 Open Access Article
Open Access ArticleStrategies for the application of metal–organic frameworks in catalytic reactions
Fei Gao
 *a,
Runhan Yan
a,
Yao Shu
d,
Qingbin Cao
*c and
Li Zhang
*b
*a,
Runhan Yan
a,
Yao Shu
d,
Qingbin Cao
*c and
Li Zhang
*b
aSchool of Physics and Materials, Nanchang University, Nanchang 330031, China. E-mail: gaofei@ncu.edu.cn; gaofeiring@163.com
bInstitute of Applied Chemistry, Jiangxi Academy of Science, Nanchang 330096, China
cThe State Key Laboratory of Chemical Engineering, Tsinghua University, Beijing 100084, China
dInstitute of New Materials, Guangdong Academy of Science, Guangzhou 510651, China
First published on 31st March 2022
Abstract
Efficient catalysts play crucial roles in various organic reactions and polymerization. Metal–organic frameworks (MOFs) have the merits of ultrahigh porosity, large surface area, dispersed polymetallic sites and modifiable linkers, which make them promising candidates for catalyzation. This review primarily summarizes the recent research progress on diverse strategies for tailoring MOFs that are endowed with excellent catalytic behavior. These strategies include utilizing MOFs as nanosized reaction channels, metal nodes decorated as catalytic active sites and the modification of ligands or linkers. All these make them highly attractive to various applications, especially in catalyzing organic reactions or polymerizations and they have proven to be effective catalysts for a wide variety of reactions. MOFs are still an evolving field with tremendous prospects; therefore, through the research and development of more modification and regulation strategies, MOFs will realize their wider practical application in the future.
1. Introduction
Enzymes in biological systems are considered as efficient catalysts for biotransformation and chemical synthesis. Due to characteristics such as high efficiency, specificity and environmental friendliness, enzymes have attracted tremendous attention in the field of catalysis and applied in diverse catalytic systems.1 Enzymes can also be encapsulated in specific nanospaces to effectively regulate catalytic products and biopolymers.2 Inspired by this, artificial metalloenzymes have been greatly developed. These are hybrid catalysts that combine transition metal catalysts with carriers such as protein or DNA.3–7 It is known that homogeneous transition metal catalysts affect the selectivity of catalytic products via electronic effects and chelating ligand steric hindrance. However, these artificial metalloenzymes as catalysts also increase the features of the biomolecular scaffold, such as hydrogen bonding and hydrophobic interactions, and provide a confined space environment, which are essential for catalytic activity and selectivity.8–11Metal–organic frameworks (MOFs) are a class of porous materials constructed by metal ions (or clusters) with organic ligands.12–15 These materials not only possess active metal sites similar to artificial metalloenzymes, but also have regular pores to provide a confined space environment.16,17 Due to their uniform dispersion of polymetallic sites, easy modification of organic ligands, easy adjustment of pore size and diverse topological structures, they are widely used in gas storage,18,19 separation,20 energy storage,21 drug delivery and catalysis.22–25 In terms of their use for catalysis, MOFs can be applied as biomimetic artificial metalloenzyme catalysts by the following strategies: (1) MOFs can utilize micro- and nano-scale pores as the catalytic reaction sites and use the recognition effects of the host and guest to regulate the activity and selectivity of the reaction. (2) They can be catalyzed using heterogeneously dispersed polymetallic sites as heterogeneous catalysts. The reaction can realize the high-efficiency utilization of the catalyst and the multi-metal synergistic effect. (3) The modified organic ligand skeleton can be used as a carrier, and diverse homogeneous metal catalysts can be supported to realize multi-metal synergistic catalysis and catalysts that are recyclable in the catalytic reaction.
An increasing number of MOFs that act as heterogeneous catalysts have been used in organic catalytic systems and polymerization systems, which are of great significance from both fundamental and practical points of view. In this paper, we review MOFs as heterogeneous catalysts, and the different catalytic methods used. The use of metal nodes as active sites, loading the metal catalyst after post-synthetic modification (PSM) of the ligand, employing MOFs as host matrices to synthesize the catalyst and regulate the selectivity of the catalytic product are summarized.26 This review primarily focuses on the three different means to achieve the application of MOFs as catalysts: (1) MOFs as nanosized reaction channels; (2) metal nodes of MOFs as active sites in catalytic systems; (3) MOFs as carriers to support metal catalysts in catalytic reactions (Fig. 2). We hope this review will be valuable and provide meaningful instructions for employing MOFs as heterogeneous catalysts, and inspire researchers with diverse methods for exploring and utilizing MOFs as efficient and economical catalysts.
2. MOFs as hosts and reaction channels
Mimicking the strategy of enzyme encapsulation in proteins, MOFs as a new class of heterogeneous catalysts can also act as carriers, encapsulating some active species like metal nanoparticles, metal–organic catalysts, metal clusters, and non-metallic catalysts. Since the cages of MOFs have windows and channels of specific sizes, the reaction substrate can be sieved by the pores of the encapsulated MOF catalyst so that the reactants that have a larger size than the windows of the MOFs cannot enter into the inner tunnels.27 Furthermore, the tunnels of MOFs also provides a specific confined space environment that regulates the structure of the product.2.1. MOFs as hosts for metal nanoparticles
MOFs act as platforms for encapsulating metal nanoparticles to form a surfactant-free active site, which allows the particles to nucleate and grow to the nanometer-scale in the restricted pores, reducing particle aggregation. MOFs can be used as a carrier for fixing metal nanoparticles because of their easy regular structures, various shapes and easy modification of the inner surface of the pores.28 Some methods such as impregnation, initial wetting, solid-phase grinding, and core–shell continuous deposition reduction are used to encapsulate the nano metal particles in the MOF and use them in the catalytic reaction.29 The pores of MOFs provide a confined space environment and have a great influence on the activity of the reaction and the selectivity of the reaction products.Chen et al. for the first time exploited an effective pre-incorporation strategy to encapsulate palladium precursors through ligand design prior to MOF assembly, which could avoid the agglomeration of palladium particles and allow precursors to be easily anchored in the pores of MOFs.30 The as-prepared Pd-in-MOF composites showed excellent and enhanced catalytic activities in the aerobic oxidation of alcohols and reduction of nitrobenzene. In addition, they also utilized another facile in situ incorporation strategy to encapsulate ultrafine Pd, Ni and PdNi alloy inside the matrices of MOFs. This one step made the preparation more convenient and effectively introduced metal precursors into the pores of the MOF.31 Moreover, PdNi-in-UiO-67 showed more superior hydrogenation activity and reuse stability as compared to the PdNi-out-UiO-67 with metal particles loaded outside the pores, which demonstrated the confinement effect provided by the MOF pores.
Uniform core–shell Pt@MOF-177 were synthesized, in which Pt nano cores were surrounded by MOF-177.32 This kind of MOF as the shell had a pore size of 2.3–2.5 nm, and powder XRD was used to determine that the crystal structure had not been destroyed after the encapsulation of the nanoparticles. The Pt-encapsulated MOF was employed to catalyse the oxidation of alcohols, and the reaction was carried out at room temperature without added alkali. The catalytic yield of benzyl alcohol could be up to 50%, and the reaction conversion frequency was 968 h−1. After the first run, the Pt@MOF-177 catalyst was deactivated because the MOF skeleton was partially collapsed under the reaction conditions; thus, using more stable MOFs as hosts for nanoparticles is crucial.
Li et al. injected Pd nanoparticles into Cr-MIL-101 for catalysing the Suzuki–Miyaura cross-coupling reaction with a 1% Pd loading.33 It could be seen from the TEM characterization that the nanoparticles of Pd were uniformly distributed in Cr-MIL-101. The average diameter was 1.9 nm, while the pore size of the MOF was 2.9 nm to 3.4 nm. When the reactants were 4-chloroanisole and phenylboronic acid, and NaOMe was used as a base, a yield of 96% of 4-methoxybiphenyl was obtained in aqueous conditions at 80 °C. As a comparison, Pd2+/Cr-MIL-101, Pd/C and Pd/ZIF-8 were used as catalysts under the same conditions. The yields were 3%, 35% and 16%, respectively, which were lower than the MOF catalyst-encapsulated Pd nanoparticles.
Pd nanoparticles could also be supported on amino-functionalized MOFs like MIL-53(Al)–NH2, which were synthesized by using anionic exchange and reduction with NaBH4.34 MIL-53(Al)–NH2 was chosen as the carrier because of its high thermal and chemical stability and was used in the Suzuki–Miyaura cross-coupling reaction. With aryl bromides and phenylboronic acid as reactants, the yield could reach 99% with TON of 198. Under the same conditions, commercial Pd/C was also used in this catalytic system, which had a 44% yield of product and TON was 88.
Li et al. utilized urea deposition–precipitation to prepare Au/MIL-53(Cr) and Au/MIL-101(Cr) (Fig. 1). These catalysts were employed to catalyse the oxidation of cyclohexane to cyclohexanone and cyclohexanol.35 In this catalytic system, Au/MIL-53(Cr) and Au/MIL-101(Cr) as catalysts had higher conversion than Au/ZSM-5 (ZSM: Zeolite Socony Mobil). Moreover, Au/MIL-101(Cr) as the catalyst had the highest KA-oil selectivity, which was 87.7%. This was due to the uniformly dispersed Au particles of less than 2 nm with the confinement effect provided by the pores of MIL-101(Cr).
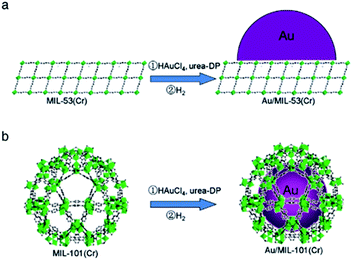 | ||
| Fig. 1 Preparations of (a) Au/MIL-53(Cr) and (b) Au/MIL-101(Cr). Adapted with permission from ref. 35. Copyright 2012, Elsevier. | ||
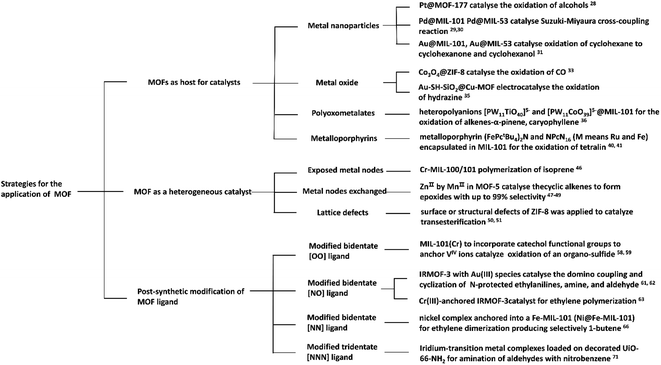 | ||
| Fig. 2 Schematic illustration of the strategies for the application of metal–organic frameworks in catalytic reactions. | ||
Another catalytic rhodium complex could also be encapsulated in the MOF tunnel. They used MIL-101 as the platform to support rhodium and catalysed the hydroformylation of linear alkenes with different chain lengths.36 The reaction substrates n-hex-1-ene, n-oct-1-ene or n-dec-1-ene were chosen; the conversions could reach 35–55% after 1 h and achieve up to 90% of conversion after 3 h of all alkenes employed as reactants. The high activity was attributed to well dispersed single site Rh species in the pores of MIL-101.
2.2. MOFs as hosts for metal oxide
Metal oxides were accommodated in the channels of MOF by Li et al. and used as catalysts in CO oxidation catalytic systems.37 The Co3O4 nanoparticles were synthesized from cobalt nitrate and dispersed uniformly in the pores of ZIF-8 as this kind of MOF has relatively high stability and large pores (11.6 Å). The metal oxide Co3O4 exhibited high catalytic activity toward the oxidation of CO and had a high reaction rate which was 58.2 mmol g−1 h−1 at 70 °C.Kim et al. developed an efficient adsorption catalysis system using a manganese dioxide/metal–organic framework (MnO2@UiO-66–NH2) for the removal of formaldehyde from the air. The catalytic activity declined with increased MnO2 loading as the specific surface area was covered proportionately by MnO2 content. The manganese dioxide and MOF exhibited excellent synergistic catalytic effects.38
Bagheri et al. utilized Au–SH–SiO2 nanoparticles immobilized on the Cu-MOF to generate Au–SH–SiO2@Cu-MOF as the electrocatalyst,39 which exhibited the electrocatalytic activity of the oxidation of hydrazine and showed a wide linear range (0.04–500 μM) and high sensitivity (0.1 μA μM−1), which also had remarkable stability and repeatability.
Parida et al. reviewed metal oxide-integrated MOFs (MO@MOF) and utilized them in photocatalytic reactions with superior activity.40 MO@MOFs can be prepared by various methods such as hydrothermal, microwave, sonochemical and mechanochemical techniques. The composite materials exhibited superior catalytic activity as compared to their pristine materials towards a wide range of photocatalytic applications such as Rhodamine B (RhB) degradation, photocatalytic water splitting (H2), photocatalytic CO2 methanation, methylene blue (MB) degradation, tetracycline (TC) degradation, benzyl alcohol (BA) oxidation, amoxicillin (AMX) degradation, malathion (MA) degradation and photo-oxidation of cyclohexane.
2.3. MOFs as hosts for polyoxometalates
Polyoxometalates (POMs) are constructed of metal–oxygen octahedra and exhibit Brønsted acid characteristics. The composites formed by encapsulating POMs in the pores of materials like zeolites can be used for catalysis and the steric environment can influence the reaction. POMs have features like thermodynamic stability and redox tunability and have, therefore, attracted a lot of research.Titanium and cobalt-based heteropolyanions [PW11TiO40]5− and [PW11CoO39]5− were successfully encapsulated in the pores of MIL-101.41 The MIL-supported POMs catalysts were used in the oxidation of alkenes; α-pinene, caryophyllene and cyclohexene. This catalytic system employed oxygen and hydrogen peroxide as oxidants, and it could get fairly good catalytic activity and selectivity. Furthermore, these heterogeneous catalysts could also be recycled without losing activity and selectivity under relatively mild reaction conditions.
Gascon et al. employed phosphotungstic acid (PTA) in the synthesis of MIL-101 and directly encapsulated polyoxometalates (POMs) inside the MOF skeleton, which were distributed uniformly and the size of the POMs were larger than the pentagonal windows of MIL-101, thus no leaching of POMs was observed.42 They utilized these composite catalysts in the Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate, which had high TOF values up to 600 h−1. The catalysts could be recycled up to eight times with no obvious loss of activity.
MOFs could encapsulate cobalt polyoxometalates (POMs) with Keggin-type [Co(BBPTZ)3][HPMo12O40]·24H2O (BBPTZ = 4,4′-bis(1,2,4-triazol-1-ylme-thyl)biphenyl) which acted as a catalyst in the oxidative desulfurization reaction.43 The system exhibited effective catalytic activity and size-selective characteristics, which were attributed to the collective effect of POM moieties and the confined space provided by MOF. In addition, the catalyst can be reused by centrifugal separation.
Hatton et al. synthesized MIL-101-NH2, MIL-53-NH2 and their composites with phosphotungstic acid (PTA) to catalyze the aldol condensation of acetaldehyde in a water-acetonitrile mixture (Fig. 3).44 The MOF/PTA catalytic system for aldol condensation met the second-order rate constant, which was 5 × 10−4 to 5 × 10−3 M−1 s−1. This value extremely exceeded the homogeneous catalysis by amino acids or sulfuric acid, which indicated that the MOF/PTA was an efficient heterogeneous catalyst for aldehyde condensation.
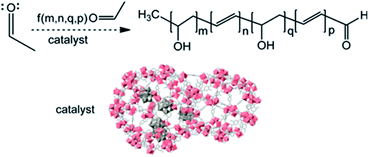 | ||
| Fig. 3 An illustration of a heteropolyacid-functionalized MOF as a reactive aldehyde sorbent and catalyst. Adapted with permission from ref. 44. Copyright 2013, American Chemical Society. | ||
2.4. MOFs as hosts for metalloporphyrins
Metalloporphyrins have been widely applied as optoelectronic materials and in chemoselective catalysis. They have the excellent characteristics of easy dimerization and self-degradation as utilized in homogeneous catalytic systems, thus immobilization or encapsulation of metalloporphyrins in porous matrices like zeolites and mesoporous silicates to avoid aggregation or leaching is an effective method. MOFs can also serve as porous carriers because of their excellent features like high porosity, stable structures and relatively high specific surface areas.45The metalloporphyrin could be successfully encapsulated in MIL-101(Cr) and used in the aerobic oxidation of tetralin with high activity.46 This high activity was caused by the dispersion of the molecular complexes as isolated monomers in the nanopore cavities from the confinement effects provided by the host porous structure.
Cai et al. used a versatile and effective platform PCN-134(Zn) (PCN = porous coordination network) to support metalloporphyrins to boost their photocatalytic performance for singlet oxygen generation and living-radical polymerization.47 They produced well-defined polymers with low polydispersities and tunable functionalities. Compared to other bulk MOF crystals, this catalytic system exhibited fast polymerization kinetics due to larger surface areas and more accessible catalytic active sites.
Abbasi et al. incorporated a cobalt-based metalloporphyrin within the UiO-66. For easy magnetic separation, Fe3O4 was introduced into the prepared composite.48 They used Fourier transform-infrared, ultraviolet-visible, and atomic absorption spectroscopy, scanning electron microscopy, and transmission electron microscopy to characterize the metalloporphyrin and MOF complex. This composite material showed excellent efficiency (95% conversion) and 67% selectivity in the oxidation of the olefins and allylic alcohols.
3. MOFs as heterogeneous catalysts
3.1. Catalysis by metal nodes
A large number of MOFs contain metal active sites generated by metal nodes after adequate thermal activation. These metal nodes expose coordinatively unsaturated metal centers that exhibit Lewis acid character and promote biological recognition and catalytic reactions (Fig. 4).49–51 As demonstrated by Yaghi and co-workers, open metal sites could stably exist because of the rigid characteristic of MOFs.52 The synergy between active metal sites and pore channels with well-understood surrounding environments greatly enhances the catalytic efficiency of organic reactions and polymer polymerizations. Férey et al. utilized chromium(III) carboxylate with giant pores, named MIL-101 (MIL for Materials of Institut Lavoisier), in the sulfoxidation of aryl sulfides after heating under vacuum at 423 K to expose trimeric chromium sites, which showed high catalytic activity and selectivity towards the corresponding sulfoxides.53 Furthermore, oxidative competition reactions promoted by chromium(III) showed that electron-releasing groups could improve reactivity.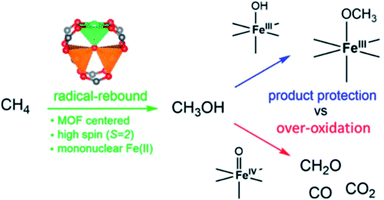 | ||
| Fig. 4 Methane oxidation to methanol catalyzed by iron species in a metal–organic framework. Adapted with permission from ref. 51. Copyright 2021, American Chemical Society. | ||
In our earlier research, we also employed Cr-MIL-100/101 as heterogeneous single-site catalysts to induce the controlled polymerization of isoprene under mild conditions.54 The additives [PhNHMe2][B(C6F5)4] could generate PhNMe2, which coordinated with chromium sites, thus forming different types of confined environments from nanochannels. The isoprene polymerization then takes place in the constrained spaces, resulting in polyisoprene with different structures. It is worth mentioning that nickel-based MOFs could also serve as high-efficiency catalysts. The nickel-based MOFs utilized steric hindrance and electronic modulation to promote the branching oligomer, and the catalytic system showed high catalytic activity. Long et al. used two kinds of MOF, Ni2(dobdc) (dobdc4− = 2,5-dioxodo-1,4-benzenedicarboxylate) and Ni2(dobpdc) (dobpdc4− = 4,4′-dioxodo-[1,1′-biphenyl]-3-3′-dicarboxylate) with coordinatively unsaturated Ni2+ sites in propene oligomerization, which exhibited relatively higher selectivity for linear oligomerization than Ni2+-exchanged aluminosilicates under the same conditions.55 This indicated that these MOFs were potential candidates for catalyzing the olefins and getting the linear polymer products.
As demonstrated by Xu and coworkers, the partial deligandation of MOFs could generate quasi-MOF,56 which could not only retain the porous characteristics of MOF to a certain extent but also exposed the inorganic nodes and showed enhanced synergistic catalysis abilities if combined with other guest species (metal nanoparticles). They synthesized Au/quasi-MIL-101 and applied them in the oxidation of carbon monoxide, which showed superior enhanced catalytic activity.57
3.2. Metal node exchange for active sites
The secondary building units (SBUs) of MOFs show biomimetic features that have various open coordination isolated sites and high-spin electronic environments. The metal nodes that connect the organic ligands of SBUs can be replaced by non-native metal ions, often called cation exchange, which was first proposed in 2007. Cation exchange is an effective method for generating new materials, especially these unavailable by direct synthesis. Ringwood's rule can be employed to explain this behavior, which means ions with identical electronegativity or lower value can be replaced more easily because the forming bonds exhibit the ionic properties. It is worth mentioning that cation exchange occurs in the channels or pores of MOFs, which is inevitably influenced by diffusion and the restricting effect of pore size. All the elaborate processes caused the exchanged metal nodes to show catalytic characteristics that facilitate multiple reactions.Partial substitution of a pristine metal node ZnII by MnII in MOF-5 (Fig. 5) was first demonstrated by Dincă et al. and they not only showed that the all-oxygen ligand had a certain degree of distortion to support the MnII site through Mn K-edge X-ray absorption spectroscopy, but they also confirmed that this MnII could shift to higher valence during selective catalyzed olefin epoxidation.58 Furthermore, this catalyst formed by metal nodes exchange could induce cyclic alkenes to form epoxides with up to 99% selectivity. This demonstrated that MOF secondary building units could serve as catalytic platforms for olefin epoxidation through metal nodes exchange. They also reported zinc-based MOFs exchanged by chromium and titanium, which facilitated the polymerization of ethylene.59 The high-density and high molecular weight of polyethylene were obtained. Copolymerization with propylene could also be achieved. As far as the polymer is concerned, the stereodefined polymers from conjugated dienes by polymerization play important roles in industrially and commercially. As a prototypical diene, 1,3-butadiene can generate different polybutadienes with diverse properties, depending on their specific structure. For instance, polybutadiene with 1,4-cis units exhibits elastomer characteristic, whereas those with 1,4-trans units show thermoplastic features.
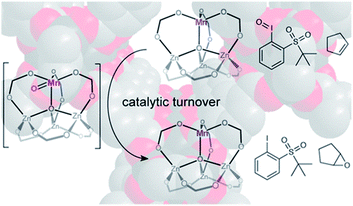 | ||
| Fig. 5 The proposed structure of selective catalytic olefin epoxidation with Mn-exchanged MOF-5. Adapted with permission from ref. 58. Copyright 2013, American Chemical Society. | ||
This requires that the catalyst must have the ability to adjust the stereoselectivity and molecular weight. Porous MOFs provided isolated single-sites which also acted as nanoreactors that could exquisitely control the stereoselective of polymerization.60 Cobalt-based MOFs were applied in the polymerization of 1,3-butadiene with relatively high stereoselectivity (>99%) and low polydispersity (PDI = 2), which indicate that MOFs as heterogeneous catalysts could stimulate the production of advanced polyolefins.
3.3. Lattice defects as active sites
For catalysis, the design of MOFs is mainly through generating low-coordinate metal sites or ligands exhibiting a large extent of chemical function features. However, some coordination saturated MOFs, as well as those that do not have such adjustable linkers, can serve as catalysts and have attracted the attention of researchers.61 This can be attributed to the formation of external surface or structural defects by different imidazolate frameworks. Chizallet and co-workers used ZIF-8 to catalyze transesterification.62 They found that the acido-basic sites on the surface or defects were ascribed to the active species as demonstrated by FTIR and DFT calculations. More specifically, acid site ZnII species and basic N− and OH− moieties located on the surface were recognized as active sites. Vermoortele et al. prepared zirconium terephthalate UiO-66(Zr) and partial terephthalates of this MOF were substituted by trifluoroacetic acid (TFA) (Fig. 6), which could generate a large number of defect sites with high catalytic ability.63 Here, TFA acted as a modulator that not only induced the crystallization of MOFs but also facilitated the production of extra Lewis acid sites. Additionally, appropriate modulators like TFA in this synthetic system do not change the size of MOF crystals. They used this coordinatively unsaturated UiO-66 as a catalyst in the conversion of citronellal, which showed high conversion efficiency.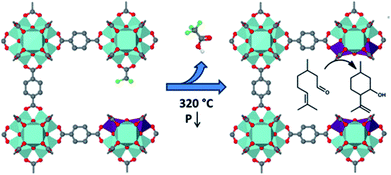 | ||
| Fig. 6 An illustration of a modulation approach to generate lattice defects of MOFs. Adapted with permission from ref. 63. Copyright 2013, American Chemical Society. | ||
4. Post-synthetic modification of MOF ligands
MOFs are well known for their structural stability and are easy to characterize in diverse reaction conditions, which also exhibited high crystallinity and modifiable structure as compared to amorphous solids. Therefore, they are ideal carriers that are applied in the field of catalysis. In order to expand their application performance, scientists utilized MOFs as the host matrix to anchor metals, which endowed the MOFs with enhanced abilities and applications such as gas adsorption, sensing and especially catalysis. The post-synthetic modification (PSM) approach (Fig. 7) is an effective strategy for chelating extraneous metals after the modification of the ligand with functional groups, and a wide range of metals can be immobilized on the modified linker.64–68 Furthermore, these ligands or linkers can be modified into different types such as bidentate [OO], [NO], [NN] and tridentate [NNN] to fix metal species by providing electronic chelation. We will discuss these different modified ligands that are incorporated into MOFs below.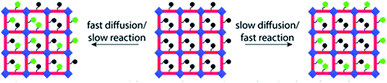 | ||
| Fig. 7 Diagram showing the expected PSM MOF products depending on the relative rates of diffusion and reaction. Left: fast diffusion relative to reaction yields a product with randomly distributed new functionality. Right: slow diffusion relative to reaction yields a product with a core–shell distribution of new functionality. Adapted with permission from ref. 68. Copyright 2021, American Chemical Society. | ||
4.1. Modified bidentate [OO] ligand-supported metal catalyst
Catalytically active sites play a crucial role in catalytic reactions. In terms of MOFs as catalysts, there are two main sources of active sites. The most common site stems from the metal nodes on the skeleton of MOFs, which can be fabricated by the hydrothermal method. Nevertheless, most of the metal nodes on MOFs are coordinated and saturated, and thus cannot expose excess sites for catalysis. Therefore, other post-synthetic strategies that generate metal-binding sites in MOFs were extensively researched. Hydroxyl groups could be introduced on the ligand of the MOF to form the bidentate [OO] ligand and the site could anchor metal complexes with catalytic properties. Lin et al. incorporated Ti(OiPr)4 into a homochiral porous MOF, which contained the bipyridyl primary functionality and orthogonal chiral 2,2′-dihydroxy secondary functionality.69 Ti(OiPr)4 could react with the chiral dihydroxy groups to form Lewis acidic compounds that were active catalysts for the addition of ZnEt2 to aromatic aldehydes to produce chiral secondary alcohols. Specifically, the composite homochiral MOFs catalyzed 1-naphthaldehyde with the addition of ZnEt2 to afford (R)-1-(1-naphthyl)propanol with complete conversion and 93% enantiomeric excess (ee). This level of ee rivals that of the homogeneous analogue under the same conditions (94% ee).The utilization of a coordination strategy to tailor the catalytic properties of MOFs is a convenient method. Nguyen and colleagues successfully modified MIL-101(Cr) to incorporate catechol functional groups. They chose MIL-101(Cr) as an ideal scaffold because of its high surface area and good thermal stability, which also contained metal centers that could be post-synthetically modified with amine and pyridyl species to support catalytically active functionalities.70 The modified MOF material was metallated with VIV ions to generate monocatecholate vanadium oxo compounds, which could not be synthesized in homogeneous solution environments. The resulting MIL-101-supported vanadyl catecholate moieties were active in the catalytic oxidation of an organo-sulfide and could be tuned to selectively afford the sulfoxide product. They also employed a single-site vanadyl monocatecholate moiety incorporated into the dipyridyl struts of a Zn-based pillared paddlewheel MOF by combining protecting groups, post-synthesis deprotection and post-synthesis metalation methods.71 The resulting metallated MOF could catalyze the benzylic oxidation of a large substrate like tetralin in the presence of a bulky oxidant with minimal metal leaching in a noncoordinating solvent.
4.2. Modified bidentate [NO] ligand-supported metal catalyst
Another type of ligand is bidentate [NO], which can be synthesized by incorporating amino group into the MOF skeleton; subsequently, reagents like anhydride can react with the amino group to form modified bidentate ligands that can bind diverse metal ions (Fig. 8).72 Cohen et al. employed 3-hydroxyphthalic anhydride and 2,3-pyrazine-dicarboxylic anhydride reacted with the amino group to generate competent metal-binding sites within a MOF.73 These sites could be metalated with divalent or trivalent transition metals like Cu2+ and Fe3+ ions. The resulting materials were found to be catalytically active for the Mukaiyama-aldol reaction over multiple catalytic cycles without the loss of activity or crystallinity.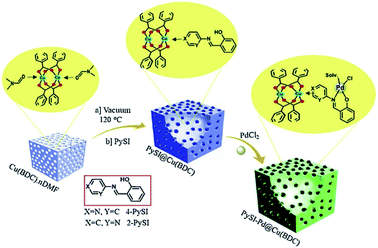 | ||
| Fig. 8 Synthetic pathway of 4-PySI–Pd@Cu(BDC) vs. 2-PySI–Pd@Cu(BDC). Adapted with permission from ref. 72. Copyright 2019, Elsevier. | ||
Corma et al. fabricated a metal–organic framework (IRMOF-3) with coordinatively unsaturated Au(III) species based on a post-synthesis strategy.74 The resulting heterogeneous MOF-based catalyst exhibited a high ability to stabilize cationic Au(III) species and the gold active sites were fully utilized. This Au(III) species-anchored MOF catalyst gave a much higher catalytic performance in the catalyzed domino coupling and cyclization of N-protected ethylanilines, amines, and aldehydes in comparison with homogeneous gold complexes. Particularly, the Au(III) species remained after the reaction and the catalyst showed cyclic regeneration performance.
Ahn and coworkers adopted a manganese(II) acetylacetonate complex immobilized on the metal–organic framework IRMOF-3 with amino groups through a one-step post-synthetic route.75 These composite materials showed high activity and selectivity in the epoxidation of alkenes using molecular oxygen and aldehyde and could be recycled several times without the loss of activity or selectivity. This type of MOF could also be decorated with organic groups to load diverse metal complexes. As demonstrated by Jie and colleagues, isoreticular metal–organic framework-3 (IRMOF-3) could also be modified to generate a Cr(III)-based heterogeneous catalyst for ethylene polymerization upon activation with various alkyl aluminum co-catalysts.76 The resulting polyethylenes featured high molecular weights and broad molecular weight distributions.
4.3. Modified bidentate [NN] ligand-supported metal catalyst
Postsynthetic modification is a powerful tool for obtaining highly sophisticated functionalized structures. It consists of the incorporation of functionalities after framework synthesis by employing various methods. In some cases, the postsynthetically introduced coordinating moieties can interact with metal nodes, and the utilization of metal salts as precursors could also lead to pore blocking with metal aggregates, which may cause unselective catalytic activity.77,78Canivet et al. anchored a molecular nickel complex into a mesoporous metal–organic framework (Ni@Fe-MIL-101) (Fig. 9), which was very efficient in the triphasic ethylene dimerization, selectively producing 1-butene and had higher selectivity than molecular nickel diamino complexes.79 The single-site isolation nickel-loaded MOF catalyst made from relatively cheap precursors may open up new perspectives for the application of MOF-based catalysts in valorization processes.
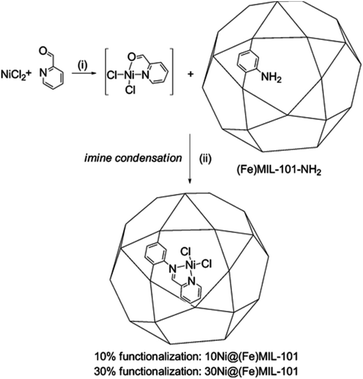 | ||
| Fig. 9 One-pot synthesis of the MOF-anchored nickel complex Ni@(Fe)MIL-101. Adapted with permission from ref. 79. Copyright 2013, American Chemical Society. | ||
Palladium-mediated catalysis has been widely investigated because of its key role in industrial processes. However, this catalytic system suffers from the waste of noble catalysts, which are too difficult to recycle and are lost in an aqueous work-up stage. Thus, the immobilization of the homogeneous complex on supports will make the catalyst separation and reuse easy. Jin et al. embedded Pd(diimine)Cl2 in the pores of MOF, which showed remarkable versatility as they were applied in catalysing Suzuki–Miyaura and Heck reactions to high yields of coupling products with little wastage of the palladium compounds.80 Koner and coworkers also anchored palladium(II) into a post-synthetically-modified metal–organic framework IRMOF-3.81 The amine group contained in IRMOF-3 was decorated upon condensation with pyridine-2-aldehyde and afforded a bidentate Schiff base moiety in the porous matrix, which could be used to anchor palladium(II) ions. The resulting catalyst exhibited high activity toward the Suzuki and Stille cross-coupling reaction in 20% H2O/EtOH and EtOH medium, respectively. The loaded complex did not leach as compared with the homogeneous catalysis.
Li et al. utilized UiO-67 as a carrier to immobilize palladium or destroy it during the catalytic reactions, thus demonstrating the complex Pd(H2bpydc)Cl2 (H2bpydc = 2,2′-bipyridine-5,5′-dicarboxylic acid) using a direct incorporation strategy.82 They used a large amount of H2bpdc ligand that could not chelate the Pd complex and allowed the generation of isolated Pd single active sites, which were dispersed uniformly in the MOF network. The palladium complex-loaded material exhibited the remarkable catalytic conversion of aryl chlorides, which had higher activity than the homogeneous Pd counterparts. The catalysts also showed high yields in the Heck and Suzuki–Miyaura coupling reactions of chloroarenes bearing different substituents with essentially identical activity after at least 5 cycles. Cohen et al. prepared a highly crystalline Zr(IV)-based metal–organic framework (MOF) containing open 2,2′-bipyridine (bpy) chelating sites through two fundamentally different strategies, direct MOF formation and postsynthetic exchange (PSE).83 Both synthetic strategies could contribute 2,2′-bipyridine (bpy) incorporation to be varied from 0% to 100%, which showed a controllable manner. The decorated MOFs exhibited efficient, heterogeneous and recyclable catalysis of Suzuki–Miyaura cross-coupling reactions. It can be seen from the above research that the homogeneous molecular Pd complex could be immobilized on diverse metal–organic frameworks to generate heterogeneous palladium catalysts for a variety of Pd-catalyzed transformations.
4.4. Modified tridentate [NNN] ligand-supported metal catalyst
Direct synthesis and post-synthetic functionalization (PSM) routes can endow MOFs with more functional and selective sites for use in catalysis and gas separation, despite the MOFs showing unusual thermal and chemical stability. These strategies are usually inseparable from one-pot type reactions, in which multistep reactions are performed in the same reaction vessel. It also has the advantages of avoiding intermediate separation, purification steps and minimizing energy consumption. Particularly, nitroarenes are one of the widely used starting compounds that can be hydrogenated into anilines, and further transformed by alkylation, and reductive alkylation and hydroamination to produce the corresponding derivatives including heterocyclics. Iglesias and colleagues developed a practical approach for the synthesis of N-alkyl amines via reductive amination in the presence of hydrogen by employing a recyclable heterogeneous catalyst that combined the high catalytic activity of Iridium-transition metal complexes with structurally stable MOF skeleton which had a modified tridentate [NNN] ligand (Fig. 10).84 This efficient method could be applied to synthesize a large range of N-alkyl amines in good to excellent yields. All of the excellent features in the procedure contribute to its application in the development of benign chemical processes and products.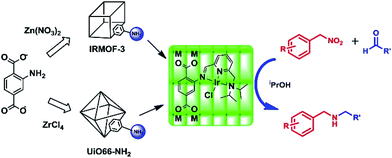 | ||
| Fig. 10 Post-modification of IRMOF-3 and UiO-66–NH2 for the synthesis of secondary amines by a one-pot three-step cascade reaction. Adapted with permission from ref. 84. Copyright 2013, Academic Press Inc. | ||
5. Conclusion and outlook
Metal–organic frameworks can be employed as heterogeneous catalysts and applied in a wide range of organic reactions and polymerizations. The designable morphology, pore size and ligand functionality of MOFs make it possible for them to be used in diverse catalytic reactions. MOFs stand out from a variety of porous materials like zeolites and activated carbons with their multifunctional organic linkers, modifiable metal sites and high surface area and pore volume, which also can be functionalized via their metal nodes and organic ligands by means of pre-synthetic and post-synthetic modifications to generate versatile properties that could not be directly synthesized under traditional synthetic methods. High porosity has been utilized not only as a reaction channel but also for the encapsulation of metal nanoparticles, metal oxides, polyoxometalates and metalloporphyrins. Loading these complexes into the pores of MOFs make them accessible to the reaction substrate, which can be sieved via a confined space environment provided by the cages of the MOFs. Sterically hindered substrates also show diminished yields as compared to small substrates, proving that the reactions mainly take place inside the pores. In addition, the metal nodes can be utilized as catalytically active sites after adequate thermal activation, which exposes coordinatively unsaturated metal centers that promote biological recognition and catalytic reactions. These metal nodes can also be exchanged by other metal cations, which is an effective strategy for generating new materials, especially these unavailable by direct synthesis. Hetero-bimetallic MOFs have exhibited enhanced catalytic performance as compared to their monometallic analogues because of the bimetallic synergy. Moreover, the external surface or structural defects of MOFs generated by different approaches also have diverse catalytic active sites for effectively catalyzing different reactions.PSM has been demonstrated to be a versatile and effective strategy for tuning the performance of MOFs. Herein, we reviewed different forms of ligand modification strategies to synthesize diverse bidentate or tridentate ligands, which provide coordinative anchoring for the extraneous metal complexes and can offer additional excellent catalytic performance that pristine MOFs do not have. Despite the benefits, it is worth pointing out that expanding ligand modification strategies, improving the efficiency of metalation and establishing protocols for precise structural characterisation of the metalated species can still promote the novel applications for MOFs.
MOFs can be sophisticatedly designed and are very suitable for heterogeneous catalytic systems. Diverse new kinds of MOFs with excellent characteristics will appear as the research of MOFs becomes more and more popular. Different MOF modification strategies can endow them with excellent catalytic properties. The research will focus on loading different compounds to generate synergistic effects and the modification of MOFs to improve catalytic performance. There will be more MOF modification methods in future research.
In summary, we have reviewed the recent progress on the strategies for the application of MOFs in catalytic reactions. Researchers have proposed many strategies for improving the catalytic properties of MOFs and they have proven to be effective catalysts for a wide variety of reactions. Despite the many problems that remain to be solved to achieve broad application, we remain optimistic that strategies for the design and modification of MOFs will continue to see rapid growth and realize their practical application in the future.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the foundation of Nanchang University, grant number: 9140/06301549.Notes and references
- F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Artificial Metalloenzymes: Reaction Scope and Optimization Strategies, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed.
- K. Wieszczycka and K. Staszak, Artificial Metalloenzymes as catalysts in non-natural compounds synthesis, Coord. Chem. Rev., 2017, 351, 160–171 CrossRef CAS.
- M. M. Pellizzoni, F. Schwizer, C. W. Wood, V. Sabatino, Y. Cotelle, S. Matile, D. N. Woolfson and T. R. Ward, Chimeric Streptavidins as Host Proteins for Artificial Metalloenzymes, ACS Catal., 2018, 8, 1476–1484 CrossRef CAS.
- V. M. Robles, E. O. Carrasco, L. A. Cotchico, J. R. Guerra, A. Lledós and J. D. Maréchal, Toward the Computational Design of Artificial Metalloenzymes: From Protein-Ligand Docking to Multiscale Approaches, ACS Catal., 2015, 5, 2469–2480 CrossRef.
- M. V. Doble, A. G. Jarvis, A. C. Ward, J. D. Colburn, J. P. Götze, M. Bühl and P. C. Kamer, Artificial Metalloenzymes as Catalysts for Oxidative Lignin Degradation, ACS Sustainable Chem. Eng., 2018, 6, 15100–15107 CrossRef CAS.
- A. G. Jarvis, L. Obrecht, P. J. Deuss, W. Laan, E. K. Gibson, P. P. Wells and P. C. Kamer, Enzyme Activity by Design: An Artificial Rhodium Hydroformylase for Linear Aldehydes, Angew. Chem., Int. Ed., 2017, 56, 13596–13600 CrossRef CAS PubMed.
- L. Villarino, K. E. Splan, E. Reddem, L. A. Cotchico, C. G. Souza, A. Lledós, J. D. Maréchal, A. W. Thunnissen and G. Roelfes, An Artificial Heme Enzyme for Cyclopropanation Reactions, Angew. Chem., Int. Ed., 2018, 57, 7785–7789 CrossRef PubMed.
- F. Nastri, M. Chino, O. Maglio, A. B. Damodaran, Y. Lu and A. Lombardi, Design and engineering of artificial oxygen-activating metalloenzymes, Chem. Soc. Rev., 2016, 45, 5020–5054 RSC.
- F. Rosati and G. Roelfes, Artificial Metalloenzymes, ChemcatChem, 2010, 2, 916–927 CrossRef CAS.
- P. J. Deuss, R. Heeten, W. Laan and P. C. Kamer, Bioinspired Catalyst Design and Artificial Metalloenzymes, Chem.–Eur. J., 2011, 17, 4680–4698 CrossRef CAS PubMed.
- J. C. Lewis, Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis, ACS Catal., 2013, 3, 2954–2975 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- G. Férey, Hybrid porous solids: past, present, future, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal–Organic Frameworks, Science, 2013, 341, 1230–1242 CrossRef PubMed.
- H. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- A. Bétard and R. Fischer, Metal–Organic Framework Thim Films: From Fundamentals to Applications, Chem. Rev., 2012, 112, 1055–1083 CrossRef PubMed.
- H. L. Nguyen, The Chemistry of Titanium-based Metal–Organic Frameworks, New J. Chem., 2017, 41, 14030–14043 RSC.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Selective Gas Adsorption and Separation in Metal–Organic Frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- D. X. Liu, J. J. Gu, Q. L. Liu, Y. W. Tan, Z. Li, W. Zhang, Y. S. Su, W. X. Li, A. Cui, C. Z. Gu and D. Zhang, Metal–Organic Frameworks Reactivate Deceased Diatoms to be Efficient CO2 Absorbents, Adv. Mater., 2014, 26, 1229–1234 CrossRef CAS PubMed.
- J. Li, J. Sculley and H. C. Zhou, Metal-Organic Frameworks for Separations, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- K. W. Nam, S. S. Park, R. D. Reis, V. P. Dravid, H. Kim, C. A. Mirkin and J. F. Stoddart, Conductive 2D Metal–Organic Framework for High-Performance Cathodes in Aqueous Rechargeable Zinc Batteries, Nat. Commun., 2019, 10, 4948–4958 CrossRef PubMed.
- I. A. Lázaro, C. J. Wells and R. S. Forgan, Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery, Angew. Chem., Int. Ed., 2020, 59, 5211–5217 CrossRef PubMed.
- K. Suresh and A. J. Matzger, Enhanced Drug Delivery by Dissolution of Amorphous Drug Encapsulated in a Water Unstable Metal–Organic Framework (MOF), Angew. Chem., Int. Ed., 2019, 58, 16790–16794 CrossRef CAS PubMed.
- A. Corma, H. García and F. X. Xamena, Engineering Metal Organic Frameworks for Heterogeneous Catalysis, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- L. Zhu, X. Liu, H. Jiang and L. Sun, Metal–Organic Frameworks for Heterogeneous Basic Catalysis, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- J. D. Evans, C. J. Sumby and C. J. Doonan, Post-synthetic Metalation of Metal–Organic Frameworks, Chem. Soc. Rev., 2014, 43, 5933–5951 RSC.
- L. Y. Chen and Q. Xu, Metal–Organic Framework Composites for Catalysis, Matter, 2019, 1, 57–89 CrossRef.
- L. Y. Chen, R. Luque and Y. W. Li, Controllable Design of Tunable Nanostructures inside Metal–Organic Frameworks, Chem. Soc. Rev., 2017, 46, 4614–4630 RSC.
- S. Abednatanzi, P. G. Derakhshandeh, H. Depauw, F. X. Coudert, H. Vrielinck, P. V. Voort and K. Leus, Mixed-Metal Metal–Organic Frameworks, Chem. Soc. Rev., 2019, 48, 2535–2565 RSC.
- L. Y. Chen, H. R. Chen, R. Luque and Y. W. Li, Metal–Organic Framework Encapsulated Pd Nanoparticles: Towards Advanced Heterogeneous Catalysts, Chem. Sci., 2014, 5, 3708–3714 RSC.
- L. Y. Chen, X. D. Chen, H. L. Liu and Y. W. Li, Encapsulation of Mono- or Bimetal Nanoparticles Inside Metal-Organic Frameworks via In Situ Incorporation of Metal Precursors, Small, 2015, 11, 2642–2648 CrossRef CAS PubMed.
- S. Proch, J. Herrmannsdörfer, R. Kempe, C. Kern, A. Jess, L. Seyfarth and J. Senker, Pt@MOF-177: Synthesis, Room-Temperature Hydrogen Storage and Oxidation Catalysis, Chem.–Eur. J., 2008, 14, 8204–8212 CrossRef CAS PubMed.
- B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, A Highly Active Heterogeneous Palladium Catalyst for The Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media, Angew. Chem., Int. Ed., 2010, 49, 4054–4058 CrossRef CAS PubMed.
- Y. Huang, Z. Zheng, T. Liu, J. Lu, Z. Lin, H. Li and R. Cao, Palladium Nanoparticles Supported on Amino Functionalized Metal–Organic Frameworks as Highly Active Catalysts for The Suzuki-Miyaura Cross-Coupling Reaction, Catal. Commun., 2011, 14, 27–31 CrossRef CAS.
- Z. G. Sun, G. Li, L. P. Liu and H. O. Liu, Au Nanoparticles Supported on Cr-Based Metal-Organic Framework as Bimetallic Catalyst for Selective Oxidation of Cyclohexane to Cyclohexanone and Cyclohexanol, Catal. Commun., 2012, 27, 200–205 CrossRef CAS.
- T. V. Vu, H. Kosslick, A. Schulz, J. Harloff, E. Paetzold, M. Schneider, J. Radnik, N. Steinfeldt, G. Fulda and U. Kragl, Selective Hydroformylation of Olefins Over the Rhodium Supported Large Porous Metal–Organic Framework MIL-101, Appl. Catal., A, 2013, 468, 410–417 CrossRef CAS.
- W. X. Wang, Y. W. Li, R. J. Zhang, D. H. He, H. L. Liu and S. J. Liao, Metal–Organic Framework as A Host for Synthesis of Nanoscale Co3O4 as An Active Catalyst for CO Oxidation, Catal. Commun., 2011, 12, 875–879 CrossRef CAS.
- K. Vikrant, K. H. Kim, C. Z. He and D. A. Giannakoudakis, Harnessing Adsorption-Catalysis Synergy: Efficient Oxidative Removal of Gaseous Formaldehyde by a Manganese Dioxide/Metal–Organic Framework Nanocomposite at Room Temperature, Adv. Funct. Mater., 2022, 32, 2107922–2107941 CrossRef.
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. R. Fakhari and M. M. Amini, Au-SH-SiO2 Nanoparticles Supported on Metal–Organic Framework (Au-SH-SiO2@Cu-MOF) as a Sensor for Electrocatalytic Oxidation and Determination of Hydrazine, Electrochim. Acta, 2013, 88, 301–309 CrossRef CAS.
- S. Subudhi, S. P. Tripathy and K. Parida, Metal Oxide Integrated Metal Organic Frameworks (MO@MOF): Rational Design, Fabrication Strategy, Characterization and Emerging Photocatalytic Applications, Inorg. Chem. Front., 2021, 8, 1619–1636 RSC.
- N. Maksimchuk, M. Timofeeva, M. Melgunov, A. Shmakov, Y. Chesalov, D. Dybtsev, V. Fedin and O. Kholdeeva, Heterogeneous Selective Oxidation Catalysts Based on Coordination Polymer MIL-101 and Transition Metal-Substituted Polyoxometalates, J. Catal., 2008, 257, 315–323 CrossRef CAS.
- J. Alcañiz, E. Ramos-Fernandez, U. Lafont, J. Gascon and F. Kapteijn, Building MOF Bottles Around Phosphotungstic Acid Ships: One-Pot Synthesis of Bifunctional Polyoxometalate-MIL-101 Catalysts, J. Catal., 2010, 269, 229–241 CrossRef.
- J. Song, Z. Luo, D. Britt, H. Furukawa, O. M. Yaghi, K. Hardcastle and C. Hill, A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate-Metal Organic Framework for Aerobic Decontamination, J. Am. Chem. Soc., 2011, 133, 16839–16846 CrossRef CAS PubMed.
- L. Bromberg, X. Su and T. Hatton, Heteropolyacid-Functionalized Aluminum 2-Aminoterephthalate Metal–Organic Frameworks As Reactive Aldehyde Sorbents and Catalysts, ACS Appl. Mater. Interfaces, 2013, 5, 5468–5477 CrossRef CAS PubMed.
- O. V. Zalomaeva, K. A. Kovalenko, Y. A. Chesalov, M. Mel'gunov, V. I. Zaikovskii, V. V. Kaichev, A. B. Sorokin, O. Kholdeeva and V. P. Fedin, Iron Tetrasulfophthalocyanine Immobilized on Metal Organic Framework MIL-101: Synthesis, Characterization and Catalytic Properties, Dalton Trans., 2011, 40, 1441–1444 RSC.
- E. Kockrick, T. Lescouet, E. V. Kudrik, A. B. Sorokin and D. Farrusseng, Synergistic Effects of Encapsulated Phthalocyanine Complexes in MIL-101 for the Selective Aerobic Oxidation of Tetralin, Chem. Commun., 2011, 47, 1562–1564 RSC.
- X. Li, Y. Huang, W. F. Wei, W. L. Guo, Z. T. Luo, J. T. Xu and T. Cai, Metalloporphyrin-anchored 2D MOF Nanosheets as Highly Accessible Heterogeneous Photocatalysts towards Cytocompatible Living Radical Polymerization, Chem. Eng. J., 2022, 434, 134692–134700 CrossRef CAS.
- S. Zamani, A. Abbasi, M. M. Farahani and S. Rayati, One-Pot, Facile Synthesis and Fast Separation of a UiO-66 Composite by a Metalloporphyrin Using Nanomagnetic Materials for Oxidation of Olefins and Allylic Alcohols, New J. Chem., 2022, 46, 654–662 RSC.
- S. M. Rogge, A. Bavykina, J. Hajek, H. Garcia, A. I. Olivos-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E. V. Ramos-Fernandez, F. X. Xamenam, V. Speybroeck and J. Gascon, Chem. Soc. Rev., 2017, 46, 3134–3184 RSC.
- A. Dhakshinamoorthy, Z. H. Li and H. Garcia, Catalysis and Photocatalysis by Metal Organic Frameworks, Chem. Soc. Rev., 2018, 47, 8134–8172 RSC.
- M. C. Simons, S. D. Prinslow, M. Babucci, A. S. Hoffman, J. Y. Hong, J. G. Vitillo, S. R. Bare, B. C. Gates, C. C. Lu, L. Gagliardi and A. Bhan, Beyond Radical Rebound: Methane Oxidation to Methanol Catalyzed by Iron Species in Metal-Organic Framework Nodes, J. Am. Chem. Soc., 2021, 143, 12165–12174 CrossRef CAS PubMed.
- B. Chen, M. Eddaoudi, T. Reineke, J. Kampf, M. O'Keeffe and O. M. Yaghi, Cu2(ATC)·6H2O: Design of Open Metal Sites In Porous Metal–Organic Crystals (ATC:1,3,5,7-Adamantane Tetracarboxylate), J. Am. Chem. Soc., 2000, 122, 11559–11560 CrossRef CAS.
- Y. Hwang, D. Hong, J. Chang, H. Seo, M. Yoon, J. Kim, S. Jhung, C. Serre and G. Férey, Selective Sulfoxidation of Aryl Sulfides by Coordinatively Unsaturated Metal Centers In Chromium Carboxylate MIL-101, Appl. Catal., A, 2009, 358, 249–253 CrossRef CAS.
- F. Gao, Z. Li, C. Yu, X. W. Yan, S. W. Zhang and X. F. Li, Controlled Polymerization of Isoprene with Chromium-Based Metal–Organic Framework Catalysts: Switching from Cyclic to Cis-1,4-Selectivity Depending on Activator, Macromol. Rapid Commun., 2018, 39, 1800002–1800008 CrossRef PubMed.
- A. N. Mlinar, B. K. Keitz, D. Gygi, E. D. Bloch, J. R. Long and A. T. Bell, Selective Propene Oligomerization with Nickel(II)-Based Metal-Organic Frameworks, ACS Catal., 2014, 4, 717–721 CrossRef CAS.
- N. Tsumori, L. Y. Chen, Q. J. Wang, Q. L. Zhu, M. Kitta and Q. Xu, Quasi-MOF: Exposing Inorganic Nodes to Guest Metal Nanoparticles for Drastically Enhanced Catalytic Activity, Chem, 2018, 4, 845–856 CAS.
- L. Y. Chen, N. Tsumori and Q. Xu, Quasi-MOF-immobilized Metal Nanoparticles for Synergistic Catalysis, Sci. China: Chem., 2020, 63, 1601–1607 CrossRef CAS.
- C. Brozek and M. Dincă, Ti3+, V2+/3+, Mn2+ and Fe2+ Substituted MOF-5 and Redox Reactivity in Cr and Fe-MOF-5, J. Am. Chem. Soc., 2013, 135, 12886–12891 CrossRef CAS PubMed.
- R. Comito, K. Fritzsching, B. Sundell, K. Schmidt-Rohr and M. Dincă, Single-Site Heterogeneous Catalysts for Olefin Polymerization Enabled by Cation Exchange In A Metal-Organic Framework, J. Am. Chem. Soc., 2016, 138, 10232–10237 CrossRef CAS PubMed.
- T. Uemura, S. Horike, K. Kitagawa, M. Mizuno, K. Endo, S. Bracco, A. Comotti, P. Sozzani, M. Nagaoka and S. Kitagawa, Conformation and Molecular Dynamics of Single Polystyrene Chain Confined in Coordination Nanospace, J. Am. Chem. Soc., 2008, 130, 6781–6788 CrossRef CAS PubMed.
- U. Ravon, M. E. Domine, C. Gaudillère, A. Desmartin-Chomel and D. Farrusseng, MOFs as Acid Catalysts with Shape Selectivity Properties, New J. Chem., 2008, 32, 937–940 RSC.
- C. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A. Quoineaud and N. Bats, Catalysis of Transesterification by A Nonfunctionalized Metal-Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and Ab Initio Calculations, J. Am. Chem. Soc., 2010, 132, 12365–12377 CrossRef CAS PubMed.
- F. Vermoortele, B. Bueken, G. L. Bars, B. V. Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. V. Speybroeck, C. Kirschhock and D. E. De Vos, Synthesis Modulation as A Tool to Increase the Catalytic Activity of Metal-Organic Frameworks: The Unique Case of UiO-66(Zr), J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed.
- C. P. Xu, R. Q. Fang, R. Luque, L. Y. Chen and Y. W. Li, Functional Metal–Organic Frameworks for Catalytic Applications, Coord. Chem. Rev., 2019, 388, 268–292 CrossRef CAS.
- J. D. Evans, C. J. Sumby and C. J. Doonan, Post-synthetic Metalation of Metal–Organic Frameworks, Chem. Soc. Rev., 2014, 43, 5933–5951 RSC.
- Z. Yin, S. Wan, J. Yang, M. Kurmoo and M. H. Zeng, Recent Advances in Post-Synthetic Modification of Metal–Organic Frameworks: New Types and Tandem Reactions, Coord. Chem. Rev., 2019, 378, 500–512 CrossRef CAS.
- S. S. Chen, Z. X. Song, J. H. Lyu, Y. Guo, B. E. Lucier, W. Luo, M. S. Workentin, X. L. Sun and Y. N. Huang, Anhydride Post-Synthetic Modification in a Hierarchical Metal–Organic Framework, J. Am. Chem. Soc., 2020, 142, 4419–4428 CrossRef CAS PubMed.
- D. R. Bois and A. J. Matzger, Reagent Reactivity and Solvent Choice Determine Metal–Organic Framework Micro-Structure during Postsynthetic Modification, J. Am. Chem. Soc., 2021, 143, 671–674 CrossRef PubMed.
- C. Wu, A. Hu, L. Zhang and W. Lin, A Homochiral Porous Metal–Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis, J. Am. Chem. Soc., 2005, 127, 8940–8941 CrossRef CAS PubMed.
- H. G. Nguyen, M. H. Weston, O. K. Farha, J. T. Hupp and S. T. Nguye, A Catalytically Active Vanadyl(catecholate)-Decorated Metal Organic Framework Via Post-Synthesis Modifications, CrystEngComm, 2012, 14, 4115–4118 RSC.
- H. G. Nguyen, M. H. Weston, A. A. Sarjeant, D. M. Gardner, Z. An, R. Carmieli, M. R. Wasielewski, O. K. Farha, J. T. Hupp and S. T. Nguyen, Design, Synthesis, Characterization, and Catalytic Properties of A Large-Pore Metal–Organic Framework Possessing Single-Site Vanadyl(monocatecholate) Moieties, Cryst. Growth Des., 2013, 13, 3528–3534 CrossRef CAS.
- H. Alamgholiloo, S. B. Zhang, A. Ahadi, S. Rostamnia, R. Banaei, Z. C. Li, X. Liu and M. Shokouhimehr, Synthesis of Bimetallic 4-PySI-Pd@Cu(BDC) via Open Metal Site Cu-MOF:Effect of Metal and Support of Pd@Cu-MOFs in H2 Generation from Formic Acid, Mol. Catal., 2019, 467, 30–37 CrossRef CAS.
- K. K. Tanabe and S. M. Cohen, Engineering a Metal–Organic Framework Catalyst by Using Postsynthetic Modification, Angew. Chem., 2009, 121, 7560–7563 CrossRef.
- X. Zhang, F. X. Xamena and A. Corma, Gold(III)-Metal Organic Framework Bridges the Gap Between Homogeneous and Heterogeneous Gold Catalysts, J. Catal., 2009, 265, 155–160 CrossRef CAS.
- S. Bhattacharjee, D. A. Yang and W. S. Ahn, A New Heterogeneous Catalyst for Epoxidation of Alkenes Via One-Step Post-Functionalization of IRMOF-3 with a Manganese(II) Acetylacetonate Complex, Chem. Commun., 2011, 47, 3637–3639 RSC.
- B. Liu, S. Y. Jie, Z. Y. Bu and B. G. Li, A MOF-Supported Chromium Catalyst for Ethylene Polymerization Through Post-Synthetic Modification, J. Mol. Catal. A: Chem., 2014, 387, 63–68 CrossRef CAS.
- S. S. Kaye and J. R. Long, Matrix Isolation Chemistry in A Porous Metal-Organic Framework: Photochemical Substitutions of N2 and H2 in Zn4O[(η6-1,4-Benzenedicarboxylate)Cr(CO)3]3, J. Am. Chem. Soc., 2008, 130, 806–807 CrossRef CAS PubMed.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications, Adv. Funct. Mater., 2021, 31, 2006291–2006313 CrossRef CAS.
- J. Canivet, S. Aguado, Y. Schuurman and D. Farrusseng, MOF-Supported Selective Ethylene Dimerization Single-Site Catalysts through One-Pot Postsynthetic Modification, J. Am. Chem. Soc., 2013, 135, 4195–4198 CrossRef CAS PubMed.
- S. L. Huang, A. Q. Jia and G. X. Jin, Pd(diimine)Cl2 Embedded Heterometallic Compounds with Porous Structures as Efficient Heterogeneous Catalysts, Chem. Commun., 2013, 49, 2403–2405 RSC.
- D. Saha, R. Sen, T. Maity and S. Koner, Anchoring of Palladium onto Surface of Porous Metal–Organic Framework through Post-Synthesis Modification and Studies on Suzuki and Stille Coupling Reactions under Heterogeneous Condition, Langmuir, 2013, 29, 3140–3151 CrossRef CAS PubMed.
- L. Y. Chen, S. Rangan, J. Li, H. F. Jiang and Y. W. Li, A Molecular Pd(II) Complex Incorporated into A MOF as A Highly Active Single-site Heterogeneous Catalyst for C–Cl Bond Activation, Green Chem., 2014, 16, 3978–3985 RSC.
- H. H. Fei and S. M. Cohen, A Robust, Catalytic Metal-Organic Framework with Open 2,2'-Bipyridine Sites, Chem. Commun., 2014, 50, 4810–4812 RSC.
- M. Pintado-Sierra, A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sánchez, Bifunctional Iridium-(2-Aminoterephthalate)-Zr-MOF Chemoselective Catalyst for The Synthesis of Secondary Amines by One-Pot Three-Step Cascade Reaction, J. Catal., 2013, 299, 137–145 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |





