Open Access Article
Open Access ArticleLuminometric dosimetry of X-ray radiation by a zwitterionic uranium coordination polymer†
Zhaofa Zhengbc,
Jie Qiu*a,
Huangjie Lu bc,
Jian-Qiang Wang
bc,
Jian-Qiang Wang *bc and
Jian Lin
*bc and
Jian Lin *a
*a
aSchool of Nuclear Science and Technology, Xi'an Jiaotong University, Xi'an, 710049, P. R. China. E-mail: qiujie1228@xjtu.edu.cn; jianlin@xjtu.edu.cn
bKey Laboratory of Interfacial Physics and Technology, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, 2019 Jia Luo Road, Shanghai 201800, P. R. China. E-mail: wangjianqiang@sinap.ac.cn
cUniversity of Chinese Academy of Sciences, No. 19(A) Yuquan Road, Shijingshan District, Beijing, 100049, P. R. China
First published on 28th April 2022
Abstract
A novel X-ray dosimeter based on a uranium coordination polymer U-Cbdcp was obtained by the judicious synergy between the luminescent uranyl centres and zwitterionic tritopic ligands. Notably, U-Cbdcp exhibits luminescence quenching upon increasing X-ray dose, which in combination with its excellent radiolytic stability, makes it suitable for X-ray dosimetry.
X-ray radiation has been extensively used in medical diagnosis and treatment, security screening, quality control inspection, scientific instrumentation, etc.1–3 Overexposure to X-ray radiation cause damage to human cells, which could result in skin burn, tissue damage, and increased incidence of cancer.4,5 Moreover, X-ray dosimetry is required in many industrial fields, including food irradiation, sterilization, and material modification.6 Thus, different types of radiation dosimeters, including ionization chamber, scintillator, semiconductor, thermoluminescence dosimeter, chemical dosimeter, and so on, have been commercialized to quantify the incident X-ray dose.6 The former three types of dosimeters are more frequently applied to record the dose-rate of incident radiation.7–10 Thermoluminescence dosimeters and chemical dosimeters are suitable for dosimetry of accumulated dose, but they suffer from critical drawbacks such as cumbersome reading processing, instrument-demand, or cost-ineffectiveness.11,12 Therefore, further development of new types of X-ray dosimeters remains essential.
Coordination polymers, which are assembled from metal ions and organic ligands, have been met with great interest in diverse fields including catalysis, sensing, sorption, separation, and luminescence.13–18 Their tunability in terms of chemical composition, structure, and more importantly photophysical property, makes them promising for radiation detection. Indeed, pioneering works by Allendorf and co-workers have demonstrated that scintillating metal–organic frameworks (MOFs) assembled from metal cations and radioluminescent organic ligands can function as a new type of radiation detection materials.13 Furthermore, coordination polymers or cluster species showing radiochromism, radio-photoluminescence, fluorochromism, and photoluminescence quenching upon accumulated doses of ionizing radiation have been documented, making them as promising candidates of radiation dosimeters.19–26 Notably, the abundance of luminescent centers or radio-responsive moieties in some of these materials renders higher saturation point in response to radiation dose. This attribute allows for wider operation ranges or higher upper limits of detection compared with those of traditional metal-ion-doped inorganic dosimeters, e.g. Ag-doped phosphate glass and Mg2+-doped LiF (LiF:Mg).27
We have recently undertaken a study focused on developing actinide-based coordination polymers or cluster materials for their promising applications in ionizing radiation detection.19,21,28 The large coordination numbers and diverse coordination geometries of actinide cations engender a myriad of topologies of these materials.29–31 Moreover, the intrinsically intense green emission from the uranyl cation can be utilized as a radio-luminescent center.23,26 In addition, the slight radioactivity of 238U (t1/2 = 4.47 billion years) can be neglected in the course of high dose radiation detection.32,33 Herein, a novel zwitterionic uranium coordination polymer is reported, showing rather unique fluorescence quenching response to X-ray radiation. This radio-responsive feature, in combination with its high radiolytic stability, points to the potential implementation of uranium-bearing materials for radiation dosimetry.
Solvothermal reaction between UO2(NO3)2·6H2O, zwitterionic N-(4-carboxybenzyl)-(3,5-dicarboxyl)pyridinium bromide (H3CbdcpBr), and CH3COOH in DMF/H2O mixed solution at 100 °C afforded yellow crystals of UO2(OH)(H2Cbdcp)(HCbdcp)·4H2O (U-Cbdcp) with a yield of 63% based on U.
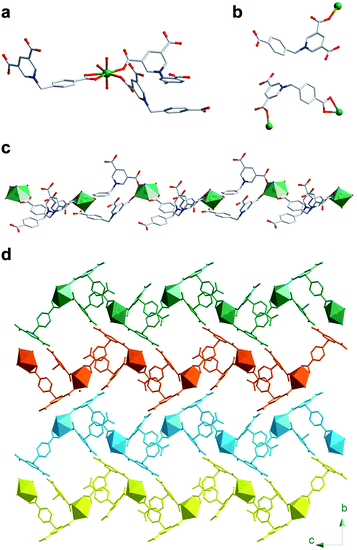
Single crystal X-ray diffraction (SCXRD) analysis revealed that U-Cbdcp crystalizes in the monoclinic P21/n space group (Table S1†). The asymmetric unit of U-Cbdcp network consists of one crystallographically independent UO22+ cation, two Cbdcp ligands, and one hydroxide group (Fig. S1†). The coordination geometry of uranyl cation can be best described as a typical pentagonal bipyramid, of which four O atoms on the pentagonal plane are donated from three Cbdcp ligand and the rest one is from a hydroxide group (Fig. 1a).34–38 One of the organic linkers coordinates with one uranyl cation in a μ1–η1 bridging mode, while the other one interconnects with two uranyl cations in a μ2–η1:η2 manner (Fig. 1b). Therefore, these two crystallographically unique ligands can be assigned as H2Cbdcp− and HCbdcp2− with one and two carboxylate group being deprotonated, respectively. The torsion angles between the carboxybenzyl and (3,5-dicarboxyl)pyridinium moieties are measured to be 110.909° and 112.447° for H2Cbdcp− and HCbdcp2−, respectively, as defined by ∠N–C–C. The assembly of uranyl cations, H2Cbdcp−, and HCbdcp2− ligands results in the formation of a one-dimensional infinite chain extending along the c axis (Fig. 1c). The afforded chains are further extended into a 3D supramolecular network via π–π interactions and hydrogen bonds between the ligands (Fig. 1d). The phase purity of bulky U-Cbdcp sample was confirmed by powder X-ray diffraction (PXRD), showing that the measured pattern matches well with the simulated one (Fig. S2†).
The solid-state luminescence spectrum (λex = 365 nm) was collected on a tablet of U-Cbdcp, that was fabricated from finely ground powder. As expected, U-Cbdcp exhibits five characteristic bands of uranyl cation centring at 488, 508, 531, 556, and 583 nm (Fig. 2a). This intense green emission can be attributed to the HOMO-LUMO transition occurring in the uranyl bonds upon UV excitation.38,39 Strikingly, the uranyl-based luminescence is strongly quenched after X-ray radiation (4.7 kGy) as shown by the photographs of U-Cbdcp tablet (Fig. 2a inset). Concomitantly, the intensities of characteristic UO22+ emission bands, which were measured from the tablet exposed to specific interval of X-ray dose, gradually diminished upon continuous X-ray irradiation (Cu-Kα, 120 Gy min−1). More specifically, approximately 44% luminescence intensity was retained after being exposed to 260 Gy X-ray radiation (Fig. 2a). Further increasing the dose to 4.7 kGy resulted in nearly 90% emission quenching. Interestingly, I0/I as a function of radiation dose can be well fitted with a linear correlation with R2 of 0.9988, where I0 and I are the luminescence intensities monitored at 508 nm before and after irradiation, respectively. This excellent linearity allows for quantifying X-ray dose in a wide dynamic range spanning from 10 to 4700 Gy via a luminescence “turn-off” manner. To obtain limit of detection (LOD), the calibration curve was established by plotting the quenching rate (I0 − I)/I0 as a function of dose at the low dose range (0–30 Gy) (Fig. S3†). The limit of detection (LOD) is calculated to be 0.093 Gy based on the method reported by Zang and coworkers.40 Markedly, this LOD is comparable to 0.047 Gy of the most sensitive photochromic sensor Htpbz@Th-SINAP-2.21
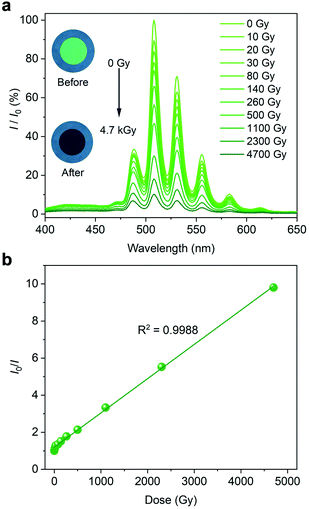 | ||
| Fig. 2 (a) X-ray dose-dependent fluorescence spectra and optical micrographs (inset) of a U-Cbdcp tablet. (b) The plot showing the linear correlation between I0/I and X-ray dose. | ||
To decipher the quenching mechanism, the structures of U-Cbdcp before and after X-ray irradiation (5 kGy) were thoroughly characterized by combined techniques including PXRD and SCXRD. The PXRD patterns of U-Cbdcp remained approximately unchanged upon irradiation, ruling out our initial speculation of radiation induced damage to the bulky sample (Fig. S4†). This supposition is additionally supported by the nearly identical FTIR spectrum of irradiated U-Cbdcp with the nonirradiated one (Fig. S5†). Furthermore, SCXRD analysis before and after X-ray radiation was conducted on the same single crystal of U-Cbdcp and revealed that the overall network derived from these two datasets retain unchanged as well (Table S1†). In detail, the local structure as represented by the bond length and bond angle of U-Cbdcp changes slightly, which can be attributed to the standard deviations of these parameters obtained from SCXRD (Table S2†). This observation further excludes the quenching mechanism via decomposition of U-Cbdcp crystal.
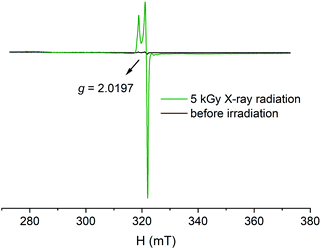
There is precedence in literature that the luminescence quenching can be associated with the generation of radicals via radio-induced bond break or electron transfer.23,26,41,42 Therefore, electron paramagnetic resonance (EPR) spectrum of irradiated U-Cbdcp was collected and indeed shows an intense EPR signal with a g-tensor of 2.0197, corresponding to the value (g = 2.0023) of a free electron (Fig. 3).43 The freshly synthesized sample, however, is EPR silent for comparison. To identify the location of radical species in the coordination polymer, EPR spectra of H3CbdcpBr ligand before and after irradiation were recorded as well. As shown in Fig. S6,† the irradiated H3CbdcpBr exhibits a relatively weak resonance with a g factor of 2.0198, which is comparable with that of U-Cbdcp. In the light of aforementioned results, we may conclude that continuous X-ray radiation generates ligand-based radical species, which functions as a quencher via a nonradiative energy transfer pathway.44–46
Encouraged by the structural integrity of U-Cbdcp upon 5 kGy X-ray irradiation, we further investigated its radiolytic stability by irradiating the sample with high dose β-ray and γ-ray radiations. The radiations were provided by a custom-built electron cyclotron (1.2 MeV) and a 60Co irradiation source (2.22 × 1015 Bq) with dose rates of 150 and 11.8 kGy per h, respectively. PXRD study indicated that no obvious changes in long-range order or loss of crystallinity of U-Cbdcp were observed after radiations, implying excellent radiation resistance of U-Cbdcp (Fig. S7†).
In summary, a new 1D uranium coordination polymer built from uranyl cations and zwitterionic Cbdcp ligands were obtained solvothermally. One of the most intriguing properties of U-Cbdcp is the occurrence of luminescence quenching upon X-ray radiation. This unique radio-induced luminometric response can be utilized as a strategy for X-ray dosimetry. Notably, the quenching response can be well fitted with a linear correlation and the detection limit was calculated to be 0.093 Gy. This finding, in conjunction with the excellent radiation resistance of U-Cbdcp, point to potential applications of uranium bearing materials for radiation detection.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22076196, 21876182, 21806127, and U1967219), the Shanghai Sailing Program (21YF1456200) and Natural Science Foundation of Shaanxi Province (2020JM-016).Notes and references
- K. Song, M. Mohseni and F. Taghipour, Water Res., 2016, 94, 341 CrossRef CAS PubMed.
- C. Decker, Macromol. Rapid Commun., 2002, 23, 1067 CrossRef CAS.
- P. Suortti and W. Thomlinson, Phys. Med. Biol., 2003, 48, R1 CrossRef CAS PubMed.
- J. Cadet, E. Sage and T. Douki, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 571, 3 CrossRef CAS PubMed.
- E. I. Azzam, J. P. Jay-Gerin and D. Pain, Cancer Lett., 2012, 327, 48 CrossRef CAS PubMed.
- P. Andreo, D. T. Burns, A. E. Nahum, J. Seuntjens and F. H. Attix, Fundamentals of Ionizing Radiation Dosimetry, Wiley, 2017 Search PubMed.
- L. Sang, M. Liao and M. Sumiya, Sensors, 2013, 13, 10482 CrossRef CAS PubMed.
- C. Sun, X.-Q. Yu, M.-S. Wang and G.-C. Guo, Angew. Chem., Int. Ed., 2019, 58, 9475 CrossRef CAS PubMed.
- Y. Wang, X. Liu, X. Li, F. Zhai, S. Yan, N. Liu, Z. Chai, Y. Xu, X. Ouyang and S. Wang, J. Am. Chem. Soc., 2019, 141, 8030 CrossRef CAS PubMed.
- H. Wei and J. Huang, Nat. Commun., 2019, 10, 1066 CrossRef PubMed.
- J. Nandha Gopal, B. Sanyal and A. Lakshmanan, Radiat. Meas., 2018, 109, 24 CrossRef CAS.
- F. Ravotti, IEEE Trans. Nucl. Sci., 2018, 65, 1440 CAS.
- F. P. Doty, C. A. Bauer, A. J. Skulan, P. G. Grant and M. D. Allendorf, Adv. Mater., 2009, 21, 95 CrossRef CAS.
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249 CrossRef CAS PubMed.
- A. M. Rice, C. R. Martin, V. A. Galitskiy, A. A. Berseneva, G. A. Leith and N. B. Shustova, Chem. Rev., 2020, 120, 8790 CrossRef CAS PubMed.
- C. R. Martin, G. A. Leith, P. Kittikhunnatham, K. C. Park, O. A. Ejegbavwo, A. Mathur, C. R. Callahan, S. L. Desmond, M. R. Keener, F. Ahmed, S. Pandey, M. D. Smith, S. R. Phillpot, A. B. Greytak and N. B. Shustova, Angew. Chem., Int. Ed., 2021, 60, 8072 CrossRef CAS PubMed.
- L. Liu, Q. Liu, R. Li, M.-S. Wang and G.-C. Guo, J. Am. Chem. Soc., 2021, 143, 2232 CrossRef CAS PubMed.
- W.-F. Wang, J. Lu, X.-M. Xu, B.-Y. Li, J. Gao, M.-J. Xie, S.-H. Wang, F.-K. Zheng and G.-C. Guo, Chem. Eng. J., 2022, 430, 133010 CrossRef CAS.
- H. Lu, J. Xie, X.-Y. Wang, Y. Wang, Z.-J. Li, K. Diefenbach, Q.-J. Pan, Y. Qian, J.-Q. Wang, S. Wang and J. Lin, Nat. Commun., 2021, 12, 2798 CrossRef CAS PubMed.
- H. Lu, Z. Zheng, Z.-J. Li, H. Bao, X. Guo, X. Guo, J. Lin, Y. Qian and J.-Q. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 2745 CrossRef CAS PubMed.
- Z. Zheng, H. Lu, X. Guo, Z. Zhou, Y. Wang, Z.-J. Li, G. Xiao, Y. Qian, J. Lin and J.-Q. Wang, Chem. Commun., 2021, 57, 8131 RSC.
- H. Liu, H. Qin, N. Shen, S. Yan, Y. Wang, X. Yin, X. Chen, C. Zhang, X. Dai, R. Zhou, X. Ouyang, Z. Chai and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 1 CrossRef.
- J. Xie, Y. Wang, W. Liu, X. Yin, L. Chen, Y. Zou, J. Diwu, Z. Chai, T. E. Albrecht-Schmitt, G. Liu and S. Wang, Angew. Chem., Int. Ed., 2017, 56, 7500 CrossRef CAS PubMed.
- X. Li, Y. Wang, J. Xie, X. Yin, M. A. Silver, Y. Cai, H. Zhang, L. Chen, G. Bian, J. Diwu, Z. Chai and S. Wang, Inorg. Chem., 2018, 57, 8714 CrossRef CAS PubMed.
- W. Liu, X. Dai, J. Xie, M. A. Silver, D. Zhang, Y. Wang, Y. Cai, J. Diwu, J. Wang, R. Zhou, Z. Chai and S. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 4844 CrossRef CAS PubMed.
- J. Xie, Y. Wang, W. Liu, C. Liang, Y. Zhang, L. Chen, D. Sheng, Z. Chai and S. Wang, Sci. China: Chem., 2020, 63, 1608 CrossRef CAS.
- F. Nakamura, T. Kato, G. Okada, N. Kawaguchi, K. Fukuda and T. Yanagida, Ceram. Int., 2017, 43, 7211 CrossRef CAS.
- H. Lu, M. Xu, Z. Zheng, Q. Liu, J. Qian, Z.-H. Zhang, M.-Y. He, Y. Qian, J.-Q. Wang and J. Lin, Inorg. Chem., 2021, 60, 18629 CrossRef CAS PubMed.
- E. A. Dolgopolova, A. M. Rice and N. B. Shustova, Chem. Commun., 2018, 54, 6472 RSC.
- C. R. Martin, G. A. Leith and N. B. Shustova, Chem. Sci., 2021, 12, 7214 RSC.
- K. Lv, S. Fichter, M. Gu, J. März and M. Schmidt, Coord. Chem. Rev., 2021, 446, 214011 CrossRef CAS.
- N. M. Edelstein, J. Fuger and L. R. Morss, The Chemistry of the Actinide and Transactinide Elements, Springer, 2010 Search PubMed.
- M. Zhang, C. Liang, G.-D. Cheng, J. Chen, Y. Wang, L. He, L. Cheng, S. Gong, D. Zhang, J. Li, S.-X. Hu, J. Diwu, G. Wu, Y. Wang, Z. Chai and S. Wang, Angew. Chem., Int. Ed., 2021, 60, 9886 CrossRef CAS PubMed.
- L. Mei, P. Ren, Q.-y. Wu, Y.-b. Ke, J.-s. Geng, K. Liu, X.-q. Xing, Z.-w. Huang, K.-q. Hu, Y.-l. Liu, L.-y. Yuan, G. Mo, Z.-h. Wu, J. K. Gibson, Z.-f. Chai and W.-q. Shi, J. Am. Chem. Soc., 2020, 142, 16538 CrossRef CAS PubMed.
- G. L. Murphy, Y. Wang, P. Kegler, Y. Wang, S. Wang and E. V. Alekseev, Chem. Commun., 2021, 57, 496 RSC.
- G. L. Murphy, E. M. Langer, O. Walter, Y. Wang, S. Wang and E. V. Alekseev, Inorg. Chem., 2020, 59, 7204 CrossRef CAS PubMed.
- H. Li, P. Kegler and E. V. Alekseev, Dalton Trans., 2020, 49, 2244 RSC.
- X. Kong, K. Hu, L. Mei, Q. Wu, Z. Huang, K. Liu, Z. Chai, C. Nie and W.-Q. Shi, Chem. - Eur. J., 2021, 27, 2124 CrossRef CAS PubMed.
- M. Azam, S. I. Al-Resayes, G. Velmurugan, P. Venuvanalingam, J. Wagler and E. Kroke, Dalton Trans., 2015, 44, 568 RSC.
- J.-M. Han, M. Xu, B. Wang, N. Wu, X. Yang, H. Yang, B. J. Salter and L. Zang, J. Am. Chem. Soc., 2014, 136, 5090 CrossRef CAS PubMed.
- J. Xie, Y. Wang, D. Zhang, C. Liang, W. Liu, Y. Chong, X. Yin, Y. Zhang, D. Gui, L. Chen, W. Tong, Z. Liu, J. Diwu, Z. Chai and S. Wang, Chem. Commun., 2019, 55, 11715 RSC.
- G. Mehlana and S. A. Bourne, CrystEngComm, 2017, 19, 4238 RSC.
- S. Tang, H. Ruan, R. Feng, Y. Zhao, G. Tan, L. Zhang and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 18224 CrossRef CAS PubMed.
- T. B. Faust and D. M. D'Alessandro, RSC Adv., 2014, 4, 17498 RSC.
- B. Lü, Y. Chen, P. Li, B. Wang, K. Müllen and M. Yin, Nat. Commun., 2019, 10, 767 CrossRef PubMed.
- Y.-J. Ma, J.-X. Hu, S.-D. Han, J. Pan, J.-H. Li and G.-M. Wang, J. Am. Chem. Soc., 2020, 142, 2682 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterizations, PXRD, FTIR, SEM-EDS, crystallographic data, selected bond distances and angles. CCDC 2141552–2141553. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra00440b |
| This journal is © The Royal Society of Chemistry 2022 |