Open Access Article
Open Access ArticleEmerging cold plasma treatment and machine learning prospects for seed priming: a step towards sustainable food production
Amruta Shelara,
Ajay Vikram Singh *b,
Paul Dietrichc,
Romi Singh Maharjanb,
Andreas Thissenc,
Pravin N. Didwald,
Manish Shindee,
Peter Laux
*b,
Paul Dietrichc,
Romi Singh Maharjanb,
Andreas Thissenc,
Pravin N. Didwald,
Manish Shindee,
Peter Laux b,
Andreas Luch
b,
Andreas Luch b,
Vikas Mathe
b,
Vikas Mathe f,
Timotheus Jahnkeg,
Manohar Chaskar*h and
Rajendra Patil
f,
Timotheus Jahnkeg,
Manohar Chaskar*h and
Rajendra Patil *i
*i
aDepartment of Technology, Savitribai Phule Pune University, Pune 411007, India
bDepartment of Chemical and Product Safety, German Federal Institute for Risk Assessment (BfR), Max-Dohrn-Strasse 8-10, 10589 Berlin, Germany. E-mail: AjayVikram.Singh@bfr.bund.de
cSPECS Surface Nano Analysis GmbH, Voltastrasse 5, 13355 Berlin, Germany
dDepartment of Materials, University of Oxford, Parks Road, Oxford OX1 3PH, UK
eCentre for Materials for Electronics Technology (C-MET), Panchawati, Pune, 411008, India
fDepartment of Physics, Savitribai Phule Pune University, Pune 411007, India
gMax Planck Institute for Medical Research, 61920 Heidelberg, Germany
hFaculty of Science and Technology, Savitribai Phule Pune University, Pune 411007, India. E-mail: chaskarmanohar@gmail.com
iDepartment of Biotechnology, Savitribai Phule Pune University, Pune 411007, India. E-mail: rpatil@unipune.ac.in
First published on 5th April 2022
Abstract
Seeds are vulnerable to physical and biological stresses during the germination process. Seed priming strategies can alleviate such stresses. Seed priming is a technique of treating and drying seeds prior to germination in order to accelerate the metabolic process of germination. Multiple benefits are offered by seed priming techniques, such as reducing fertilizer use, accelerating seed germination, and inducing systemic resistance in plants, which are both cost-effective and eco-friendly. For seed priming, cold plasma (CP)-mediated priming could be an innovative alternative to synthetic chemical treatments. CP priming is an eco-friendly, safe and economical, yet relatively less explored technique towards the development of seed priming. In this review, we discussed in detail the application of CP technology for seed priming to enhance germination, the quality of seeds, and the production of crops in a sustainable manner. Additionally, the combination treatment of CP with nanoparticle (NP) priming is also discussed. The large numbers of parameters need to be monitored and optimized during CP treatment to achieve the desired priming results. Here, we discussed a new perspective of machine learning for modeling plasma treatment parameters in agriculture for the development of synergistic protocols for different types of seed priming.
1. Introduction
The global community has decided to improve people's lives by 2030, and the second goal is to end hunger. The World Food Program is also screening methods for improving nutrition and promoting sustainable agriculture to end hunger by 2030.1 We need to ramp up food production to feed the billion people in this world amid the rapidly growing world population. A large percentage of the world's food is grown via agriculture, and seeds are vital elements of sustainable farming and food production. Emerging technologies in the agriculture sector are playing an increasingly important role in seed quality improvement, triggering a new revolution in seed technology.2 Cold plasma technology is one of the innovative and emerging technologies in the seed industry; it is an important technology to address many grave issues related to seed quality and storability. Treatment of seeds is a modern eco-agricultural technology reputed to boost plant growth.3–6 CP treatment improves seed performance and crop yield in a fast, economical, and pollution-free way.6 It plays an essential role in several developmental and physiological processes in plants, including decreasing the bacterial load on seeds, altering seed coat structures, increasing seed germination, as well as stimulating seedling growth.7–9 Seed priming is a feasible and economical method of obtaining uniform seed development in field crop production and improves nutrient uptake, water use efficiency, photo- and thermo-dormancy, and crop yield, therefore seed priming with CP enhances stress resistance and reduces cultivation costs. CP mediated seed priming increases the seed germination rates, plant growth, as well as agricultural yields.7–9 CP priming in agriculture have several advantages over conventional priming methods, including short treatment times, easy accessibility, and low temperatures during operation. Reactive chemical species in plasma change surface chemistry, which can improve surface wettability, water absorption and, consequently, initiate the complex signaling pathways in seeds.10,11 The CP priming treatment conditions can also affect DNA, enzyme activity, hormone balance, etc. The results of plasma-induced seed priming changes may improve the germination, growth, and development of plants, as well as their resistance to biotic and abiotic stresses, and synergistically promote yield.9 Furthermore, CP priming is able to treat seeds and crops by applying ambient conditions without harming their essential architecture. In comparison to thermal plasma, CP or non-thermal plasma is better suited to the treatment of thermally unstable biological samples since the samples are heated to ambient levels. To ensure desired seed priming responses, parameters of CP treatment needs to be monitored or optimized. The use of machine learning (ML) methods in data analytics of CP can help to optimize the parameters. Based on ML methods, we demonstrated that data analytics can be used to estimate operation-relevant parameters in a simple and effective manner to achieve expected responses in seed behavior. The present review will focus on the benefits and application of cold plasma as a next-generation priming agent to improve seed metabolism, seed vigor, abiotic and biotic stress resistance, enhance seed germination, and seedling establishment. The use of ML methods is especially helpful in curtailing the irreproducible effects of CP in seed priming.12 The aim and scope of this review discusses briefly how ML methods or algorithms could serve as an effective means of optimizing parameters of CP as part of real-time monitoring to develop the best priming treatment for different types of seeds.2. Major challenges associated with seed germination
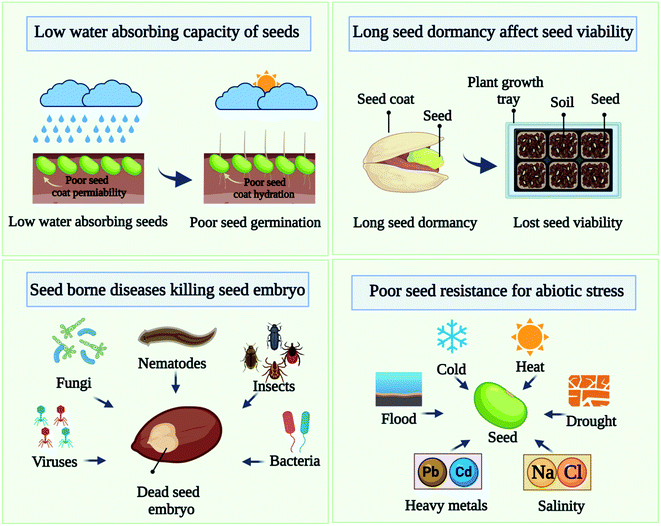
For the maintenance and expansion of plant populations as well as their recovery from disturbances, seed germination and establishment are essential. The use of chemical fertilizers poses serious challenges to balancing sustainable seed germination and growth. Many factors can contribute to seeds not germinating or seedlings dying before they can become healthy plants. Some major issues associated with seeds are listed below in Fig. 1:2.1 Low water-absorbing capacity of seeds
Seed water uptake is assumed to be a major factor affecting seed germination.13 Water absorption is the first step in germination, increasing the rate of water absorption should increase germination. Even in agricultural situations with different soil temperatures, water absorption may be influenced by soil temperature even if the supply of water is adequate. In order to understand the seed coat hydration behavior, the permeability of the seed coats has been extensively examined. A seed with poor hydration kinetics exhibits a barrier to water penetration and could be considered an impermeable seed.14 When water is available to a permeable seed (normal), it readily absorbs it. However, an impermeable seed (hard) may not absorb water for hours, days, and even weeks, resulting in a poor germination rate.2.2 Seed dormancy
Seed dormancy is one of the key traits altered during the domestication process since it is crucial for crop establishment. Dormancy is an adaptable trait that enables seeds of many species to remain dormant until the conditions are favorable for germination. Conversely, seed dormancy is a complex phenomenon that is influenced by both endogenous and external factors; it is often considered one of the least understood aspects of seed biology. In this process, both intrinsic and extrinsic cues are involved, which result in a complex set of physiological and biochemical processes. Seed dormancy and germination are influenced by ethylene production, nitric oxide production, brassinosteroids production, and reactions to light, temperature, and other environmental factors, among others. In seed germination, dormancy occurs when the seed coat is not permeable or when enzymes are insufficient (internal dormancy).15–17 Most field crops are affected by dormancy, which limits crop production. It is possible for seeds to lose viability during dormancy. To preserve the genetic integrity of seeds, seed viability needs to be preserved for a longer duration. Dormancy of the seeds delays the seedling, which can lead to non-uniform germination and difficulty maintaining plant populations.2.3 Seed borne diseases
It is important to use the healthy seed in order to successfully farm all types of crops. A healthy seed or one that is pathogen-free is essential. A good harvest depends on the health of the seed population. The health of a seed lot is determined by the presence or absence of disease-causing organisms in the seeds, such as fungi, bacteria, viruses, nematodes, and insects. Among the factors contributing to low germination rates are epiphytic and phytopathogenic bacteria, as well as filamentous fungi contaminating seeds. These infections may weaken or kill embryos, storage fungi kill and weaken seed embryos over time. The quality of seed is affected by several factors, including seed-borne pathogens that not only inhibit seed growth but also reduce seed vigor, which leads to low yields.18 Plant-borne pathogens reduce plant germination ability not only by affecting the morphology of the plant in the field, but they can reduce yields by 15 to 90%.19,20 Crop yields can be increased significantly when seeds are healthy, in addition to aiding in successful cultivation.212.4 Poor resistance to stress
Seed growth and development are adversely affected by abiotic stresses or constant changes in the external environment. Drought, salinity, heat, cold, and heavy metals are some of the abiotic stresses that seeds experience that cause complex response that result in reduced crop yield as well as reduced growth.22 There is a significant reduction in average crop yield of >50% as a result of these stresses. By activating different signaling cascades and regulating physiological and growth processes abscisic acid biosynthesis is able to mediate stress adaptation responses.23 Many different levels are being studied regarding the impact of abiotic stress on seed performance, including metabolic/physiological responses, molecular signaling pathways, ecophysiology, and crop breeding. Abiotic stress results in altered biosynthesis and nutrient acquisition, which can inhibit plant growth. Multiple signaling pathways involve many genes, proteins, and post-translational modifications. A number of stress-responsive transcription factors orchestrate the downstream responses required to mount a defense against specific abiotic problems, including MAPK, ABF/bZIP, Ca2+-CBL-CIPK, and CBF/DREB pathways. It is important that these molecular signaling pathways can anticipate and balance the effects of abiotic stress. Abiotic stress responses are mediated by signal transduction pathways mediated by phytohormone. It has been found that abscisic acid plays a major role in stress reactions, while auxin is key to plant growth.New technologies are in high demand to overcome these challenges and to improve seed performance because conventional methods have many limitations. Cold plasma priming technology, an environmentally friendly, non-thermal method that offers unique advantages over traditional processing technologies, has recently been tested by researchers for its potential to improve germination.3–6 A cold plasma priming treatment can cause seed coat erosion. It has been observed that, as a consequence of the cold plasma treatment, seeds often have a slightly damaged surface. Germination occurs when the hard seed coat is scratched or nicked to allow moisture within the seed to enter. In order to break seed dormancy, plasma treatment generates reactive species, which are formed during plasma discharge, including nitric oxide, which breaks seed dormancy and speeds up germination. Inactivating bacteria and fungi with cold plasma treatment allows seeds to be less likely to breed disease and cause economic losses.24 Plasma-treated seeds are less likely to harbor bacterial contamination and therefore pose fewer health risks.
3. Overview of cold plasma technology
The plasma is a fourth fundamental state of matter which consists of electrons, positively charged ions, radicals, gas atoms, molecules (in ground or excited state), and photons from a range of energies, including ultraviolet and vacuum ultraviolet radiation.25 The energy and electrical density of plasma are the most significant characteristics to consider. Different types of natural or artificial plasmas can be recognized based on the values of microscopic parameters, and are divided into two categories: hot plasmas and cold plasmas. CPs are artificial plasmas produced by a low frequency (0.02 to 0.4 MHz), radio frequency (RF) reduction of flux infiltration (<500 MHz), or microwave (0.5 to few GHz) persist or alternate discharge at relatively low pressure (10−2–10 Torr).25 The energy and electronic density of CPs are equal to 1–10 eV and ∼1010 cm−3, respectively. Their ionization degree is less than 10−3, therefore the gas phase is mostly made up of excited neutral species or radicals of neutral species.26 The absence of thermodynamic equilibrium between the electronic temperature (∼1000 °C) and gas temperatures (close to ambient) is the main feature of these plasmas. CPs are often created using different type of reactors such as tubular-type and bell-jar-type reactors.27 The plasma generated reduces reactive oxygen and nitrogen species (ROS and RNS), as well as changes the physical and chemical properties of the solution, such as pH, electrical conductivity, and oxidation–reduction potential.3.1 Type of cold plasmas
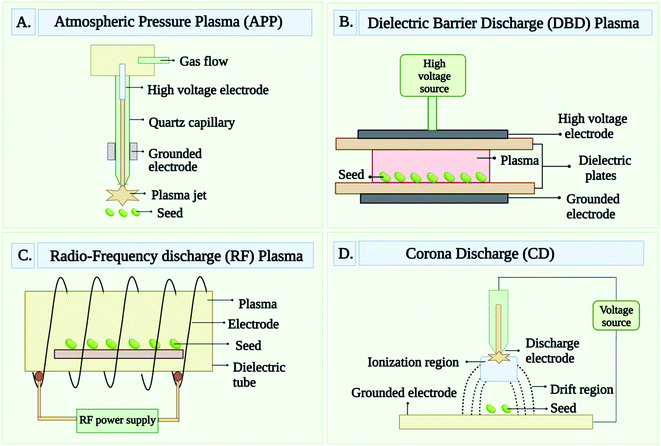
Different type of plasma techniques has been used to generate the CPs. However, among all plasma techniques, atmospheric pressure plasma, dielectric-barrier discharge, glow discharge or radio frequency discharge, and corona discharge are the most common techniques.28 These all techniques that are used to generate CPs are briefed below and well-drawn in Fig. 2(A–D). Atmospheric Pressure Plasma (APP) provides the maximum plasma density. It is a one-of-a-kind nonthermal discharge plasma that can operate at atmospheric pressure. The feed gas, which is primarily an inert carrier gas like He with a minor amount of additives like CF4, O2, and H2O, is discharged at a high flow rate.29 Dielectric-Barrier Discharge (DBD) is generated by passing a pulsed voltage between two electrodes, one of which is covered by a dielectric solid substance.30 Dielectric coatings prevent the generation of thermal plasma that would result from arc discharges and enhance treatment uniformity.31 One electrode is usually operated at less frequency (∼100 kHz) or ac current while the other is grounded. Due to the DBD plasma's extremely short time duration, typically measured in microseconds, the temperature of the gas does not typically raise much.30,31 Glow Discharge or Radio Frequency Discharge (GD or RFD) is produced at a low pressure which ensures the best homogeneity throughout treatment. DC current, RF frequency (0.04 to 13.56 MHz), or low frequency (50 Hz) voltage is applied to both electrodes to create plasma.32,33 Using a microwave (2.45 GHz) power supply, an electrodeless microwave glow discharge can also be accomplished.34 This plasma can be categorized into two types such as capacitive-coupled plasmas (CCP) and inductively coupled plasmas (ICP), which is depends on the generation of plasma inside the coil. Under low-pressure conditions, an RF set-up can generate a relatively large volume of high-density plasma with minimal heating.35 When a pulsed high voltage or low frequency is applied to an electrode pair at atmospheric pressure, the Corona Discharge (CD) forms. The size of the electrodes varies greatly. A series of tiny lightning-like discharges comprise this plasma. Because of its inhomogeneity and high local energy levels, this is not a desirable method for treating materials.3.2 Characterization of cold plasma
Plasmas are formed by electrons being produced, their density rising to about ne = 1011–1016 cm−3 and then they are being accelerated by an electric field in a gas medium.36 An important parameter of plasmas is its electron density. When electrons travel at high speeds and densities, they collide a lot with each other and with atoms and molecules. In plasmas, a wide variety of reactive oxygen and nitrogen species are produced through a variety of chemical reactions. Through electric discharge in a gas, which is often defined as a partially ionized gas containing both charged and neutral particles, CPs are generated. In CPs, ions, radicals, and excited molecules are also found along with photons emitted from dissociating electrically excited molecules. As shown in Fig. 3, CPs can generate short-lived species with half-lives in the s range (H2O2, NO2−, NO3−, O3) and relatively long-lived species with half-lives in the millisecond range (O, ˙OH, O2−, 1O2, NO˙, NO2˙).37 Relative species concentrations of various charged and neutral species produced by gas-phase CPs plasmas are as follows (Table 1). Using an electric field, gases such as Ar, O2, N2, He, and/or air can be ionized to produce electrons, ions, UV, thermal radiation, and reactive species. The air plasmas contain ROS such as superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH˙), singlet oxygen (1O2), ozone (O3), and RNS such as nitric oxide (NO˙), peroxynitrite and nitrogen dioxide radical (NO2˙). The CPs can be generated by using neon, argon, or nitrogen as the working gas. Inert gases like Ne, Ar, and N2 can have their meta-stable forms formed by electric discharges. Ozone, or 1O2, is created when a small amount of O2 is added to another working gas.38 The results are other reactive particles and OH radicals. Adding water to CP-treated samples leads to the formation of very reactive OH radicals, the by-product of water in the sample. NO and other nitrogen oxide species may be produced in air or when air is present as part of the working gas. However, the final composition of CPs is dependent on more parameters than just working gas. CPs composition can be affected by discharge characteristics (DBD, CD, microwave discharge, gliding arc, plasma jet, etc.), input parameters (frequency, voltage, power density, etc.), and gas flow.37 Changing the physical input parameters (amount of energy supplied) of plasma devices can lead to differing CPs compositions or particle counts. Because of this, it can be challenging to compare the results of different devices that use plasma.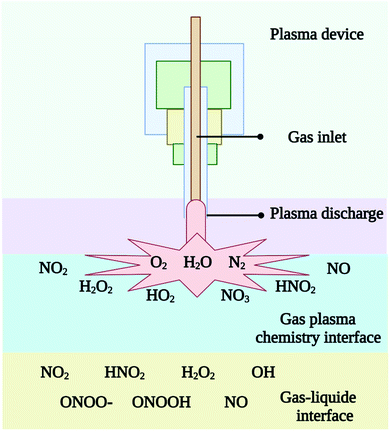 | ||
| Fig. 3 Different reactive species originate from gas plasma, both in the atmosphere and at the gas–liquid interfaces. | ||
| Plasma generated species | Chemical formula | Density (cm−3) |
|---|---|---|
| Superoxide radicals | O2˙− | 1010–1012 |
| Hydroxyl radicals | OH˙ | 1015–1017 |
| Hydrogen peroxide | H2O2 | 1014–1016 |
| Singlet oxygen | 1O2 | 1014–1016 |
| Ozone | O3 | 1015–1017 |
| Nitric oxide | NO | 1013–1014 |
| Electrons | e− | 109–1011 |
| Positive ions | M+ | 1010–1012 |
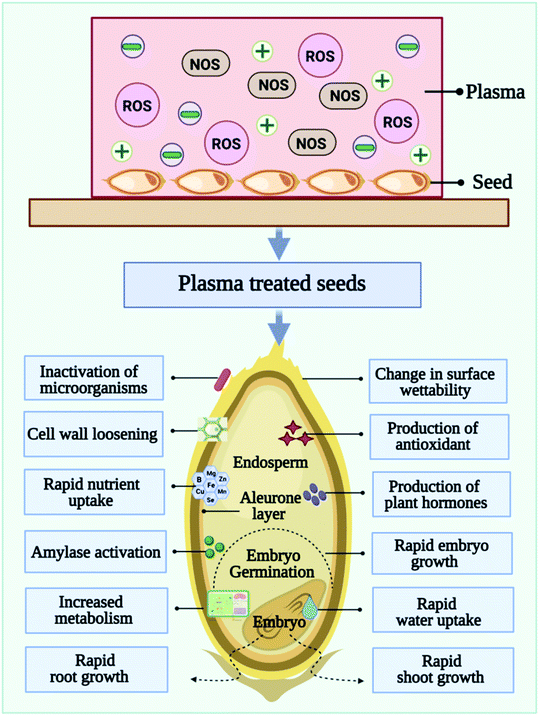
4. Cold plasma effects on seed germination
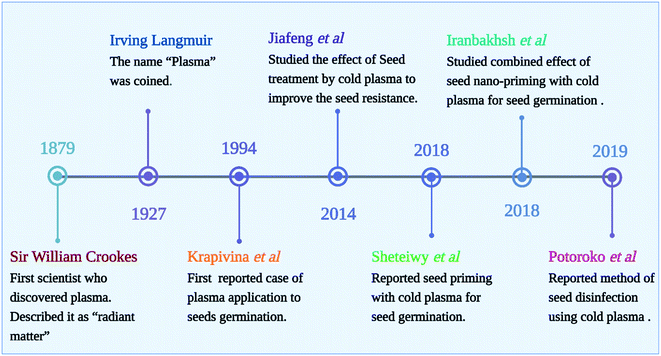
Depending on the plasma system, different modes of CP can be applied to the seeds. The treatment can be performed in direct mode or glow mode, or indirect mode (afterglow). Plasma particles (ions, electrons, and other excited atoms and molecules) are emitted from the surface exposed to the discharge (direct plasma treatment) and is UV and/or VUV radiation sources.39,40 While indirect treatment uses non-equilibrium gas, the samples are still exposed to long-lived radicals without being exposed to the glowing plasma region. Usually, indirect plasma treatments are weaker and require longer exposure times in order to achieve similar results to direct plasma treatments. The physical factors such as heat, ultraviolet light, electromagnetism fields, and mechanical scarification are the first points of contact with the seed coat, triggering downstream consequences. As depicted in Fig. 4, the beginnings of the use of cold plasma in seed quality improvement can be traced back to Sir William Crookes, who observed plasma for the first time in 1879, describing it as “radiant matter”. In 1927, Irving Langmuir gained the Nobel Prize for Chemistry for coining the term “plasma”. Considering that Langmuir discovered the plasma sheath phenomenon, he may be considered the first plasma physicist in history. Krapivina et al. published the first US patent on plasma application to seeds, which enhanced the germination and growth of soybean seeds with cold atmospheric pressure plasma generated from a mixture of inorganic gases.Scientists have observed the effects of CP exposure on seed germination and later growth and development of seedlings. Moreover, plasma treatment could also increase plant metabolism, which significantly increases crop yields. The agents generated by plasma may trigger signaling cascades and exert various biochemical and structural effects. The treatment of seeds with CP can be effective in increasing germination rates and promoting faster germination in various crops.6 CP treated seeds grew better roots and shoots while control seeds developed differently. Moreover, root branching development varied depending on the plasma type, treatment conditions, plant species, and even plant variety. Thus, systematic studies relating seed type and variety to treatment conditions are needed. A systematic study correlating treatment conditions with seed types and varieties is thus needed.3,9 Although CP has been shown to improve germination and viability of seeds, we are still unsure what mechanisms are behind the effect. In order to understand plasma–seed interactions, it is important to note that different research groups use different experimental setups, parameters, and types of seeds. All of these factors contribute to the results. In this review, all their results are summarized in Table 2, and in Table 3, summarizes a few of the findings regarding the use of cold plasma in seed treatment found in patent databases and literature.
| No. | Seed type | Plasma treatment | Exposure time | Results | References |
|---|---|---|---|---|---|
| 1 | Broccoli | Corona discharge plasma jet; atm. pressure: air (voltage: 20 kV DC, frequency: 58 kHz) | 0–3 minutes (min) | Improved seed metabolism, reduced microbial load on seeds | 41 |
| 2 | Cotton | DBD; atm. pressure; air, N2 (voltage: 19 kV, frequency: 1 kHz) | 3 min, 9 min, and 27 min | Improved seed coat for healthy germination | 42 |
| 3 | Pepper & lentil | RF inductive; low pressure; air (frequency: 13.56 MHz; pressure 0.5 Torr; power: 18 W) | 60 seconds (s) | Improved seed coat for healthy germination | 43 |
| 4 | Soybean | DBD; atm. pressure; Ar (voltage: 22.1 kV) | 12 s | Improved seed germination and seedling growth | 44 |
| 5 | Barley | DBD; atm. pressure; N2 and air (plasma power: 400 W) | 0 to 80 s | Improved seed germination and plant growth | 45 |
| 6 | Norway spruce | CCP RF; low pressure (60 Pa); air (frequency: 5.28 MHz, specific power of 0.35 W cm−1) | 5, 10, and 15 min | Improved seed germination and seed growth | 46 |
| 7 | Chili pepper | DBD; atm. pressure; Ar (voltage: 11 kV, frequency: 23 kHz) | 0, 60 and 120 s | Improved seed germination and seed growth | 47 |
| 8 | Wheat | DBD; atm. pressure; Ar (plasma power: 80 W) | 15 s, 30 s, 60 s, 90 s and 120 s | Improved wheat growth and salinity resistance | 48 |
| 9 | Cucumber & pepper | DBD; atm. pressure; air (frequency: 15 kHz and power: 400 W) | 4 s for pepper, 20 s for cucumber | Improved seeds germination and reduced disease | 49 |
| 10 | Tomato | DBD (fluidized); atm. pressure; air (voltage: 13–17 kV amplitude: 50 Hz) | 5, 15, 30 and 45 min | Improved seed germination and seed growth | 50 |
| 11 | Arabidopsis | (1) DBD; atm. pressure; air | 15 min | Improved seed germination and seed growth | 51 |
| (2) Plasma jet; atm. pressure; He (voltage: 10 kV, frequency: 9.7 kHz) | |||||
| 12 | Sunflower | Plasma flashlight; atm. pressure Ar, O2 (voltage: 8, 10, 12, and 14 kV) | 1, 3, and 5 min | Improved seed germination and seed growth | 52 |
| 13 | Arabidopsis, radish | RF; low pressure (20–80 Pa); O2, Ar (frequency: 13.56 MHz, power: 60 W) | — | Improved seed germination and seed growth | 53 |
| 14 | Thuringian mallow | Gliding arc; atm. pressure; N2 (voltage: 3.7 kV, frequency: 17 kHz) | 1, 2, 5, 10 and 15 minutes | Improved seed germination and seed growth | 54 |
| 15 | Wheat | DBD; low pressure (10 Torr); air, Ar, O2 (voltage: 5–10 kV, frequency: 3–8 kHz) | — | Modified seed coat, improved seed germination and growth | 55 |
| 16 | Pea & zucchini | Gliding arc; atm. pressure; air | 30 and 60 s | Improved seed germination | 56 |
| 17 | Maize, peppers, wheat, soybeans, tomatoes, eggplants, pumpkins | CCP glow RF; low pressure (30–200 Pa); air, He (frequency: 13.56 MHz, power: 50–1000 W) | 5–90 s | Improved seed germination | 57 |
| 18 | Coffee and grape seeds | DBD; atm. pressure; He (frequency: 10 kHz) | 30 s, 60 s, 120 s and 240 s | Improved seed germination | 58 |
| 19 | Artichoke | CCP RF; low pressure; N2 (plasma power: 10 W) | 3, 10, and 15 min | Improved seed germination and seed growth | 59 |
| 20 | Tomato | ICCP RF; low pressure (150 Pa); He (frequency: 13.56 MHz) | 15 s | Improved seed germination | 60 |
| 21 | Asparagus | RF; low pressure (800 mTorr); N2, O2 (frequency: 13.56 MHz, plasma power: 50 W) | 1, 15 and 30 min | Improved seed germination | 61 |
| 22 | Wheat | RF capacitive; low pressure; Ar (voltages: 200–800, frequency: 13.56 MHz) | 1–8 min | Improved seed germination, pesticidal effect against red flour beetles | 62 |
| 23 | Wheat | DBD; low pressure (10 Torr); air, Ar, O2 (voltage: 5–10 kV, frequency: 3–8 kHz) | 90 s | Reduced toxicity of cadmium. Improved seed germination | 63 |
| 24 | Rapeseed, mustard | DBD; low pressure (10 Torr); air, Ar, O2 (voltage: 3–6 kV, frequency: 3–10 kHz) | — | Improved metabolism | 64 |
| 25 | Wheat | Not clear; low pressure; Ar (voltage: 800 V, frequency: 10 Hz) | 1–4 min | Improved seed germination and seed sterilization | 65 |
| 26 | Basil | RF; low pressure; (0.40 mbar) O2 and Ar (frequency: 13.56 MHz, power: 300 W) | 10 min | Improved seed growth and seedling establishment | 66 |
| 27 | Arabidopsis | DBD; atm. pressure; air (voltage: 10 kV frequency: 10 kHz) | — | Improved seed germination and salinity resistance | 67 |
| 28 | Wheat | Plasma jet; atm. pressure; N2 (frequency: 20 kHz, voltage: 2.6 kV) | 2, 4, 6, 8 and 10 min | Improved water uptake and germination | 68 |
| 29 | Melissa officinalis | DBD; atm. pressure; Ar (voltage: 10 kV, frequency: 13 kHz) | 0, 50 or 90 s | Synergistic effect of cold plasma and nanoparticles to improve seed germination | 69 |
| 30 | Soybean | Needle to plane DBD; atm. pressure; N2, O2 (voltage: 25 kV, frequency: 50 Hz) | 60 to 180 s | Improved seed germination and yield | 70 |
| 31 | Astragalus fridae | DBD; atm. pressure; Ar | 0, 30, 60, and 90 s | Improved seed germination, physiology and growth | 71 |
| 32 | Wheat | DBD; atm. pressure; air (voltage: 80 kV, frequency: 50 Hz) | 30, 60, or 180 s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Improved seed germination and seed surface chemistry | 72 |
| 33 | Radish, mung bean, wheat, tomato, lettuce, mustard, Dianthus and sticky bean | DBD jet; atm. pressure; N2, O2, air (voltage: 0–18 kV, frequency: 500 Hz) | — | Improved seed germination | 73 |
| 34 | Arabidopsis | DBD; atm. pressure; air (voltage: 8.47 kV, frequency: 7.95 kHz) | 0.5, 1, and 3 min | Improved seed germination | 24 |
| 35 | Pine | DBD; atm. pressure; air (voltage: 10 kV, frequency: 14 kHz) | 5, 10, 60, 180, and 300 s | Improved seed growth and sterilization of seeds | 74 |
| 36 | Sunflower | CCP RF; low pressure (200 Pa); air (voltage: 17.96 kV, frequency: 5.28 MHz) | 2, 5, and 7 m | Improved seed germination and seedling development | 75 |
| 37 | Pea | DBD; atm. pressure; air (plasma power: 15 W) | 1–10 min | Improved seed growth | 76 |
| 38 | Bitter melon | DBD; atm. pressure; Ar (voltage: 10 kV, frequency: 13 kHz) | 0, 60, and 120 s | Improved seed germination, growth and yield | 77 |
| 39 | Basil | DBD; atm. pressure; air | 30 s and 3 m | Improved seed germination and seedling development | 78 |
| 40 | Moringa | RF; low pressure; Ar (frequency: 13.56 MHz, power: 0–200 W) | 1, 5, 10, and 15 min | Improved seed germination and growth | 79 |
| 41 | Arabidopsis (seedlings) | DBD; atm. pressure; air (voltage: 6 kV and frequency: 20 kHz) | 2, 5, 10, 15, or 20 s | Improved seed germination and growth | 80 |
| 42 | Maize, wheat, lupine | CCP RF; low pressure (200 Pa); air (frequency: 5.28 MHz) | 2, 4, 5 and 7 min | Improved seed growth and sterilization of seeds | 81 |
| 43 | Grape cultivar Muscat of Alexandria | DBD; atm. pressure; He and O2 (plasma power: 30 W) | 2, 5 and 10 min | Improved seed germination and growth | 82 |
| 44 | Catharanthus roseus | DBD; atm. pressure; Ar (power: 80 W; voltage: 10 kV frequency: 13 kHz) | 0, 30, 60, and 90 s | Improved seed germination and seedling growth | 83 |
| 45 | Radish | DBD; atm. pressure; humid air (frequency: 14.4 kHz) | 3 min | Modified seed coat, improved seed germination and growth | 84 |
| 46 | Hyssop | Not clear; atm. pressure; air (voltage: 23 kV) | 1, 5, and 10 min | Tissue modification | 85 |
| 47 | Black gram | DBD; low pressure (400 Torr); air (voltage: 5 kV, frequency: 4.5 kHz) | 20 to 180 s | Modified seed coat, improved seed germination and growth | 86 |
| 48 | Rice | (1) Arc discharge; low or atm. pressure; underwater | 10–30 min | Improved seed germination and disease resistance | 87 |
| (2) DBD; low and atm. pressure; (0.6–1 atm); not clear (frequency: 12 Hz) | |||||
| 49 | Hemp | DBD; atm. pressure; Ar (frequency: 13 kHz; power: 80 W) | 0, 40, and 80 s | Improved seed germination | 88 |
| 50 | Arabidopsis | DBD; atm. pressure; air (voltage: 7 V, frequency: 500 Hz) | — | Improved seed germination | 89 |
| 51 | Coriander | (1) DBD; atm. pressure; Ar, N2, air | 30 s, 1 and 3 min | Improved seed germination and development | 90 |
| (2) Microwave plasma torch for NO; N2, O2 (frequency: 15.4 kHz, power: 400 W) | |||||
| 52 | Clover | Not clear; not clear; not clear (plasma power: 20–280 W) | — | Improved seed germination and seed growth | 91 |
| 53 | Pea | DBD; atm. pressure; air (voltage: 10 kV, frequency: 14 kHz) | 60 to 600 s | Improved seed germination and metabolism | 92 |
| 54 | Wheat | DBD; atm. pressure; air (frequency: 50 Hz) | 5, 15, 30 min | Improved seed germination and early growth | 93 |
| 55 | Radish | DBD; atm. pressure; air, O2, NO, He, Ar, N2 (voltage: 9.2 kV, frequency: 10 kHz) | 3 min | Improved seed growth | 94 |
| 56 | Radish | Plasma flashlight; atm. pressure; Ar (voltage: 0–30 kV) | 2, 4 and 6 min | Improved seed germination | 95 |
| 57 | Ajwain | CCP RF; low pressure; air (plasma power: 50 W) | 2 min | Improved seed germination | 96 |
| 58 | Peanut | CCP RF; low pressure (150 Pa); He (frequency: 13.56 MHz, power: 60–140 W) | 15 s | Improved seed germination, growth and yield | 97 |
| 59 | Rice | Hybrid microcorona discharge; atm. pressure; air, Ar; (frequency: 700 Hz) | ∼1 min | Improved seed germination and disease resistance | 98 |
| 60 | Mung bean | Plasma jet array; atm. pressure; He, N2, air, O2 (voltage: 0–20 kV frequency: 9.0 kHz) | 3 min | Improving seed germination and seedling growth | 99 |
| 61 | Wheat | DBD; atm. pressure; air (voltage: 20 kV, frequency: 14 kHz) | 10–600 s | Improved seed germination and disease resistance | 100 |
| 62 | Mulungu | Plasma jet DBD; atm. pressure; He (voltage: 10 kV, frequency: 750 Hz, power: 150 W) | 60 s | Modification of seed coat, improved seed germination | 101 |
| 63 | Bell pepper | Glow discharge; low pressure (0.2 mbar); O2 | 0, 3, 6, 9, 12, 15 min | Seed coat modification, growth parameters | 102 |
| 64 | Wheat | DBD; atm. pressure; air, Ar, O2, N2 (voltage: 13.0 kV, frequency: 50 Hz) | 2 min | Improved seed germination and seedling growth | 103 |
| 65 | Mimosa | DBD; atm. pressure; air (voltage: 17.5 kV, frequency of 990 Hz) | 3, 9 and 15 min | Increased wettability and seed germination | 104 |
| 66 | Soybean | Needle to plane DBD; atm. pressure; Ar (voltage: 22.1 kV, frequency: 60 Hz) | 12 s | Improved seed growth and seed metabolism | 105 |
| 67 | Wheat | DBD; atm. pressure; air (voltage: 0–50 kV, frequency of 50 Hz) | 4 min | Improved seed germination and drought resistance | 106 |
| 68 | Quinoa | (1) DBD; low pressure (500 mbar); dry air | 15 min | Improved seed germination | 107 |
| (2) RF; low pressure (0.1 mbar); dry air (voltage: 8.2 kV, frequency: 1 kHz) | |||||
| 69 | Radish | Corona discharge plasma jet; atm. pressure air (voltage: 20 kV, frequency: 58 kHz) | 2 min | Improved seed germination and disease resistance | 108 |
| 70 | Rapeseed | Corona discharge plasma jet; atm. pressure; air (voltage: 20 kV, frequency: 58 kHz) | 3 min | Improved seed germination and disease resistance | 109 |
| 71 | Cultivars of hemp | (1) Gliding arc; atm. pressure; humid air | 0, 180, 300 and 600 s | Improved seed treatment | 110 |
| (2) Microwave plasma discharge; low pressure (140 Pa); Ar, O2 (frequency 50 Hz) | |||||
| 72 | Wheat | DBD; atm. pressure; air (voltage: 0–50 kV, frequency: 50 Hz) | 0, 1, 4, 7, 10, and 13 min | Improved seed germination and growth | 111 |
| 73 | Purple coneflower | CCP RF; low pressure (60 Pa); air (frequency: 5.28 MHz) | 2–7 min | Growth parameters | 112 |
| No. | Inventors | Patent year | Patent registration no. | Title of patent | Description of invention |
|---|---|---|---|---|---|
| 1 | Yuanhua Dong et al. | 2018 | US20150327430A1US20150327430A1 | Cold plasma seed treatment device | The present invention belongs to the cold plasma seed treatment field, and particularly relates to a cold plasma seed treatment device |
| 2 | Ferencz S. Denes et al. | 2000 | WO2014086129A1 | Cold-plasma treatment of seeds to remove surface materials | Plasma processing of materials and particularly to plasma treatment of seeds |
| 3 | Edward Bormashenko et al. | 2013 | WO2013168038A1 | Processing seeds by cold plasma treatment to reduce an apparent contact angle of seeds coat surface methods | Methods for reduction on apparent contact angle of seeds coat surface by cold plasma treatment, agricultural devices for said treatment and seeds obtained by methods thereof |
5. Cold plasma and seed priming technology
Seed priming is a pre-sowing and further drying treatment, this approach activates pre-germination metabolism and speeds up the seed germination process. The seed priming technique is an established and reliable technique for enhancing germination efficiency and plant growth.113 It improves yield by improving vigor, reducing germination time, and reducing seedling mortality. Among the many techniques for priming seeds, hydropriming, halopriming, osmopriming, biological priming, chemical priming, and hormonal priming are among the most popular.114 Priming makes it possible for the seed to take up water (imbibition), which triggers a series of actions called pre-germinative metabolism. DNA repair and oxidative stress are two critical events that occur before pre germinative metabolism is fully activated. Reactive oxygen species act as signaling molecules in the germination cascade. During germination, antioxidant mechanisms also are induced to maintain reactive oxygen species accumulation.115 Cold plasma induces reactive species and charged particles that affect the seed coat, causing the surface to crack and making it easier for water to penetrate.116 While a small number of studies have examined the long-standing effects of cold plasma seed priming on plant systems, these processes break dormancy and accelerate seed germination. It is important to study the long-term effects of cold plasma seed priming on plants to evaluate its versatility. The mechanisms of molecular changes and their regulation after cold plasma seed priming yet remain unclear and is a potential target for future research.5.1 Seed priming with cold plasma
Adhikari et al. (2020)117 assessed the germination and subsequent growth of tomato plants (Solanum lycopersicum) by cold plasma priming. By priming seeds with cold plasma, the seed coat is modified and free radicles are generated. Radicles of reactive oxygen and nitrogen regulate epigenetic levels in tomato seedlings, promoting germination at the seedling stage and promoting germination at the seed stage. Various antioxidant, phytohormone, and stress resistance-related genes are affected by epigenetic expression, resulting in morphological and biochemical changes in tomato seedlings. Moreover, cold plasma primed seedlings developed the ability to cope with drought stress induced by polyethylene glycol (PEG). The effects of cold plasma on tomato seed priming were examined in terms of its growth, development, and its stress capacity. Cold plasma seed priming will probably have a wide application in tomato crop improvement in the future according to this study. Ghaemi et al. (2020)118 have investigated the transcriptional changes following seed priming with cold plasma and electromagnetic fields in Salvia nemorosa L. An analysis of the effects of cold plasma or electromagnetic fields on early growth, biomass accumulation, and expression of SnWRKY1, SnAREB1, SnCCR2, and SnRAS genes has been conducted in S. nemorosa. Here, the authors propose that the plasma or electromagnetic field-mediated modulation of WRKY1 and AREB1 genes may play a crucial role in the improvement of plant protection under stress. Further, primed seeds with cold plasma or electromagnetic fields modulated the expression of SnCCR2 and SnRAS genes, which are involved in secondary metabolism. Using these findings, seed and agriculture technologies may improve knowledge of plants' reactions to cold plasma and electromagnetic fields. According to Ghasempour et al. (2019), seed priming with cold plasma improved seedling performance, secondary metabolism, and expression of the deacetylvindoline O-acetyltransferase gene in Catharanthus roseus.83 The present study indicated that cold plasma influenced growth, photosynthetic pigments, antioxidant enzymes, and proline concentration in response to the exposure time. Using cold plasma to seed prime led to improvements in total soluble phenols, PAL activity, DAT gene expression (which contributed to the synthesis of alkaloids), and an alkaloid concentration. In Catharanthus roseus, as a valuable pharmaceutical plant, a molecular analysis of plasma influences on secondary metabolism is potentially presented. Using cold plasma therapy and exogenous salicylic acid (SA) priming, Sheteiwy et al. (2018) studied Oryza sativa seedling salinity tolerance.119 Chlorophyll fluorescence, photosynthetic pigments, and photosynthetic gas exchange were improved by cold plasma treatment and SA priming improved seed performance under salinity stress in comparison with untreated seeds. Cultivars of rice under salinity stress significantly improved their antioxidant enzyme activities when prioritizing SA with cold plasma treatment. A combination of cold plasma and SA priming under salinity stress increased the activities of enzymes involved in secondary metabolism assimilation. Under salinity stress, the activities of enzymes involved in secondary metabolism assimilation were up-regulated with either cold plasma alone or cold plasma combined with SA priming. Hence, the cold plasma treatment and SA priming can be helpful in improving the growth of rice in high salinity soil.5.2 Integrating cold plasma priming with nanomaterials
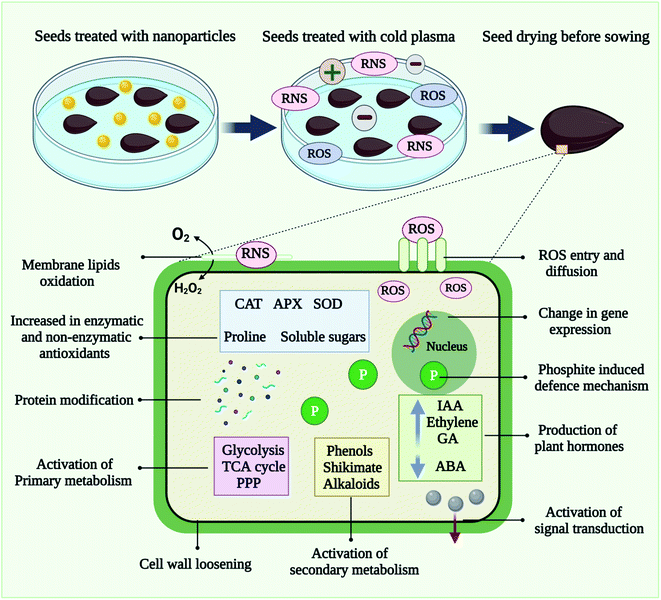
Plant species from across the globe have benefited from nanopriming involving various nanomaterials in terms of seedling growth and germination.120,121 As shown in Fig. 5, cold plasma and nanoparticles work together as a seed priming treatment at the molecular level. In seed treated with nanoscale particles and cold plasma, the enhancement of antioxidant enzyme systems and the boosting of defense response improve physiological processes and contribute to pathogen inactivation.Seed priming with cold plasma and multi-walled carbon nanotubes (MWCNT), Seddighinia et al. (2020) examined bitter melon (Momordica charantia) growth, tissue differentiation, anatomy, and yield.77 Treatments with plasma and MWCNT concurrently amplified their individual effects. The study examined the potential benefits of seed priming with plasma and MWCNTs on Momordica charantia's growth rates, tissue differentiation, anatomy, and reproductive efficiency, as well as possible contributing mechanisms. Several positive phenotypic, physiological, developmental, and anatomical alterations were observed for both individuals and combinations of plasma and CNT treatment. A dose of MWCNTs of the highest applied level did not result in any toxicity. MWCNT may hold promise for cultivating crops even under long-term exposures. Cold plasma and CNT technologies can be applied to the plant, agriculture, and food sciences according to the findings. In a study by Abedi et al. (2020), seed priming with cold plasma improved Cichorium intybus early growth, flowering, and protection against selenium nanoparticles. This result provides further insight into the potential advantages of cold plasma in terms of improving plant growth and protection. This finding demonstrates that plasma can be used to improve plant tolerance to stress conditions by enhancing plant defense mechanisms, particularly antioxidant release mechanisms. Further, plasma priming in combination with nano-selenium at a very low optimum dose can be utilized as an efficient protocol to support plant growth, biochemistry, and protection. Using a non-thermal plasma, Babajani et al. (2019) investigated seed priming with modifications regarding plant reactions to selenium oxide or zinc oxide nanoparticles.69 A study was conducted to determine whether seed priming with non-thermal plasma could modify the responses of Melissa officinalis nanoparticles to zinc oxide (nZnO) or selenium (nSe) nanoparticles. Plasma was used as a primer for germinating seeds, and then the seeds were cultured in Petri dishes containing a Hoagland nutrient solution modulated by nSe and nZnO. The plasma treatment not only enhanced growth-related traits (say, stem, root, and leaf width) and led to more biomass accumulation, but also reduced the toxicity signs of nSe. It has been reported that M. Moghanloo et al. (2019) discovered differential expression and physiology of phenylalanine ammonia-lyase (PAL) and universal stress protein (USP) in endangered species.122 Using cold plasma for priming seeds as well as silica nanoparticles for treating culture medium-induced peroxidase activities in roots and leaves of the plants. The simultaneous application of plasma and nSI enhanced the expression rate of phenylalanine ammonia lyase gene. USP expression levels in plasma- and nSi-treated seedlings were not significantly different, but nSi-treated seedlings displayed higher levels of USP. Following plasma and nSi exposure, leaf thicknesses and vascular development (xylem and phloem) were reinforced. In the study, evidence demonstrates the potential benefits of plasma and nSi against phytotoxicity, which may serve as the basis for possible commercial exploitation. Cold plasma on Capsicum annum cayenne restored all signs of toxicity from nano zinc oxide, as shown by Iranbakhsh et al. (2018).47 The present study examined the plant's (Capsicum annuum) responses to cold plasma and zinc oxide nanoparticles (nZnO) in vitro and in-pot, using functional scientific devices and metal-based nanoparticles. It was found that nZnO and/or plasma treatments played an important role in eliciting peroxidase activity in both culture media. We also found that both roots and leaves of the plasma and nZnO groups exhibited significantly higher activities of phenylalanine ammonia-lyase and soluble phenols. The plasma treatments alleviated the inhibiting effects of nZnO on xylem differentiation. During the pot experiment, soaking the seeds before plasma treatment was the most effective way to stimulate plant growth.
6. Role of reactive species in cold plasma seed priming
As discussed earlier, a CP is a mixture of neutral gas, ionized gas, reactive oxygen species (ROS) and reactive nitrogen species (RNS), and positively charged particles. As shown in Fig. 6, in CP- mediated seed priming, ROS, and RNS play a wide range of regulatory roles during seed development and growth processes including germination, metabolism, signal transduction, nutrient uptake, senescence, and the ability to tolerate biotic and abiotic stress.123 During the past three decades, it has been discovered that ROS can act as helpful signaling molecules to regulate stress response processes in primed seeds.113 ROS are commonly produced and associated with the theory of oxidative stress, resulting in pathological changes to DNA, proteins, and lipids because of stress conditions. A significant amount of ROS, which primarily includes superoxide anion (O2˙−), singlet oxygen (1O2), hydroxyl radicals (˙OH), and hydrogen peroxide (H2O2), is produced during CP priming treatment.117 Findings clearly indicated that seed priming with CP could trigger molecular mechanisms, in seeds.124 In a recent study, Adhikari et al., proposed that cold plasma-primed seeds exhibited greater levels of H2O2, NOx, and O2˙− than control seedlings of tomatoes.124 Present study reveals that oxygen radicals such as O2+ cause the etching of seed coats and stimulate germination chemistry. By modifying the seed coat and inducing water and gaseous exchange inside the seed, CP seed priming results in the generation of reactive oxygen species. The reactive oxygen species (H2O2, O2˙−) and water uptake modulate the phytohormone ratio, activate the amylase enzyme, and stimulate the germination signal. However, CP priming also affects the growth, development, and sustainability of seedlings at the early vegetative stage, and improves the level of reactive oxygen and nitrogen species. In seed cells, reacting species serve as signaling molecules that stimulate oxidative signaling. During seedling development, reactive oxygen species also cause the expression of histone modification genes that are responsible for epigenetic modification. Various antioxidant, phytohormone, and stress resistance-related genes are modulated by these epigenetic alterations and translate to improvement in morphological and biochemical traits. The up-regulated reactive species activate the antioxidant machinery to maintain redox homeostasis in seedlings. Plasma-primed seedlings showed higher superoxide activity, indicating a greater rate of superoxide radical conversion into H2O2. Additionally, superoxide radical serves as a precursor to highly metabolically active molecules such as H2O2 and ˙OH. This study found that CP seed treatment modulated tomato growth, redox homeostasis, and osmotic stress responses by producing ROS. Additionally, Adhikari et al. observed increased antioxidant enzyme activity in CP primed seedlings and asserted that plasma plays an important role in redox regulation. As compared to control seedlings, biochemical profiling of tomato seedlings suggests that accumulated reactive species activate antioxidant machinery, lower oxidative damage, and induce phytohormone synthesis in CP primed seeds.117 Other studies also showed that CP seed priming had a positive impact on physiological and biochemical characteristics of seeds at the germination stages, including antioxidants, sugars and proteins. Hydrogen peroxide triggers the synthesis of salicylic acid and jasmonic acid in plants. CP primed seedlings accumulate H2O2 in the form of salicylic acid and jasmonic acid, therefore salicylic acid and jasmonic acid levels are induced.117Some studies also indicated that CP treatment enhances seed water uptake ability by modifying the seed surface.125 CP seed priming breaks the dormancy and speeds up the germination process. CP-induced reactive species and charged particles interact with seed coats, creating cracks on the surface that facilitate imbibition.126 Therefore, CP seed priming induces seed germination through modification of the seed coat. The studies by Li et al.,127 examined the interaction between morphological, physiological, biochemical, molecular, genetic, and hormonal factors in tomato seedlings to demonstrate modulation of cold stress tolerance at the molecular and molecular levels induced by CP seed priming treatments. In the case of tomato seeds exposed to CP treatment, complex physical and chemical reactions between neutral gas, ionized gas, ROS, and RNS molecules, electrons and positively charged particles possess key role in the generation and stimulation of the embryo. Seed priming with CP is in its initial stages. Few studies have been published describing the proper mechanism of CP priming. For a better understanding of the cold plasma seed priming effects at different stages of the plant, further studies need to be conducted at the cellular and metabolic levels.
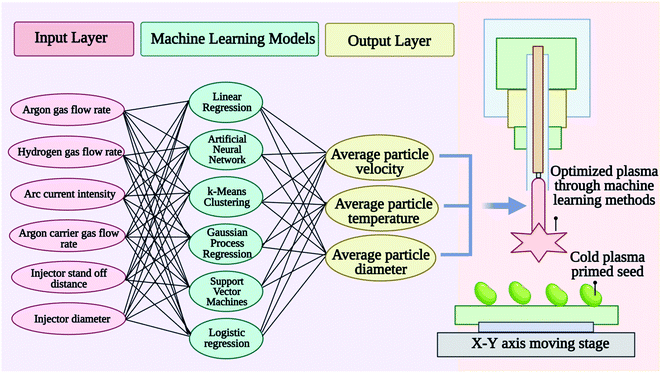
7. Modeling, simulation, and diagnostics of cold plasmas for seed priming treatment using a machine-learning approach
7.1 Machine learning and cold plasma
The branch of artificial intelligence called machine learning (ML) is concerned with analyzing statistical or probabilistic data in order to identify patterns.128,129 ML system is a collection of tools that can analyze and utilize large amounts of data and be used for a variety of purposes. In ML, learning is said to be supervised when input data is provided to the machine for matching with output data. By observing many inputs and outputs of a complex system, researchers can train a computer algorithm that can predict or discover patterns in its behavior much more effectively than programming a set of rules. CP modeling, simulation, diagnostics, and control are potentially transformed by machine learning.130 Plasma applications can range from chemical, physical, and electrical properties of a target surface to plasma properties such as degree of molecular gas dissociation, plasma density, electron energy, the rotational and vibrational temperature of neutral, species or energetic and angle distributions of sputtered particles.131 Applications of machine learning can analyze, for example, optical emission spectrums, current and voltage signals, electroacoustic emission measurements, laser-induced fluorescence measurements, mass spectrometry measurements, and visual images.132,133 Any information about the plasma state can be used as input data for predicting various properties of the plasma or its effects on adjacent surfaces as output data. Understanding and minimizing irreproducible plasma effects can be achieved by monitoring plasma characteristics in real-time. By monitoring plasma characteristics in real-time, it is possible to detect abnormal or undesirable operating conditions. To enhance CP treatment reliability as well as reduce variability, real-time plasma diagnostics are essential for advanced process control.Several new models, diagnoses, and control tools are emerging based on machine learning that can be used to model, diagnose, and control cold plasma systems applied to complex systems such as surfaces or even biological systems. As shown in Fig. 7 modeling cold plasma treatment properties, parameters, and effects on seed priming through machine learning offers the possibility of developing treatment protocols for different seed types aimed at improving their germination and vigor with cold plasma treatments.130
7.2 An approach to modeling and simulating cold plasma by machine learning
ML has been highly successful in modeling and simulating CP behavior in order to understand the chemical and physical mechanisms of the interactions between the plasma and complex surfaces. Two primary ways ML can help develop predictive models for CPs are as follows: (1) using surrogate models for physics-based predictions, and (2) learning models for plasma–surface interactions and plasma induced surface effects from experiments when comprehensive theoretical models are not available for the fundamental plasma–surface interaction mechanisms. CPs can be modeled and simulated in several different ways, such as fluid, particle, or hybrid fluid-particle models. Based on such models, it is possible to determine the spatiotemporal distributions of the density and energy of charged particles, the self-consistent electric field and currents, and the densities and temperatures of neutral species. Surrogate models can be developed using supervised learning methods such as artificial neural networks (ANN), support vector machines (SVMs), and kriging models, which are much more compact and cost much less to evaluate than their high-fidelity counterparts. A surrogate model of the interface of fundamental plasma and surface processes can be used for modeling and simulation of CPs to bridge time- and length-scale differences between them. Data-driven modeling of CPs is also a promising application of machine learning. As opposed to physics-based models, data-driven modeling builds models from experimental data. A data-driven model is essentially a ‘black box’ model, which draws on enormous amounts of input and output measurements. A multiscale model allows for the prediction of the complex effects of ‘plasma outputs’ (for instance reactive species fluxes, charges, electric field emission, photon flow, or localized heating) on the surface response. In this way, supervised learning of plasma–surface interface models can be useful for understanding fundamental surface mechanisms, for instance, in plasma catalysis or plasma medicine, in which the complex surface effects of CP are generally not fully understood.7.3 A real-time diagnostic approach for cold plasma based on machine learning
Due to the requirement for expensive equipment and complex analysis, real-time diagnostics of CP sources can be challenging. This challenge can be addressed with data analytics based on machine learning algorithms predicting the quantitative structure–activity relationship (QSAR) modeling,134 in silico Discriminant Function Analysis135 and absorption, distribution, metabolism, and excretion (ADME) calculations.136 The QSAR and ADME profiling are being used to classify the effect of CP on the seed viability. Data analytics utilizing ML techniques can provide a method for estimating real-time parameters relevant to operations, including temperature, vibration, and substrate characteristics.137 We have found that machine learning holds great potential as a real-time diagnostic tool for CPs. Transitions between modes are also frequently observed in CP sources, for example, streamer-to-spark transitions in corona-like air discharges. Research and applications in CP are challenged by such variability, particularly where the plasma interacts with complex systems, as in plasma treatment to biological systems. ML can monitor variations in plasma characteristics, which could be especially useful for both analyzing and minimizing irreproducible plasma effects. Diagnoses can detect unstable operating conditions as well as drifts in plasma characteristics in real-time. Real-time plasma monitoring is essential, as it reduces (partly) the variability of CP sources and improves their operating reliability.138 Traditionally, extracting spectra information has been accomplished with pure physical approaches. With data analytics, this can be done differently. ML algorithms, which discover patterns in data through detecting patterns, have gained traction as data analytics tools because data sets are larger than ever. An ML analysis can be used to infer physical quantities, which would otherwise be difficult to obtain in real-time because of instrumentation limitations or the length of time, required for analysis. There are two considerations relating to the output data that are important to consider: (1) continuous or discrete output variables. (2) Whether the output data is used for training. When an ML method uses the output data for training, it is known as supervised learning. The goal is to predict continuous output variables (e.g., regression) or discrete output variables (e.g., classification). By contrast; unsupervised learning is only concerned with discovering patterns in the data. Following Table 4 is a brief overview of selected ML methods, as tools for real-time CP diagnostics.The application of machine learning in plasma can create enormous opportunities for modelling the results of plasma treatment, allowing the development of personalized treatment protocols.
8. Conclusion and future prospects
The development and application of plasma in agriculture are just beginning internationally, and it is one of the current hotspots in Agro-research. It combines low operating costs, no variation, no pollution, and strong practicability with plasma seed processing technology to use in agricultural production. Cold plasma seed priming is believed to trigger a variety of biological, biochemical, and molecular events. By moderating seed coats with cold plasma, free radicles are generated in the seed, which significantly increases the yield. Oxygen and nitrogen reactive species are likely to play a major role in the CP priming of seeds. In addition, it is important to determine which properties and parameters of the CP were responsible for the changes. With machine learning methods, the potential for modeling parameters, properties, and end effects of plasma treatment in agriculture is greatly enhanced, allowing for the development of treatment protocols for different seed types.Future advancements of CP treatment must take into account, the treatment parameters need to optimize, in order to achieve a reproducible beneficial effect of priming on seeds. By optimizing the parameters of CP, it is possible to apply CP to seeds for getting significant priming effects. CP-priming also needs to be understood over time to gain an understanding of the genotoxicity effect on seed and plant growth, it is important to find out at which parameters CP-priming is showing toxicity to seeds and how they will affect future plant generations. There is no clear knowledge of the mechanisms of changes occurring in seeds during and after CP treatment. There are few studies published on the molecular mechanism of CP- mediated seed priming and toxicity effects, for the future it needs to study the plasma priming treatment effect on seed at the molecular level. These plasma treatments and biological effects must be reproduced consistently, not only that, but the plasma priming treatments must be scaled up for industrial applications as well. We may eventually be able to learn how plasma–seed priming works in detail to turn this into a viable seed processing technology. Plasma priming treatments will hopefully be another useful technology in the agriculture community.
Author contributions
Conceptualization, A. S., A. V. S. and R. P.; data curation, A. S., V. M., M. S., P. D. and A. V. S.; writing – original draft preparation, A. S. and A. V. S. and R. P.; writing – review and editing, A. S. A. V. S., R. S. M., P. N. D., T. J. and R. P.; graphic design and visualization, A. S., R. S. M. and A. V. S.; supervision, R. P., A. V. S. and V. M.; project administration, A. V. S., M. C. and R. P.; funding acquisition A. L. & P. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by BfR SFP 1322-725; BfR SFP 1322-735 (A. V. S.).https://Biorender.comReferences
- S. N. Garcia, B. I. Osburn and M. T. Jay-Russell, One health for food safety, food security, and sustainable food production, Front. Sustain. Food Syst., 2020, 4, 1 CrossRef.
- H. Xiong, T. Dalhaus, P. Wang and J. Huang, Blockchain technology for agriculture: applications and rationale, Front. Blockchain, 2020, 3, 7 CrossRef.
- A. Waskow, A. Howling and I. Furno, Mechanisms of plasma-seed treatments as a potential seed processing technology, Front. Phys., 2021, 2, 3 Search PubMed.
- B. Adhikari, M. Adhikari and G. Park, The effects of plasma on plant growth, development, and sustainability, Appl. Sci., 2020, 10, 6045 CrossRef CAS.
- M. Domonkos, P. Tichá, J. Trejbal and P. Demo, Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry, Appl. Sci., 2021, 11, 4809 CrossRef CAS.
- J.-S. Song, S. B. Kim, S. Ryu, J. Oh and D.-S. Kim, Emerging plasma technology that alleviates crop stress during the early growth stages of plants: a review, Front. Plant Sci., 2020, 11, 988 CrossRef PubMed.
- B. Adhikari, K. Pangomm, M. Veerana, S. Mitra and G. Park, Plant disease control by non-thermal atmospheric-pressure plasma, Front. Plant Sci., 2020, 11, 77 CrossRef PubMed.
- L. Ling, J. Jiafeng, L. Jiangang, S. Minchong, H. Xin, S. Hanliang and D. Yuanhua, Effects of cold plasma treatment on seed germination and seedling growth of soybean, Sci. Rep., 2014, 4, 1–7 Search PubMed.
- P. Starič, K. Vogel-Mikuš, M. Mozetič and I. Junkar, Effects of nonthermal plasma on morphology, genetics and physiology of seeds: a review, Plants, 2020, 9, 1736 CrossRef PubMed.
- C. Varilla, M. Marcone and G. A. Annor, Potential of cold plasma technology in ensuring the safety of foods and agricultural produce: a review, Foods, 2020, 9, 1435 CrossRef CAS PubMed.
- S. K. Pankaj, Z. Wan and K. M. Keener, Effects of cold plasma on food quality: a review, Foods, 2018, 7, 4 CrossRef PubMed.
- P. Attri, K. Ishikawa, T. Okumura, K. Koga and M. Shiratani, Plasma agriculture from laboratory to farm: a review, Processes, 2020, 8, 1002 CrossRef CAS.
- Y. Tian, B. Guan, D. Zhou, J. Yu, G. Li and Y. Lou, Responses of seed germination, seedling growth, and seed yield traits to seed pretreatment in maize (Zea mays L.), Sci. World J., 2014, 2014, 834630 Search PubMed.
- A. Mohammad Hasan Dad, L. Santosh, M. K. Raviraj, L. S. Prabhakar, P. Vaibhav, G. Subodh, Y. Sanjay, L. Peter, L. Andreas, G. Donato, Z. Paolo and S. Ajay Vikram, Recent Advances in Plant Nanobionics and Nanobiosensors for Toxicology Applications, Curr. Nanosci., 2020, 16, 27–41 CrossRef.
- W. E. Finch-Savage and G. Leubner-Metzger, Seed dormancy and the control of germination, New Phytol., 2006, 171, 501–523 CrossRef CAS PubMed.
- P. Smýkal, V. Vernoud, M. W. Blair, A. Soukup and R. D. Thompson, The role of the testa during development and in establishment of dormancy of the legume seed, Front. Plant Sci., 2014, 5, 351 Search PubMed.
- C. Baskin and J. Baskin, Variation in seed dormancy and germination within and between individuals and populations of a species, Seeds, 2014, 4, 1913–1923 Search PubMed.
- E. B. Nelson, The seed microbiome: origins, interactions, and impacts, Plant Soil, 2018, 422, 7–34 CrossRef CAS.
- M. Panth, S. C. Hassler and F. Baysal-Gurel, Methods for management of soilborne diseases in crop production, Agriculture, 2020, 10, 16 CrossRef CAS.
- M. Gebeyaw, Review on: impact of Seed-Borne Pathogens on Seed Quality, Am. J. Plant Biol., 2020, 5, 77–81 CrossRef.
- T. N. Liliane and M. S. Charles, Factors affecting yield of crops, Agronomy-Climate Change & Food Security, 2020, vol. 9 Search PubMed.
- S. Yadav, P. Modi, A. Dave, A. Vijapura, D. Patel and M. Patel, Effect of abiotic stress on crops, Sustainable Crop Production, 2020 Search PubMed.
- K. Chen, G.-J. Li, R. A. Bressan, C.-P. Song, J.-K. Zhu and Y. Zhao, Abscisic acid dynamics, signaling, and functions in plants, J. Integr. Plant Biol., 2020, 62, 25–54 CrossRef CAS PubMed.
- D. Cui, Y. Yin, J. Wang, Z. Wang, H. Ding, R. Ma and Z. Jiao, Research on the physio-biochemical mechanism of non-thermal plasma-regulated seed germination and early seedling development in Arabidopsis, Front. Plant Sci., 2019, 10, 1322 CrossRef PubMed.
- F.-G. C. Ekezie, D.-W. Sun and J.-H. Cheng, A review on recent advances in cold plasma technology for the food industry: Current applications and future trends, Trends Food Sci. Technol., 2017, 69, 46–58 CrossRef.
- B. Günaydin Daşan, B. Önal Ulusoy, J. Pawlat, J. Diatczyk, Y. Sen and M. Mutlu, A New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma, Food Bioprocess Technol., 2017, 10, 650–661 CrossRef.
- R. Wang, S. Zheng and Y. Zheng, Interface of polymer matrix composites, Polymer matrix composites and technology, Elsevier, 2011, pp. 169–548 Search PubMed.
- K. Vaideki, Antimicrobial textiles, Elsevier, 2016, pp. 73–86 Search PubMed.
- C. Tendero, C. Tixier, P. Tristant, J. Desmaison and P. Leprince, Atmospheric pressure plasmas: A review, Spectrochim. Acta, Part B, 2006, 61, 2–30 CrossRef.
- R. Brandenburg, Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments, Plasma Sources Sci. Technol., 2017, 26, 053001 CrossRef.
- U. Kogelschatz, Collective phenomena in volume and surface barrier discharges, J. Phys.: Conf. Ser., 2010, 257, 012015 CrossRef.
- L. Wilken, V. Hoffmann and K. Wetzig, Radio frequency glow discharge source with integrated voltage and current probes used for evaluation of discharge parameters, J. Appl. Phys., 2006, 99, 063305 CrossRef.
- M. R. Winchester and R. Payling, Radio-frequency glow discharge spectrometry: a critical review, Spectrochim. Acta, Part B, 2004, 59, 607–666 CrossRef.
- A. Bogaerts and R. Gijbels, Description of the argon-excited levels in a radio-frequency and direct current glow discharge, Spectrochim. Acta, Part B, 2000, 55, 263–278 CrossRef.
- A. Bogaerts and R. Gijbels, Similarities and differences between direct current and radio-frequency glow discharges: a mathematical simulation. Invited Lecture Presented at the 2000 Winter Conference on Plasma Spectrochemistry, Fort Lauderdale, FL, USA, January 10–15, 2000, J. Anal. At. Spectrom., 2000, 15, 1191–1201 RSC.
- M. Laroussi, Cold plasma in medicine and healthcare: the new frontier in low temperature plasma applications, Front. Phys., 2020, 8, 74 CrossRef.
- A. Grill, Cold Plasma Generation, 1994 Search PubMed.
- A. Grill, Fundamentals of Plasma, 1994 Search PubMed.
- J. Benedikt, M. M. Hefny, A. Shaw, B. Buckley, F. Iza, S. Schäkermann and J. Bandow, The fate of plasma-generated oxygen atoms in aqueous solutions: non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O (aq), Phys. Chem. Chem. Phys., 2018, 20, 12037–12042 RSC.
- M. Kong and G. Shama, Cold Atmospheric Gas Plasmas, 2014 Search PubMed.
- J. W. Kim, P. Puligundla and C. Mok, Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds, J. Sci. Food Agric., 2017, 97, 128–134 CrossRef CAS PubMed.
- X.-Q. Wang, R.-W. Zhou, G. de Groot, K. Bazaka, A. B. Murphy and K. K. Ostrikov, Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- Y. Shapira, G. Chaniel and E. Bormashenko, Surface charging by the cold plasma discharge of lentil and pepper seeds in comparison with polymers, Colloids Surf., B, 2018, 172, 541–544 CrossRef CAS PubMed.
- J. J. Zhang, T. Kwon, S. B. Kim and D. K. Jeong, Plasma farming: non-thermal dielectric barrier discharge plasma technology for improving the growth of soybean sprouts and chickens, Plasma, 2018, 1, 285–296 CrossRef.
- Y. Park, K. S. Oh, J. Oh, D. C. Seok, S. B. Kim, S. J. Yoo and M. J. Lee, The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley, Plasma Processes Polym., 2018, 15, 1600056 CrossRef.
- G. Pauzaite, A. Malakauskiene, Z. Nauciene, R. Zukiene, I. Filatova, V. Lyushkevich, I. Azarko and V. Mildaziene, Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: short-term versus long-term effects, Plasma Processes Polym., 2018, 15, 1700068 CrossRef.
- A. Iranbakhsh, Z. O. Ardebili, N. O. Ardebili, M. Ghoranneviss and N. Safari, Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology, Acta Physiol. Plant., 2018, 40, 1–11 CrossRef CAS.
- A. Iranbakhsh, N. O. Ardebili, Z. O. Ardebili, M. Shafaati and M. Ghoranneviss, Non-thermal plasma induced expression of heat shock factor A4A and improved wheat (Triticum aestivum L.) growth and resistance against salt stress, Plasma Chem. Plasma Process., 2018, 38, 29–44 CrossRef CAS.
- V. Štěpánová, P. Slavíček, J. Kelar, J. Prášil, M. Smékal, M. Stupavská, J. Jurmanová and M. Černák, Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement, Plasma Processes Polym., 2018, 15, 1700076 CrossRef.
- M. Măgureanu, R. Sîrbu, D. Dobrin and M. Gîdea, Stimulation of the germination and early growth of tomato seeds by non-thermal plasma, Plasma Chem. Plasma Process., 2018, 38, 989–1001 CrossRef.
- M. Bafoil, A. Jemmat, Y. Martinez, N. Merbahi, O. Eichwald, C. Dunand and M. Yousfi, Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth, PLoS One, 2018, 13, e0195512 CrossRef PubMed.
- K. Matra, Atmospheric non-thermal argon–oxygen plasma for sunflower seedling growth improvement, Jpn. J. Appl. Phys., 2017, 57, 01AG03 CrossRef.
- N. Hayashi, R. Ono, R. Nakano, M. Shiratani, K. Tashiro, S. Kuhara, K. Yasuda and H. Hagiwara, DNA microarray analysis of plant seeds irradiated by active oxygen species in oxygen plasma, Plasma Med., 2016, 6, 3–4 Search PubMed.
- J. Pawłat, A. Starek, A. Sujak, P. Terebun, M. Kwiatkowski, M. Budzeń and D. Andrejko, Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds' germination, PLoS One, 2018, 13, e0194349 CrossRef PubMed.
- M. M. Rahman, S. A. Sajib, M. S. Rahi, S. Tahura, N. C. Roy, S. Parvez, M. A. Reza, M. R. Talukder and A. H. Kabir, Mechanisms and signaling associated with LPDBD plasma mediated growth improvement in wheat, Sci. Rep., 2018, 8, 1–11 Search PubMed.
- S. Khatami and A. Ahmadinia, Increased germination and growth rates of pea and Zucchini seed by FSG plasma, J. Theor. Appl. Phys., 2018, 12, 33–38 CrossRef.
- B. Zhang, R. Li and J. Yan, Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment, Arch. Biochem. Biophys., 2018, 655, 37–42 CrossRef CAS PubMed.
- T. Tounekti, Z.-u.-I. Mujahid and H. Khemira, Non-thermal dielectric barrier discharge (DBD) plasma affects germination of coffee and grape seeds, AIP Conf. Proc., 2018, 1976, 020029 CrossRef.
- S. I. Hosseini, S. Mohsenimehr, J. Hadian, M. Ghorbanpour and B. Shokri, Physico-chemical induced modification of seed germination and early development in artichoke (Cynara scolymus L.) using low energy plasma technology, Phys. Plasmas, 2018, 25, 013525 CrossRef.
- J. Jiang, L. Jiangang and D. Yuanhua, Effect of cold plasma treatment on seedling growth and nutrient absorption of tomato, Plasma Sci. Technol., 2018, 20, 044007 CrossRef.
- C. L. Porto, L. Sergio, F. Boari, A. F. Logrieco and V. Cantore, Cold plasma pretreatment improves the germination of wild asparagus (Asparagus acutifolius L.) seeds, Sci. Hortic., 2019, 256, 108554 CrossRef.
- S. Afsheen, U. Fatima, T. Iqbal, M. Abrar, S. Muhammad, A. Saeed, M. Isa, M. Malik and S. Shamas, Influence of cold plasma treatment on insecticidal properties of wheat seeds against red flour beetles, Plasma Sci. Technol., 2019, 21, 085506 CrossRef CAS.
- A. H. Kabir, M. M. Rahman, U. Das, U. Sarkar, N. C. Roy, M. A. Reza, M. R. Talukder and M. A. Uddin, Reduction of cadmium toxicity in wheat through plasma technology, PLoS One, 2019, 14, e0214509 CrossRef CAS PubMed.
- S. Islam, F. B. Omar, S. A. Sajib, N. C. Roy, A. Reza, M. Hasan, M. R. Talukder and A. H. Kabir, Effects of LPDBD plasma and plasma activated water on germination and growth in rapeseed (Brassica napus), Gesunde Pflanz., 2019, 71, 175–185 CrossRef.
- T. Iqbal, M. Farooq, S. Afsheen, M. Abrar, M. Yousaf and M. Ijaz, Cold plasma treatment and laser irradiation of Triticum spp. seeds for sterilization and germination, J. Laser Appl., 2019, 31, 042013 CrossRef.
- R. Singh, P. Prasad, R. Mohan, M. K. Verma and B. Kumar, Radiofrequency cold plasma treatment enhances seed germination and seedling growth in variety CIM-Saumya of sweet basil (Ocimum basilicum L.), J. Appl. Res. Med. Aromat. Plants, 2019, 12, 78–81 Search PubMed.
- M. Bafoil, A. Le Ru, N. Merbahi, O. Eichwald, C. Dunand and M. Yousfi, New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses, Sci. Rep., 2019, 9, 1–10 CAS.
- K. Lotfy, N. A. Al-Harbi and H. Abd El-Raheem, Cold atmospheric pressure nitrogen plasma jet for enhancement germination of wheat seeds, Plasma Chem. Plasma Process., 2019, 39, 897–912 CrossRef CAS.
- Z. O. Ardebili, B. Eslami, A. Babajani and A. Iranbakhsh, Plasma Chem. Plasma Process., 2019, 39, 21–34 CrossRef.
- M. C. Pérez-Pizá, L. Prevosto, P. E. Grijalba, C. G. Zilli, E. Cejas, B. Mancinelli and K. B. Balestrasse, Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status, Heliyon, 2019, 5, e01495 CrossRef PubMed.
- M. Moghanloo, A. Iranbakhsh, M. Ebadi, T. N. Satari and Z. O. Ardebili, Seed priming with cold plasma and supplementation of culture medium with silicon nanoparticle modified growth, physiology, and anatomy in Astragalus fridae as an endangered species, Acta Physiol. Plant., 2019, 41, 1–13 CrossRef CAS.
- A. Los, D. Ziuzina, D. Boehm, P. J. Cullen and P. Bourke, Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics, Plasma Processes Polym., 2019, 16, 1800148 CrossRef.
- B. Liu, B. Honnorat, H. Yang, J. Arancibia, L. Rajjou and A. Rousseau, Non-thermal DBD plasma array on seed germination of different plant species, J. Phys. D: Appl. Phys., 2018, 52, 025401 CrossRef.
- B. Šerá, A. Zahoranová, H. Bujdakova and M. Šerý, Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O’Donnell using non-thermal plasma treatment, Rom. Rep. Phys., 2019, 71, 701–712 Search PubMed.
- V. Mildažienė, V. Aleknavičiūtė, R. Žūkienė, G. Paužaitė, Z. Naučienė, I. Filatova, V. Lyushkevich, P. Haimi, I. Tamošiūnė and D. Baniulis, Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression, Sci. Rep., 2019, 9, 1–12 Search PubMed.
- X. Gao, A. Zhang, P. Héroux, W. Sand, Z. Sun, J. Zhan, C. Wang, S. Hao, Z. Li and Z. Li, Effect of dielectric barrier discharge cold plasma on pea seed growth, J. Agric. Food Chem., 2019, 67, 10813–10822 CrossRef CAS PubMed.
- F. S. Seddighinia, A. Iranbakhsh, Z. O. Ardebili, T. N. Satari and S. Soleimanpour, Seed priming with cold plasma and multi-walled carbon nanotubes modified growth, tissue differentiation, anatomy, and yield in bitter melon (Momordica charantia), J. Plant Growth Regul., 2020, 39, 87–98 CrossRef CAS.
- P. F. Ambrico, M. Šimek, M. Ambrico, M. Morano, V. Prukner, A. Minafra, I. Allegretta, C. Porfido, G. S. Senesi and R. Terzano, On the air atmospheric pressure plasma treatment effect on the physiology, germination and seedlings of basil seeds, J. Phys. D: Appl. Phys., 2019, 53, 104001 CrossRef.
- N. Dawood, Effect of RF plasma on Moringa seeds germination and growth, J. Taibah Univ. Sci., 2020, 14, 279–284 CrossRef.
- M. Kobayashi, Y. Wang, S. Kumagai, Y. Uraoka and T. Ito, Effects of cold atmospheric plasma irradiation on Arabidopsis seedlings, Jpn. J. Appl. Phys., 2019, 59, SAAB09 CrossRef.
- I. Filatova, V. Lyushkevich, S. Goncharik, A. Zhukovsky, N. Krupenko and J. Kalatskaja, The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields, J. Phys. D: Appl. Phys., 2020, 53, 244001 CrossRef CAS.
- Y. Meiqiang, H. Mingjing, M. Buzhou and M. Tengcai, Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield, Plasma Sci. Technol., 2005, 7, 3143 CrossRef.
- M. Ghasempour, A. Iranbakhsh, M. Ebadi and Z. Oraghi Ardebili, Seed priming with cold plasma improved seedling performance, secondary metabolism, and expression of deacetylvindoline O-acetyltransferase gene in Catharanthus roseus, Contrib. Plasma Phys., 2020, 60, e201900159 CrossRef CAS.
- K. Koga, P. Attri, K. Kamataki, N. Itagaki, M. Shiratani and V. Mildaziene, Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment, Jpn. J. Appl. Phys., 2020, 59, SHHF01 CrossRef CAS.
- S. Rezaei, B. Ghobadian, M. T. Ebadi, F. Jangi and H. Ghomi, Effects of cold plasma on the color parameters of Hyssop (Hyssopus officinalis L.) using color imaging instrumentation and spectrophotometer, Color Res. Appl., 2020, 45, 29–39 CrossRef.
- M. Billah, S. Sajib, N. Roy, M. Rashid, M. Reza, M. Hasan and M. Talukder, Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo l) seed germination and growth, Arch. Biochem. Biophys., 2020, 681, 108253 CrossRef CAS PubMed.
- M.-H. Kang, M. Veerana, S. Eom, H.-S. Uhm, S. Ryu and G. Park, Plasma mediated disinfection of rice seeds in water and air, J. Phys. D: Appl. Phys., 2020, 53, 214001 CrossRef CAS.
- A. Iranbakhsh, Z. O. Ardebili, H. Molaei, N. O. Ardebili and M. Amini, Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in Hemp (Cannabis sativa L.), Plasma Chem. Plasma Process., 2020, 40, 527–537 CrossRef CAS.
- K. Kadowaki and N. Kurisaka, Stimulation and Inhibition of Arabidopsis Seed Germination with Repetitive Barrier Discharges Produced by Polarity-Reversed Voltage Pulses, Electron. Commun. Jpn., 2014, 97, 1–8 CrossRef.
- S. H. Ji, T. Kim, K. Panngom, Y. J. Hong, A. Pengkit, D. H. Park, M. H. Kang, S. H. Lee, J. S. Im and J. S. Kim, Assessment of the effects of nitrogen plasma and plasma-generated nitric oxide on early development of Coriandum sativum, Plasma Processes Polym., 2015, 12, 1164–1173 CrossRef CAS.
- N. Munkhuu, C. Shao, D. Wang, L. Liu, I. Muhammad, C. He, S. Qian, R. Jia and J. Feng, Stimulating Effect of Low-Temperature Plasma on Seed Germination Characteristics of Trifolium repens, Int. Conf. Comput. Commun. Technol. Agric. Eng., 2014, 452, 167–174 Search PubMed.
- T. Stolárik, M. Henselová, M. Martinka, O. Novák, A. Zahoranová and M. Černák, Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.), Plasma Chem. Plasma Process., 2015, 35, 659–676 CrossRef.
- D. Dobrin, M. Magureanu, N. Mandache and M. Ionita, Innovative Food Sci. Emerging Technol., 2015, 29, 255–260 CrossRef CAS.
- T. Sarinont, T. Amano, P. Attri, K. Koga, N. Hayashi and M. Shiratani, Effects of plasma irradiation using various feeding gases on growth of Raphanus sativus L, Arch. Biochem. Biophys., 2016, 605, 129–140 CrossRef CAS PubMed.
- K. Matra, Non-thermal plasma for germination enhancement of radish seeds, Procedia Comput. Sci., 2016, 86, 132–135 CrossRef.
- A. Gholami, N. N. Safa, M. Khoram, J. Hadian and H. Ghomi, Effect of low-pressure radio frequency plasma on ajwain seed germination, Plasma Med., 2016, 6, 3–4 Search PubMed.
- L. Ling, L. Jiangang, S. Minchong, H. Jinfeng, S. Hanliang, D. Yuanhua and J. Jiafeng, Improving seed germination and peanut yields by cold plasma treatment, Plasma Sci. Technol., 2016, 18, 1027 CrossRef.
- N. Khamsen, D. Onwimol, N. Teerakawanich, S. Dechanupaprittha, W. Kanokbannakorn, K. Hongesombut and S. Srisonphan, Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma, ACS Appl. Mater. Interfaces, 2016, 8, 19268–19275 CrossRef CAS PubMed.
- R. Zhou, R. Zhou, X. Zhang, J. Zhuang, S. Yang, K. Bazaka and K. K. Ostrikov, Effects of atmospheric-pressure N2, He, air, and O2 microplasmas on mung bean seed germination and seedling growth, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- A. Zahoranová, M. Henselová, D. Hudecová, B. Kalináková, D. Kovácik, V. Medvecká and M. Černák, Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface, Plasma Chem. Plasma Process., 2016, 36, 397–414 CrossRef.
- C. A. Junior, J. de Oliveira Vitoriano, D. L. S. da Silva, M. de Lima Farias and N. B. de Lima Dantas, Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma, Sci. Rep., 2016, 6, 1–7 CrossRef PubMed.
- C. Nalwa, A. K. Thakur, A. Vikram, R. Rane and A. Vaid, Studies on plasma treatment and priming of seeds of bell pepper (Capsicum annuum L.), J. Appl. Nat. Sci., 2017, 9, 1505–1509 CrossRef CAS.
- Y. Meng, G. Qu, T. Wang, Q. Sun, D. Liang and S. Hu, Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources, Plasma Chem. Plasma Process., 2017, 37, 1105–1119 CrossRef CAS.
- A. Da Silva, M. Farias, D. Da Silva, J. Vitoriano, R. de Sousa and C. Alves-Junior, Using atmospheric plasma to increase wettability, imbibition and germination of physically dormant seeds of Mimosa caesalpiniafolia, Colloids Surf., B, 2017, 157, 280–285 CrossRef CAS PubMed.
- J. J. Zhang, J. O. Jo, R. K. Mongre, M. Ghosh, A. K. Singh, S. B. Lee, Y. S. Mok and D. K. Jeong, Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- Q. Guo, Y. Wang, H. Zhang, G. Qu, T. Wang, Q. Sun and D. Liang, Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment, Sci. Rep., 2017, 7, 1–14 CrossRef PubMed.
- P. Gómez, A. M. Kist, P. Schlegel, D. A. Berry, D. K. Chhetri, S. Dürr, M. Echternach, A. M. Johnson, S. Kniesburges and M. Kunduk, Bagls, a multihospital benchmark for automatic glottis segmentation, Sci. Data, 2020, 7, 1–12 CrossRef PubMed.
- P. Puligundla, J.-W. Kim and C. Mok, Effects of nonthermal plasma treatment on decontamination and sprouting of radish (Raphanus sativus L.) seeds, Food Bioprocess Technol., 2017, 10, 1093–1102 CrossRef CAS.
- P. Puligundla, J.-W. Kim and C. Mok, Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds, Food Control, 2017, 71, 376–382 CrossRef.
- B. Sera, M. Sery, B. Gavril and I. Gajdova, Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.), Plasma Chem. Plasma Process., 2017, 37, 207–221 CrossRef CAS.
- Y. Li, T. Wang, Y. Meng, G. Qu, Q. Sun, D. Liang and S. Hu, Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed, Plasma Chem. Plasma Process., 2017, 37, 1621–1634 CrossRef CAS.
- V. Mildaziene, G. Pauzaite, Z. Naucienė, A. Malakauskiene, R. Zukiene, I. Januskaitiene, V. Jakstas, L. Ivanauskas, I. Filatova and V. Lyushkevich, Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves, Plasma Processes Polym., 2018, 15, 1700059 CrossRef.
- A. Shelar, A. V. Singh, R. S. Maharjan, P. Laux, A. Luch, D. Gemmati, V. Tisato, S. P. Singh, M. F. Santilli and A. Shelar, Sustainable Agriculture through Multidisciplinary Seed Nanopriming: Prospects of Opportunities and Challenges, Cells, 2021, 10, 2428 CrossRef CAS PubMed.
- S. Lutts, P. Benincasa, L. Wojtyla, S. Kubala, R. Pace, K. Lechowska, M. Quinet and M. Garnczarska, Seed priming: new comprehensive approaches for an old empirical technique, New challenges in seed biology-basic and translational research driving seed technology, 2016, pp. 1–46 Search PubMed.
- M. Hasanuzzaman, M. Bhuyan, F. Zulfiqar, A. Raza, S. M. Mohsin, J. A. Mahmud, M. Fujita and V. Fotopoulos, Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator, Antioxidants, 2020, 9, 681 CrossRef CAS PubMed.
- N. Misra, B. Yadav, M. Roopesh and C. Jo, Cold plasma for effective fungal and mycotoxin control in foods: mechanisms, inactivation effects, and applications, Compr. Rev. Food Sci. Food Saf., 2019, 18, 106–120 CrossRef CAS PubMed.
- B. Adhikari, M. Adhikari, B. Ghimire, B. C. Adhikari, G. Park and E. H. Choi, Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum), Free Radicals Biol. Med., 2020, 156, 57–69 CrossRef CAS PubMed.
- M. Ghaemi, A. Majd and A. Iranbakhsh, Transcriptional responses following seed priming with cold plasma and electromagnetic field in Salvia nemorosa L, J. Theor. Appl. Phys., 2020, 14, 323–328 CrossRef.
- M. S. Sheteiwy, J. An, M. Yin, X. Jia, Y. Guan, F. He and J. Hu, Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings, Protoplasma, 2019, 256, 79–99 CrossRef CAS PubMed.
- A. do Espirito Santo Pereira, H. Caixeta Oliveira, L. Fernandes Fraceto and C. Santaella, Nanotechnology potential in seed priming for sustainable agriculture, Nanomaterials, 2021, 11, 267 CrossRef CAS PubMed.
- M. A. Khalaki, M. Moameri, B. A. Lajayer and T. Astatkie, Influence of nano-priming on seed germination and plant growth of forage and medicinal plants, Plant Growth Regul., 2021, 93, 13–28 CrossRef.
- M. Moghanloo, A. Iranbakhsh, M. Ebadi and Z. O. Ardebili, Differential physiology and expression of phenylalanine ammonia lyase (PAL) and universal stress protein (USP) in the endangered species Astragalus fridae following seed priming with cold plasma and manipulation of culture medium with silica nanoparticles, 3 Biotech., 2019, 9, 1–13 CrossRef PubMed.
- B. Gong, Y. Yan, L. Zhang, F. Cheng, Z. Liu and Q. Shi, Unravelling GSNOR-Mediated S-Nitrosylation and Multiple Developmental Programs in Tomato Plants, Plant Cell Physiol., 2019, 60, 2523–2537 CrossRef CAS PubMed.
- T. Finkel, Signal transduction by reactive oxygen species, J. Cell Biol., 2011, 194, 7–15 CrossRef CAS PubMed.
- Z. Zhou, Y. Huang, S. Yang and W. Chen, Introduction of a new atmospheric pressure plasma device and application on tomato seeds, Agric. Sci., 2011, 2, 23–27 Search PubMed.
- M. Selcuk, L. Oksuz and P. Basaran, Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment, Bioresour. Technol., 2008, 99, 5104–5109 CrossRef CAS PubMed.
- K. Li, C. Zhong, Q. Shi, H. Bi and B. Gong, Cold plasma seed treatment improves chilling resistance of tomato plants through hydrogen peroxide and abscisic acid signaling pathway, Free Radicals Biol. Med., 2021, 172, 286–297 CrossRef CAS PubMed.
- A. V. Singh, M. H. D. Ansari, D. Rosenkranz, R. S. Maharjan, F. L. Kriegel, K. Gandhi, A. Kanase, R. Singh, P. Laux and A. Luch, Artificial intelligence and machine learning in computational nanotoxicology: unlocking and empowering nanomedicine, Adv. Healthcare Mater., 2020, 9, 1901862 CrossRef CAS PubMed.
- S. Borderud, Y. Li, J. Burkhalter and C. Sheffer, Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes, Cancer, 2015, 121, 800 CrossRef PubMed.
- D. Gidon, X. Pei, A. D. Bonzanini, D. B. Graves and A. Mesbah, Machine learning for real-time diagnostics of cold atmospheric plasma sources, IEEE Trans. Radiat. Plasma Med. Sci., 2019, 3, 597–605 Search PubMed.
- A. Mesbah and D. B. Graves, Machine learning for modeling, diagnostics, and control of non-equilibrium plasmas, J. Phys. D: Appl. Phys., 2019, 52, 30LT02 CrossRef CAS.
- A. V. Singh, V. Chandrasekar, P. Janapareddy, D. E. Mathews, P. Laux, A. Luch, Y. Yang, B. Garcia-Canibano, S. Balakrishnan and J. Abinahed, Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers, ACS Chem. Neurosci., 2021, 448–455 Search PubMed.
- A. V. Singh, R.-S. Maharjan, A. Kanase, K. Siewert, D. Rosenkranz, R. Singh, P. Laux and A. Luch, Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells, ACS Appl. Mater. Interfaces, 2020, 13, 1943–1955 CrossRef PubMed.
- A. V. Singh, A. Romeo, K. Scott, S. Wagener, L. Leibrock, P. Laux, A. Luch, P. Kerkar, S. Balakrishnan and S. P. Dakua, Emerging Technologies for In Vitro Inhalation Toxicology, Adv. Healthcare Mater., 2021, 2100633 CrossRef CAS PubMed.
- A. V. Singh, R. S. Maharjan, H. Jungnickel, H. Romanowski, Y. U. Hachenberger, P. Reichardt, F. Bierkandt, K. Siewert, A. Gadicherla and P. Laux, Evaluating Particle Emissions and Toxicity of 3D Pen Printed Filaments with Metal Nanoparticles As Additives: In Vitro and in Silico Discriminant Function Analysis, ACS Sustainable Chem. Eng., 2021, 9, 11724–11737 CrossRef CAS.
- A. V. Singh, R. S. Maharjan, C. Kromer, P. Laux, A. Luch, T. Vats, V. Chandrasekar, S. P. Dakua and B.-W. Park, Advances in Smoking Related In Vitro Inhalation Toxicology: A Perspective Case of Challenges and Opportunities from Progresses in Lung-on-Chip Technologies, Chem. Res. Toxicol., 2021, 9, 1984–2002 Search PubMed.
- M. Khalili, L. Daniels, A. Lin, F. C. Krebs, A. E. Snook, S. Bekeschus, W. B. Bowne and V. Miller, Non-thermal plasma-induced immunogenic cell death in cancer, J. Phys. D: Appl. Phys., 2019, 52, 423001 CrossRef CAS PubMed.
- T. van der Gaag, H. Onishi and H. Akatsuka, Arbitrary EEDF determination of atmospheric-pressure plasma by applying machine learning to OES measurement, Phys. Plasmas, 2021, 28, 033511 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |