Open Access Article
Open Access ArticleFabric phase sorptive extraction combined with gas chromatography-mass spectrometry as an innovative analytical technique for the determination of selected polycyclic aromatic hydrocarbons in herbal infusions and tea samples†
Natalia Manousi ab,
Abuzar Kabir
ab,
Abuzar Kabir cd,
Kenneth G. Furton
cd,
Kenneth G. Furton c,
Erwin Rosenberg
c,
Erwin Rosenberg *b and
George A. Zachariadis
*b and
George A. Zachariadis a
a
aLaboratory of Analytical Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, Thessaloniki 54124, Greece
bInstitute of Chemical Technologies and Analytics, Vienna University of Technology, Getreidemarkt 9/164, 1060 Vienna, Austria. E-mail: erosen@mail.zserv.tuwien.ac.at
cInternational Forensic Research Institute, Department of Chemistry and Biochemistry, Florida International University, Miami, FL, USA
dDepartment of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka-1207, Bangladesh
First published on 1st March 2022
Abstract
This study presents a fabric phase sorptive extraction (FPSE) protocol for the isolation and preconcentration of four selected polycyclic aromatic hydrocarbons from tea samples and herbal infusions, followed by their separation and quantification by gas chromatography-mass spectrometry (GC-MS). In FPSE, extraction of the target analytes is performed utilizing a flexible fabric substrate that is coated with a highly efficient sol–gel sorbent. In this work, eighteen different FPSE membranes were examined, with the highest extraction recoveries being observed with the sol–gel C18 coated FPSE membrane. The main parameters that influence the adsorption and desorption of the PAHs were optimized and the proposed method was validated. The detection limits and the quantification limits were 0.08–0.17 ng mL−1 and 0.25–0.50 ng mL−1, respectively, for the different target compounds with a 10 mL sample. The relative standard deviations for intra-day and inter-day repeatability were less than 7.9% and 8.5%, respectively. The sol–gel C18 coated FPSE membrane could be used for at least 5 subsequent sample preparation cycles. Finally, the proposed protocol was successfully employed for the determination of PAHs in a wide range of tea and herbal infusion samples.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of chemicals that consist of two or more fused benzene rings. These chemical compounds are hydrophobic, and consequently exhibit low water solubility. PAHs are usually derived from the incomplete combustion of organic materials, and they can originate both from anthropogenic and from natural processes. Sixteen PAHs have been classified as priority pollutants by the US Environmental Protection Agency (EPA), since they exhibit toxicity, as well as potential carcinogenic and mutagenic action.1–3Food consumption is among the major sources of exposure of humans to PAHs. These pollutants can be present in both fresh and processed food such as fruit, vegetables, and sugars, as well as in meat, fish, milk, and beverages. Dried teas have been identified by the European Food Safety Authority (EFSA) as a food category that exhibits consistently high content of PAHs.4 PAHs that occur in water, air or soil might accumulate in tea plants, while the enforced heating and drying of the tea leaves during processing may also result in contamination with PAHs. Three-ring and four-ring PAHs are those PAHs that are most likely to be detected in tea samples. Thus, the development of accurate and sensitive methodologies that are simple to use, cost effective and robust in order to assess exposure to PAHs due to tea consumption is of utmost importance.5
High-performance liquid chromatography (HPLC) and gas chromatography (GC) are widely used for the determination of PAHs. Among the available detection systems, GC coupled to flame ionization detectors (FID), mass spectrometry (MS) detectors or tandem MS detectors, as well as HPLC coupled to fluorescence detectors (FLD) and ultraviolet detectors (UV) are the most common instrumental set-ups used for this purpose. For GC applications, MS and tandem MS detectors are widely used for PAHs' determination in real samples since they provide enhanced sensitivity and reduced detection limits.2
Generally, since the concentration of PAHs in tea leaves is at considerably low levels, a preconcentration technique is required. Solid-phase extraction and liquid–liquid extraction are two widely used sample preparation techniques which are traditionally employed for the extraction and preconcentration of PAHs from tea samples. These sample preparation techniques have some significant limitations including significant consumption of hazardous solvents and long extraction time.5 To overcome these disadvantages, a plethora of novel sample preparation techniques and novel sorbents have been developed. Examples of novel sorbents include molecularly imprinted polymers,6 functionalized graphene oxide,7 metal–organic frameworks8 and covalent organic frameworks.9 Examples of novel miniaturized and microextraction techniques for the determination of PAHs in herbal infusions and tea samples are solid-phase microextraction,10 micro-solid phase extraction (μSPE),5 dispersive liquid–liquid extraction,11 and stir bar sorptive extraction.12
Fabric phase sorptive extraction (FPSE) is a novel sample extraction technique that was proposed by Kabir and Furton.13 In FPSE, the analytes are extracted by a sol–gel sorbent that is bonded chemically to a fabric substrate. The combination of the porous architecture of the sol–gel network with the open geometry of the FPSE substrate promotes the fast extraction and elution of organic compounds. For assisting the diffusion of the analytes, magnetic stirring or mechanical shaking can be employed. The FPSE membrane exhibits high chemical stability which enables the utilization of any organic solvent for the desorption of the analytes. Moreover, only a small elution volume is required, resulting in high enhancement factors for the desired compounds. Therefore, solvent evaporation and/or reconstitution are not required and the eluent can be directly analyzed by an instrumental technique.14 Other advantages of the FPSE technique are its simplicity, regarding sample handling, and its cost-effectiveness.15 Therefore, FPSE is a powerful sample preparation technique, which can significantly simplify the overall extraction process and eliminate time-consuming steps that are prone to errors, resulting in high extraction efficiency.14
Until now, FPSE has been utilized for the extraction of a variety of analytes, i.e. alkyl phenols,16 penicillins,14,17 sulfonamides,15 parabens,18–20 tetracyclines,21 estrogenic endocrine disrupting chemicals and bisphenol A,22 pirimicarb and fenitrothion pesticides23 etc. from complex food, biological and environmental matrices. PAHs including anthracene, phenanthrene, pyrene and fluoranthene have been extracted with a sol–gel C18 coated FPSE medium from water samples followed by analysis by HPLC-FLD.24 These FPSE media have been already evaluated for their performance for the extraction of a wide range of compounds including parabens,19 β-blockers,25 adamantine26 and solar UV filters27 etc.
The aim of this work was to develop a fast and facile protocol for the separation and quantification of selected PAHs (i.e., phenanthrene, naphthalene, fluorene, and pyrene) in tea samples and herbal infusions. For this purpose, the FPSE technique using a sol–gel C18 coated FPSE membrane was explored as an innovative analytical tool for the enrichment of the target analytes for the development of a green sample preparation protocol.
Experimental
Chemicals and reagents
Methanol (MeOH), toluene and NaCl were obtained from Merck and they were of analytical grade (Merck KGaA, Darmstadt, Germany). Phenanthrene (97%), pyrene (98%), fluorene (98%) and naphthalene (99%), as well as hexane (analytical grade) were obtained from Sigma-Aldrich (St. Louis, MO; United States). Fig. S1† presents the structure of the four PAHs. Stock solutions of the target analytes (100 mg L−1) were made in toluene and MeOH and stored at 4 °C. Working solutions were prepared daily through serial dilutions of the stock standards in MeOH or toluene. Methanolic solutions were used for the preparation of the spiked water samples. On the other hand, the solutions prepared in toluene were used for establishing the calibration curves and for the calculation of the recovery of the selected PAHs.For the preparation of FPSE membranes, Muslin 100% cotton cellulose substrate was obtained from Jo-Ann Fabric (Miami, FL, USA), while the sol–gel synthesis precursors were obtained from Sigma-Aldrich. NaOH and HCl were obtained from Thermo Fisher Scientific (Milwaukee, WI, USA).
Eight different herbal infusions and tea samples were purchased from Thessaloniki, Greece: CH-1 (chamomile), CH-2 (chamomile), CH-3 (chamomile), GMT (Greek mountain tea), IN-1 (herbal infusion with nettle, meadowsweet, rosemary and grapefruit flavour), IN-2 (herbal infusion with apple and cinnamon flavour), IN-3 (herbal infusion with strawberry and raspberry flavour) and GT (green tea).
Instrumentation
For the analysis of the infusions, a gas chromatograph (Agilent 6890 N) coupled to a Quadrupole MS Detector (Agilent 5973 K) (Hewlett Packard, Waldbronn, Germany) was employed. An HP-5 capillary column (30 m × 0.32 mm, 0.25 μm; Agilent Technologies, Santa Clara, CA, USA) was employed in this study to separate the target analytes. Helium (99.999%) delivered at a flow rate of 1.5 mL min−1 was used as the mobile phase. The oven temperature program was as follows: 60 °C initial temperature, raised to 210 °C at a rate of 45 °C min−1 and it was held constant at 210 °C for 6 min. The oven temperature was further raised to 320 °C at a rate of 50 °C min−1. The solvent delay and the total analysis time were 2.0 min and 12.5 min, respectively. The temperature of the injection port was 250 °C, while the MS source and the MS Quad were operated at 230 °C and 150 °C, respectively. Injection was performed in splitless mode and an aliquot of 2 μL of each sample was injected in the GC-MS system. Quantification of the selected PAHs was performed in selected ion monitoring mode (SIM) using the following m/z ratios for the quantification: m/z 128 (naphthalene), 165 (fluorene), 178 (phenanthrene) and 202 (pyrene). A representative chromatogram is shown in Fig. S2.†Preparation of sol–gel C18 coated FPSE membrane
The preparation of the sol–gel C18 coated FPSE media was previously reported.24 In brief, deionized water was used for the cleaning of the cellulose fabric and the cleaning process was assisted with ultrasonication. Subsequently, the membrane was extensively rinsed with DI water in order to remove starch and other finishing chemicals that might be present in the commercially available cellulose fabric. Then, the fabric surface was activated to maximize the number of available –OH groups that are necessary for the efficient binding with the sol–gel network through a condensation reaction. This was achieved by immersing the fabric in 1 mol L−1 sodium hydroxide solution for 60 min under ultrasonic radiation. Afterwards, the fabric was thoroughly rinsed with water, and treated with a 0.1 mol L−1 hydrochloric acid solution for 60 min for the neutralization of the residual base that could potentially be present on the surface of the cellulose fabric. Finally, drying of the cellulose substrate took place and the dried membranes were kept in an air-tight containers before their coating with the sol–gel sorbent.The sol solution that was used for the fabrication of the sol–gel C18 coated FPSE membrane was created through mixing methyl trimethoxysilane (MTMS, sol–gel precursor); dichloromethane and acetone (organic solvents as porogens), octadecyl trimethoxysilane (functionalized sol–gel precursor), trifluoroacetic acid (sol–gel catalyst) and H2O at the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33
0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.94
1.94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3
2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, as described in ref. 24. The function of the MTMS is that of a networking precursor. While both, tetramethoxysilane (TMOS) or methyl trimethoxysilane could potentially be used as networking precursors, the use of the latter was reported to produce a softer gel and an open, crack-free surface coating.28 The open porous and crack-free morphology of the surface coating obtained with MTMS as a precursor is particularly advantageous in FPSE, as it supports fast extraction kinetics. Octadecyl silane was used in the sol solution as the polymeric component to randomly integrate into the growing sol–gel network during polycondensation. Likewise, the use of the two organic solvents (methylene chloride and acetone) allows to more precisely control the solubility of the precursor compounds which, as they become insoluble, form the gel structure, and thus the obtained domain size. After the addition of each ingredient, vortex mixing took place to ensure homogenization. The resulting solution was centrifuged, and the supernatant was collected, followed by sonication for the removal of potentially trapped molecules of gases.
3, as described in ref. 24. The function of the MTMS is that of a networking precursor. While both, tetramethoxysilane (TMOS) or methyl trimethoxysilane could potentially be used as networking precursors, the use of the latter was reported to produce a softer gel and an open, crack-free surface coating.28 The open porous and crack-free morphology of the surface coating obtained with MTMS as a precursor is particularly advantageous in FPSE, as it supports fast extraction kinetics. Octadecyl silane was used in the sol solution as the polymeric component to randomly integrate into the growing sol–gel network during polycondensation. Likewise, the use of the two organic solvents (methylene chloride and acetone) allows to more precisely control the solubility of the precursor compounds which, as they become insoluble, form the gel structure, and thus the obtained domain size. After the addition of each ingredient, vortex mixing took place to ensure homogenization. The resulting solution was centrifuged, and the supernatant was collected, followed by sonication for the removal of potentially trapped molecules of gases.
Accordingly, the fabric substrate was placed in the sol solution, for the initiation of the coating through a dip-coating process. The cellulose fabric remained in the sol solution for two hours, to form a three-dimensional sol–gel network. Subsequently, the sol solutions were discarded, and the coated fabrics were dried. The obtained sol–gel C18 coated FPSE medium was aged/conditioned under He flow for 24 h at 50 °C, followed by sequential cleaning with dichloromethane and methanol. After drying under a flow of He at 50 °C for one hour, the obtained membranes were cut and stored in airtight glass containers until future use.
Pretreatment of the herbal infusions and tea samples
The preparation of the herbal infusion and the tea samples was described by Shi et al.5 In brief, 0.6 g of weighed herbal or tea sample was placed into a beaker that contained 10 mL of boiling H2O and the herbal material was soaked for 30 min. Afterwards, the obtained herbal infusions or tea samples were filtered, followed by 5-fold dilution, and subjected to the FPSE protocol described below.Fabric phase sorptive extraction of PAHs
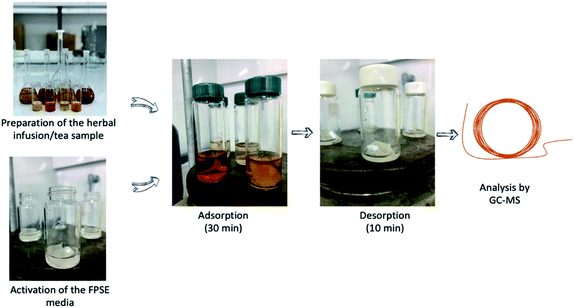
Fig. 1 presents the major steps of the FPSE procedure. Initially, activation of the sol–gel C18 coated FPSE membrane (2 × 2 cm) was performed by immersion in a 2 mL mixture of MeOH and ACN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) for 5 min, followed by immersion in 2 mL of deionized H2O for another 5 min.
50, v/v) for 5 min, followed by immersion in 2 mL of deionized H2O for another 5 min.
Subsequently, 10 mL of herbal infusion/tea was placed in a 40 mL glass vial and a magnetic was added in the vial. The FPSE membrane was added in the sample and extraction took place within 30 min under stirring at 200 rpm. Afterwards, the aqueous sample was discarded, and 1 mL of toluene was added for the desorption of the adsorbed analytes. Desorption took place within 10 min and after this timespan the eluent was filtered with a 0.22 μm nylon syringe filter and analysis was done by GC/MS.
After the FPSE procedure, the sol–gel C18 coated FPSE membrane was washed with 2 mL of a mixture of MeOH and ACN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) for 5 min. The membranes were left to dry and stored for future use secluded from air.
50, v/v) for 5 min. The membranes were left to dry and stored for future use secluded from air.
Results and discussion
Investigation of FPSE conditions
Eighteen different FPSE membranes (see ESI, Table S1†) were examined to select the most efficient extraction medium. For this purpose, standard solutions of the selected PAHs were employed. The results are shown in Fig. 3.Considerably high extraction efficiencies for the four selected PAHs were observed when using sol–gel mixed mode/C, sol–gel CW 20/C, sol–gel C18/C, sol–gel PTHF/C and sol–gel PPO–PEO–PPO/C fabric phases. Aiming to find the most appropriate FPSE medium, these five FPSE membranes were investigated in more detail for their performance regarding the extraction of the PAHs from spiked herbal infusions samples. The highest extraction efficiency for real samples was observed with the sol–gel C18/C FPSE membrane, and thus this was selected for the present study. The C18 sorbent is a hydrophobic sorbent that, in accordance with our expectations, is an appropriate choice for the extraction of PAHs which are inherently nonpolar, hydrophobic compounds. The sol–gel C18 FPSE media that contain long hydrophobic octadecyl chains were previously used for the quantification of PAHs from water samples.24 Afterwards, different dimensions of the C18 coated FPSE membrane were evaluated (i.e., 1 cm × 1.5 cm, 2 cm × 2 cm and 2 cm × 2.5 cm). The extraction performance increased by increasing the dimensions of the FPSE membrane from 1 cm × 1.5 cm to 2 cm × 2 cm. Further increase of the dimensions of the FPSE media led to a slight reduction in terms of extraction efficiency, which might be a limitation of insufficient contact of the medium with the elution solvent. Thus, a sol–gel C18 coated FPSE membrane with dimensions 2 cm × 2 cm was finally chosen.
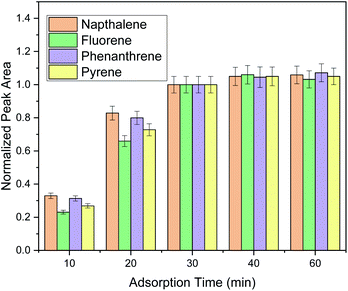
Sufficient adsorption time must be provided to allow the partitioning equilibrium be reached between the selected PAHs and the FPSE membrane and to provide high extraction efficiency. Aiming to find the optimum adsorption time, five timespans (i.e., 10, 20, 30, 40 and 60 min) were studied (Fig. 2). It was observed that thirty minutes were sufficient for the extraction of all the selected PAHs included in this study. Moreover, the performance of the extraction procedure for the monitored PAHs did not increase more than 10% when the extraction time was increased up to 60 min. Thus, a time span of 30 min was chosen as the optimum extraction time.
The optimization of sample volume was investigated in the next step. As shown in Fig. S4,† the best performance was obtained using 10 mL sample volume. Further increase of the sample volume resulted in lower extraction efficiencies. Therefore, further experiments were conducted using 10 mL of sample. The extraction efficiency is depending on both, the capacity of the sorbent and the volume-to-surface ratio of sample and fabric phase. At larger sample volumes, apparently either the capacity of the sorbent phase becomes the limiting factor, or the volume-to-surface ratio becomes unfavorable, limiting the amount of sample that is absorbed by the fabric phase under the same conditions.
Subsequently, three different stirring rates in the range of 100–300 rpm were evaluated. The results are presented in Fig. S5.† Mechanical agitation is needed to increase the turbulence of the sample solution and allow the PAHs to more intensively come into contact with the extraction medium, resulting in high extraction efficiency. The stirring rate must be sufficient to enable the mass transfer of PAHs to the FPSE.29,30 In this case, an increase of the stirring rate from 100 rpm to 200 rpm enhanced the performance of the extraction for all PAHs, while an increase to 300 rpm did not enhance the extraction efficiency. Thus, a stirring rate of 200 rpm was used in further experiments.
For the investigation of the ionic strength, different concentrations of NaCl (0–20% w/v) were evaluated. It was found that the presence of salt had a negative impact on the FPSE procedure as shown in Fig. S6.† This can be attributed to an enhancement of the viscosity of the sample that can potentially hinder the mass transfer of the desired compounds and leads to low extraction efficiencies for a given extraction time. This phenomenon was more intense for fluorene, phenanthrene and pyrene, most likely due to their bigger size and consequently lower diffusion coefficient compared to naphthalene. Moreover, salt addition possibly reduces the interactions of the sorbent and the organic compounds and thus reduces the overall extraction efficiency. As a result, no addition of salt was used in further experiments.31
Three different organic solvents, i.e., toluene, methanol and n-hexane were examined to find the most appropriate elution solvent for the adsorbed PAHs. When choosing the optimum elution membrane, the polarity of the solvent is the key factor to obtain good extraction efficiency. As it can be observed from Fig. S7,† methanol resulted in the lowest extraction efficiency, since it was the most polar solvent among the three examined solvents.32 Satisfactory extraction efficiencies were observed with toluene due to its low polarity and its aromatic character that results in π–π interactions among the solvent and the PAHs.33 A slight enhancement of the extraction efficiency of pyrene was observed with the use of n-hexane as an elution solvent. However, n-hexane belongs to the undesirable solvents for laboratory use, while toluene is considered “usable” based on the Pfizer solvent selection guide.34 All things considered, the use of toluene as an elution solvent is a reasonable compromise.
After choosing the optimum elution solvent, different elution volumes (0.25–1 mL) were investigated, and the obtained data are presented in Fig. S8.† The eluent amount must be sufficient to enable the desorption of the adsorbed PAHs. In addition, the utilization of the lowest possible volume of the eluent is preferred in order to achieve high sample preconcentration and to meet the requirements of Green Chemistry.35 As it can be observed, 1 mL of solvent resulted in the highest desorption efficiency for the selected PAHs. Further increase of the elution volume would decrease the sensitivity of the method and was therefore not investigated.
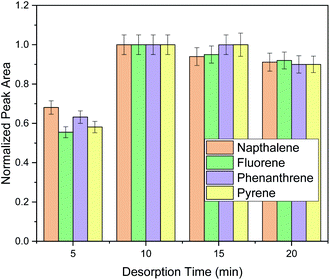
Finally, elution time was investigated in order to ensure complete elution of PAHs. Generally, the elution time span must be enough to enable the desorption of the adsorbed PAHs. For this purpose, desorption timespans of 5–20 min were investigated as shown in Fig. 3.
As it can be observed, ten minutes under stirring were sufficient for analyte's elution and the prolongation of the extraction time did not have any benefit in the extraction performance. Therefore, 10 min were chosen for the elution of the target analytes. After method optimization, extraction recoveries (ER%) were 35.3% for naphthalene, 47.5% for fluorene, 39.1% for phenanthrene and 71.3% for pyrene.
Figures of merit
The results for the validation of the FPSE-GC-MS method protocol are shown in Table 1. For the evaluation of the linearity of the proposed method, least square linear regression analysis was used. Therefore, calibration curves were prepared for all analytes and the linear ranges were 0.25–25 ng mL−1 for naphthalene, phenanthrene and pyrene and 0.50–50 ng mL−1 for fluorene. The coefficients of determination for all analytes were in the range 0.9988–0.9999, which points to good linearity for the investigated working range. The lowest point of each calibration curve was considered to be the LOQ value of the respective analyte, while the LOD values were the LOQ values divided by 3.3. In this case, the LODs and LOQs for the PAHs were 0.08–0.17 ng mL−1 and 0.25–0.50 ng mL−1, respectively.| Target analyte | Regression analysis | R2 | Linear range [ng mL−1] | LOD [ng mL−1] | LOQ [ng mL−1] |
|---|---|---|---|---|---|
| Naphthalene | y = 3263x + 90 | 0.9988 | 0.25–25 | 0.08 | 0.25 |
| Fluorene | y = 2713x + 807 | 0.9991 | 0.50–50 | 0.17 | 0.50 |
| Phenanthrene | y = 5078x + 5079 | 0.9996 | 0.25–25 | 0.08 | 0.25 |
| Pyrene | y = 6289x + 1358 | 0.9999 | 0.25–25 | 0.08 | 0.25 |
Table 2 presents the data for the intra-day repeatability and for inter-day precision and trueness. For this purpose, a chamomile infusion was employed to prepare spiked samples at 1 ng mL−1 and 10 ng mL−1. Intra-day repeatability expressed as RSD% values, was determined within the same day thrice, while inter-day precision and trueness was calculated from triplicate analysis on four different days at the same concentrations.17,36 As shown in Table 2, the RSDs for the target analytes were lower than 7.9% for intra-day repeatability and lower than 8.5% for inter-day precision. Finally, the relative recoveries were 91.9–109.8%, showing good method trueness.
| Analyte | Added (ng mL−1) | Within-day (n = 5) | Between-day (n = 5 × 3) | ||||
|---|---|---|---|---|---|---|---|
| Found (ng mL−1) | RSD% | RR% | Found (ng mL−1) | RSD% | RR% | ||
| Naphthalene | 1.00 | 0.98 ± 0.03 | 3.2 | 97.8 | 0.96 ± 0.04 | 4.0 | 95.9 |
| 10.00 | 9.77 ± 0.28 | 2.9 | 97.7 | 9.34 ± 0.68 | 7.3 | 93.4 | |
| Fluorene | 1.00 | 0.96 ± 0.04 | 3.7 | 96.0 | 0.95 ± 0.05 | 5.2 | 95.2 |
| 10.00 | 10.33 ± 0.61 | 5.9 | 103.3 | 10.86 ± 0.86 | 7.9 | 108.6 | |
| Phenanthrene | 1.00 | 0.92 ± 0.07 | 7.9 | 91.9 | 1.07 ± 0.06 | 5.9 | 107.2 |
| 10.00 | 10.65 ± 0.57 | 5.3 | 106.5 | 9.85 ± 0.73 | 7.4 | 98.5 | |
| Pyrene | 1.00 | 1.10 ± 0.09 | 7.9 | 109.8 | 1.04 ± 0.09 | 8.5 | 104.1 |
| 10.00 | 10.07 ± 0.16 | 1.5 | 100.7 | 10.71 ± 0.56 | 5.2 | 107.1 | |
Reusability of the extraction phase
The evaluation of the potential reusability of the herein used FPSE media was performed in order to study its overall performance using a spiked chamomile infusion sample (c = 10.0 ng mL−1). For this purpose, the FPSE membrane was washed by the addition of 2 mL of a mixture containing MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN (50
ACN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v), after one complete implementation of the FPSE protocol. Washing was performed within 5 min, without magnetic stirring. Following the washing step, the membranes were left to dry and stored in air-tight vials, prior to their next use. After the desorption of PAHs, no significant carry-over was observed. Moreover, as shown in Fig. S9,† each FPSE medium can be potentially used for at least 5 times.
50, v/v), after one complete implementation of the FPSE protocol. Washing was performed within 5 min, without magnetic stirring. Following the washing step, the membranes were left to dry and stored in air-tight vials, prior to their next use. After the desorption of PAHs, no significant carry-over was observed. Moreover, as shown in Fig. S9,† each FPSE medium can be potentially used for at least 5 times.
Determination of PAHs in real samples
The optimized and validated FPSE protocol was implemented for the analysis of various herbal infusions and tea samples. Table S2† presents the results regarding the determination of PAHs in real herbal infusions and tea samples.The recoveries for the monitored PAHs were calculated by preparing spiked sample solutions (c = 5 ng mL−1) for the infusions and tea samples and by comparing the experimental found concentration to the nominal concentration of the PAHs in the samples. As it can be observed from Table S2,† very satisfactory trueness was observed since relative recoveries were in the range of 94.3–109.8%.
Naphthalene and fluorene were not found in any of the examined herbal infusions and tea samples. Phenanthrene was detected in the green tea samples. Pyrene was detected in two chamomile samples and in the Greek mountain tea sample. The existence of these PAHs in tea samples at similar concentration ranges has been previously reported.6,30
Comparison with other methodologies
The FSPE-GC-MS method was compared in terms of extraction time, adsorption volume, RSD% values, ER% and LOD values, with other published studies about the monitoring of PAHs in herbal infusions and teas, as shown in Table 3.| Sample preparation | Detection system | Sample volume (mL) | Extraction time (min) | RSD% | ER% | LODs (ng mL−1) | Ref. |
|---|---|---|---|---|---|---|---|
| QuEChERS | GC-MS | 10 | >20 | <20 | 50–120 | 0.2–0.4 | 31 |
| DLLME | HPLC-UV | 10 | 12 | <19 | 20.2–117.0 | 0.010–0.600 | 11 |
| SPE | HPLC-FLD | 100 | 10 | ≤18 | 54–100 | 0.05–0.09 | 37 |
| μSPE | HPLC-UV | 10 | 40 | ≤13.53 | NA | 0.549–0.673 | 32 |
| Dispersive μSPE | GC-MS | 10 | 5 | <6.7 (intra-day), <7.8 (inter-day) | 85.0–93.5 | 0.012–0.014 | 5 |
| FPSE | GC-MS | 10 | 30 | <7.9 (intra-day), <8.5 (inter-day) | 35.3–71.3 | 0.08–0.17 | This study |
It can be observed that 10 mL of the aqueous samples was required in most studies. The adsorption time of the herein developed method was lower than that reported in ref. 32 but slightly higher than in other studies. The RSD% values of these studies were similar to those reported in ref. 5 and better than the values of the other studies. The extraction recoveries reported in ref. 5 were better than the ER% values of this method, while similar ER% with current method was reported in the other studies. Finally, the detection limits of this study were lower than those of ref. 32, similar to the values reported in ref. 37 but higher than those of ref. 5 and 11. However, compared to the SPE and d-μSPE methods (ref. 5 and 37), lower consumption of organic solvent was required. In SPE approaches, the sorbent must be packed into cartridges for in house-prepared specialty sorbents which is a laborious process and may lead to high back-pressure which becomes a limiting factor for fast enrichment. This problem can be overcome using FPSE. Consequently, the use of the FPSE membrane with its far more favourable aspect ratio when compared to enrichment phases in SPE columns eliminates potential limitations of classical SPE, such as column back-pressure build-up and long extraction times. As a result, the overall performance of the FPSE method can be considered highly satisfactory.
Conclusions
A facile and fast FPSE protocol for PAHs' extraction from herbal infusions and teas followed by GC-MS analysis was developed. After optimization of the main parameters that affect the steps of the FPSE process, the method was validated. Acceptable extraction recovery, wide linear range, very satisfactory LODs and LOQs and good precision were achieved. The use of FPSE as a sample preparation protocol efficiently reduces the consumption of organic solvents in comparison with conventional techniques (e.g., LLE). The sol–gel C18 coated FPSE media could be reused for at least 5 times. The overall protocol was simple and economic and able to clean-up demanding samples. Finally, the FPSE methodology was utilized for the analysis of various tea samples. Among the examined analytes, pyrene was the most commonly detected PAHs in the herbal infusions and tea samples, while phenanthrene was also detected occasionally.Author contributions
N. M. – conceptualization, investigation, writing – original draft, writing – review & editing; A. K. – conceptualization, resources, investigation, writing – review & editing, supervision; K. G. F. – supervision; E. R. – conceptualization, resources, supervision, writing – review & editing; G. A. Z. –conceptualization, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the HFRI PhD Fellowship grant (Fellowship Number: 138). The authors would like to acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Programme.References
- R. A. Pérez, B. Albero, J. L. Tadeo, M. V. Fraile and C. Sánchez-Brunete, Anal. Methods, 2014, 6, 1941–1950 RSC.
- N. Manousi and G. A. Zachariadis, Molecules, 2020, 25, 1–31 Search PubMed.
- X. Zhang, S. Xie, M. C. Paau, B. Zheng, H. Yuan, D. Xiao and M. M. F. Choi, J. Chromatogr. A, 2012, 1247, 1–9 CrossRef CAS PubMed.
- F. Rolle, F. Pennecchi, S. Perini and M. Sega, Measurement, 2017, 98, 290–299 CrossRef.
- Z. Shi, J. Jiang, W. Pang, H. Ma, X. Chu, C. Zhou and H. Zhang, Microchem. J., 2019, 151, 104209 CrossRef CAS.
- A. Azizi, F. Shahhoseini and C. S. Bottaro, J. Chromatogr. A, 2020, 1610, 460534 CrossRef CAS PubMed.
- A. Amiri, M. Baghayeri and E. Hamidi, New J. Chem., 2018, 42, 16744–16751 RSC.
- P. Rocío-Bautista, V. Pino, J. H. Ayala, J. Pasán, C. Ruiz-Pérez and A. M. Afonso, J. Chromatogr. A, 2016, 1436, 42–50 CrossRef PubMed.
- J. Yu, S. Di, T. Ning, H. Yang, G. T. Zhu, P. Chen, H. Yu, J. Wang and S. Zhu, Microchim. Acta, 2020, 187, 531 CrossRef CAS PubMed.
- P. Viñas, N. Campillo, N. Aguinaga, E. Pérez-Cánovas and M. Hernández-Córdoba, J. Chromatogr. A, 2007, 1164, 10–17 CrossRef PubMed.
- M. Germán-Hernández, P. Crespo-Llabrés, V. Pino, J. H. Ayala and A. M. Afonso, J. Sep. Sci., 2013, 36, 2496–2506 CrossRef PubMed.
- V. G. Zuin, L. Montero, C. Bauer and P. Popp, J. Chromatogr. A, 2005, 1091, 2–10 CrossRef CAS PubMed.
- R. Kumar, Gaurav, Heena, A. K. Malik, A. Kabir and K.G. Furton, J. Chromatogr. A, 2014, 1359, 16–25 CrossRef CAS PubMed.
- V. Samanidou, K. Michaelidou, A. Kabir and K. G. Furton, Food Chem., 2017, 224, 131–138 CrossRef CAS PubMed.
- E. Karageorgou, N. Manousi, V. Samanidou, A. Kabir and K. G. Furton, Food Chem., 2016, 196, 428–436 CrossRef CAS PubMed.
- R. Kumar, Gaurav, A. Kabir, K. G. Furton and A. K. Malik, J. Sep. Sci., 2015, 38, 3228–3238 CrossRef CAS PubMed.
- V. Alampanos, A. Kabir, K. G. Furton, V. Samanidou and I. Papadoyannis, Microchem. J., 2019, 149, 103964 CrossRef CAS.
- G. Rigkos, V. Alampanos, A. Kabir, K. G. Furton, Ž. Roje, I. V. Vrček, I. Panderi and V. Samanidou, Biomed. Chromatogr., 2020, 1–11 Search PubMed.
- V. Alampanos, A. Kabir, K. G. Furton, Ž. Roje, I. V. Vrček and V. Samanidou, J. Chromatogr. A, 2020, 1630, 461530 CrossRef CAS PubMed.
- S. Gülle, H. I. Ulusoy, A. Kabir, A. Tartaglia, K. G. Furton, M. Locatelli and V. F. Samanidou, Anal. Methods, 2019, 11, 6136–6145 RSC.
- E. Agadellis, A. Tartaglia, M. Locatelli, A. Kabir, K. G. Furton and V. Samanidou, Microchem. J., 2020, 159, 105437 CrossRef CAS.
- R. Mesa, A. Kabir, V. Samanidou and K. G. Furton, J. Sep. Sci., 2019, 42, 598–608 CrossRef CAS PubMed.
- H. İ. Ulusoy, K. Köseoğlu, A. Kabir, S. Ulusoy and M. Locatelli, Microchim. Acta, 2020, 187, 337 CrossRef CAS PubMed.
- S. S. Saini, A. Kabir, A. L. J. Rao, A. K. Malik and K. G. Furton, Separations, 2017, 4, 22 CrossRef.
- K. Mazaraki, A. Kabir, K. G. Furton, K. Fytianos, V. F. Samanidou and C. K. Zacharis, J. Pharm. Biomed. Anal., 2021, 199, 114053 CrossRef CAS PubMed.
- G. Sidiropoulou, A. Kabir, K. G. Furton, F. S. Kika, K. Fytianos, P. D. Tzanavaras and C. K. Zacharis, Microchem. J., 2022, 176, 107250 CrossRef CAS.
- M. Locatelli, K. G. Furton, A. Tartaglia, E. Sperandio, H. I. Ulusoy and A. Kabir, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1118–1119, 40–50 CrossRef CAS PubMed.
- S. L. Chong, D. Wang, J. D. Hayes, B. W. Wilhite and A. Malik, Anal. Chem., 1997, 69, 3889–3898 CrossRef CAS PubMed.
- E. Rianawati and R. Balasubramanian, Phys. Chem. Earth, 2009, 34, 857–865 CrossRef.
- M. T. Hamed Mosavian, Z. Es'Haghi, N. Razavi and S. Banihashemi, J. Pharm. Anal., 2012, 2, 361–365 CrossRef CAS PubMed.
- M. Safari, Y. Yamini, A. Mani-Varnosfaderani and H. Asiabi, J. Iran. Chem. Soc., 2017, 14, 623–634 CrossRef CAS.
- Y. Jia, Y. Zhao, M. Zhao, Z. Wang, X. Chen and M. Wang, J. Chromatogr. A, 2018, 1551, 21–28 CrossRef CAS PubMed.
- L. Pang, W. Zhang, W. Zhang, P. Chen, J. Yu, G. T. Zhu and S. Zhu, RSC Adv., 2017, 7, 53720–53727 RSC.
- D. R. Joshi and N. Adhikari, J. Pharm. Res. Int., 2019, 28, 1–18 CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- N. Manousi, B. Gomez-Gomez, Y. Madrid, E. A. Deliyanni and G. A. Zachariadis, Microchem. J., 2020, 152, 104428 CrossRef CAS.
- M. N. Kayali-Sayadi, Analyst, 1998, 123, 2145–2148 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00408a |
| This journal is © The Royal Society of Chemistry 2022 |