Open Access Article
Open Access ArticleThree-component assembly of stabilized fluorescent isoindoles†
Vladimir A. Maslivetc a,
James J. La Clair
a,
James J. La Clair *b and
Alexander Kornienko
*b and
Alexander Kornienko *a
*a
aDepartment of Chemistry and Biochemistry, Texas State University, San Marcos, Texas 78666, USA. E-mail: a_k76@txstate.edu
bXenobe Research Institute, P.O. Box 3052, San Diego, CA 92163-1052, USA. E-mail: i@xenobe.org
First published on 2nd March 2022
Abstract
The tandem addition of an amine and a thiol to an aromatic dialdehyde engages a selective three-component assembly of a fluorescent isoindole. While an attractive approach for diversity-based fluorophore discovery, isoindoles are typically unstable and present considerable challenges for their practical utility. We found that introduction of electron-withdrawing substituents into the dialdehyde component affords stable isoindole products in one step with acceptable yields and high purity.
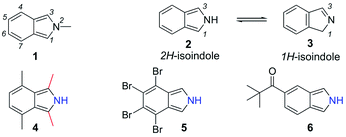
Since the preparation of the first isoindole (1, Fig. 1) in 1951 (ref. 1) and the isolation of the parent unsubstituted isoindole (2) in 1972,2 the relative instability of this heterocyclic ring system has been an important impediment to the discovery of new chemical transformations and biological applications. The position of the equilibrium between the two tautomeric forms 2 and 3, respectively 2H- and 1H-isoindoles, could be invoked to assess the stability of the 2H-form. It has been found that substituents on the isoindole ring system play a key role. For example, it appears that electron-donating groups, such as methyl groups in 4, destabilize the 2H-isoindole,3 whereas electron acceptors in 54 and 65 improve stability.
 | ||
| Fig. 1 N-methylisoindole (1) and a selection of the first isoindoles prepared 4–6 along with a depiction of the substituent-based tautomeric preferences. | ||
Multicomponent reactions6,7 that enable three or more discrete molecules to combine into one product not only curtail synthetic operations but also advance the complexity viable within a single operation. To date, many of our fluorescent probes are prepared by two-component processes wherein moiety A is attached to moiety B to generate a fluorescent probe. Conventionally, this is achieved by adding dye A to a biological molecule B, however methods have been established that generate the probe motif as part of the coupling process.8,9 The latter, referred to as turn-on labeling, advantageously removes potential non-fluorescent impurities as only the desired product displays the proper fluorescence. While rare, advance of multicomponent turn-on labeling strategies offers a robust ability to improve the selectivity of labeling as well as to further expand the diversity possible within a labeling reaction. Here, we turn our attention to explore a three-component strategy to prepare isoindoles with improved stability.
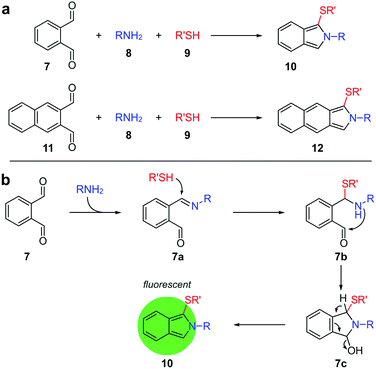
An early report of the formation of fluorescent species when o-diacetylbenzene was exposed to proteins10 led to the discovery of a multicomponent reaction between o-phthalaldehyde (7), amines 8 and thiols 9, which yields highly fluorescent isoindole 10 (Fig. 2).11–16 This reaction now forms the basis for the quantitative determination of amino acids and is used in commercial amino acid analyzers.17 The method is characterized by high sensitivity, although the lack of mechanistic understanding has led researchers to optimize the conditions primarily empirically.
An important drawback of the method is the lack of stability of the product isoindoles 10 (Fig. 2), which, once form, undergo further conversions introducing inaccuracies due to unstable fluorescence. In general, the highest fluorescence must be achieved within 5–25 min and remain time-independent for another 20–30 min, the conditions which are hard to fulfill as the rate of isoindole formation and its stability depend on an individual amino acid.18,19 Naphthalene-1,2-dicarboxaldehyde (11) was recommended as an alternative reagent with claims that the product isoindoles 12 would be more stable, but the proposal seemingly has not received acceptance from the scientific community as the increases in stability are probably not significant to justify the cost of this reagent.17
Our initial attempt to characterize the product of the three-component reaction between phthalaldehyde 7, amines and thiols (Fig. 3) met with significant synthetic challenges, as evident by the low stability of 10a. This compound is unstable at room temperature and rapidly decomposed when column chromatography purification was attempted. In order to obtain an analytical sample, 10a was repeatedly recrystallized from cold CH3CN, dried in vacuum at 0 °C and immediately analyzed by NMR. Isoindole 12a, derived from 2,3-naphthalenedicarboxaldehyde (11), turned out to be only slightly more stable in comparison to product 10a and also rapidly decomposed when column chromatography purification was attempted.
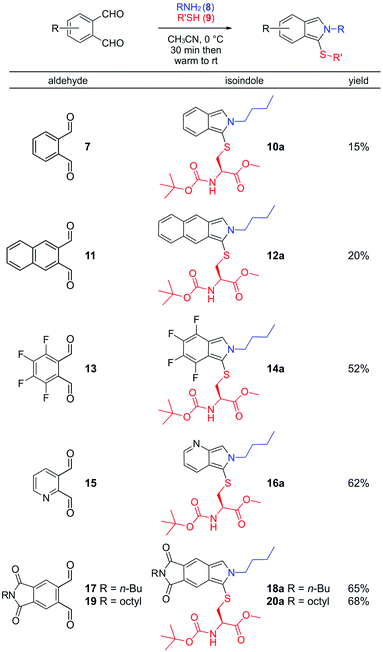 | ||
| Fig. 3 One-pot synthesis of fluorescent 1-thio-2H-isoindoles and related structures from aromatic dialdehydes, butylamine and N-(tert-butoxycarbonyl)-L-cysteine methyl ester (Boc-Cys-OMe). | ||
Electron-deficient dialdehydes 13, 15, 17 and 19 (Fig. 3) on the other hand, all gave stable isoindoles 14a, 16a (structure assigned by NOESY analyses, see ESI†), 18a and 20a respectively (Fig. 3), when reacted with butyl amine and protected cysteine. These isoindoles could be purified by column chromatography and were considerably easier to handle.
Next, we turned our attention to evaluate if n-butyl- and n-octylphthalimidic phthalaldehydes 17 and 19 produced readily isolated products from a variety of aliphatic or aromatic amines and thiols. As shown in Fig. 4, isoindoles 18b–d and 20b–f can be obtained by adding a thiol and amine to the solution of 17 or 19 in CH3CN at 0 °C. Subsequent simple removal of the volatiles on the rotary evaporator and purification of the product, facilitated by its fluorescence on TLC, gave the desired 18b–d and 20b–f in a straightforward manner. These reactions could be conducted with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of dialdehyde, amine, and thiol in acceptable isolated yields (48–66%, Fig. 4). The yields did not seem to be affected by the electronic properties of the reacting amines or thiols and were similar for reactions involving electron-rich (18b, c, 20c–f) vs. electron-deficient (18d, 20b) amines or electron-rich (18c, 20d) vs. electron-deficient (18b, d, 20b, c, e, f) thiols.
1 ratio of dialdehyde, amine, and thiol in acceptable isolated yields (48–66%, Fig. 4). The yields did not seem to be affected by the electronic properties of the reacting amines or thiols and were similar for reactions involving electron-rich (18b, c, 20c–f) vs. electron-deficient (18d, 20b) amines or electron-rich (18c, 20d) vs. electron-deficient (18b, d, 20b, c, e, f) thiols.
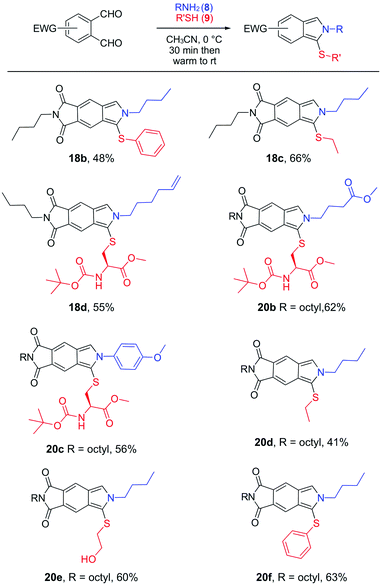 | ||
| Fig. 4 One-pot synthesis of fluorescent 1-thio-2H-isoindoles from n-butyl- and n-octylphthalimidic phthalaldehydes 17 and 19, aliphatic and aromatic amines, and aliphatic and aromatic thiols. | ||
While an attractive analytical tool and fluorophore, practical applications of isoindoles suffer due to their rapid degradation. Proposed early on by Simons and Johnson,20 the problem arises from nucleophilic attack by ROH (water or alcohols) at C1 (Fig. 5) resulting in the loss of the thiol and formation of the corresponding γ-lactam 21b.21 This is further complicated by the potential for self-dimerization by a Diels–Alder reaction as well as cycloaddition with oxygen, the latter of which results in the incipient formation of a rapidly degraded endoperoxide. In 1981, a team led by Simmons and Ammon reported the first stable isoindole by the reaction with dimethylene acetylenedicarboxylate.22 Here, the product of the Diels–Alder cycloaddition opened to deliver a stabilized isoindole by indirect functionalization at C3. While a practical discovery, the overall analytical utility of isoindoles would benefit from discovery of a material with bench stability.
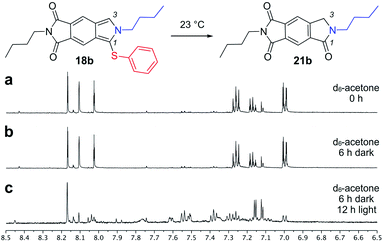 | ||
| Fig. 5 Stability analyses on isoindole 18b were evaluated using time course NMR studies. Selected spectra are shown with expansion of the aromatic region from 6.50–8.50 ppm. The predominant product of this degradation step found to be 21b. Expansions and spectra from treating 18a, 18c and 18d under the same conditions are provided in the ESI.† | ||
Overall, we were able to prepare and collect spectral data on 13 new isoindoles (Fig. 3 and 4). These materials were sufficiently stable for purification and spectral analyses. While we were able to prepare and isolate 10a and 12a, spectra on these materials needed to be collected immediately after preparation. NMR analyses were most challenging due to the fact that trace acidic or basic materials in the solvents led to rapid decomposition ultimately leading to the use of acetone-d6 for NMR data collection.
To further evaluate their utility, samples of 18a–d were monitored for their stability neat and in solution. Subjecting these samples to the same protocol (see ESI†), isoindoles 18c and 18d were not sufficiently stable in neat form to endure >30 days at −20 °C followed by 48 h at 23 °C (conditions that modelled compound storage). Under the same conditions, 18a and 18b (Fig. 5a) were stable when stored (>30 days at −20 °C followed by 48 h at 23 °C) and when dissolved acetone-d6 (Fig. 5a) and kept in the dark (Fig. 5b). Exposure to light, however, led to decomposition of both 18a and 18b (Fig. 5c). These observations indicated that steric bulk within the amine and thiol components contributed to the products stability. Additionally, the presence of alkene functionality within the amine was not tolerated, as given by the comparison of unstable 18d to stable 18a.
Synthesis of isoindoles23 through a three-component coupling provides a robust tool to rapidly access diverse fluorescent materials as recently demonstrated by adaptation for a Click-like processes24 or crosslinking,25 called Flick. Here, we describe how the addition of electron withdrawing groups effectively stabilizes the materials as demonstrated by 18a and 18b. While this data suggests that stability can be achieved, the light sensitivity of these agents suggests a future potential as photochemical sensors or modifiers. Efforts are now underway to explore this application.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Wittig, H. Tenhaeff, W. Schoch, G. Koenig and G. Einige, Justus Liebigs Ann. Chem., 1951, 572, 1–22 CrossRef CAS.
- R. Bonnett and R. F. C. Brown, J. Chem. Soc., Chem. Commun., 1972, 7, 393–395 RSC.
- C. O. Bender and R. Bonnett, J. Chem. Soc., Chem. Commun., 1966, 7, 198–199 RSC.
- R. Kreher and K. J. Herd, Angew. Chem., Int. Ed. Engl., 1974, 13, 739–740 CrossRef.
- R. Kreher, N. Kohl and G. Use, Angew. Chem., Int. Ed. Engl., 1982, 21, 621 CrossRef.
- P. S. G. Nunes, H. D. Vidal and A. G. Correa, Org. Biomol. Chem., 2020, 18, 7751–7773 RSC.
- J. Fairoosa, S. Saranya, S. Radhika and G. Anillkumar, ChemistrySelect, 2020, 5, 5180–5197 CrossRef CAS.
- V. Jamsheena, R. K. Mishra, K. S. Veena, S. Sini, P. Jayamurthy and R. S. Lankalapalli, ACS Omega, 2018, 3, 856–862 CrossRef CAS PubMed.
- S. B. Wagh, V. A. Maslivetc, J. J. La Clair and A. Kornienko, ChemBioChem, 2021, 22, 3109–3139 CrossRef CAS PubMed.
- G. Hillmann, Hoppe-Seyler's Z. für Physiol. Chem., 1943, 277, 222–232 CrossRef CAS.
- R. Roth, Anal. Chem., 1971, 43, 880–882 CrossRef PubMed.
- S. S. Simons Jr and D. F. Johnson, J. Org. Chem., 1978, 43, 2886–2891 CrossRef.
- S. S. Simons Jr and D. F. Johnson, J. Am. Chem. Soc., 1976, 98, 7098–7099 CrossRef.
- Z. Yao, X. Feng, C. Li and G. Shi, Chem. Commun., 2009, 5886–5888 RSC.
- R. G. Carlson, K. Srinivasachar, R. S. Givens and B. K. Matuszewski, J. Org. Chem., 1986, 51, 3978–3983 CrossRef CAS.
- O. S. Wong, L. A. Sternson and R. N. Schowen, J. Am. Chem. Soc., 1985, 107, 6421–6422 CrossRef CAS.
- P. Zuman, Anal. Lett., 2005, 38, 1213–1220 CrossRef CAS.
- E. Trepman and R. F. Chen, Arch. Biochem. Biophys., 1980, 204, 524–532 CrossRef CAS PubMed.
- J. R. Cronin, S. Pizzarello and W. E. Gandy, Anal. Biochem., 1979, 93, 174–179 CrossRef CAS PubMed.
- S. S. Simons Jr and D. F. Johnson, Anal. Biochem., 1978, 90, 705–725 CrossRef CAS.
- M. C. G. Alvarez-Coque, M. J. M. Hernández, R. M. V. Camañas and C. M. Fernández, Anal. Biochem., 1989, 178, 1–7 CrossRef.
- S. S. Simons Jr, H. H. Ammon, R. Doherty and D. F. Johnson, J. Org. Chem., 1981, 46, 4739–4744 CrossRef.
- D. Grosheva and N. Cramer, Angew. Chem., Int. Ed. Engl., 2018, 57, 13644–13647 CrossRef CAS PubMed.
- M. Todorovic and D. M. Methods, Enzymol, 2020, 639, 313–332 CAS.
- M. Todorovic, K. D. Schwab, J. Zeisler, C. Zhang, F. Bénard and D. M. Perrin, Angew. Chem., Int. Ed. Engl., 2019, 58, 14120–14124 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00505k |
| This journal is © The Royal Society of Chemistry 2022 |