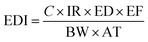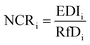Open Access Article
Open Access ArticleOccurrence and exposure risk assessment of phthalate esters in edible plant oils with a high-frequency import rate in west China
Zhentao Tanga,
Zhiguo Gongb,
Wei Jiac,
Wenxuan Shenb,
Qingrong Hand,
Fang Fang*b and
Cheng Peng*d
aKey Laboratory of Southwestern Chinese Medicine Resources, Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
bUrumqi Customs District P. R. China, Urumqi, China. E-mail: fangfanguc@163.com
cSchool of Food and Biological Engineering, Shaanxi University of Science & Technology, Shaanxi, China
dKey Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
First published on 4th March 2022
Abstract
Phthalate esters (PAEs) are ubiquitous pollutants in the environment with toxicological and epidemiological effects for humans. As one of the daily necessities, edible plant oil is an important exposure source of PAEs, due to the inevitable contact with PAE-containing materials and the intrinsic lipid solubility of PAEs. However, limited information is currently available on the exposure risk of PAEs in commercial plant oil. This study was aimed at investigating the occurrence and risk assessment of PAEs in plant oils with a high-frequency import rate in west China. The analysis method was referenced to the Chinese national standard for the determination of PAEs in food. Results indicated that PAEs (mainly including DBP and DEHP) were ubiquitous contaminants in imported plant oils with the detectable rate being up to 56.83% in 366 samples. The detected concentrations were in the range of 0.10–3.20 mg kg−1 (median 0.28 mg kg−1) for dibutyl phthalate (DBP) and 0.25–1.95 mg kg−1 (median 0.44 mg kg−1) for bis(2-ethylhexyl)phthalate (DEHP). Based on an integrated probabilistic analysis method, the values of non-carcinogenic risk were lower than 1 in all cases, indicating that there would be an unlikely incremental non-carcinogenic risk to humans. Generally, the carcinogenic risk of DEHP was lower than the upper acceptable carcinogenic risk level (<10−4), while 50.40% of the carcinogenic risk exceeded the lower acceptable carcinogenic risk level (>10−6). Besides, diverse health risks were obviously shown and discussed for different categories of plant oils. The obtained results in this study could provide valuable information to understand the contamination status and health risk of PAEs in plant oil and improve the relative supervision and regulation. And the proposed strategy suggests a potential application for health risk assessment of other contaminants in food or even environments.
1. Introduction
Phthalate esters (PAEs) are plasticizers and additives that are widely used in daily products, including furnishings for households and transportation vehicles, drug coatings, personal care products, food packages, and numerous other products.1,2 They are ubiquitous pollutants in the environment, on account of the fact that PAEs normally exist as free phases that are able to be gradually released from PAE-containing products.3,4 With the widespread pollution of PAEs, human beings have been inevitably exposed to these compounds through pathways of respiration, ingestion and dermal contact, etc.5 Several toxicological and epidemiological studies have demonstrated that some PAEs are endocrine disruptors and have potential teratogenic, carcinogenic and mutagenic effects.4,6 As a result, PAEs are classified as priority pollutants by the European Union (EU), the United States Environmental Protection Agency (USEPA) and several other countries.7 Bis(2-ethylhexyl)phthalate (DEHP) and benzyl butyl phthalate (BBP) are respectively defined as Group 2B and Group 3 carcinogen by the International Agency for Research on Cancer (IARC). Due to the multiple exposure sources and potential physiological toxicities, PAEs contamination has attracted great concern for human health and ecological environment.8,9 There is a large number of references relating to PAEs distribution and exposure assessment in environmental media and the food chain.10–17 Along with the research for PAEs contamination and exposure risk assessment, regulations have been gradually established by more countries and international organizations to control the use of PAEs.4Although the use of PAEs has been restricted worldwide, they are still frequently detected in human bodies and the environment.18 As one of the most important exposure pathways for humans, dietary exposure has attracted lots of attention in recent years.5 Edible plant oil, one of the daily necessities, also encounters PAEs pollution during manufacturing, packaging or storage procedure.10,19 It is well known that PAEs contamination is more prone to happen in food with matrix abundant in lipid, due to the intrinsic lipid solubility.12,20 There are numerous studies exploring the determination of PAEs in edible oil.21–25 However, limited studies have been conducted for exposure assessment of PAEs in commercial plant oil. Up to now, the Maximum Residue Limits (MRLs) of PAEs in food are mostly referenced to the migration limits for food contact materials, regardless of the foods' categories. Taking DBP, DEHP and DINP as representatives, the MRLs have been regulated respectively to be 0.3 mg kg−1, 1.5 mg kg−1 and 9.0 mg kg−1 by China and the European Union (EU). Consequently, a lack of specific MRLs of PAEs exists for edible plant oil, while some temporarily regulated MRLs are slightly defective.
Especially with the improvement of people's living and the change of residents' diet structure, the demand and consumption of edible plant oil are increasing year by year in China. The import of plant oil is accordingly raised, along with the diversified categories.26 Statistically, the import volume of plant oil was 11.53 million tons in 2020, which was speculated to have a further increase in the future.27 With a high-frequency import rate, imported plant oil occupies a relatively large market share in China. To guarantee the quality safety of imported plant oils, monitoring of PAEs and formulation of specific residue limits are of equal importance. They are also advantageous for the promotion of the harmonious and orderly development of trade and economic cooperation between nations. With the gradual maturity of analysis methods for PAEs, more investigations regarding the exposure risk assessment of PAEs in plant oil are vital and required to comprehensively address the relevant scientific issues of PAEs in edible oil.
The objective of this study is to (i) determine the occurrence of PAEs in edible plant oils with a high-frequency import rate in west China; (ii) estimate the health risk of dietary exposure to PAEs in the imported plant oils with a comprehensive method; (iii) to characterize the health risk based on the methodology of carcinogenic risk and non-carcinogenic risk; (iv) to better understand the health risk associated with the consumption of imported plant oils and in order to provide valuable information for effective prevention and control.
2. Materials and methods
2.1 Chemicals and reagents
A standard mixture solution of 17 PAEs was acquired from Anpel Laboratory Technology Co. Ltd (Shanghai, China), including benzyl butyl phthalate (BBP), diallyl phthalate (DAP), dibutyl phthalate (DBP), bis(2-n-butoxyethyl)phthalate (DBEP), dicyclohexyl phthalate (DCHP), diethyl phthalate (DEP), bis(2-ethoxyethyl)phthalate (DEEP), bis(2-ethylhexyl)phthalate (DEHP), dihexyl phthalate (DHP), diisobutyl phthalate (DiBP), dimethyl phthalate (DMP), bis(2-methoxyethyl)phthalate (DMEP), bis(4-methyl-2-pentyl)phthalate (DMPP), dinonyl phthalate (DNP), di-n-octyl phthalate (DnOP), dipentyl phthalate (DPP), diphenyl phthalate (DPhP). N-Hexane was purchased from Dikma Co. (Beijing, China). Acetonitrile, acetone, dichloromethane and Si/PSA SPE glass cartridges were from CNW Technology (Germany). All the reagents were of chromatographic grade.2.2 Instrumental conditions
Instrumental analysis was performed on a 7890A gas chromatography – 5975C mass spectrometry system (GC-MS, Agilent, USA) in electron ionization and selective ion monitoring mode. Chromatographic separation was carried out on a DB-5 MS capillary column (30 m × 0.25 mm diameter, 0.25 μm film thickness, Agilent, USA). The column temperature program was as follows: initiated at 60 °C for 1 min, increased to 220 °C at the rate of 15 °C min−1, held for 1 min, then to 250 °C at the rate of 5 °C min−1, held for another 1 min, finally increased to 290 °C at the rate of 20 °C min−1, and held at this temperature for 7.5 min. The injection port temperature, transfer line and ion source temperature were set at 260 °C, 280 °C and 230 °C, respectively. The injection volume was 1 μL with split-less injection mode. Helium (purity > 99.999%) was used as carrier gas at a flow rate of 1.0 mL min−1.Ultrasonic bath and vortex mixer respectively supplied by Shanghai Kedao Co. (SK8210LHC, China) and IKA Co. (MS 3, Germany) were employed in this study. Deionized water prepared by a Milli-Q system (Millipore Co., USA) was used throughout the analysis.
2.3 Sample collection and preparation
A total of 366 plant oil samples with a high-frequency import rate were gathered from three provinces in west China, including Xinjiang, Sichuan and Shaanxi. The samples were categorized into 8 species of plant oils, with the classified distribution as follows: 20 crude rapeseed oil samples, 23 crude sunflower seed oil samples, 26 linseed oil samples, 34 safflower oil samples, 82 sunflower seed oil samples, 54 virgin linseed oil samples, 87 virgin rapeseed oil samples and 40 virgin sunflower seed oil samples.The sample pre-treatment procedure was referenced to the second method of the standard operating procedures regulated in China.28 Briefly, 0.5 g of each sample was weighed accurately in a centrifugal tube and mixed successively with 100 μL n-hexane and 2 mL acetonitrile. The mixture was vortexed for 1 min and subsequently ultrasonicated for 20 min. After being centrifuged at 4000 rpm for 5 min, the supernatant was collected. Another 2 mL acetonitrile was added into the residue, followed by vortex for 1 min and centrifugation for 5 min, successively. The extraction process of residue was repeated once. Then the supernatant was combined for further purification.
The clean-up procedure was realized by the Si/PSA SPE glass cartridge. Briefly, the SPE column was pre-washed with 5 mL dichloromethane and 5 mL acetonitrile. Then the sample extract prepared by the above-mentioned procedure was transferred through the glass cartridge and collected. After being eluted with 5 mL acetonitrile, the eluate was combined and mixed with 1 mL acetone. The final solution was nearly dried under a mild stream of nitrogen at 40 °C. The residue was re-dissolved with 2 mL n-hexane for GC-MS analysis.
2.4 Risk assessment
The mathematical approach, Monte Carlo simulation (probabilistic analysis), was used for the computation of the health risk assessment of PAEs in plant oil samples. In this study, the health risk was assessed in the form of non-carcinogenic risk and carcinogenic risk, which were estimated according to the method recommended by USEPA.29 Only daily intake through the oral ingestion pathway was concerned, due to the fact that the health risk of PAEs in plant oil samples was mainly induced by dietary exposure. To comprehensively evaluate the health risk, categories of populations and plant oils were respectively considered. The exposure risk was calculated as follows.| CR = EDI × CFS |
| NCR = ∑NCRi |
EDI (μg (kg day)−1) represents the estimated daily intake via dietary route; C is the concentration of PAEs in plant oil, mg kg−1; IR (g day−1) is the daily intake rate of plant oil; EF (day year−1) is exposure frequency; ED (year) is exposure duration; AT (day) is average life time; BW (kg) is the body weight; CR (unitless) is the carcinogenic risk; CFS is the slope factor of carcinogenic ((kg day) mg−1); NCRi is the non-carcinogenic risk of specific chemical; RfD (mg (kg day)−1) is defined as the daily maximum permissible dose; i represents the different PAEs (DBP and DEHP in this study); NCR is the total non-carcinogenic risk induced by detected PAEs. In this study, CFS and RfD were obtained from the Integrated Risk Information System (IRIS) of USEPA or OAKRIDGE National Laboratory of the US Department of Energy (ORNL). Specifically, RfDoral values for two PAE congeners are respectively 100 (μg (kg day)−1, DBP) and 20 (μg (kg day)−1, DEHP). CFS of DEHP is 1.4 × 10−5 (kg day) μg−1. In this study, the time factor (ED × EF/AT) was assumed to be one since edible oil is inevitable in the daily life of the Chinese.
2.5 Statistical analysis
Data was processed by Origin 2021, Microsoft Office Excel 2019 and SPSS 26 (IBM, USA) for Windows. Monte Carlo simulation was recommended as the probabilistic analysis tool, which was performed by @RISK software package. The probability distribution of PAEs concentrations was divided into two parts: the PAEs concentrations below limits of detection (LODs) were considered as a uniform distribution (0 − LODs). The best fit distribution of the PAEs concentrations higher than LODs was formed by SPSS with the assistance of the Kolmogorov–Smirnov Test. A combination of 1000 iterator results was obtained based on the integrated data, referring to the method described in.30 The consumption data of plant oil was also simulated by the Monte Carlo approach as distribution.3. Results and discussion
3.1 Validation of the detection method
For quality control purposes, procedural blank, matrix-spiked sample, and duplicates were analyzed for each batch of samples. The external standard method was employed for quantitative analysis of the target PAEs. A good linear relationship (R2 ≥ 0.999) was obtained with the concentration range of 0.02–1.00 μg mL−1. The limits of detection (LODs) were obtained as 0.10 mg kg−1 for DBP and 0.25 mg kg−1 for the others, respectively. Recovery was tested at three different concentration levels, resulting in the range of 70–110%. And acceptable precision (n = 3) was validated to be no more than 10%. As a representative, the recovery of PAEs in spiked sunflower seed oil was shown in Table 1. Supported by the results, the method was proved to be sensitive, reliable and accurate for determination of the contamination levels of PAEs in plant oils.| PAEs | Low (0.5 mg kg−1) | Medium (1.5 mg kg−1) | High (3.0 mg kg−1) | |||
|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%, n = 3) | Recovery (%) | RSD (%, n = 3) | Recovery (%) | RSD (%, n = 3) | |
| BBP | 82.63 | 5.37 | 85.81 | 5.90 | 90.72 | 6.39 |
| DAP | 90.20 | 6.10 | 91.63 | 7.21 | 100.09 | 8.93 |
| DBP | 106.41 | 7.56 | 94.25 | 6.13 | 95.77 | 5.35 |
| DBEP | 87.80 | 6.12 | 86.37 | 8.36 | 91.35 | 6.94 |
| DCHP | 78.09 | 9.69 | 75.83 | 6.30 | 76.96 | 8.02 |
| DEP | 83.20 | 5.18 | 87.60 | 6.39 | 93.38 | 5.77 |
| DEEP | 83.10 | 7.35 | 87.80 | 5.31 | 98.51 | 6.57 |
| DEHP | 77.56 | 7.10 | 75.50 | 7.02 | 80.10 | 6.35 |
| DHP | 80.07 | 6.16 | 84.12 | 4.47 | 84.33 | 5.39 |
| DiBP | 81.85 | 6.74 | 86.05 | 6.67 | 91.10 | 5.79 |
| DMP | 75.06 | 8.37 | 85.71 | 8.53 | 90.23 | 6.37 |
| DMEP | 79.80 | 7.15 | 83.93 | 8.19 | 99.31 | 4.33 |
| DMPP | 79.11 | 8.17 | 85.80 | 9.21 | 90.20 | 7.50 |
| DNP | 88.80 | 5.25 | 80.43 | 4.36 | 77.82 | 4.65 |
| DnOP | 76.77 | 6.31 | 75.51 | 6.15 | 79.27 | 6.53 |
| DPP | 79.25 | 6.30 | 84.80 | 4.26 | 88.01 | 6.28 |
| DPhP | 83.70 | 5.29 | 79.43 | 8.70 | 83.90 | 7.52 |
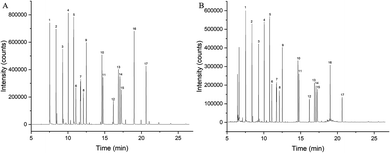
Fig. 1 showed the total ion chromatograms of standard solution and spiked oil sample. As can be seen, good chromatographic separation was achieved for 17 PAEs.
3.2 Occurrence and distribution of PAEs detected in plant oil samples
No other phthalate ester was detected in the investigated samples, except for DBP, DEHP and DMP. In general, the detectable rate (>LODs) was 56.83% for 366 samples. For individual PAE congener, the detectable rate was noticeably different, ranging from 0.27% to 56.83%. The contamination frequency of three PAE congeners declined in the order of DBP, DEHP and DMP. Thereinto, DMP was detected in only one sunflower seed oil sample with the concentration of 3.64 mg kg−1. Since DMP is non-carcinogenic without any available risk assessment parameter, the statistical analysis of DBP and DEHP was conducted and described in the following sections. According to the results in Table 2, DBP was the most frequently detected phthalate ester in the plant oil samples, which was in accordance with its widespread use in plastics manufacture. In addition, DBP spanned the larger concentration range (0.10–3.20 mg kg−1), while the median/mean value of DEHP concentration (0.44 mg kg−1/0.58 ± 0.40 mg kg−1) was higher on the contrary. The results indicated that the occurrence and distribution of PAEs varied greatly from each other.| PAEs | Min. | Max. | Mean ± SD | Median | Detectable rate (%) |
|---|---|---|---|---|---|
| DBP | 0.10 | 3.20 | 0.41 ± 0.40 | 0.28 | 56.83 |
| DEHP | 0.25 | 1.95 | 0.58 ± 0.40 | 0.44 | 21.58 |
Distribution of the PAEs concentrations were verified to follow log-normal distribution with the Kolmogorov–Smirnov test (p > 0.05). Corresponding parameters of the probability distribution were listed in Table 3.
Furtherly, the classificatory distribution of the detected PAEs (concentrations higher than LODs) in different categories of plant oils was shown in Table 4. As can be seen, all kinds of plant oils were contaminated with at least one kind of phthalate ester, indicating that PAEs contamination was easy to occur in commercial plant oils. Furthermore, significant variation in the occurrence and contamination levels of the PAEs was presented for different plant oils. Co-occurrence of DBP and DEHP has been observed in most of the plant oil species, implying the potential co-exposure to PAE congeners through daily consumption of commercial plant oils. On the other hand, the incidence of DBP was 100% for 8 categories of plant oils. And DEHP was detected in more than half of the categories, except for crude oil and linseed oil. This phenomenon might be induced by the manufacturing procedure of oil products. As for the contamination concentrations of PAEs in different oil species, the average concentrations of ∑PAEs descended in the following sequence: safflower oil (1.66 ± 1.11 mg kg−1), virgin rapeseed oil (0.87 ± 0.60 mg kg−1), sunflower seed oil (0.74 ± 0.46 mg kg−1), virgin linseed oil (0.74 ± 0.28 mg kg−1), virgin sunflower seed oil (0.59 ± 0.30 mg kg−1), linseed oil (0.35 ± 0.30 mg kg−1), crude rapeseed oil (0.27 ± 0.15 mg kg−1), crude sunflower seed oil (0.25 ± 0.13 mg kg−1). High concentration of PAEs means a potential threat to human health. It was clear that safflower oil and virgin rapeseed oil exhibited relatively high contamination frequency and concentration levels of PAEs, which might result in human health risk.
| Plant oil | DBP | DEHP | ||||
|---|---|---|---|---|---|---|
| Detected number/sample number | Concentration range (mg kg−1) | Mean ± SD (mg kg−1) | Detected number/sample number | Concentration range (mg kg−1) | Mean ± SD (mg kg−1) | |
| Crude rapeseed oil | 10/20 | 0.11–0.48 | 0.26 ± 0.15 | 0/20 | — | — |
| Crude sunflower seed oil | 5/23 | 0.13–0.44 | 0.25 ± 0.13 | 0/23 | — | — |
| Linseed oil | 6/26 | 0.16–0.95 | 0.35 ± 0.30 | 0/26 | — | — |
| Safflower oil | 28/34 | 0.12–2.09 | 0.65 ± 0.59 | 24/34 | 0.48–1.95 | 1.01 ± 0.52 |
| Sunflower seed oil | 54/82 | 0.10–2.20 | 0.33 ± 0.32 | 8/82 | 0.25–0.76 | 0.41 ± 0.15 |
| Virgin linseed oil | 27/54 | 0.10–0.66 | 0.30 ± 0.16 | 6/54 | 0.27–0.58 | 0.44 ± 0.11 |
| Virgin rapeseed oil | 58/87 | 0.10–3.20 | 0.47 ± 0.46 | 10/87 | 0.25–0.58 | 0.40 ± 0.14 |
| Virgin sunflower seed oil | 20/40 | 0.10–1.28 | 0.35 ± 0.30 | 1/40 | 0.25 | 0.25 |
3.3 Risk assessment
According to the toxicologic research of PAEs, DBP was recognized as a non-cancer related compound, while DEHP was regarded as a potential carcinogen. In consequence, carcinogenic risk as well as non-carcinogenic risk derived from the detected PAEs were evaluated for risk assessment in this study. Generally, health risk assessment can be conducted by probabilistic approach and point estimate methodology (deterministic approach). Deterministic analysis is prone to over-estimate the real health risk induced by hazard exposure, while probabilistic analysis is commonly applied to account for the ignorance of the variability and uncertainty of the data available in a deterministic approach.31,32 Therefore, the probabilistic approach was used for the risk assessment of the PAE congeners in this study. To conduct this strategy, the probabilistic model was established by Monte Carlo simulation. The probabilistic approach simulated the consumption data of plant oil and the concentration levels of PAEs as distribution. Based on the distribution, the health risk was evaluated according to the computation formulae in section 2.4.A more “holistic” approach was expected in consideration of the variability of oil consumption, the human body weight and the PAEs concentration level. It is worth mentioning that data of oil consumption was referenced to “the investigation of the status of cooking oil and salt consumption in adults among 15 provinces in China in 2015”.33 In this investigation, significant differences in the oil consumption data were observed for different populations. Therefore, representative classification parameters were considered in this study, including gender and age. The specific consumption data was listed in Table 5. With respect to human body weight, the average body weights of adults in China are respectively 68.9 (±8.9) kg for male and 59.7 (±5.59) kg for female,30 which were applied in the subsequent evaluation of human health risk.
| Group | Mean (S) | P5 | P25 | P50 | P75 | P95 |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 45.16 (37.12) | 7.91 | 22.25 | 36.15 | 57.8 | 110.37 |
| Female | 37.93 (29.97) | 6.39 | 18.5 | 30.81 | 48 | 94.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Age | ||||||
| 18–44 | 39.74 (32.76) | 6.09 | 18.97 | 32.02 | 50.83 | 97.01 |
| 45–59 | 42.89 (34.58) | 8.15 | 21.25 | 34.23 | 53.38 | 104.64 |
| Total | 41.36 (33.74) | 7.08 | 20.02 | 33.1 | 52.25 | 101.2 |
3.4 Risk assessment for different populations
The health risk associated with exposure to DBP and DEHP was firstly assessed for different populations classified by gender and age. The resulting EDI values of individual PAE congener were listed in Table 6. According to the result, the mean and maximum EDI values of DBP were both higher than those of DEHP, which was contrary to the median values. It was indicated that DBP and DEHP were both important contributors to human health risk. The maximum EDI value for two PAE congeners was up to 7942.13 ng (kg day)−1, implying the potential of adverse health effects.| PAEs | Male | Female | 18–44 | 45–59 | No classification | |
|---|---|---|---|---|---|---|
| DBP | Mean | 178.26 | 169.59 | 152.57 | 168.77 | 171.61 |
| 5th | 4.72 | 4.80 | 4.06 | 5.02 | 4.72 | |
| 50th | 63.23 | 63.32 | 60.91 | 66.06 | 63.97 | |
| 95th | 683.69 | 627.70 | 641.03 | 639.04 | 622.38 | |
| Maximum | 6364.77 | 7499.63 | 2943.61 | 4868.44 | 7942.13 | |
| DEHP | Mean | 148.04 | 143.09 | 141.89 | 146.84 | 150.35 |
| 5th | 6.33 | 5.91 | 6.07 | 6.49 | 6.24 | |
| 50th | 71.67 | 69.87 | 67.19 | 76.12 | 72.43 | |
| 95th | 556.73 | 485.02 | 501.77 | 585.56 | 534.10 | |
| Maximum | 5023.31 | 2498.50 | 2793.78 | 2541.92 | 3211.91 | |
Based on the resulting EDI values, the distribution of carcinogenic risk was computed and displayed in Fig. 2. The 5th to 95th percentile of carcinogenic risk was calculated to be 8.74 × 10−8–7.48 × 10−6, despite the populations' classification. Although the maximum values of carcinogenic risk were all at the acceptable level (10−6–10−4) according to USEPA,29 relatively high probabilities of CR values exceeding the lower acceptable carcinogenic risk level (10−6) were obtained. The possibility of CR values higher than 10−6 for different populations was speculated to be 50.10% (male), 49.40% (female), 48.50% (age group of 18–44) and 51.70% (age group of 45–59), respectively. On the other side, there was no significant difference in the median values of carcinogenic risk among different populations. It's noteworthy that the maximum carcinogenic risk was close to the upper acceptable carcinogenic risk level (10−4), especially for male with the highest CR value (7.03 × 10−5). Overall, the exposure to DEHP in the plant oil was considered to be acceptable. And similar distribution of carcinogenic risk induced by DEHP were observed for different populations.
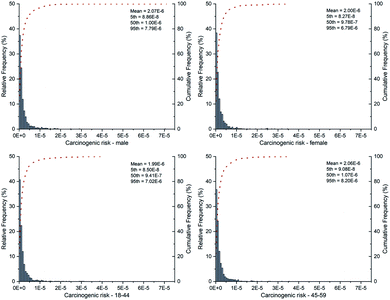 | ||
| Fig. 2 The probability and cumulative probability distribution of carcinogenic risk for different populations. | ||
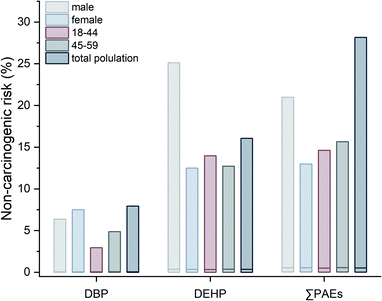
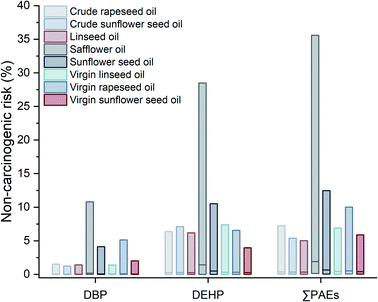
The individual and total non-carcinogenic risk of PAE congeners were also evaluated by the Monte Carlo simulation process. Fig. 3 illustrated the distribution of non-carcinogenic risk induced by DBP and DEHP for different populations. Although extremely low non-carcinogenic risk was observed on the whole (NCR value < 1), DEHP exhibited more potential of non-carcinogenic risk in comparison to DBP. Similar to the result of carcinogenic risk, no considerable difference was obtained for median values of non-carcinogenic risk among different populations. Nevertheless, the maximum values existed in diversity for single PAEs, with the highest NCR value of DBP (7.94%) for the total population and the one of DEHP (25.12%) for male. As for the 95th percentiles, the maximum values were obtained for male (DBP, 0.68%) and age group of 45–59 (DEHP, 2.93%), respectively. These phenomena might result from the composite effect of different populations' body weight and consumption habit of cooking oil. Since the probability of occurrence had been considered in the simulation model, the total non-carcinogenic risk of PAEs was assessed in a synergetic combination of DBP and DEHP. The integrated outcome of the non-carcinogenic risk of ∑PAEs was realized by random sampling of 1000 iterators in Monte Carlo simulation. It can be seen from the result that the total non-carcinogenic risk was not simply summing up the individual NCR values point-to-point. And DEHP obviously contributed most to the total non-carcinogenic risk. A decrease of the maximum values was observed in the sequence of total population, male, age group of 45–59, age group of 18–44 and female. Combined with the results of individual non-carcinogenic risk, the values of PAEs' non-carcinogenic risk were lower than 1 in all cases, suggesting that the additional hazardous health effects were unlikely to happen by daily consumption of the investigated plant oil.
3.5 Risk assessment for different categories of plant oils
With regard to the health risk associated with different categories of plant oils, the carcinogenic risk and non-carcinogenic risk of PAEs were similarly investigated in spite of the populations' classification. The probability distribution of carcinogenic risk for different categories of plant oils was displayed in Fig. 4. As shown in the figure, safflower oil presented relatively high values of carcinogenic risk in the 5th to 95th percentile of 2.51 × 10−7–2.18 × 10−5. The median values of carcinogenic risk descended in the following sequence: safflower oil (3.99 × 10−6) > sunflower seed oil (1.38 × 10−6) > virgin linseed oil (9.03 × 10−7) > virgin rapeseed oil (8.99 × 10−7) > crude rapeseed oil (7.78 × 10−7) > virgin sunflower seed oil (7.77 × 10−7) > crude sunflower seed oil (7.58 × 10−7) > linseed oil (7.28 × 10−7). It was also revealed that the CR values were lower than 10−4 for all kinds of plant oils. The median values were below the lower acceptable level of carcinogenic risk (10−6), except for those of safflower oil and sunflower seed oil. From another perspective, the probability of CR values exceeding 10−6 was 39.90%, 41.60%, 38.40%, 80.40%, 62.40%, 46.00%, 46.40% and 40.70% for crude rapeseed oil, crude sunflower seed oil, linseed oil, safflower oil, sunflower seed oil, virgin linseed oil, virgin rapeseed oil and virgin sunflower seed oil, respectively. The results implied that the exposure of DEHP in each investigated species of plant oil was considered to be safe, while safflower oil and sunflower seed oil exhibited a relatively high potential of carcinogenic risk.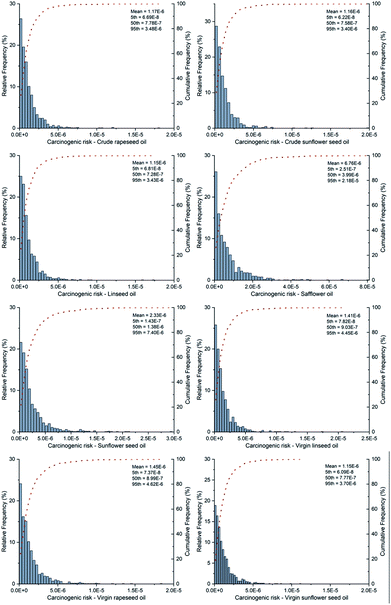 | ||
| Fig. 4 The probability and cumulative probability distribution of carcinogenic risk for different categories of plant oils. | ||
The distribution of non-carcinogenic risk for different kinds of plant oils was presented in Fig. 5. Initially, the values of individual and total non-carcinogenic risk were far below l for all plant oil species. Obvious variance, meanwhile, was displayed in the distribution of non-carcinogenic risk among the 8 categories of plant oils. It was especially outstanding that safflower oil had the highest median and maximum values of non-carcinogenic risk, which were up to 9 times of those of other plant oils. Individual non-carcinogenic risk induced by DEHP was higher than those induced by DBP, just the same as the situation for the populations' classification. As for total non-carcinogenic risk, the 5th to 95th percentiles were 0.11–1.48% (crude rapeseed oil), 0.08–1.38% (crude sunflower seed oil), 0.09–1.34% (linseed oil), 0.51–8.00% (safflower oil), 0.18–2.86% (sunflower seed oil), 0.12–1.65% (virgin linseed oil), 0.14–1.93% (virgin rapeseed oil) and 0.12–1.44% (virgin sunflower seed oil), respectively. Relatively high total non-carcinogenic risk was obtained at the maximum level for safflower oil (35.59%), while the maximum values for other plant oils were all below 15%.
To sum up, the health risk of PAEs induced by consumption of the imported plant oils appeared to be generally low. Noteworthily, extreme cases of non-carcinogenic risk and carcinogenic risk existed in consumption of plant oil, implying that potential of hazardous effects may be posed by exclusive consumption of one variety of plant oil with a relatively high PAEs contamination rate/level over a long period.
3.6 Uncertainty
In this study, there were still several uncertainties and data deficiency. Primarily, a relatively limited number of samples (n = 366) were investigated, which may have an influence on the statistical prediction ability of the assessment model. Furthermore, the time parameter (ED × EF/AT) was considered to be equal to 1 by default. In this way, the risk was unlikely to vary over exposure time. Therefore, deviation more or less existed in the assessment of health risk in this study.4. Conclusions
According to the results of this study, conclusions were summarized as the following points:Firstly, contamination of PAEs, mainly including DBP and DEHP, occurred frequently in the plant oil samples with wide concentration range, which indicated a potential of human health risk. Besides, an obvious distinction was observed in the occurrence and contamination levels of PAEs for different categories of plant oils, in accordance with their usage conditions in plastics manufacture.
Secondly, relatively low health risk associated with DBP and DEHP was obtained from the plant oil samples. There could be less concern about the non-carcinogenic risk of PAEs induced by daily consumption of plant oil. It's worth noting that more than half of the carcinogenic risk exceeded the lower acceptable limit (>10−6), although the carcinogenic risk of DEHP was generally considered to be safe (<10−4).
Thirdly, obviously diverse health risks were associated with different categories of plant oils. Particularly for safflower oil, there was relatively high health risk index. Accordingly, long-term or exclusive consumption of plant oil with relatively high PAEs contamination rate/level may induce potential health risk for humans.
Last but not least, intensive routine monitoring of PAEs in imported plant oil is recommended on account of the high occurrence frequency and diverse contamination levels of PAEs. Simultaneously, a call for specialized maximum residue limits (MRLs) of PAEs in plant oil is inevitable, comprehensively considering the health risk related to PAEs in the plant oil samples.
Author contributions
Z. Tang: methodology, visualization, funding acquisition, writing – original draft. Z. Gong: investigation, writing – original draft. W. Jia: methodology, writing – review & editing. W. Shen: investigation, visualization. Q. Han: investigation, visualization. F. Fang: methodology, supervision, writing – review & editing. C. Peng: supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts of interest.Acknowledgements
This work was supported by China Postdoctoral Science Foundation (grant no. 2019M663458), Natural Science Foundation of Science & Technology Department of Xinjiang Uygur Autonomous Region (grant no. 2021D01B14), National Natural Science Foundation of China (grant no. 81891012) and the “Xinglin scholars” program of Chengdu university of TCM (grant no. BSH2019014).References
- L. Zhang, J. Liu, H. Liu, G. Wan and S. Zhang, Ecotoxicology, 2015, 24, 967–984 CrossRef CAS.
- Q. Zhang, Y. Sun, Q. Zhang, J. Hou, P. Wang, X. Kong and J. Sundell, Sci. Total Environ., 2020, 719, 136965–1369671 CrossRef CAS PubMed.
- M. M. Abdel daiem, J. Rivera Utrilla, R. Ocampo Perez, J. D. Mendez Diaz and M. Sanchez Polo, J. Environ. Manage., 2012, 109, 164–178 CrossRef CAS.
- S. Net, R. Sempere, A. Delmont, A. Paluselli and B. Ouddane, Environ. Sci. Technol., 2015, 49, 4019–4035 CrossRef CAS.
- M. Wittassek, H. M. Koch, J. Angerer and T. Bruning, Mol. Nutr. Food Res., 2011, 55, 7–31 CrossRef CAS PubMed.
- T. T. Bui, G. Giovanoulis, A. P. Cousins, J. Magner, I. T. Cousins and C. A. de Wit, Sci. Total Environ., 2016, 541, 451–467 CrossRef CAS PubMed.
- S. Net, R. Sempere, A. Delmont, A. Paluselli and B. Ouddane, Environ. Sci. Technol., 2015, 49, 4019–4035 CrossRef CAS PubMed.
- O. Anne and T. Paulauskiene, Sustainability, 2021, 13, 529–542 CrossRef CAS.
- M. V. Russo, P. Avino, L. Perugini and I. Notardonato, RSC Adv., 2015, 5, 37023–37043 RSC.
- N. Nanni, K. Fiselier, K. Grob, M. Pasquale, L. Fabrizi, P. Aureli and E. Coni, Food Control, 2011, 22, 209–214 CrossRef CAS.
- I. Ostrovsky, R. Cabala, R. Kubinec, R. Gorova, J. Blasko, J. Kubincova, L. Rimnacova and W. Lorenz, Food Chem., 2011, 124, 392–395 CrossRef CAS.
- Y. Ling, W. Jia, P. Jiang, J. Huang and X. Chu, Food Sci., 2013, 34, 155–159 CAS.
- C. Li, J. Chen, J. Wang, P. Han, Y. Luan, X. Ma and A. Lu, Sci. Total Environ., 2016, 568, 1037–1043 CrossRef CAS PubMed.
- G. S. Javier, M. A. G. Curbelo, B. S. Rodriguez, J. H. Borges and M. A. Rodriguez Delgado, Chemosphere, 2018, 201, 254–261 CrossRef PubMed.
- B. S. Rodriguez, J. G. Salamo, A. V. H. Herrera, A. S. Mayor and J. H. Borges, Anal. Bioanal. Chem., 2018, 410, 5617–5628 CrossRef PubMed.
- C. O. Zamora, J. G. Salamo, C. H. Sanchez and J. H. Borges, ACS Sustainable Chem. Eng., 2020, 8, 8783–8794 CrossRef.
- E. Cheshmazar, L. Arfaeinia, Y. Vasseghian, B. Ramavandi and A. M. Khaneghah, J. Food Compos. Anal., 2021, 99, 103880–103887 CrossRef CAS.
- J. Wang, G. Chen, P. Christie, M. Zhang, Y. Luo and Y. Teng, Sci. Total Environ., 2015, 523, 129–137 CrossRef CAS PubMed.
- J. Yang, Y. Li, Y. Wang, J. Ruan, J. Zhang and C. Sun, TrAC, Trends Anal. Chem., 2015, 72, 10–26 CrossRef CAS.
- Y. Huang, J. Anal. Sci., 2012, 28, 217–221 CAS.
- P. Wu, D. Yang, L. Zhang, X. Shen, X. Pan, L. Wang, J. Zhang, Y. Tan, L. Feng and Y. Ying, J. Sep. Sci., 2012, 35, 2932–2939 CrossRef CAS PubMed.
- W. Li, J. Cao, J. Jin, Q. Jin and X. Wang, Zhongguo Youzhi, 2015, 40, 62–65 CAS.
- F. He, Y. Tian, Z. Xu, L. Luo, J. Yang, H. Wang, Y. Sun, Q. Du and Y. Shen, J. Toxicol. Environ. Health, Part A, 2018, 81, 80–88 CrossRef CAS.
- X. Li, Q. Zhang, L. Chen, J. Zhao and H. Li, Anal. Methods, 2018, 10, 3197–3206 RSC.
- J. Zhao, N. Wang and Y. Dong, Xiandai Yufang Yixue, 2020, 47, 608–611 Search PubMed.
- Z. Lu and S. Na, Agric. Outlook, 2017, 94–98 Search PubMed.
- L. Zhang, Hei Long Jiang, Liang Shi, 2021, 32–34.
- GB, National Standards of the People's Republic of China, GB 5009.271—2016, Determination of Phthalate Esters in Food, National Health and Family Planning Commission of China, China Food and Drug Administration, 2016, http://down.foodmate.net/standard/yulan.php?itemid=50428, accessed 20 November 2021 Search PubMed.
- USEPA, Guidelines for Exposure Assessment, EPA/600/Z-92/001 the United States Environmental Protection Agency, 1992,https://www.epa.gov/sites/default/files/2014-11/documents/guidelines_exp_assessment.pdf, accessed 20 November 2021 Search PubMed.
- W. L. Wang, Q. Y. Wu, C. Wang, T. He and H. Y. Hu, Environ. Sci. Pollut. Res., 2015, 22, 3620–3630 CrossRef CAS PubMed.
- Z. Han, D. Nie, E. N. Ediage, X. Yang, J. Wang, B. Chen, S. Li, S. L. On, S. De Saeger and A. Wu, Food Chem. Toxicol., 2014, 74, 334–342 CrossRef CAS PubMed.
- K. Nakazawa, O. Nagafuchi, U. Otede, J. Chen, K. Kanefuji and K. Shinozuka, RSC Adv., 2020, 10, 18296–18304 RSC.
- H. Jiang, J. Zhang, C. Su, J. Zhang, B. Zhang and H. Wang, Acta Nutr. Sin., 2018, 40, 27–31 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |