Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boosting the photocatalytic H2 evolution activity of type-II g-GaN/Sc2CO2 van der Waals heterostructure using applied biaxial strain and external electric field
Francis Opoku *a,
Samuel Osei-Bonsu Oppong
*a,
Samuel Osei-Bonsu Oppong b,
Albert Aniagyei
b,
Albert Aniagyei c,
Osei Akoto
c,
Osei Akoto a and
Anthony Apeke Adimado
a and
Anthony Apeke Adimado a
a
aDepartment of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. E-mail: ofrancis2010@gmail.com; francisopoku@knust.edu.gh
bMarine Engineering Department, Regional Maritime University, P.O. Box GP 1115, Accra, Ghana
cDepartment of Basic Sciences, University of Health and Allied Sciences, Ho, Ghana
First published on 4th March 2022
Abstract
Two-dimensional (2D) van der Waals (vdW) heterostructures are a new class of materials with highly tunable bandgap transition type, bandgap energy and band alignment. Herein, we have designed a novel 2D g-GaN/Sc2CO2 heterostructure as a potential solar-driven photocatalyst for the water splitting process and investigate its catalytic stability, interfacial interactions, and optical and electronic properties, as well as the effects of applying an electric field and biaxial strain using first-principles calculation. The calculated lattice mismatch and binding energy showed that g-GaN and Sc2CO2 are in contact and may form a stable vdW heterostructure. Ab initio molecular dynamics and phonon dispersion simulations show thermal and dynamic stability. g-GaN/Sc2CO2 has an indirect bandgap energy with appropriate type-II band alignment relative to the water redox potentials. Meanwhile, the interfacial charge transfer from g-GaN to Sc2CO2 can effectively separate electron–hole pairs. Moreover, a potential drop of 3.78 eV is observed across the interface, inducing a built-in electric field pointing from g-GaN to Sc2CO2. The heterostructure shows improved visible-light optical absorption compared to the isolated g-GaN and Sc2CO2 monolayers. Our study demonstrates that tunable electronic and structural properties can be realised in the g-GaN/Sc2CO2 heterostructure by varying the electric field and biaxial strain. In particular, the compressive strain and negative electric field are more effective for promoting hydrogen production performance. Since it is challenging to tune the electric field and biaxial strain experimentally, our research provides strategies to boost the performance of MXene-based heterojunction photocatalysts in solar harvesting and optoelectronic devices.
I. Introduction
There are increased power and energy demands in applications, including industrial and household energy management, hybrid electric vehicles and cordless electric tools.1 To date, new approaches have been designed to generate alternative energy sources owing to the eventual depletion of fossil fuel.2 Photocatalysis has been an effective technology for producing sustainable and storable fuels using solar energy.3 Water is broadly regarded as the only accessible electron and proton source for fuel synthesis reactions, and this makes the hydrogen evolution reaction (HER) a critical component of solar energy technology.4 Due to the high energy efficiency and the practical and economic advantages, producing H2 via the HER to replace the depleted fossil fuel is regarded as the most promising alternative clean energy carrier.5 Nonetheless, the low abundance and high cost of Pt-based H2 generation materials substantially restrict their use as an effective HER photocatalyst. Therefore, there is a higher challenge in developing a highly active, lower cost and more abundant noble metal free HER catalyst materials.6 Semiconductor-based photocatalyst materials, particularly those with strong structure stability and enhanced photoactivity, are promising solar energy conversion and storage materials.7Lately, several two-dimensional (2D) have received much interest as potential materials for next-generation nanoelectronic, optoelectronic and solar conversion devices owing to their unique structures and outstanding properties.8–11 MXene with a hexagonal lattice has attracted much attention due to its promising applications in electronic devices.12 MXenes, such as 2D transition-metal carbonitrides, nitrides and carbides, have been prepared by isolating elementary MX layers from the bulk MAX materials,13 where M is an early transition metal, A is a group 13 or 14 element and X represents N or C element.14 MXenes have been studied as a possible energy storage solution for supercapacitors, CO2 conversion, photocatalytic hydrogen evolution and battery anodes in recent years.15–18 Most bulk MXenes show metallic features without bandgap energy, limiting their potential application.13 Nevertheless, they can tune from a metallic to a semiconducting state by surface functionalisation with groups, such as OH, F and O. For example, Sc2CO2, Sc2C(OH)2, Sc2CF2, Zr2CO2, Hf2CO2 and Ti2CO2 monolayers were predicted to be semiconductor materials.19 Materials with oxygen functional groups, such as Hf2CO2, Sc2CO2, Zr2CO2 and Ti2CO2, are typically the most stable and have excellent semiconductor properties.20,21 Particularly, Sc2CF2 and Sc2CO2 monolayers are demonstrated to have visible light optical absorption and high carrier mobilities.22 Sc2CO2, on the other hand, is distinguished from other semiconductor MXenes by its asymmetric oxygen-terminated structure.23 Because of its nonvanishing electric dipole moment,24 Sc2CO2 is a good material when investigating the effect of polarity on heterostructure band alignment. Because it has a stable structure with an indirect bandgap and can resist high temperature,25 graphene-like gallium nitride (g-GaN) is one of the next generation 2D materials.26,27 Recently, g-GaN was synthesised using the migration-enhanced encapsulated growth technique,28 and its electronic properties may be easily controlled using a stacking effect or an external electric field.29
Surprisingly, recent theoretical studies30–32 suggested that several 2D-based materials may be suitable for water splitting applications. Nonetheless, because they are atomically thin, photogenerated charge carriers have a short lifetime,30,33 limiting their practical applicability. As a result, employing 2D monolayer as photocatalyst for water splitting is difficult and complicated. 2D van der Waals (vdW) heterostructures, which combine the characteristics of their individual components, are now regarded as a potential approach of fabricating nanoelectronic, optoelectronic and solar energy conversion devices.34,35 In the vdW heterostructure, band alignments are classified as type-I, type-II, or type-III, with each having a distinct application that allows for the fabrication of various electronic devices.36 Many newly designed 2D-based vdW heterostructures37,38 have type-II band alignment with a reduced recombination rate of photogenerated charge carriers with the help of the valence-band offset (VBO) and conduction-band offset (CBO),38 extending photogenerated charge carrier lifetimes and enhancing water splitting efficiency.
More crucially, g-GaN sharing nearly identical lattice constants and a similar hexagonal crystal structure with Sc2CO2 is beneficial to the fabrication of g-GaN/Sc2CO2 heterostructure, which are highly expected and of great interest.20,39 Therefore, we systematically investigate the underlying mechanism of charge transfer, interface interaction, electronic structures, relative stability, band alignments and optical absorption properties of g-GaN/Sc2CO2 heterostructure as a possible water splitting photocatalyst using density functional theory (DFT) calculations. Considering that g-GaN and Sc2CO2 monolayers have been reported to show outstanding electronic properties under strain,29 the effect of biaxial strain and electric field on the structural and electronic (bandgaps and band edge positions) properties of g-GaN/Sc2CO2 heterostructure was also investigated. Our findings should open the way for a new generation of optoelectronic devices and develop vdW heterostructure as viable candidates for photocatalytic application.
II. Computational details
First-principles calculations were carried out within the plane-wave DFT framework, as implemented in the Quantum Espresso package,40 with optimised norm-conserving Vanderbilt pseudopotentials.41 For the exchange–correlation energy, structural optimisations were done using the generalised gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional.42 For wavefunctions and charge density, the plane wave kinetic energy cutoffs were set to 80 and 520 Ry, respectively. The interlayer vdW interactions and dipole were treated using the dispersion corrections method of Grimme (DFT-D3(BJ)).43 To minimise spurious interaction between periodic pictures, a vacuum spacing of 30 Å was used. By fully relaxing both ionic locations and cell vectors until the maximum residual force and energy were less than 10−3 Ry per bohr and 10−8 Ry, respectively, optimal lattice parameters were achieved. The Brillouin zone (BZ) for g-GaN, Sc2CO2 and g-GaN/Sc2CO2 heterostructure was sampled using a Monkhorst–Pack44 with k-points of 14 × 14 × 1, 12 × 12 × 1, and 12 × 12 × 1, respectively. To retain the same number of k-points within the irreducible BZ for all structural models, no symmetry was used in all computations. The Methfessel–Paxton smearing method was used with a broadening of 0.002 Ry along the high symmetry zone. Because the PBE functional underestimates the bandgap energy of semiconductors, the Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional (mixing parameter 0.25, screening parameter 0.2 Å−1)45 was used. HSE06 has been shown to be a dependable functional for calculating optical and electronic properties.46 To analyse the kinetic stability of the heterostructure and monolayers, phonon dispersion calculations were done using the density functional perturbation theory47 within the Phonopy package48 as implemented in the Quantum ESPRESSO code. The real-space force constants were computed using the finite displacement technique from the Hellmann–Feynman forces by introducing displacements to supercells.49 The force constants were then used to calculate the dynamical matrices and phonon frequencies. Besides, the thermal stability was evaluated using ab initio molecular dynamics (AIMD) simulations with the Nosé–Hoover scheme50,51 at an ambient temperature of 300 K for 70 ps within each 1 fs time step. To consider the constraints of lattice translational, we design 4 × 4 supercells of Sc2CO2/g-GaN heterostructure for the AIMD simulations. Visualisations of charge density difference, electron localisation function and optimised structures were obtained with the XCrySDen package.52III. Results and discussion
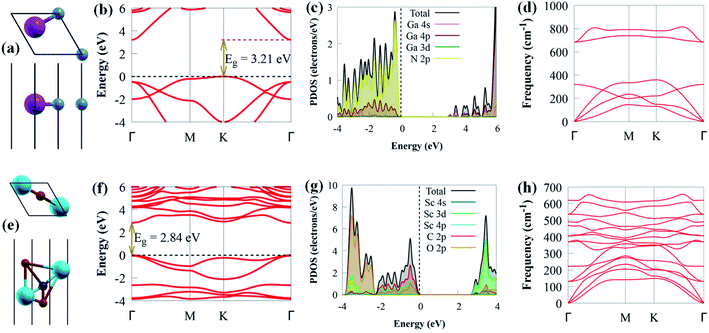
Before delving into the optical and electronic properties of g-GaN/Sc2CO2 heterostructure, we first examine the lattice constants of g-GaN and Sc2CO2 monolayers, which are optimised as 3.438 and 3.253 Å, respectively. Our optimised lattice parameters agreed with earlier results,8,39 signifying the dependability of our computational method. The relaxed crystal structures, projected density of states (PDOS), electronic band structures and phonon of g-GaN and Sc2CO2 monolayers are depicted in Fig. 1. The indirect bandgap of g-GaN monolayer is 3.21 eV. The conduction band minimum (CBM) and valence band maximum (VBM) of g-GaN monolayer are positioned at the Γ and K points, respectively. However, the direct bandgap in Sc2CO2 monolayer (2.84 eV) is situated at Γ point of the BZ. These findings are consistent with previously reported values.8,53 The PDOS of g-GaN monolayer showed that the VBM mainly comprises N 2p states, whereas the CBM is dominated by Ga 4s state (Fig. 1c). The PDOS results agreed with earlier studies.54 Analysis of PDOS further reveals that the CBM of Sc2CO2 monolayer is dominated by the Sc 3d state, while C 2p states mainly contribute to the VBM (Fig. 1g). The PDOS results of Sc2CO2 are in agreement with earlier study.53g-GaN/Sc2CO2 heterostructure was designed from the unit cell of g-GaN and Sc2CO2 monolayers, as shown in Fig. 2a.
Generally, a stable heterostructure may favourably synthesise when the lattice mismatch is 5%.55 The interlayer lattice mismatch between g-GaN and Sc2CO2 monolayers is 5.38%, which is acceptable and accessible in the experimental synthesis because their interfaces are flexible and can accommodate this mismatch.53 Thus, the small lattice mismatch within 5.38% in all directions means high possibility to grow heterostructure among these compounds. For example, both experimental and theoretical studies on CsPbI3/GaN heterostructure confirm that the small lattice mismatch (2.1%) contributes to the in-plane growth of CsPbI3 on the mica substrate.56 Moreover, the lattice mismatch between MoS2 and Nb2CO2 monolayers is only about 1.5%, which indicates that the fabricated heterostructure is desirable. Expectedly, MoS2/Nb2CO2 heterostructure has been synthesised through a facile hydrothermal chemical method.57 Also, a lattice mismatch of less than 0.16% offers the experimental synthesis of PtSe2/GaN heterostructure. Zhuo et al.58 have successfully synthesised the PtSe2/GaN vdW heterostructure experimentally that is used for self-powered deep ultraviolet photodetector. In the supercell, the positive sign reflects a small lattice constant of the g-GaN monolayer with respect to the Sc2CO2 monolayer. The interface binding energy (Eb) of g-GaN/Sc2CO2 heterostructure is computed using the following equation to analyse the structural stability as follows:59
| Eb = Eg-GaN/Sc2CO2 − Eg-GaN − ESc2CO2/S, | (1) |
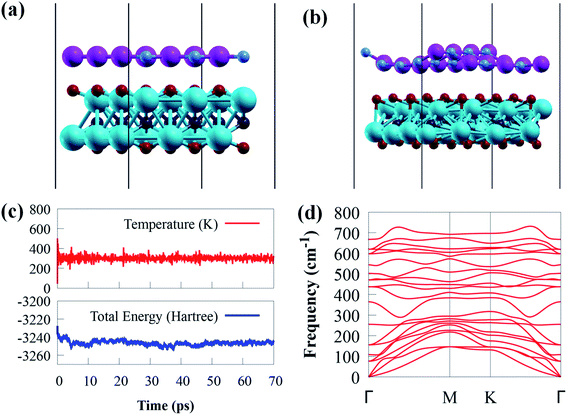
In addition, the change in total energy and temperature during the AIMD simulation is depicted in Fig. 2b and c. The results show that no visible bond breaking occurs after 70 ps at room temperature, and the overall energy and temperature variations are within a limited range, showing the thermal stability of g-GaN/Sc2CO2 heterostructure. This also confirmed that experimental fabrication is highly expected.67,68
The phonon dispersion spectrum is a trustworthy method for determining the stability of a structure.69 For all modes along the high symmetry BZ direction, a dynamically stable structure has no imaginary phonon frequencies. The phonon dispersion spectra of g-GaN/Sc2CO2 heterostructure, as well as g-GaN and Sc2CO2 monolayers, are calculated to analyse their kinetic stabilities. The phonon spectrum of Sc2CO2 (g-GaN) monolayer in Fig. 1d and h consist of 15 (6) branches, including 3 (3) acoustic and 12 (3) optical modes composed of five (two) atoms in the unit cell. The out-of-plane acoustic (ZA), transversal acoustic (TA) and longitudinal (LA) branches are linear near the Γ point of the BZ. It is worth noting that all the frequencies of g-GaN/Sc2CO2 heterostructure (Fig. 2d), g-GaN and Sc2CO2 monolayers are positive, confirming the dynamic stability.
Having established the dynamic and thermal stability, we then investigated the projected band structure of g-GaN/Sc2CO2 heterostructure, as given in Fig. 3a.
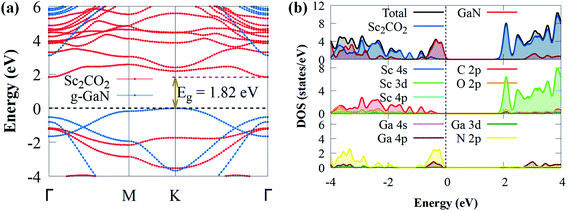
When a g-GaN/Sc2CO2 heterostructure is built, the band structures of both the Sc2CO2 and g-GaN monolayers are relatively well conserved. Compared to the direct bandgap of Sc2CO2 monolayer, the g-GaN/Sc2CO2 heterostructure has an indirect bandgap (1.82 eV) with VBM and CBM positioned at the K and Γ points of the BZ, respectively. The indirect bandgap semiconductor corresponds with other vdW heterostructures.26,70,71 The bandgap energy was lower than g-GaN/BSe (2.268 eV),72 MoSSe/g-GaN (2 eV),73 GaN/SiS (2.45 eV),74 WSSe/g-GaN (2.14),73 GeC/GaN (2.76 eV)75 and BlueP/Sc2CO2 (1.91 eV)76 vdW heterostructures. The smaller bandgap may be more conducive to the transfer and separation of photogenerated charge carriers, highlighting their potential application as visible light photocatalysts for H2 generation compared to the above vdW heterostructures. Furthermore, the heterostructure exhibited a lower bandgap than the g-GaN and Sc2CO2 monolayers, implying that electron excitation from the VBM to the CBM is simpler when the heterostructure is exposed to visible light. Furthermore, it had a much higher bandgap than the water splitting redox potential energy (1.23 eV), showing that the electronic structure of g-GaN/Sc2CO2 heterostructure makes it a visible light photocatalyst.74 The projected DOS and band structure shows that the Sc2CO2 monolayer dominates the CBM, whereas the g-GaN monolayer dominates the VBM, indicating that the VBM and CBM states are spatially separated. As a result, at the interface, an inherent type-II (staggered) band alignment is formed, which could aid in the effective separation of photoexcited electron–hole pairs once the vdW heterostructure is illuminated by sunlight. Also, because staggered type II band alignment heterostructures allow larger offsets on one side, substantial carrier confinement is always present.36 As a result, the heterostructure has potential applications in light-emitting diodes, lasers and solar energy conversion devices.77 The PDOS was computed and shown in Fig. 3b to help comprehend the band alignment. The VBM is predominantly derived from the N 2p orbitals, while the Sc 3d orbitals contribute to CBM. This validates the type-II band alignment, which effectively separates electron–hole pairs in both layers.78
As mentioned above, the g-GaN/Sc2CO2 heterostructure exhibit type-II band alignment with promising application in photocatalytic water splitting. The built-in electric field has a vital influence on the lifetime of photogenerated charge carriers, which is well recognised. As a result, we evaluate the interfacial properties of g-GaN/Sc2CO2 heterostructures to check if a built-in electric field exists by analysing the charge transfer, as illustrated in Fig. 4a.
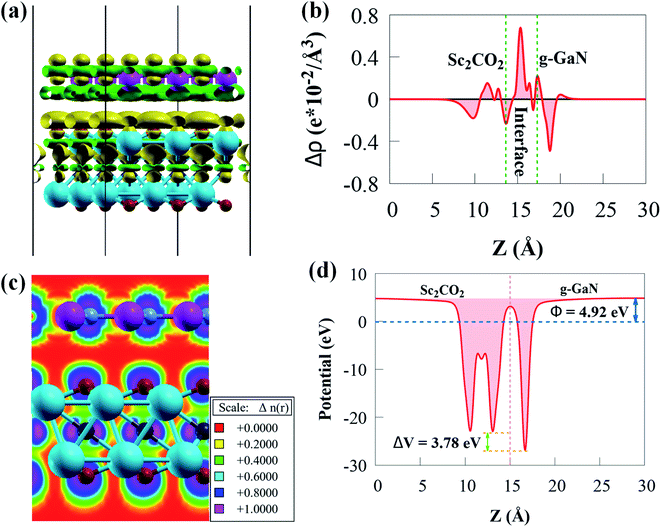
The green and yellow colours represent charge depletion and accumulation, respectively. Because of the vdW interaction at the interface, charge transfer is redistributed at the interface of g-GaN/Sc2CO2 heterostructure. The g-GaN monolayer donates electrons, whereas Sc2CO2 monolayer receives electrons. Thus, charge redistributions indicate interfacial charge transfer from g-GaN to Sc2CO2, which induces a built-in electric field pointing from g-GaN to Sc2CO2. The built-in electric field can cause photogenerated electrons and holes to separate spatially on various monolayers. According to the Löwdin charge population analysis,79 about 0.033 e per unit cell are transported from the g-GaN monolayer to the Sc2CO2 monolayer, resulting in a weak built-in electric field pointing from g-GaN monolayer to Sc2CO2 monolayer. The weak interaction between g-GaN and Sc2CO2 monolayers is indicated by such a small charge transfer. Even though this built-in electric field is small, we cannot overlook its vital significance in preventing charge carrier recombination rate, which can play an important influence in enhancing carrier mobility and prolonging their lifetime.
The Δρ(z) in Fig. 4b is negative and positive at the interface near g-GaN and Sc2CO2 monolayers, respectively. This confirms that electrons are transferred from g-GaN monolayer to Sc2CO2 monolayer through the vdW gap, which is primarily responsible for the band offset. Because the average electrostatic potential of g-GaN monolayer is lower than that of Sc2CO2 monolayer, as shown in Fig. 4c, a potential drop of 4.18 eV is observed across the interface, generating a built-in electric field. The potential drop correlates to a strong electrostatic field across the interface, which may have a significant impact on carrier dynamics and charge transfer if the g-GaN monolayer is used as an electrode. As a result, a strong driving force between the two monolayers is produced and electrons may be propelled across the interface from g-GaN monolayer to Sc2CO2 monolayer. The band offset is principally caused by the significant potential drop, which will aid in the separation and transfer of electron–hole pairs.80
The electron localisation function (ELF) may also be used to visualise the weak interfacial contact in the g-GaN/Sc2CO2 heterostructure. The spatial localisation extent of reference electrons is described by ELF, which is a continuous variable with values ranging from 0 to 1.81 ELF of 0 denotes total electron decentralisation, whereas ELF of 1 represents complete electron localisation.81 As seen in Fig. 4d, no electron is localised in the interface area, confirming the existence of vdW force.
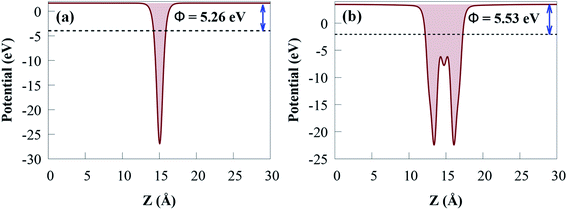
The charge density distribution at the heterostructure interface can influence the band alignment of semiconductor photocatalyst materials. The work function (Φ) of a photocatalyst material is an important electronic property that provides an in-depth knowledge of charge transfer and the relative location of the Fermi energy level.82 Φ is affected by the vacuum energy (Evacuum) and the Fermi energy (EF) as follows:
| Φ = Evacuum − EFermi | (2) |
For g-GaN and Sc2CO2 monolayers shown in Fig. 5, the work functions are 5.26 and 5.53 eV, respectively, which agreed with previous reported values.53,83
As Sc2CO2 coupled with g-GaN, the Φ difference between g-GaN and Sc2CO2 caused electrons in the g-GaN monolayer to spontaneously transfer to Sc2CO2 monolayer, and similarly, holes transfer to g-GaN, bringing the Fermi levels of the two monolayers to level up. Accordingly, electrostatics contribution makes the g-GaN and Sc2CO2 potentials positively and negatively charged, respectively, across the interfacial region. After the two monolayers have reached an equalised Fermi energy level, a built-in electric field pointing from g-GaN to Sc2CO2 will be established. Therefore, the electrons accumulated in Sc2CO2 surface cannot transfer back to g-GaN, thus limiting the direct recombination rate of charge carriers. Because of interface formation and charge transfer, the Φ of g-GaN/Sc2CO2 heterostructure (4.92 eV) in Fig. 4c is lower than that of the constituent monolayers.73 Because of the low Φ, light irradiation would make the electron transfer between the VBM and CBM edges easier.76
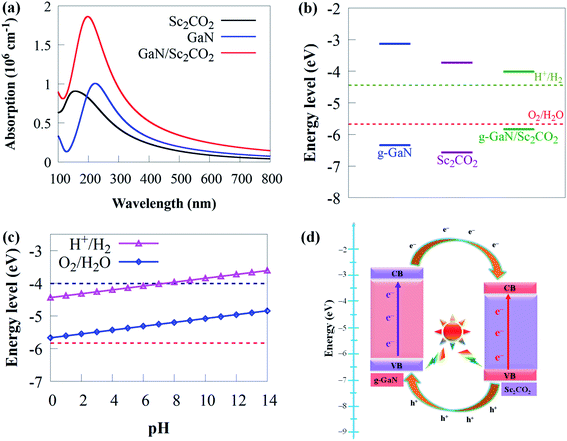
Because it can catch visible light to trigger photocatalytic redox processes, the optimal bandgap for a semiconductor photocatalyst is ∼2 eV.84,85 To promote photoactivity, rational modulation of bandgap is vital for efficient use of visible light absorption. Hence, it is significant to tune the bandgap and the band potential to prolong the visible light response to a broader wavelength region. Therefore, the novel 2D g-GaN/Sc2CO2 photocatalyst material designed should have enough optical absorption properties to capture more solar energy. As a result, as shown in Fig. 6, comparisons of optical absorption spectra are carried out between the novel 2D g-GaN/Sc2CO2 heterostructure and individual g-GaN and Sc2CO2 monolayers.
Both g-GaN and Sc2CO2 monolayers exhibit a weak absorption edge and intensity. The visible light absorption edge and intensity of g-GaN/Sc2CO2 heterostructure is higher than that of the individual monolayers. Because it results in type-II band alignment and benefits the effective separation of charge carriers, interlayer coupling between g-GaN and Sc2CO2 monolayers may be responsible for the enhanced redshift of absorption, thereby improving the photocatalytic performance. As a result, g-GaN/Sc2CO2 heterostructure can function as high-performance solar energy absorbers, which is advantageous for achieving a much better photocatalyst than the monolayers.
The band edge of photocatalytic water splitting materials must be at a suitable position in addition to having ideal bandgap energy. The valence band (VB) and the conduction band (CB) edge potentials of g-GaN and Sc2CO2 monolayers are evaluated using the bandgap and the absolute electronegativity of the atoms as follows:86
| EVB = χ − Ee + 0.5Eg | (3) |
| ECB = EVB − Eg | (4) |
Increasing the pH value from 0 (strong acid) to 3 (relatively weak acid) and eventually to 7 (neutral) retains the CBM more positive than the reduction potential, showing that the g-GaN/Sc2CO2 heterostructure has a favourable response for photocatalytic water splitting (Fig. 6c). We predict that novel 2D g-GaN/Sc2CO2 heterostructure straddling the redox potential of water at pH of 0–7 are beneficial for water splitting for large-scale and cost-efficient hydrogen production. However, when the pH is raised to 8, the CBM is pushed lower than the reduction potential, indicating that the overall photocatalytic performance has decreased. Thus, given the overpotential factor, it may not have been adequate for oxidation or reduction in a strong acid or base environment. These findings suggest that water splitting processes benefit from a mild acid environment similar to GaTe/C2N90 and SiC (g-SiC)/MoS2 (ref. 91) vdW heterostructure.
The schematic illustration of charge transfer at g-GaN/Sc2CO2 interface is given in Fig. 6d. Arrows represent the transfer of photogenerated electron–hole pairs at the interface. When the g-GaN/Sc2CO2 heterostructure is irradiated by sunlight, photogenerated electron–hole pairs are generated by absorbing energy larger than the bandgap energy. Subsequently, electrons will transfer from the VB to the CB of the respective monolayers. Moreover, driven by the CBO of 0.6 eV, electrons staying in the CB of g-GaN monolayer tend to transfer to the CB of Sc2CO2 monolayer. Meanwhile, the photogenerated holes in the Sc2CO2 monolayer move to the VB of g-GaN monolayer, driven by VBO of 0.23 eV. Consequently, this process can promote charge separation, resulting in low electron–hole pair recombination rate and allowing water redox reactions to be efficiently performed on the respective components. We predicted that H2 reduction would come from Sc2CO2 monolayer, while O2 oxidation from g-GaN monolayer under sunlight irradiation.
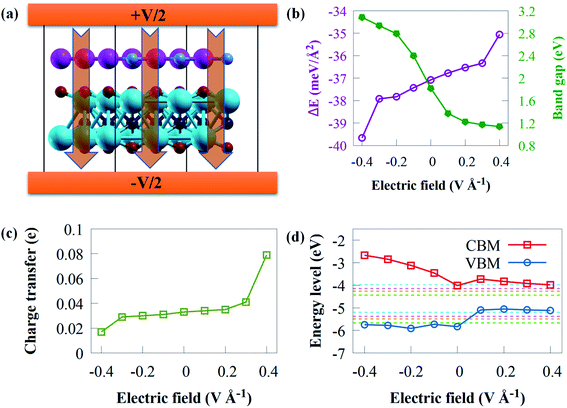
The use of an electric field is frequently simple theoretically, and it offers the advantages of reversibility and high operability since the nature of the system does not alter when electric field is withdrawn.92 Applying an external electric field is an effective technique of modifying the electronic property of vdWs heterostructure,93 which can be used in nanoelectronic devices. Recent theoretical studies94 have reported that the introduction of an electric field results in a tunable electronic property. Practically, materials are normally affected by an external electric field when used in electronic devices.95 Herein, the influence of an external electric field on the electronic and structural properties of g-GaN/Sc2CO2 vdW heterostructure are carefully studied since a tunable bandgap engineering is experimentally meaningful.96 Two opposite electric fields (+z, −z) vertical to the stacking layers were examined. A positive external electric field (+z) corresponds to the direction of the intrinsic built-in electric field in g-GaN/Sc2CO2 vdW heterostructure. In this study, the external negative electric field (−z) points from Sc2CO2 monolayer to the g-GaN monolayer, while the external positive electric field direction points from g-GaN monolayer to the Sc2CO2 monolayer. Herein an external electric field varying from −0.4 to +0.4 V Å−1 with steps of 0.1 V Å−1 is applied, as shown in Fig. 7a.
To gain fundamental insight into the influence of external electric field on the interfacial interaction, the Eb is shown in Fig. 7b. Eb remains negative within the entire external electric field range, demonstrating the thermodynamic stability of g-GaN/Sc2CO2 heterostructure under an external electric field.
The bandgap energy against applied external electric field is given in Fig. 7b. The bandgap energy is sensitive to the intensity and direction of external electric field. The results indicated that when the electric field strength increased, the bandgap energy decreased linearly, where +0.1 V Å−1 causes a steeply decrease in the bandgap energy. However, a gradual decrease is observed from +0.2 to +0.4 V Å−1, where the bandgap decreases from 1.33 eV with +0.2 V Å−1 to 1.14 eV with +0.4 V Å−1. This indicates that g-GaN/Sc2CO2 heterostructure retains its semiconducting character. The highly narrow bandgap induced by an external positive electric field might not be suitable for the overall water splitting, whereas a wide bandgap might reduce the performance to absorb solar energy. From +0.2 to +0.4 V Å−1, the bandgap energies varying from 1.33 to 1.14 eV are lower than the minimum bandgap energy (1.23 eV) essential for photocatalytic water splitting application. However, these bandgap energies fall within photovoltaic range (1.1 to 1.5 eV);97,98 hence, g-GaN/Sc2CO2 heterostructure may be good for photovoltaic cells with applied electric field of +0.2 to +0.4 V Å−1. Similar tunable bandgap energies under positive electric field in other heterostructures has also been reported.99 On the other hand, when a positive electric field is applied, the bandgap energy increase from 1.82 eV at 0 V Å−1 to 3.08 eV at −0.4 V Å−1. The results reveal that both the magnitude and the direction of the external electric field could be used to tune the bandgap energy of g-GaN/Sc2CO2 heterostructure. Based on the above discussion, g-GaN/Sc2CO2 heterostructure is a potential candidate for catalysis and optoelectronic devices.
Apart from the tunable bandgap, the applied electric field can considerably boost the electron redistributions at the interface and the interactions between the isolated monolayers.94 The charge transfer against electric field are calculated as given in Fig. 7c. The direction of external positive electric field can boost the transfer of electrons from g-GaN to Sc2CO2. When Δq increases monotonically under external positive electric field, continuous charge transfer from g-GaN to Sc2CO2 occurs. This attenuated charge accumulation suggests that the increasing external negative electric field interferes with interfacial interactions. However, compared with the case of an external electric field of 0 V Å−1, the number of electrons transferring from g-GaN to Sc2CO2 decreases when the applied negative external electric field increases.
To offer a fundamental understanding of band edge potentials of g-GaN and Sc2CO2 monolayers in the heterostructure, we plotted the potentials against external electric field at pH of 0 to 7 (Fig. 7d). The CBM and VBM composed of Sc2CO2 and g-GaN monolayers move upward as the negative electric field increases, particularly the CBM band. The CBM and VBM potentials of g-GaN/Sc2CO2 heterostructure under an electric field of −0.1 to −0.4 V Å−1 are all well aligned to straddle the redox potentials of water, and this can practically be employed as photocatalytic water splitting at pH 0 to 7. However, g-GaN/Sc2CO2 heterostructure with positive external electric field exhibit a more negative potential with a higher reduction ability to generate H2 via water splitting process, but the VBM could not straddle the water oxidation potential (EO2/H2O) of water.
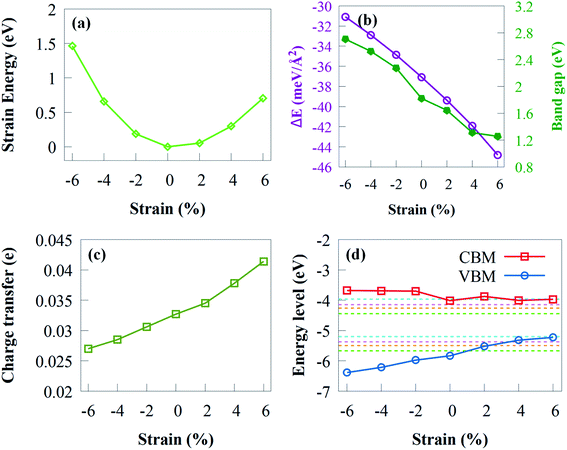
Electronic structures of 2D materials can be tuned by mechanical strain.100 Compressive or tensile strain has been demonstrated in experiments by several techniques, such as lattice mismatch, bending, external load, etc.101 It is generally known that strain may change the band edge positions and band gaps of 2D photocatalytic materials and reduce the energy of hydrogen absorption.102 Here, we have systematically investigated the influence of strain on electronic and structural properties by applying a biaxial strain in the range of −6% (compressive) to +6% (tensile). By changing the lattice parameters, the in-plane biaxial strain, which is defined as ε = (a1 − a0)/a0 × 100% is simulated. Here a0 and a1 represent the lattice constant of unstrained and strained system, respectively.
The strain energy (Es) under biaxial strain is first discussed before analysing the electronic properties as follows:
| ES = ET(ε1) − ET(ε0), |
It is noteworthy that the Eb in Fig. 8b is always negative, implying the stability of g-GaN/Sc2CO2 vdW heterostructure under strain of −6 to +6%. The Eb decreases rapidly when biaxial strain changes from −2 to −6%. Nevertheless, the Eb under tensile strain increases monotonically when tensile strain increase from +2 to +6%. It is worth noting that Eb is less/more negative with increasing compressive/tensile strain, which indicates that enhanced stability is achieved when tensile biaxial strains are applied.
The curve regarding the bandgap under strain shows a linear variation. As shown from Fig. 8b, the bandgap decreased monotonically with tensile biaxial strain changing from +2 to +6%. The bandgap drops rapidly, indicating that high tensile biaxial strain may not favour water splitting process when g-GaN/Sc2CO2 is used as a photocatalyst. However, when the compressive biaxial strain varies from −2% to −6%, the bandgap monotonically increases. By applying biaxial strain, the bandgap energies exhibit very sharp change, which indicates that the electronic properties are sensitive to compressive or tensile strain.
The charge transfer from g-GaN monolayer to Sc2CO2 monolayer of various biaxial strained g-GaN/Sc2CO2 heterostructure is shown in Fig. 8c. The electron transfer reaches its maximum and minimum value at biaxial strain of +8% and −8%, respectively. The amount of transferred electrons increased as the strength of tensile strain increases. The enhanced charge transfer implies that the interaction between the g-GaN and Sc2CO2 monolayers is stronger compared with compressive strain, which results in the reduced bandgap energy.
We have also estimated the band edge positions of g-GaN/Sc2CO2 heterostructure under biaxial strain (Fig. 8d). When tensile strain is applied, the VBM is lower than the oxidation potential, which indicates that the heterostructure is not suitable for generating O2 but can serve as a potential photocatalyst to generate H2; thus, the reduction potential is satisfied. As a result of tensile strain, the VBO decreases, lowering the driving power for the holes moving from Sc2CO2 to g-GaN. This means that the overall photocatalytic water splitting cannot be achieved when tensile biaxial strain varies from +2% to +6%. In contrast, when compressive biaxial strain varies from −2% to −6%, the VBM and CBM gradually move away from the oxidation and reduction potentials, respectively. This result indicates that compressive strain can improve the photocatalytic efficiency of g-GaN/Sc2CO2 vdW heterostructure to some extent.
IV. Conclusions
In summary, we have systematically investigated the interfacial interactions, charge density difference, band alignment, work function, electronic, structural and photocatalytic properties of novel 2D g-GaN/Sc2CO2 heterostructure through first-principles DFT calculations. The significant role of external electric field and biaxial strain on the structural and electronic properties were investigated in detail. The results showed that g-GaN and Sc2CO2 were in contact and formed a stable GaN/Sc2CO2 heterostructure. Using phonon dispersion and AIMD simulations, the thermal and dynamic stability of g-GaN/Sc2CO2 heterostructure was confirmed and easy to synthesise in experiments. g-GaN/Sc2CO2 heterostructure exhibits an indirect bandgap semiconductor with an inherent type-II band alignment, which can effectively separate and transfer charge carriers, thereby enhancing the photocatalytic activity. Besides, a large built-in electric field was found at the heterostructure interface, which will enhance the lifetime of charge carriers. Both charge density difference and electrostatic potential reveal electrons transferring from g-GaN monolayer to Sc2CO2 monolayer. Compared with the monolayers, g-GaN/Sc2CO2 vdW heterostructure possessed improved visible-light optical absorption intensity, ensuring the high-efficiency photocatalytic activity. The band alignment reveals that g-GaN/Sc2CO2 vdW heterostructure achieve overall water splitting at pH 0 to 7. Changing the strength and direction of external electric field and biaxial strain, the bandgap energy and band alignment of g-GaN/Sc2CO2 heterostructure could be effectively tuned in a broader range. Compressive strain and negative electric field were found to retain the reduction capability of Sc2CO2 and the oxidation capability of g-GaN, and this could significantly promote the hydrogen production from the overall water splitting. Most importantly, because of the influence of applied electric field and biaxial strain, g-GaN/Sc2CO2 heterostructure offer fundamental insights into the design of efficient photocatalyst with potential applications in solar energy conversion, high-performance nanoelectronics and optoelectronics devices.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors are grateful to the Centre for High Performance Computing (CHPC), Cape Town, for the computational resources provided.References
- U. M. Patil, M. S. Nam, S. Kang, J. S. Sohn, H. B. Sim, S. Kang and S. C. Jun, RSC Adv., 2016, 6, 43261–43271 RSC.
- M. E. Kahn, Science, 2015, 347, 239 CrossRef CAS.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- F. E. Osterloh and B. A. Parkinson, MRS Bull., 2011, 36, 17–22 CrossRef CAS.
- P. A. Russo, N. Donato, S. G. Leonardi, S. Baek, D. E. Conte, G. Neri and N. Pinna, Angew. Chem., Int. Ed., 2012, 51, 11053–11057 CrossRef CAS PubMed.
- J. Li and G. Zheng, Adv. Sci., 2017, 4, 1–15 Search PubMed.
- L. Zhang, W. Wang, S. Sun, Y. Sun, E. Gao and Z. Zhang, Appl. Catal., B, 2014, 148, 164–169 CrossRef.
- Z. Cui, K. Ren, Y. Zhao, X. Wang, H. Shu, J. Yu, W. Tang and M. Sun, Appl. Surf. Sci., 2019, 492, 513–519 CrossRef CAS.
- S. Li, M. Sun, J.-P. Chou, J. Wei, H. Xing and A. Hu, Phys. Chem. Chem. Phys., 2018, 20, 24726–24734 RSC.
- S. Sattar, Y. Zhang and U. Schwingenschlögl, Adv. Theory Simul., 2018, 1, 1800083 CrossRef.
- F. Opoku, K. K. Govender, C. G. C. E. van Sittert and P. P. Govender, New J. Chem., 2017, 41, 8140–8155 RSC.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. W. Barsoum and M. Radovic, Annu. Rev. Mater. Res., 2011, 41, 195–227 CrossRef CAS.
- X. Li, Y. Dai, Y. Ma, Q. Liu and B. Huang, Nanotechnology, 2015, 26, 135703 CrossRef PubMed.
- N. K. Chaudhari, H. Jin, B. Kim, D. San Baek, S. H. Joo and K. Lee, J. Mater. Chem. A, 2017, 5, 24564–24579 RSC.
- Y. Wen, T. E. Rufford, X. Chen, N. Li, M. Lyu, L. Dai and L. Wang, Nano Energy, 2017, 38, 368–376 CrossRef CAS.
- X. Sun, Y. Li, X.-F. Wang, R. Fujii, Y. Yamano, O. Kitao and S.-i. Sasaki, New J. Chem., 2022, 46(5), 2166–2177 RSC.
- E. Gong, S. Ali, C. B. Hiragond, H. S. Kim, N. S. Powar, D. Kim, H. Kim and S.-I. In, Energy Environ. Sci., 2022 10.1039/D1EE02714J.
- M. Khazaei, M. Arai, T. Sasaki, C.-Y. Chung, N. S. Venkataramanan, M. Estili, Y. Sakka and Y. Kawazoe, Adv. Funct. Mater., 2013, 23, 2185–2192 CrossRef CAS.
- L. Li and W. Shi, J. Mater. Chem. C, 2017, 5, 8128–8134 RSC.
- H. Zhang, G. Yang, X. Zuo, H. Tang, Q. Yang and G. Li, J. Mater. Chem. A, 2016, 4, 12913–12920 RSC.
- K. Xiong, P. Wang, G. Yang, Z. Liu, H. Zhang, S. Jin and X. Xu, Sci. Rep., 2017, 7, 15095 CrossRef PubMed.
- Y. Lee, S. B. Cho and Y.-C. Chung, ACS Appl. Mater. Interfaces, 2014, 6, 14724–14728 CrossRef CAS PubMed.
- L. Li, J. Phys. Chem. C, 2016, 120, 24857–24865 CrossRef CAS.
- A. Onen, D. Kecik, E. Durgun and S. Ciraci, Phys. Rev. B, 2016, 93, 085431 CrossRef.
- M. Sun, J.-P. Chou, J. Yu and W. Tang, Phys. Chem. Chem. Phys., 2017, 19, 17324–17330 RSC.
- M. Sun, J.-P. Chou, Q. Ren, Y. Zhao, J. Yu and W. Tang, Appl. Phys. Lett., 2017, 110, 173105 CrossRef.
- Z. Y. Al Balushi, K. Wang, R. K. Ghosh, R. A. Vilá, S. M. Eichfeld, J. D. Caldwell, X. Qin, Y.-C. Lin, P. A. DeSario and G. Stone, Nat. Mater., 2016, 15, 1166–1171 CrossRef CAS PubMed.
- D. Xu, H. He, R. Pandey and S. P. Karna, J. Phys.: Condens. Matter, 2013, 25, 345302 CrossRef PubMed.
- K. Ren, M. Sun, Y. Luo, S. Wang, J. Yu and W. Tang, Appl. Surf. Sci., 2019, 476, 70–75 CrossRef CAS.
- K. Ren, M. Sun, Y. Luo, S. Wang, Y. Xu, J. Yu and W. Tang, Phys. Lett. A, 2019, 383, 1487–1492 CrossRef CAS.
- Y. Luo, S. Wang, K. Ren, J.-P. Chou, J. Yu, Z. Sun and M. Sun, Phys. Chem. Chem. Phys., 2019, 21, 1791–1796 RSC.
- Z. Ma, Z. Hu, X. Zhao, Q. Tang, D. Wu, Z. Zhou and L. Zhang, J. Phys. Chem. C, 2014, 118, 5593–5599 CrossRef CAS.
- Q. Fang, X. Zhao, Y. Huang, K. Xu, T. Min and F. Ma, J. Mater. Chem. C, 2019, 7, 3607–3616 RSC.
- Y.-Q. Zhao, X. Wang, B. Liu, Z.-L. Yu, P.-B. He, Q. Wan, M.-Q. Cai and H.-L. Yu, Org. Electron., 2018, 53, 50–56 CrossRef CAS.
- V. O. Özcelik, J. G. Azadani, C. Yang, S. J. Koester and T. Low, Phys. Rev. B, 2016, 94, 035125 CrossRef.
- Z. Guan, C.-S. Lian, S. Hu, S. Ni, J. Li and W. Duan, J. Phys. Chem. C, 2017, 121, 3654–3660 CrossRef CAS.
- S. Wang, H. Tian, C. Ren, J. Yu and M. Sun, Sci. Rep., 2018, 8, 1–6 Search PubMed.
- X.-H. Li, B.-J. Wang, G.-D. Wang and S.-H. Ke, Sustainable Energy Fuels, 2020, 4, 5277–5283 RSC.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. Buongiorno Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. Dal Corso, S. de Gironcoli, P. Delugas, R. A. DiStasio, A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H. Y. Ko, A. Kokalj, E. Küçükbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H. V. Nguyen, A. Otero-de-la-Roza, L. Paulatto, S. Poncé, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, J. Phys.: Condens. Matter, 2017, 29, 465901 CrossRef CAS PubMed.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892–7895 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- J. D. Pack and H. J. Monkhorst, Phys. Rev. B: Solid State, 1977, 16, 1748–1749 CrossRef.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- H. Zhang, D. Wu, Q. Tang, L. Liu and Z. Zhou, J. Mater. Chem. A, 2013, 1, 2231–2237 RSC.
- X. Gonze and C. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 10355–10368 CrossRef CAS.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- K. Parlinski, Z. Q. Li and Y. Kawazoe, Phys. Rev. Lett., 1997, 78, 4063–4066 CrossRef CAS.
- S. Nosé, J. Chem. Phys., 1984, 81, 511–519 CrossRef.
- W. G. Hoover, Phys. Rev. A, 1985, 31, 1695–1697 CrossRef PubMed.
- A. Kokalj, J. Mol. Graphics Modell., 1999, 17, 176–179 CrossRef CAS PubMed.
- G. Rehman, S. Ali Khan, R. Ali, I. Ahmad, L.-Y. Gan and B. Amin, J. Appl. Phys., 2019, 126, 143101 CrossRef.
- H. Huang, J. Peng, H. Dong, L. Huang, M. Wen and F. Wu, Phys. Chem. Chem. Phys., 2021, 23, 20901–20908 RSC.
- L. Xu, Z. Ma, Q. Li, T. Chen, B. Peng, J. Zeng, Y. Zhang, K.-W. Luo, L.-L. Wang and C. Shuai, New J. Chem., 2020, 44, 15439–15445 RSC.
- X. Liu, D. Cao, Y. Yao, P. Tang, M. Zhang, X. Chen and H. Shu, J. Mater. Chem. C, 2022, 10, 1984–1990 RSC.
- L. Hu, Y. Sun, S.-J. Gong, H. Zong, K. Yu and Z. Zhu, New J. Chem., 2020, 44, 7902–7911 RSC.
- R. Zhuo, L. Zeng, H. Yuan, D. Wu, Y. Wang, Z. Shi, T. Xu, Y. Tian, X. Li and Y. H. Tsang, Nano Res., 2019, 12, 183–189 CrossRef CAS.
- S. Wang, C. Ren, H. Tian, J. Yu and M. Sun, Phys. Chem. Chem. Phys., 2018, 20, 13394–13399 RSC.
- J. Liao, B. Sa, J. Zhou, R. Ahuja and Z. Sun, J. Phys. Chem. C, 2014, 118, 17594–17599 CrossRef CAS.
- X. Meng, Y. Shen, J. Liu, L. Lv, X. Yang, X. Gao, M. Zhou, X. Wang, Y. Zheng and Z. Zhou, Appl. Catal., A, 2021, 624, 118332 CrossRef CAS.
- Z. Chen, X. Ma, J. Hu, F. Wan, P. Xu, G. Wang, M. Wang, S. Deng and C. Huang, New J. Chem., 2021, 45, 16520–16528 RSC.
- D. Gao, Y. Li, Z. Guo, Z. Liu, K. Guo, Y. Fang, Y. Xue, Y. Huang and C. Tang, J. Alloys Compd., 2021, 887, 161273 CrossRef CAS.
- A. A. Attia and H. R. Jappor, Chem. Phys. Lett., 2019, 728, 124–131 CrossRef CAS.
- T. Björkman, A. Gulans, A. V. Krasheninnikov and R. M. Nieminen, Phys. Rev. Lett., 2012, 108, 235502 CrossRef PubMed.
- X. Chen, F. Tian, C. Persson, W. Duan and N.-x. Chen, Sci. Rep., 2013, 3, 1–5 Search PubMed.
- T. Li, C. He and W. Zhang, J. Energy Chem., 2021, 52, 121–129 CrossRef.
- W. Zhang, Y. Yin and C. He, J. Phys. Chem. Lett., 2021, 12, 5064–5075 CrossRef CAS PubMed.
- Q. Li, D. Zhou, W. Zheng, Y. Ma and C. Chen, Phys. Rev. Lett., 2013, 110, 136403 CrossRef PubMed.
- K. Ren, S. Wang, Y. Luo, Y. Xu, M. Sun, J. Yu and W. Tang, RSC Adv., 2019, 9, 4816–4823 RSC.
- F. Opoku, O. Akoto, S. O.-B. Oppong and A. A. Adimado, New J. Chem., 2021, 45, 20365–20373 RSC.
- K. Ren, Y. Luo, S. Wang, J.-P. Chou, J. Yu, W. Tang and M. Sun, ACS Omega, 2019, 4, 21689–21697 CrossRef CAS PubMed.
- M. Idrees, C. V. Nguyen, H. D. Bui, I. Ahmad and B. Amin, Phys. Chem. Chem. Phys., 2020, 22, 20704–20711 RSC.
- S. S. Ullah, M. Farooq, H. U. Din, Q. Alam, M. Idrees, M. Bilal and B. Amin, RSC Adv., 2021, 11, 32996–33003 RSC.
- P. T. Huong, M. Idrees, B. Amin, N. N. Hieu, H. V. Phuc, L. T. Hoa and C. V. Nguyen, RSC Adv., 2020, 10, 24127–24133 RSC.
- X.-H. Li, B.-J. Wang, H. Li, X.-F. Yang, R.-Q. Zhao, X.-T. Jia and S.-H. Ke, New J. Chem., 2020, 44, 16092–16100 RSC.
- Y. Li, Y.-L. Li, B. Sa and R. Ahuja, Catal. Sci. Technol., 2017, 7, 545–559 RSC.
- F. Opoku, K. K. Govender, C. G. C. E. van Sittert and P. P. Govender, New J. Chem., 2017, 41, 11701–11713 RSC.
- D. Sanchez-Portal, E. Artacho and J. M. Soler, Solid State Commun., 1995, 95, 685–690 CrossRef CAS.
- D. D. Vo, T. V. Vu, T. H. T. Nguyen, N. N. Hieu, H. V. Phuc, N. T. Binh, M. Idrees, B. Amin and C. V. Nguyen, RSC Adv., 2020, 10, 9824–9832 RSC.
- A. D. Becke and K. E. Edgecombe, J. Chem. Phys., 1990, 92, 5397–5403 CrossRef CAS.
- J. Liu, B. Cheng and J. Yu, Phys. Chem. Chem. Phys., 2016, 18, 31175–31183 RSC.
- J. Tian, L. Liu, S. Xia, Y. Diao and F. Lu, Phys. Lett. A, 2019, 383, 3018–3024 CrossRef CAS.
- Y. Liang, Y. Dai, Y. Ma, L. Ju, W. Wei and B. Huang, J. Mater. Chem. A, 2018, 6, 2073–2080 RSC.
- K. Cheng, Y. Guo, N. Han, X. Jiang, J. Zhang, R. Ahuja, Y. Su and J. Zhao, Appl. Phys. Lett., 2018, 112, 143902 CrossRef.
- L. Shi, L. Liang, F. Wang, M. Liu and J. Sun, RSC Adv., 2015, 5, 101843–101849 RSC.
- R. G. Pearson, Inorg. Chem., 1988, 27, 734–740 CrossRef CAS.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC.
- T. A. Pham, D. Lee, E. Schwegler and G. Galli, J. Am. Chem. Soc., 2014, 136, 17071–17077 CrossRef CAS PubMed.
- X.-H. Li, B.-J. Wang, X.-L. Cai, W.-Y. Yu, Y.-Y. Zhu, F.-Y. Li, R.-X. Fan, Y.-S. Zhang and S.-H. Ke, Nanoscale Res. Lett., 2018, 13, 1–10 CrossRef PubMed.
- X. Gao, Y. Shen, Y. Ma, S. Wu and Z. Zhou, Phys. Chem. Chem. Phys., 2019, 21, 15372–15379 RSC.
- Z. Ma, Y. Wang, Y. Wei, C. Li, X. Zhang and F. Wang, Phys. Chem. Chem. Phys., 2019, 21, 21753–21760 RSC.
- X. Chen, C. Tan, Q. Yang, R. Meng, Q. Liang, J. Jiang, X. Sun, D. Yang and T. Ren, Phys. Chem. Chem. Phys., 2016, 18, 16229–16236 RSC.
- Y. Li, F. Li and Z. Chen, J. Am. Chem. Soc., 2012, 134, 11269–11275 CrossRef CAS PubMed.
- C. Xia, Q. Zhang, W. Xiao, W. Xiong, J. Du and J. Li, J. Phys. D: Appl. Phys., 2018, 51, 215303 CrossRef.
- S. Zhang, N. Wang, S. Liu, S. Huang, W. Zhou, B. Cai, M. Xie, Q. Yang, X. Chen and H. Zeng, Nanotechnology, 2016, 27, 274001 CrossRef PubMed.
- P. D. Antunez, J. J. Buckley and R. L. Brutchey, Nanoscale, 2011, 3, 2399–2411 RSC.
- D. J. Wehenkel, K. H. Hendriks, M. M. Wienk and R. A. Janssen, Org. Electron., 2012, 13, 3284–3290 CrossRef CAS.
- X. Chen, J. Jiang, Q. Liang, R. Meng, C. Tan, Q. Yang and X. Sun, J. Mater. Chem. C, 2016, 4, 7004–7012 RSC.
- C. Lei, Y. Ma, X. Xu, T. Zhang, B. Huang and Y. Dai, J. Phys. Chem. C, 2019, 123, 23089–23095 CrossRef CAS.
- Y. Jiao, L. Zhou, F. Ma, G. Gao, L. Kou, J. Bell, S. Sanvito and A. Du, ACS Appl. Mater. Interfaces, 2016, 8, 5385–5392 CrossRef CAS PubMed.
- D. Voiry, J. Yang and M. Chhowalla, Adv. Mater., 2016, 28, 6197–6206 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |