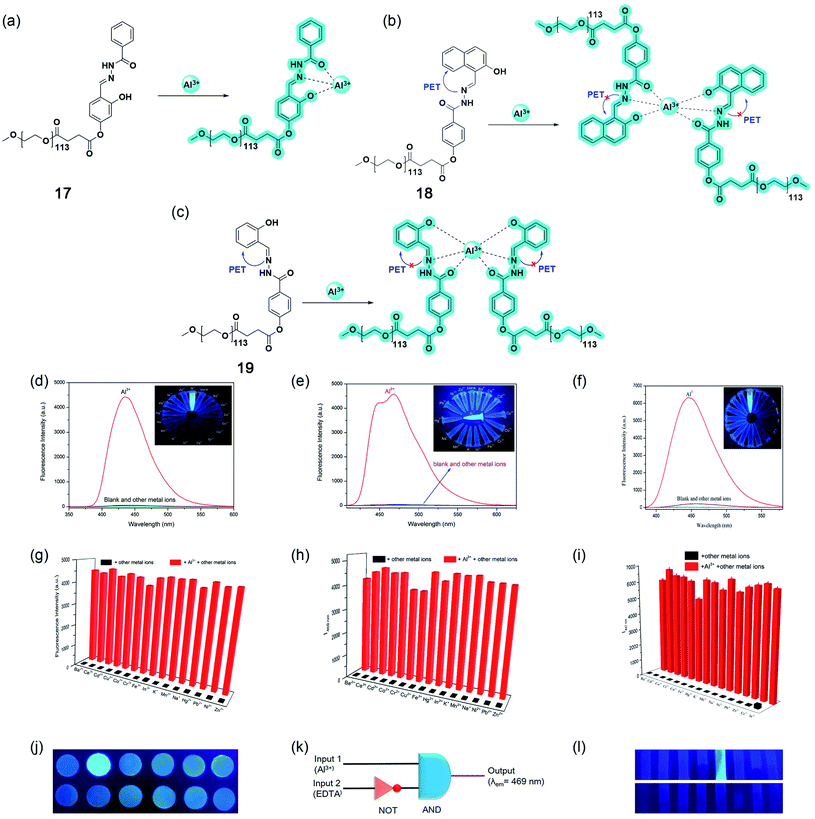
Nanomaterials-based sensing platforms are gaining immense attention. In this direction, plasmonic, semiconductor-based, and carbonaceous nanoparticles are mostly getting preference compared to other metal oxide nanoparticles. Generally, the plasmonic band of the novel metals displays a visible color due to the interaction of free electrons on metals and electromagnetic fields. Most importantly, the optical absorption property of plasmonic particles is sensitive to the local dielectric of the surrounding medium. Such environment-sensitive plasmonic properties of the most frequently utilized copper, silver, and gold nanoparticles have shown a great promise for sensing bioanalytes, metal ions, and environmentally important small molecules. On the other hand, semiconductor-based luminescent nanoparticles, popularly known as quantum dots, are also employed for sensing applications. In the latter case, the turn on–off or ratiometric responses are utilized for designing the sensing platform.In 2013, Wu and coworkers have developed a sensing method for Al3+ in water at pH 7.4 utilizing silver nanoparticles (Ag-NPs, 20).71 The synthesized Ag-NPs were suitably decorated with an Al3+ binding ligand, namely, 8-hydroxyquinoline-5-sulfonate. Upon the addition of Al3+ ions, these modified Ag-NPs show a distinct color change from yellow to deep orange as well as a strong enhancement in the fluorescence emission. The absorption and emission-based titrations showed high nanomolar sensitivity and good selectivity toward Al3+ ions over other metal ions. Further, the authors determined Al3+ ions in living mouse myeloma cells via fluorescence imaging.
The mechanism of colorimetric and fluorometric detection is associated with the aggregation of Ag-NPs (as shown in Fig. 15a–d) mediated by the 8-hydroxyquinoline-5-sulfonate ligand, a selective binder for Al3+. The authors have observed a ratiometric response in the optical absorption upon the addition of different concentrations of Al3+. Such changes in the optical absorption result in a visible color change of the solution. The plasmonic band at 392 nm shifted toward red and a new peak at 530 nm appeared due to the Al3+-induced aggregation of Ag-NPs. Upon the addition of Al3+, the emission intensity got enhanced by 3 times due to the inhibition of photoinduced electron transfer from the involvement of lone-pair of electrons from the quinolone nitrogen atom due to Al3+ binding.
 | ||
| Fig. 15 Silver-nanoparticle (Ag-NPs)-mediated sensing platform (20) for Al3+ ions in water. (a) Schematic representation of the sensing principle, (b) UV-Vis titration of Ag-NPs with increasing concentration of Al3+ ions; insets show linear and ratiometric response of optical absorption and color change of the solution before and after the addition of Al3+ ions, (c) shows the selectivity for Al3+ ions and the inset shows ratiometric response upon the addition of Al3+, Fe3+, and Cr3+ ions, (d) bar diagram representing the selectivity from the ratiometric optical response for different metal ions. Reprinted with permission from ref. 71 copyright 2013. Springer Nature. | ||
Later, in 2014, Kaur and colleagues developed 1,8-napthalimide-based organic nanoparticles (ONPs, 21) for the sensing of Al3+ in aqueous media, as shown in Fig. 16a.72 The molecule itself has an emission maximum at 390 nm in DMF. However, after the formation of ONPs, due to the J-type nanoaggregation, a new peak at 494 nm was observed along the existing peak at 394 nm (Fig. 16b). The morphology and size of ONPs were assessed using TEM and dynamic light scattering experiments, respectively. A representative TEM image is shown in Fig. 16c. The developed sensor is highly selective toward Al3+ and sensitive with a detection limit of 0.67 μM. A significant enhancement of both the emission band was observed upon the addition of only Al3+ and metal ions have a negligible effect on the emission property (as shown in Fig. 16d and e). The intensity-based kinetic measurement shows a very fast response time of the sensor and within 120 seconds, a saturation of the emission signal was obtained (Fig. 16f). The sensor system was further utilized to determine the Al3+ content of river water and acidic soil. The fluorescence enhancement of ONPs with the addition of Al3+ was due to the reduction of photoinduced electron transfer (PET) from the central nitrogen of the diethylenetriamine linker part to electron-deficient Al3+. The sensing mechanism was validated using DFT calculation.
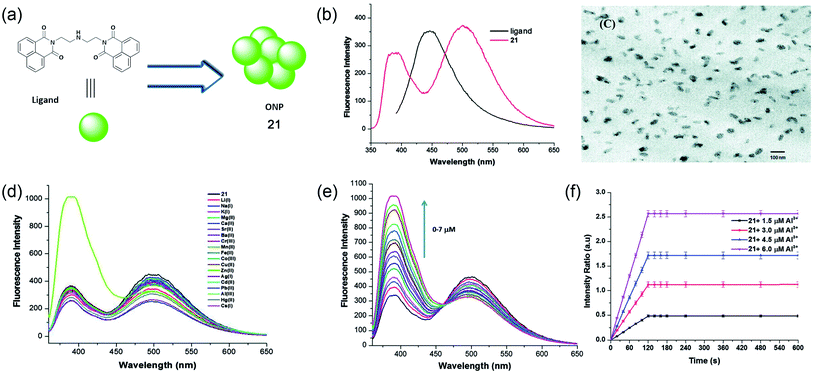 | ||
| Fig. 16 Water-soluble Al3+ sensing platform using fluorescent organic nanoparticle 21. (a) Structure of 1,8-napthalimide ligand used for the synthesis of ONPs, (b) fluorescence properties of 1,8-napthalimide ligand and self-assembled ONPs, (c) TEM image of ONPs, (d) fluorescence response of ONPs using different metals ions, (e) titration of ONPs with increasing concentration of Al3+ ions, and (f) kinetic response of fluorescence intensity ratio with different concentration of Al3+ ions. Reprinted with permission from ref. 72 copyright 2014. Royal Society of Chemistry. | ||
Recently, Wang and coworkers presented a fluorescence-based Al3+ sensing method using thiolated fluorescence gold nanocluster (Au NCs, 22).73 The synthetic method was an easy, green, and one-pot synthesis in an aqueous solution. The synthesized GHS-Au NCs were highly stable, ultrasmall, and monodisperse in size with a diameter of 1.4 nm. The large Stokes-shifted emission of GSH-Au NCs shows a fluorogenic response with ∼40-fold enhancement upon binding with Al3+ (as shown schematically in Fig. 17a). The designed probe has shown a great selectivity for Al3+ ions and the digital photo at the inset of Fig. 17b also demonstrates the selectivity of Al3+ ions over other ions in an aqueous solution. The fluorescence intensity of GSH-AU NCs also displays a linear increase in the fluorescence intensity with increasing concentration of Al3+ from 0–600 μM (Fig. 17c). Furthermore, the authors demonstrated the suitability of the probe for imaging a high concentration of Al3+ in live cells, as shown in Fig. 17d and e.
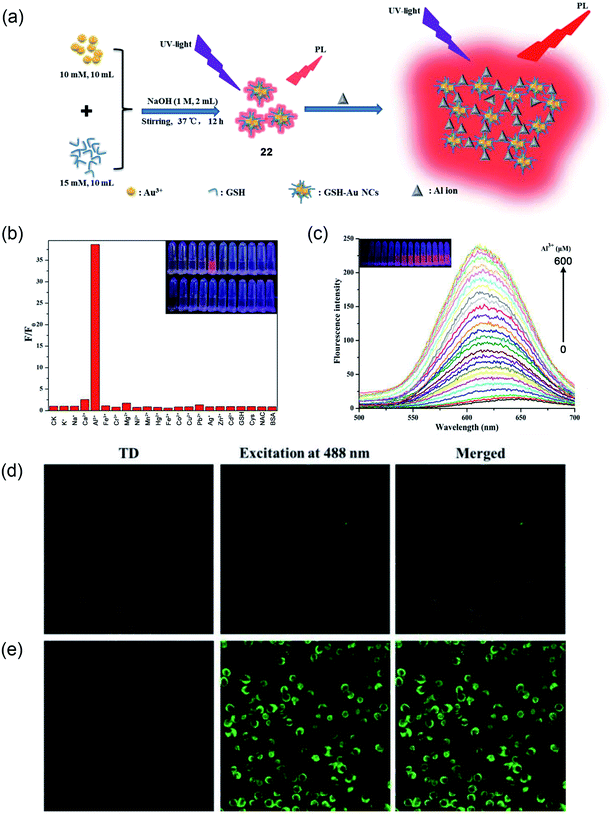 | ||
| Fig. 17 (a) Schematic illustration of GSH-Au NCs (22) synthesis and the fluorescence enhancement of GSH-Au NCs upon the addition of Al3+ ion due to the Al3+-triggered aggregation of the GSH-Au NCs. (b) Selectivity study of GSH-Au NCs toward Al3+ against other metal ions, (c) PL emission spectra of GSH-Au NCs solution after adding different concentrations of Al3+. Inset: digital photos of different concentrations of Al3+ processed GSH-Au NCs under UV light. (d and e) Confocal fluorescence images of AT-II cells co-incubated with the as-prepared Au NCs solution without (A) and with (B) 400 μM Al3+. Reprinted with permission from ref. 73 copyright 2019. Elsevier. | ||
2.6 Water-soluble aggregation induced emission (AIE)-based Al3+ sensors
AIE-based sensing platforms are becoming popular due to their synthetic variation, suitable tunability, and high sensitivity. Recently, in 2019, Li and coworkers have reported a Schiff's base derivative (23), which shows a marked AIE response and specific Al3+ ion binding property.74 The weakly-fluorescent Schiff's base compound in DMF turns into a strong green-emissive solution with an emission maximum of about 510 nm upon the addition of more than 40% water (schematically, the AIE process and Al3+ sensing are demonstrated in Fig. 18a and b). The weak fluorescence emission of probe 23 is due to the fast rotation of the furyl group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. Upon aggregation, these paths of non-radiative de-activation get restricted and cause a turn-on AIE response. Further, upon the addition of Al3+ ions in this green-emission solution, the fluorescence emission showed a significant blue shift from 520 nm to 455 nm (as shown in Fig. 18c). The blue-shifted and enhanced emission of the 1
N isomerization. Upon aggregation, these paths of non-radiative de-activation get restricted and cause a turn-on AIE response. Further, upon the addition of Al3+ ions in this green-emission solution, the fluorescence emission showed a significant blue shift from 520 nm to 455 nm (as shown in Fig. 18c). The blue-shifted and enhanced emission of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of probe 23 and Al3+ is due to restricted intramolecular movements. Other metal ions did not cause any change in the fluorescence emission. This result indicated an excellent specificity for Al3+ ions over other metal ions. The developed method has very fast response time (within few minutes) and a suitable pH window for Al3+ detection. As an application of this method, the authors performed live-cell laser confocal microscopy using A549 lung cancer cells. Cells treated with Al3+ showed a strong enhancement in the fluorescence emission coming from the cells compared to the same without Al3+, indicating good cellular permeability and confirming excellent fluorescence imaging capabilities.
1 complexation of probe 23 and Al3+ is due to restricted intramolecular movements. Other metal ions did not cause any change in the fluorescence emission. This result indicated an excellent specificity for Al3+ ions over other metal ions. The developed method has very fast response time (within few minutes) and a suitable pH window for Al3+ detection. As an application of this method, the authors performed live-cell laser confocal microscopy using A549 lung cancer cells. Cells treated with Al3+ showed a strong enhancement in the fluorescence emission coming from the cells compared to the same without Al3+, indicating good cellular permeability and confirming excellent fluorescence imaging capabilities.
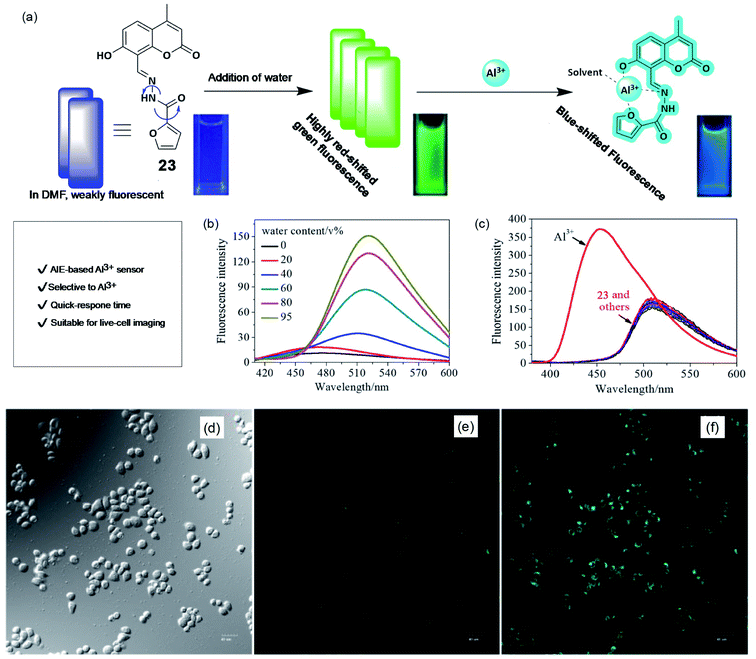 | ||
| Fig. 18 AIE-based sensing of Al3+. (a) Schematically showing the AIE process and Al3+ sensing. Cuvette picture show fluorescence color change upon AIE and Al3+ binding. (b) Effect of water addition in DMF solution of the probe causes emission enhancement due to AIE, (c) effect of metal ions on the emission property to show the selectivity toward Al3+ ions. (d–f) Fluorescence images of A549 cells (d) brightfield, in the presence (f), and absence of Al3+ (e). Reprinted with permission from ref. 74 copyright 2019. Wiley-VCH. | ||
Leray and coworkers in 2019 reported 4-propoxysulfonate salicylaldehyde azine (PSSA, 24)-based water-soluble fluorescent sensor based on the AIE enhancement (AIEE) effect.75 The structure of PSSA is provided in Fig. 19a. PSSA is highly soluble due to the presence of negatively charged sulfonate groups. However, with increasing concentration of ethanol content, there is a dramatic enhancement of the emission intensity, as shown in Fig. 19b. Upon the addition of Al3+ ions in PSSA solution, there is a significant ca. 100-fold enhancement in the emission intensity due to the complexation-assisted aggregation of PSSA, as schematically shown in Fig. 19c and d. PSSA has also shown good specificity over other metal ions (Fig. 19e) with rapid response time.
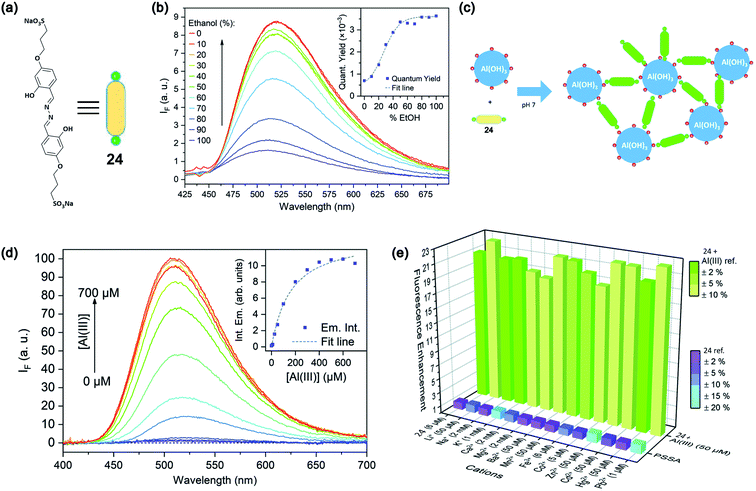 | ||
| Fig. 19 (a) Structure of PSSA, 24 (b) AIE effect upon the addition of ethanol content; the inset shows the increase in the emission quantum yield with an increasing percentage of ethanol. (c) Schematic representation of the sensing principle. (d) Effect of Al3+ addition on the emission intensity and the inset shows a plot of intensity variation with increasing concentration of Al3+ ions. (e) Bar diagram shows the selectivity of detection toward Al3+ over other metals. Reprinted with permission from ref. 75 copyright 2019. Royal Society of Chemistry. | ||
3. Conclusions
The springing number of optical probes holds promise to overcome the challenges for the effective, convenient, and rapid detection of Al3+ sensing. Water solubility is the desideratum for efficient fluorescent probes in biological applications. The preceding sections illustrated the different classes of water-soluble fluorescent sensors to detect Al3+ in real-time biological application. The strategies used based on PET, ESIPT, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, and CHEF effect are deciphered for the development of turn-on fluorescent probes. This review summarized the findings of several probe's photophysical properties, detection limit, selectivity, and detection mechanism.
N isomerization, and CHEF effect are deciphered for the development of turn-on fluorescent probes. This review summarized the findings of several probe's photophysical properties, detection limit, selectivity, and detection mechanism.
Water being the most common medium for the mobilization of aluminium to the biosphere is strictly regulated and quality controlled by health organizations. The Environmental Protection Agency (EPA) and WHO recommend a Secondary Maximum Contaminant Level (SMCL) of 0.05–0.2 μg mL−1 and 0.2 μg mL−1, respectively, for aluminium in drinking water.76 The optical sensors discussed in this review can detect Al3+ much lower than the SMCL. However, many of them are not utilized for practical applications, and their biocompatibility remains a question to employ in biological systems. Although various probes with certain modifications have been developed, there is still a requirement for highly photostable, selective, and sensitive probes with good aqueous solubility to avoid the usage of mixed aqueous-organic solvent systems. To date, the detection of Al3+ was achieved in real water samples and live-cell imaging, but clinical utility requires deep tissue and in vivo imaging. In this regard, there is significant scope for developing red-emitting fluorescent probes to provide deep tissue penetration depth with negligible autofluorescence. Moreover, interference from other metal ions restricted the exploitation of optical sensors for potential applications in complex biological systems. Future research toward the innovation of highly selective, sensitive, and water-soluble fluorescent probes opens the door for practical applications. The authors believe that this review furnishes in-depth mechanistic information to readers who are fascinated by the emerging field of Al3+ sensors for biological application.
Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
We wish to acknowledge the financial support from the host institute IISER Bhopal (IISERB). ARUP thanks DST for INSPIRE fellowship during his BS-MS studies at IISERB. KP and AS are thankful to IISER Bhopal and CSIR, respectively, for their doctoral fellowship. The authors also thank Mr Vishnu K for assisting in the design of illustrations.References
- E. Monosson, in Evolution in a Toxic World: How Life Responds to Chemical Threats, Island Press/Center for Resource Economics, Washington, DC, 2012, pp. 48–64, DOI:10.5822/978-1-61091-221-1_4.
- C. D. Foy, R. L. Chaney and M. C. White, Annu. Rev. Plant Physiol., 1978, 29, 511–566 CrossRef CAS.
- M. Schmitt, T. Watanabe and S. Jansen, AoB Plants, 2016, 8, plw065 CrossRef PubMed.
- R. A. Yokel, Neurotoxicology, 2000, 21, 813–828 CAS.
- C. Poschenrieder, B. Gunse, I. Corrales and J. Barcelo, Sci. Total Environ., 2008, 400, 356–368 CrossRef CAS PubMed.
- E. Bojorquez-Quintal, C. Escalante-Magana, I. Echevarria-Machado and M. Martinez-Estevez, Front. Plant Sci., 2017, 8, 1767 CrossRef PubMed.
- F. P. Veitch, J. Am. Chem. Soc., 2002, 26, 637–662 CrossRef.
- M. A. Qifu, Z. Rengel and J. Kuo, Ann. Bot., 2002, 89, 241–244 CrossRef CAS PubMed.
- L. V. Kochian, M. A. Pineros and O. A. Hoekenga, Plant Soil, 2005, 274, 175–195 CrossRef CAS.
- E. Delhaize and P. R. Ryan, Plant Physiol., 1995, 107, 315–321 CrossRef CAS PubMed.
- D. L. Jones, E. B. Blancaflor, L. V. Kochian and S. Gilroy, Plant Cell Environ, 2006, 29, 1309–1318 CrossRef CAS PubMed.
- X. B. Zhang, P. Liu, Y. Yang and G. D. Xu, Bot. Stud., 2007, 48, 435–444 CAS.
- X. J. Zhao, E. Sucoff and E. J. Stadelmann, Plant Physiol., 1987, 83, 159–162 CrossRef CAS PubMed.
- S. Ishikawa and T. Wagatsuma, Plant Cell Physiol., 1998, 39, 516–525 CrossRef CAS.
- J. W. Huang, D. L. Grunes and L. V. Kochian, Plant Soil, 1995, 171, 131–135 CrossRef CAS.
- E. Olivares, E. Pena, E. Marcano, J. Mostacero, G. Aguiar, M. Benitez and E. Rengifo, Environ. Exp. Bot., 2009, 65, 132–141 CrossRef CAS.
- I. O. Igbokwe, E. Igwenagu and N. A. Igbokwe, Interdiscip. Toxicol., 2019, 12, 45–70 CrossRef CAS PubMed.
- C. Exley and E. Clarkson, Sci. Rep., 2020, 10, 7770 CrossRef CAS PubMed.
- K. E. Page, K. N. White, C. R. McCrohan, D. W. Killilea and G. J. Lithgow, Metallomics, 2012, 4, 512–522 CrossRef CAS PubMed.
- J. Kamalov, D. O. Carpenter and I. Birman, J. Toxicol., 2011, 2011, 796719 Search PubMed.
- S. Biswas, V. Sharma, P. Kumar and A. L. Koner, Sens. Actuator B-Chem., 2018, 260, 460–464 CrossRef CAS.
- R. Roy, R. Bhowal, V. Sharma, D. Chopra and A. L. Koner, J. Mater. Chem. C, 2021, 9, 1778–1785 RSC.
- R. Roy, N. R. Sajeev, V. Sharma and A. L. Koner, ACS Appl. Mater. Interfaces, 2019, 11, 47207–47217 CrossRef CAS PubMed.
- P. Kumar, S. Biswas and A. L. Koner, New J. Chem., 2020, 44, 10771–10775 RSC.
- S. Mallick, F. Chandra and A. L. Koner, Analyst, 2016, 141, 827–831 RSC.
- A. Silswal, A. Kanojiya and A. L. Koner, Front. Chem., 2022, 10, 840297 CrossRef PubMed.
- D. Das, R. Alam, A. Katarkar and M. Ali, Photochem. Photobiol. Sci., 2019, 18, 242–252 CrossRef CAS PubMed.
- S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 10639–10643 RSC.
- B. Liu, P. F. Wang, J. Chai, X. Q. Hu, T. Gao, J. B. Chao, T. G. Chen and B. S. Yang, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2016, 168, 98–103 CrossRef CAS PubMed.
- A. Barba-Bon, A. M. Costero, S. Gil, M. Parra, J. Soto, R. Martinez-Manez and F. Sancenon, Chem. Commun., 2012, 48, 3000–3002 RSC.
- S. Goswami, K. Aich, S. Das, A. K. Das, D. Sarkar, S. Panja, T. K. Mondal and S. Mukhopadhyay, Chem. Commun., 2013, 49, 10739–10741 RSC.
- P. Roy, Dalton Trans., 2021, 50, 7156–7165 RSC.
- D. Ezhumalai, I. Mathivanan and A. Chinnadurai, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2018, 199, 209–219 CrossRef CAS PubMed.
- G. Balamurugan, S. Velmathi, N. Thirumalaivasan and S. P. Wu, Analyst, 2017, 142, 4721–4726 RSC.
- W. H. Ding, D. Wang, X. J. Zheng, W. J. Ding, J. Q. Zheng, W. H. Mu, W. Cao and L. P. Jin, Sens. Actuators, B, 2015, 209, 359–367 CrossRef CAS.
- A. K. Saini, V. Sharma, P. Mathur and M. M. Shaikh, Sci. Rep., 2016, 6, 34807 CrossRef CAS PubMed.
- H. Tian, X. Qiao, Z. L. Zhang, C. Z. Xie, Q. Z. Li and J. Y. Xu, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2019, 207, 31–38 CrossRef CAS PubMed.
- H. Xiao, K. Chen, N. Jiang, D. Cui, G. Yin, J. Wang and R. Wang, Analyst, 2014, 139, 1980–1986 RSC.
- L. Wang, J. Yang, H. Wang, C. Ran, Y. Su and L. Zhao, Sensors, 2019, 19, 2423 CrossRef CAS PubMed.
- K. Baksi and B. K. Pal, Talanta, 1994, 41, 81–87 CrossRef CAS PubMed.
- D. P. Roek, J. E. Chateauneuf and J. F. Brennecke, Ind. Eng. Chem. Res., 2000, 39, 3090–3096 CrossRef CAS.
- F. P. Schwarz and S. P. Wasik, Anal. Chem., 1976, 48, 524–528 CrossRef CAS PubMed.
- I. H. Hwang, Y. W. Choi, K. B. Kim, G. J. Park, J. J. Lee, L. Nguyen, I. Noh and C. Kim, New J. Chem., 2016, 40, 171–178 RSC.
- J. Jun Lee, G. Jin Park, Y. Sung Kim, S. Young Lee, H. Ji Lee, I. Noh and C. Kim, Biosens. Bioelectron., 2015, 69, 226–229 CrossRef CAS PubMed.
- E. Destandau, V. Alain and E. Bardez, Anal. Bioanal. Chem., 2004, 378, 402–410 CrossRef CAS PubMed.
- S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marek and P. Chattopadhyay, Analyst, 2012, 137, 3975–3981 RSC.
- V. P. Singh, K. Tiwari, M. Mishra, N. Srivastava and S. Saha, Sens. Actuators, B, 2013, 182, 546–554 CrossRef CAS.
- S. Kim, J. Y. Noh, S. J. Park, Y. J. Na, I. H. Hwang, J. Min, C. Kim and J. Kim, RSC Adv., 2014, 4, 18094–18099 RSC.
- Z. C. Liu, Y. X. Li, Y. J. Ding, Z. Y. Yang, B. D. Wang, Y. Li, T. R. Li, W. Luo, W. P. Zhu, J. P. Xie and C. J. Wang, Sens. Actuators, B, 2014, 197, 200–205 CrossRef CAS.
- G. Y. Yeap, Y. H. Chan and W. A. K. Mahmood, J. Fluoresc., 2017, 27, 2017–2022 CrossRef CAS PubMed.
- J. Y. Noh, S. Kim, I. H. Hwang, G. Y. Lee, J. Kang, S. H. Kim, J. Min, S. Park, C. Kim and J. Kim, Dyes Pigm., 2013, 99, 1016–1021 CrossRef CAS.
- Y. S. Kim, J. J. Lee, Y. W. Choi, G. R. You, L. Nguyen, I. Noh and C. Kim, Dyes Pigm., 2016, 129, 43–53 CrossRef CAS.
- J. Wang, Y. Li, K. Li, X. Meng and H. Hou, Chem. –Eur. J., 2017, 23, 5081–5089 CrossRef CAS PubMed.
- L. Q. Yan, Y. Ma, M. F. Cui and Z. J. Qi, Anal. Methods, 2015, 7, 6133–6138 RSC.
- Y. X. Song, Z. Chen and H. Q. Li, Curr. Org. Chem., 2012, 16, 2690–2707 CrossRef CAS.
- L. Wang, Y. F. Li, G. P. Li, Z. K. Xie and B. X. Ye, Anal. Methods, 2015, 7, 3000–3005 RSC.
- M. A. Rohman, P. Baruah, S. O. Yesylevskyy and S. Mitra, Chem. Phys., 2019, 517, 67–79 CrossRef CAS.
- L. Q. Yan, M. F. Cui, Y. Zhou, Y. Ma and Z. J. Qi, Anal. Sci., 2015, 31, 1055–1059 CrossRef CAS PubMed.
- M. Jakubek, Z. Kejik, V. Parchansky, R. Kaplanek, L. Vasina, P. Martasek and V. Kral, Supramol. Chem., 2017, 29, 1–7 CrossRef CAS.
- D. Divya and S. Thennarasu, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2020, 243, 118796 CrossRef CAS PubMed.
- P. Wang, L. Liu, P. Zhou, W. Wu, J. Wu, W. Liu and Y. Tang, Biosens. Bioelectron., 2015, 72, 80–86 CrossRef CAS PubMed.
- X. Yang, Q. Zhang, L. Li and R. Shen, J. Inorg. Biochem., 2007, 101, 1242–1250 CrossRef CAS PubMed.
- M. Kilyen, P. Forgo, A. Lakatos, G. Dombi, T. Kiss, N. Kotsakis and A. Salifoglou, J. Inorg. Biochem., 2003, 94, 207–213 CrossRef CAS PubMed.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- W. S. P. Carvalho, M. Wei, N. Ikpo, Y. Gao and M. J. Serpe, Anal. Chem., 2018, 90, 459–479 CrossRef CAS PubMed.
- B. In, G. W. Hwang and K. H. Lee, Bioorg. Med. Chem. Lett., 2016, 26, 4477–4482 CrossRef CAS PubMed.
- S. Ramezanpour, H. Barzinmehr, P. Shiri, C. Meier, S. A. Ayatollahi and M. Mehrazar, Anal. Bioanal. Chem., 2021, 413, 3881–3891 CrossRef CAS PubMed.
- L. Bai, G. Li, L. Li, M. Gao, H. Li, F. Tao, A. Deng, S. Wang and L. Wang, React. Funct. Polym., 2019, 139, 1–8 CrossRef CAS.
- L. Bai, Y. Xu, G. Li, S. Tian, L. Li, F. Tao, A. Deng, S. Wang and L. Wang, Polymers, 2019, 11, 573 CrossRef PubMed.
- L. Bai, F. Tao, L. Li, A. Deng, C. Yan, G. Li and L. Wang, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2019, 214, 436–444 CrossRef CAS PubMed.
- Y. Shang, D. Gao, F. Y. Wu and X. F. Wan, Microchim. Acta, 2013, 180, 1317–1324 CrossRef CAS.
- A. Saini, J. Singh, R. Kaur, N. Singh and N. Kaur, New J. Chem., 2014, 38, 4580–4586 RSC.
- P. Luo, Y. Zheng, Z. Qin, C. Li, H. Jiang and X. Wang, Talanta, 2019, 204, 548–554 CrossRef CAS PubMed.
- Y. Xie, X. Li, L. Yan and J. Li, Luminescence, 2020, 35, 156–162 CrossRef CAS PubMed.
- H. L. Nguyen, N. Kumar, J. F. Audibert, R. Ghasemi, J. P. Lefevre, M. H. Ha-Thi, C. Mongin and I. Leray, New J. Chem., 2019, 43, 15302–15310 RSC.
- I. Krupińska, Molecules, 2020, 25, 641 CrossRef PubMed.
Footnote |
| † Contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |