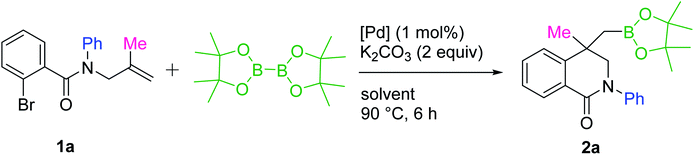Open Access Article
Open Access ArticleSynthesis of dihydroisoquinolinone-4-methylboronic esters via domino Heck/borylation using a structurally characterized palladacycle as a catalyst †
Jhansi Rani Morla and
Dastagiri Reddy Nareddula *
*
Department of Chemistry, Pondicherry University, Pondicherry 605014, India. E-mail: ndreddy.che@pondiuni.edu.in
First published on 28th February 2022
Abstract
Synthesis of dihydroisoquinolinone-4-methylboronic esters from N-allylcarboxamides and B2(Pin)2 via domino Heck/borylation approach is reported. A quinoxaline-based NHC-palladacycle [Pd(C∧C:)PPh3Cl], which has been structurally characterized, is used as a catalyst. The scope of the substrate with a wide range of substituents is explored. In addition to the synthesis of title compounds, a few examples of methylboronic esters of indoline and benzofuran motifs have also been prepared using the same protocol.
Introduction
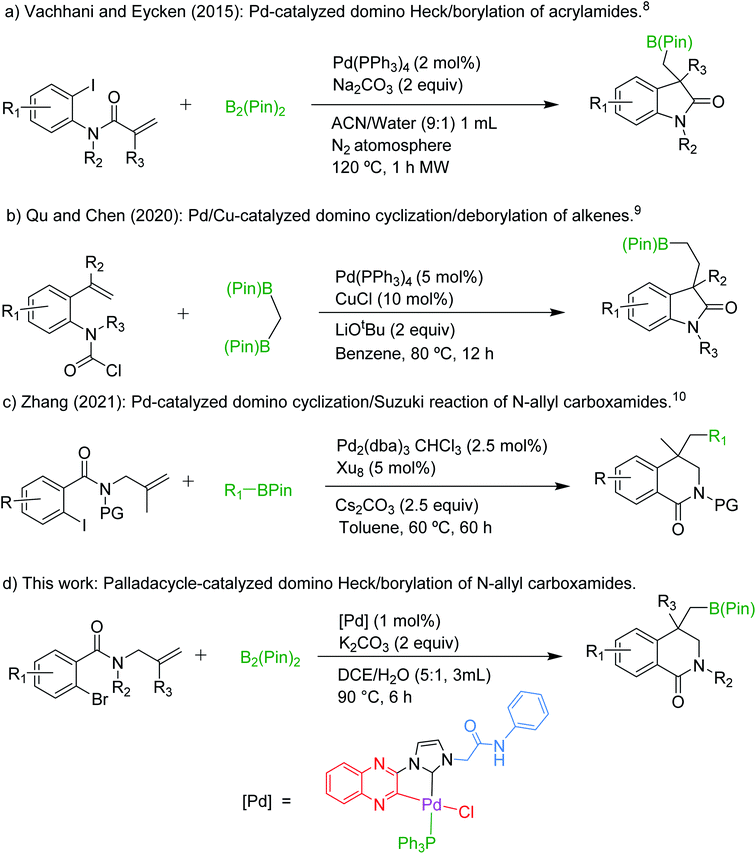
Compounds containing dihydroisoquinolinone core have been found to be highly biologically active.1 They have widespread applications as drugs in various therapies including cancer treatment.2 Owing to the importance of the dihydroisoquinolinone motif there has been a plethora of synthetic methodologies reported in the literature, which have been reviewed in detail recently.3 Most of these methods do not directly lead to the synthesis of targeted drug, necessitating the building of the remaining parts of the drug. Hence, the synthesis of easily functionalizable dihydroisoquinolinone core is very important.4 Recent advances in Pd-catalyzed domino reactions demonstrate an intramolecular carbopalladation followed by the trapping of the alkyl-Pd species by various nucleophiles.5 Boronic ester moiety is one of the best tools for constructing C–C, C–N, C–O and C–halogen bonds.6 Incorporation of such an indispensable moiety into the isoquinolinone core can make it a valuable building block in the synthesis of drugs and natural products.7 Recently, Vachhani and Eycken have demonstrated the synthesis of indolinone-3-methylboronic esters via domino Heck/borylation reactions of acryl amide and bis(pinacolato)diborane B2(Pin)2 (Scheme 1a).8 In spite of potential hurdles such as direct Miyaura-type borylation, hydroarylation of the alkene and the cross-coupling products of the targeted boronic ester as detailed in the article, the authors were quite successful in obtaining the desired indolinone-3-boronic esters.Motivated by this work we designed a strategy for the synthesis of dihydroisoquinolinone-4-methylboronic esters. Most recently, Qu and Chen have reported the synthesis of indolinone-3-ethylboronic esters from alkene substituted carbamoyl chloride and 1,1-[bis(pinacolato)boryl]methane and successfully transformed the boronic ester moiety into various functional groups without affecting the indolinone core (Scheme 1b).9 While preparing the manuscript we have also come across a report on the synthesis of dihydroisoquinolinones using a similar strategy that we used in this work (Scheme 1c).10 However, the synthesis of dihydroisoquinolinone-4-methylboronic esters, which can be further functionalized easily on sp3 carbon, is more challenging and important. Herein we report their synthesis using a structurally characterized palladacycle as a catalyst in a domino Heck/borylation approach.
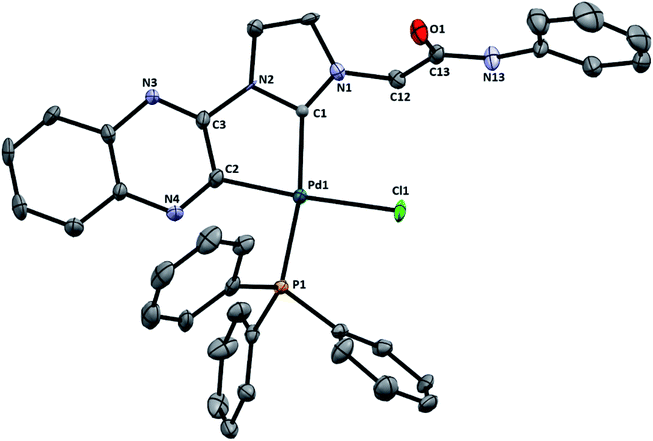
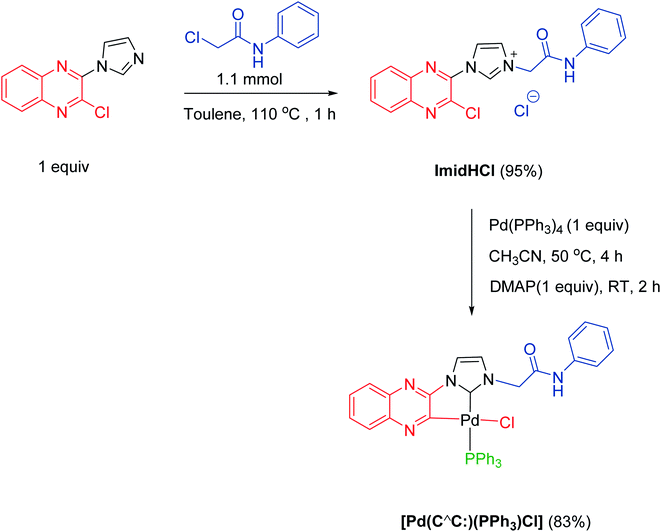
Result and discussion
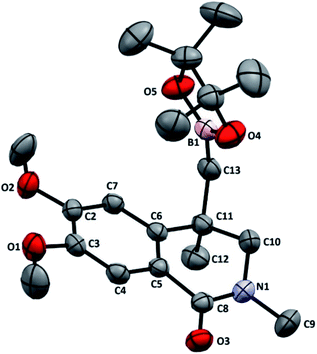
We have recently reported the synthesis of zwitterionic palladium complexes and their catalytic efficiency in Suzuki–Miyaura cross coupling (SMC) reactions.11 In order to explore the mechanistic details of those reactions two quinoxaline based NHC-palladacycles were synthesized. It was found that these palladacycles were less efficient than the precursor imidazolium zwitterionic complexes in catalyzing the SMC reactions.11a However, with a slight modification we were able to successfully utilize them as catalysts for the synthesis of dihydroisoquinolinone-4-methylboronic esters. The palladacycle [Pd(C∧C:)(PPh3)Cl] has been prepared as shown in Scheme 2 and characterized thoroughly using NMR and single crystal X-ray techniques (Fig. 1) supported by elemental analysis and ESI mass spectral data (see ESI†).A model reaction was probed between an N-allylcarboxamide 1a and bis(pinacolato)diboron (B2(Pin)2) using [Pd(C∧C:)(PPh3)Cl] as a catalyst, and K2CO3 as a base in water at 90 °C under nitrogen. After 6 h the desired borylated product 2a was obtained in 16% yield. Optimization studies for a suitable solvent revealed that dichloroethane (DCE) was the best among all the solvents probed (Table S1, ESI†). Hence, further optimization reactions such as employing commercially available Pd reagents as catalysts and screening of various bases, and controlled experiments were conducted in DCE. The results of these reactions, which are listed in Tables 1 and S1 (ESI†), suggest that the best combination is [Pd(C∧C:)(PPh3)Cl] as catalyst and K2CO3 as base in DCE as a solvent at 90 °C under nitrogen atmosphere, which gave 91% yield of 2a (Table 1, entry 3). The yields were further improved when a mixture of DCE and water in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was used instead of neat DCE (94%, entry 4). Among the commercially available Pd complexes only Pd(PPh3)2Cl2 afforded good yield (82%). The others either gave low yields (entries 5 and 9) or were not active at all (entries 6 and 7). We also probed for a possible C–H activation by employing a carboxamide without Br (Br replaced by H in 1a), which resulted in no reaction (entry 10). Controlled experiments (entries 1 and 2) disclosed the importance of the catalyst and the base. Reaction between 1a and B2(Pin)2 was also probed using various bases and found that K2CO3 and Na2CO3 performed equally good and better than the other bases. The detailed results are listed in Table S1, ESI.†
1 ratio was used instead of neat DCE (94%, entry 4). Among the commercially available Pd complexes only Pd(PPh3)2Cl2 afforded good yield (82%). The others either gave low yields (entries 5 and 9) or were not active at all (entries 6 and 7). We also probed for a possible C–H activation by employing a carboxamide without Br (Br replaced by H in 1a), which resulted in no reaction (entry 10). Controlled experiments (entries 1 and 2) disclosed the importance of the catalyst and the base. Reaction between 1a and B2(Pin)2 was also probed using various bases and found that K2CO3 and Na2CO3 performed equally good and better than the other bases. The detailed results are listed in Table S1, ESI.†
| entry | [Pd] | Solvent | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1 (0.5 mmol), B2(Pin)2 (1.0 mmol), [Pd] (1 mol%), K2CO3 (1.0 mmol), and DCE (3 mL) or DCE/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 3 mL) at 90 °C under N2 for 6 h.b Isolated yield. 1; 3 mL) at 90 °C under N2 for 6 h.b Isolated yield. |
|||
| 1 | No catalyst | DCE | 0 |
| 2 | [Pd(C∧C:)(PPh3)Cl] without K2CO3 | DCE | 0 |
| 4 | [Pd(C∧C:)(PPh3)Cl] | DCE/H2O | 94 |
| 5 | Pd(PPh3)4 | DCE/H2O | 30 |
| 6 | Pd2(dba)3 | DCE/H2O | 0 |
| 7 | Pd(OAc)2 | DCE/H2O | 0 |
| 8 | Pd(PPh3)2Cl2 | DCE/H2O | 82 |
| 9 | PdCl2 | DCE/H2O | 43 |
| 10 | [Pd(C∧C:)(PPh3)Cl] Br replaced by H in 1a | DCE/H2O | 0 |
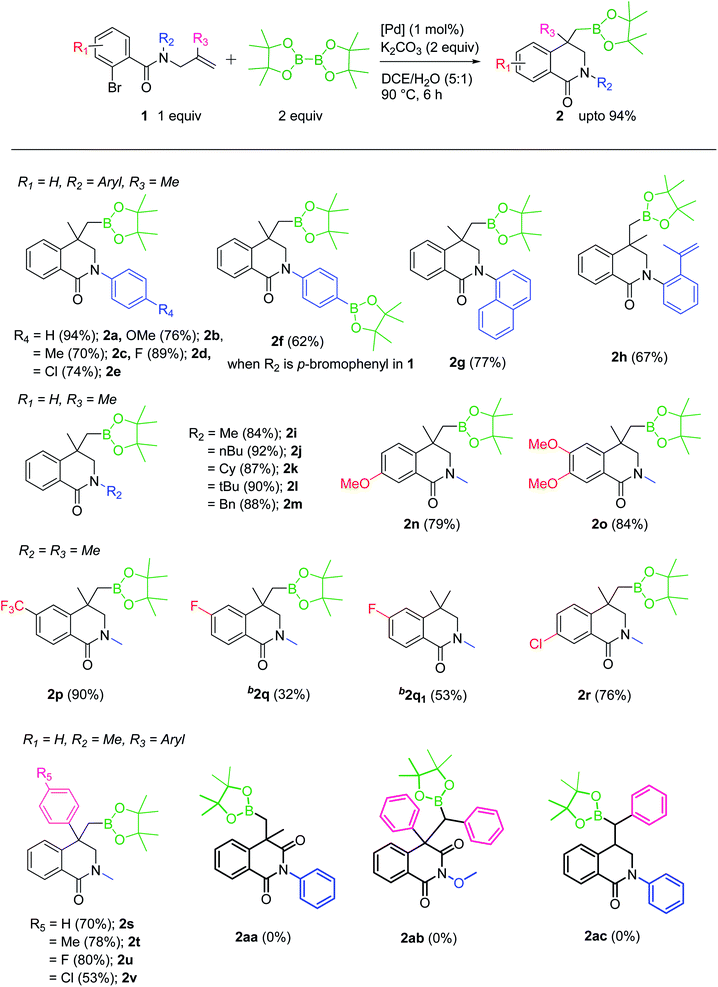
After optimizing the reaction conditions, substrate scope of the reaction was explored by varying R1, R2 and R3. Both aryl and alkyl substituents on the N-allyl carboxamide were well tolerated and the corresponding products (2a–e and 2g–m) were obtained in good to excellent yields. However, in case of N-p-bromophenyl substitution, a diborylated product 2f was obtained in 62% yield. Even when one equivalent of B2(Pin)2 was used the formation of only 2f was observed. It is noteworthy that the additional alkenyl substitution in 1h is intact and the desired dihydroisoquinolinone-4-methylboronic ester 2h was produced in 67% yield. Further studies on the substrate scope by changing the substituents (R1) on the bromobenzoyl moiety of carboxamides (1n–r) were carried out and observed the facile formation of 2n–r (Table 2). The molecular structure of 2o was determined by single crystal X-ray technique and an ORTEP diagram is given in Fig. 2. Though a slight difference in the yields based on electron withdrawing or donating nature of the substituents can be seen, it is not enough to draw any conclusions. Unexpectedly, in case of fluoro substitution (1q) a mixture of the targeted boronic ester (2q) and a product of hydroaylation of alkene (2q1) was formed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (based on NMR of the crude product. The isolated yields were 32% and 53% respectively). Aromatic substitution having either electron donor or acceptor groups on C2 carbon of the allyl group (R3) was also found to be highly compatible and afforded the corresponding products 2s–v in moderate to good yields (53–80%).
2 ratio (based on NMR of the crude product. The isolated yields were 32% and 53% respectively). Aromatic substitution having either electron donor or acceptor groups on C2 carbon of the allyl group (R3) was also found to be highly compatible and afforded the corresponding products 2s–v in moderate to good yields (53–80%).
Our attempts to synthesize isoquinoline-1,3-dionemethylboronic esters 2aa and 2ab under similar reaction conditions were unsuccessful. When an N-allylcarboxamide with phenyl substitution on the terminal alkene carbon was employed, in order to make the boronic ester 2ac, the reaction did not occur. Surprisingly, not even the Heck coupling product was formed though R3 = H. Synthesis of compound 2a was also accomplished in gram-scale, which demonstrates the importance of this protocol in large scale preparation of readily functionalizable dihydroisoquinolinone-4-methylboronic esters.
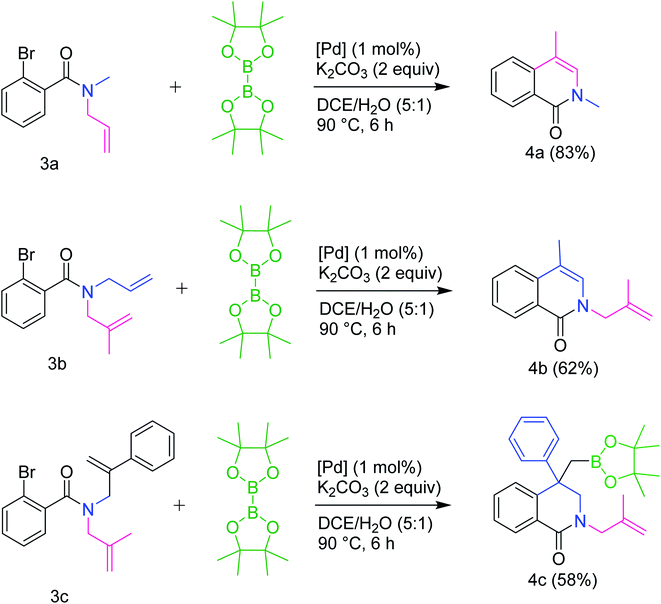
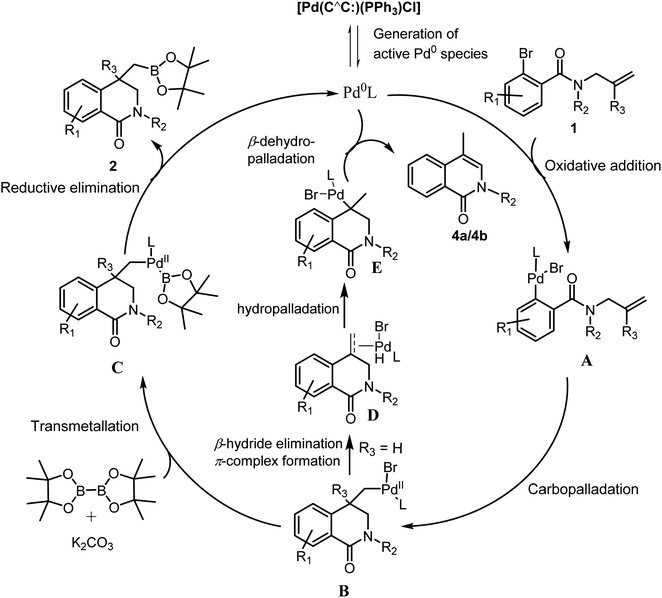
Interestingly, when there was a competition between methyl and phenyl substituted allyl groups on the N (basically when there were two allyl groups, one with phenyl substitution and another with Me substitution, present on the N), only the allyl group with phenyl substitution on C2 carbon was found to undergo carbopalladation producing the boronic ester 4c in 58% yield (Scheme 3). This can be explained in terms of the stability of the Pd-alkene π-complex involved in the transition state, which is more stable when phenyl substitution is present due to delocalization. The trapping of σ-alkylpalladium intermediate with boronic ester moiety did not occur when a simple allyl group is present on the N as in 3a and 3b. Instead, the corresponding intramolecular Heck coupling products 4a and 4b were formed in 83% and 62% yields respectively. This behavior can be attributed to the facile 1,2-Pd migration, which is possible only in case of allyl group (R3 = H), followed by β-dehydropalladation. A detailed plausible mechanistic path for all these transformations is given in Scheme 4. After carbopalladation, the resultant alkyl-Pd species undergoes 1,2-Pd migration via a sequence of β-hydride elimination/π-complex formation/hydropalladation. The isomerized alkyl-Pd finally undergoes β-dehydropalladation to give 4a and 4b. It is noteworthy that allyl group undergoes carbopalladation preferentially over 2-methylallyl group.
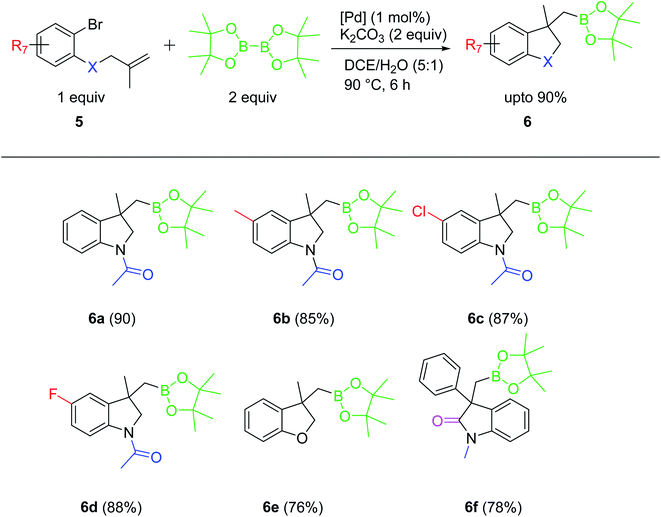
Encouraged by these results, this protocol was also applied to access methylboronic esters of indoline and benzofuran motifs, which are well known for their biological activity and their applications in pharmaceutical industry. The indoline-3-methylboronic esters 6a–d were prepared from corresponding N-(2-bromoaryl)-N-(2-methylallyl) acetamide and the 2-oxoindoline-3-methylboronic ester 6f was obtained by employing N-(2-bromophenyl)-N-(methyl)-2-phenylacrylamide (Scheme 5). All these compounds were produced in very good yields. Under similar conditions, 2-methylallyl-2-bromophenylether afforded benzofuran-3-methylboronic ester 6e in good yield. These results clearly emphasize the versatility and efficiency of the palladacycle [Pd(C∧C:)(PPh3)Cl] as a catalyst in domino Heck/borylation approach for the synthesis of heterocyclic methylboronic esters.
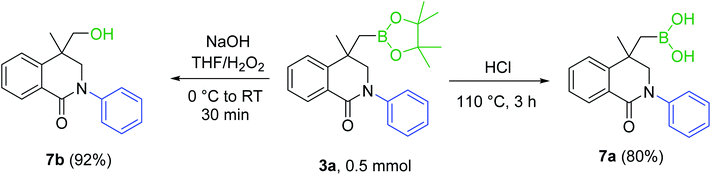
Transformation of methylboronic ester moiety into various functional groups has been reported in the literature.8,9 As shown in Scheme 6, 3a was converted to dihydroisoquinolinone boronic acid 7a and hydroxymethyl-substituted dihydroisoquinolinone 7b in good yields.
Conclusion
We have synthesized and structurally characterized a palladacycle [Pd(C∧C:)(PPh3)Cl], which has been found to be an efficient catalyst in domino Heck/borylation reactions leading to the synthesis of dihydroisoquinolinone-4-methylboronic esters. The reaction protocol, which works with aryl bromides, involves mild reaction conditions and requires less catalyst loading (1 mol%). Tolerance to a wide range of N-allylcarboxamides and employability in the synthesis of indoline and benzofuran derivatives demonstrate the versatility of the method.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Department of Science and Technology (DST), New Delhi for financial support and Alexander von Humboldt Stiftung, Germany for donation of a glove box. We thank the Central Instrumentation Facility, Pondicherry University for the NMR spectra and DST-FIST for a single crystal X-ray diffraction facility and the ESI-MS facility. MJR also thanks the CSIR, India, for a senior research fellowship.References
- (a) C.-S. Marques, D. Peixoto and A.-J. Burke, Transition-metal-catalyzed intramolecular cyclization of amido(hetero)arylboronic acid aldehydes to isoquinolinones and derivatives, RSC Adv., 2015, 5, 20108–20114 RSC; (b) F. Ji, W. Yi, M. Sun, M. Lv and C. Cai, Synthesis of novel isoquinolinone and 1,2-dihydroisoquinoline scaffolds via Ugi reaction and ring opening reaction of furans, Mol. Diversity, 2013, 17, 295–305 CrossRef CAS PubMed; (c) D.-S. Deshmukh, N. Gangwar and B. M. Bhanage, Rapid and Atom Economic Synthesis of Isoquinolines and Isoquinolinones by C–H/N–N Activation Using a Homogeneous Recyclable Ruthenium Catalyst in PEG Media, Eur. J. Org. Chem., 2019, 2919–2927 CrossRef CAS; (d) G. Saini, P. Kumar, G. S. Kumar, A. R. K. Mangadan and M. Kapur, Palladium-Catalyzed α-Arylation of Silylenol Ethers in the Synthesis of Isoquinolines and Phenanthridines, Org. Lett., 2018, 20, 441–444 CrossRef CAS PubMed; (e) X. Kou and K. G. M. Kou, α-Arylation of Silyl Enol Ethers via Rhodium(III)-Catalyzed C–H Functionalization, ACS Catal., 2020, 10, 3103–3109 CrossRef CAS.
- (a) L. Xin, W. Wan, Y. Yu, Q. Wan, L. Ma and X. Huang, Construction of Protoberberine Alkaloid Core through Palladium Carbene Bridging C–H Bond Functionalization and Pyridine Dearomatization, ACS Catal., 2021, 11, 1570–1577 CrossRef CAS; (b) A. Mondal, P. Kundu, M. Jash and C. Chowdhury, Palladium-catalysed stereoselective synthesis of 4-(diarylmethylidene)-3,4-dihydroisoquinolin-1(2H)-ones: expedient access to 4-substituted isoquinolin-1(2H)-ones and isoquinolines, Org. Biomol. Chem., 2018, 16, 963–980 RSC.
- M. R. Kulkarni and N. D. Gaikwad, Recent Advances in Synthesis of 3,4 Dihydroisoquinolin-1(2H)-one, ChemistrySelect, 2020, 5, 8157–8184 CrossRef CAS.
- (a) J. Mo, A. M. Messinis, J. C. A. Oliveira, S. Demeshko, F. Meyer and L. Ackermann, Iron-Catalyzed Triazole-Enabled C−H Activation with Bicyclopropylidenes, ACS Catal., 2021, 11, 1053–1064 CrossRef CAS; (b) C. Mayer, C. L. Ladd and A. B. Charette, Utilization of BozPhos as an Effective Ligand in Enantioselective C−H Functionalization of Cyclopropanes: Synthesis of Dihydroisoquinolones and Dihydroquinolones, Org. Lett., 2019, 21, 2639–2644 CrossRef CAS PubMed; (c) A. Dieudonné-Vatran, M. Azoulay and J.-C. Florent, A new access to 3-substituted-1(2H)-isoquinolone by tandem palladium-catalyzed intramolecular aminocarbonylation annulation, Org. Biomol. Chem., 2012, 10, 2683–2691 RSC; (d) K. Yan, J. Jin, Y. Kong, B. Li and B. Wang, Palladium-Catalyzed Inert C– H Bond Activation and Cyclocarbonylation of Isoquinolones with Carbon Dioxide Leading to Isoindolo[2,1-b]isoquinoline-5,7-diones., Adv. Synth. Catal., 2019, 361, 3080–3085 CrossRef CAS; (e) S. Guo, L. Sun, Y. Liu, N. Ma, X. Zhang and X. Fan, Rh(III)-Catalyzed Oxidative Spirocyclization of Isoquinolones with α-Diazo-1,3-indandiones, Org. Lett., 2019, 21, 4082–4086 CrossRef CAS PubMed; (f) X. Zhou, Z. Zhang, H. Zhao, P. Lu and Y. Wang, Rh-Catalyzed Annulations of N-Methoxybenzamides and Ketenimines: Sterically and Electronically Controlled Synthesis of Isoquinolinones and Isoindolinones, J. Org. Chem., 2017, 82, 3787–3797 CrossRef CAS PubMed.
- (a) Y. Ping, Y. Li, J. Zhu and W. Kong, Construction of Quaternary Stereocenters by Palladium-Catalyzed Carbopalladation-Initiated Cascade Reactions, Angew. Chem., Int. Ed., 2019, 58, 1562–1573 CrossRef CAS PubMed; (b) M. P-Gómez, S. H-Ponte, D. Bautista and J.-A. G-López, Synthesis of spiro-oxoindoles through Pd-catalyzed remote C–H alkylation using α-diazocarbonyl compounds, Chem. Commun., 2017, 53, 2842–2845 RSC; (c) A. D. Marchese, E. M. Larin, B. Mirabi and M. Lautens, Metal-Catalyzed Approaches toward the Oxindole Core, Acc. Chem. Res., 2020, 53, 1605–1619 CrossRef CAS PubMed; (d) H. A. Döndas, M. G. Retamosa and J. M. Sansano, Recent Development in Palladium-Catalyzed Domino Reactions: Access to Materials and Biologically Important Carbo- and Heterocycles, Organometallics, 2019, 38, 1828–1867 CrossRef; (e) H. Lu, X. Yang, L. Zhou, W. Li, G. Deng, Y. Yang and Y. Liang, Palladium-catalyzed domino Heck-disilylation and - borylation of alkene-tethered 2-(2-halophenyl)-1H-indoles: access to diverse disilylated and b orylated indolo[2,1-a]isoquinolines, Org. Chem. Front., 2020, 7, 2016–2021 RSC; (f) K. Ishu, D. Kumar, N. K. Maurya, S. Yadav, D. Chaudharya and M. R. Kuram, Dicarbofunctionalization of unactivated alkenes by palladium-catalyzed domino Heck/intermolecular direct hetero arylation with heteroarenes, Org. Biomol. Chem., 2021, 19, 2243–2253 RSC; (g) J. Bajohr, A. G. Diallo, A. Whyte, S. Gaillard, J.-L. Renaud and M. Lautens, Palladium-Catalyzed Domino Heck/Sulfination: Synthesis of Sulfonylated Hetero- and Carbocyclic Scaffolds Using DABCO−Bis(sulfur dioxide), Org. Lett., 2021, 23, 2797–2801 CrossRef PubMed; (h) R. Karu and S. Gedu, Microwave assisted domino heck cyclization and alkynylation: synthesis of alkyne substituted dihydrobenzofurans, Green Chem., 2018, 20, 369–374 RSC; (i) J. Ma, T.-X. Liu, P. Zhang, C. Zhang and G. Zhang, Palladium-catalyzed domino spirocyclization of [60]fullerene: synthesis of diverse [60]fullerene-fused spiro[4,5]/[5,5] derivatives, Chem. Commun., 2021, 57, 49–52 RSC; (j) S. Guo, J. Chen, M. Yi, L. Dong, A. Lin and H. Yao, An approach to unsymmetrical 3,3’- diindolylmethanes through Pd-catalyzed cascade Heck cyclization of allenamides and o-ethynylanilines, Org. Chem. Front., 2021, 8, 1783–1788 RSC; (k) M. Shrestha, X. Wu, W. Huang, J. Qu and Y. Chen, Recent advances in transition metal-catalyzed reactions of carbamoyl chlorides, Org. Chem. Front., 2021, 8, 4024–4045 RSC.
- (a) P. Zheng, X. Han, J. Hu, X. Zhao and T. Xu, Enantioselective Copper-Catalyzed Desymmetrization of 1,3-Diketones Involving Borylation of Styrenes, Org. Lett., 2019, 21, 6040–6044 CrossRef CAS PubMed; (b) A. Whyte, K. I. Burton, J. Zhang and M. Lautens, Enantioselective Intramolecular Copper-Catalyzed Borylacylation, Angew. Chem., Int. Ed., 2018, 57, 13927–13930 CrossRef CAS; (c) A. Whyte, A. Torelli, B. Mirabi and M. Lautens, Enantioselective Copper-Catalyzed Borylative Cyclization with Cyclic Imides, Org. Lett., 2019, 21, 8373–8377 CrossRef CAS PubMed; (d) J. Chen, J.-H. Li, Y.-P. Zhu and Q.-A. Wang, Copper-catalyzed enantioselective arylboronation of activated alkenes leading to chiral 3,3’-disubstituted oxindoles, Org. Chem. Front., 2021, 8, 2532–2536 RSC; (e) R. Sakae, N. Matsuda, K. Hirano, T. Satoh and M. Miura, Highly Stereoselective Synthesis of (Borylmethyl)cyclopropylamines by Copper-Catalyzed Aminoboration of Methylenecyclopropanes, Org. Lett., 2014, 16, 1228–1231 CrossRef CAS; (f) H. Iwamoto, Y. Ozawa, K. Kubota and H. Ito, Copper(I)-Catalyzed Regio- and Stereoselective Intramolecular Alkylboration of Propargyl Ethers and Amines, J. Org. Chem., 2017, 82, 10563–10573 CrossRef CAS PubMed; (g) Y. Li, K. Wang, Y. Ping, Y. Wang and W. Kong, Nickel-Catalyzed Domino Heck Cyclization/Suzuki Coupling for the Synthesis of 3,3-Disubstituted Oxindoles, Org. Lett., 2018, 20, 921–924 CrossRef CAS PubMed; (h) K. Kubota, E. Yamamoto and H. Ito, Copper(I)-Catalyzed Borylative exo-Cyclization of Alkenyl Halides Containing Unactivated Double Bond, J. Am. Chem. Soc., 2013, 135, 2635–2640 CrossRef CAS PubMed; (i) K. Wang, Z. Ding, Z. Zhou and W. Kong, Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling, J. Am. Chem. Soc., 2018, 140, 12364–12368 CrossRef CAS PubMed.
- (a) L. Zhou, S. Li, B. Xu, D. Ji, L. Wu, Y. Liu, Z. –M. Zhang and J. Zhang, Enantioselective Difunctionalization of Alkenes by a Palladium-Catalyzed Heck/Sonogashira Sequence, Angew. Chem., Int. Ed., 2020, 59, 2769–2775 CrossRef CAS PubMed; (b) C. Cheng, B. Wan, B. Zhou, Y. Gu and Y. Zhang, Enantioselective synthesis of quaternary 3,4-dihydroisoquinolinones: Via Heck carbonylation reactions: Development and application to the synthesis of Minalrestat analogues, Chem. Sci., 2019, 10, 9853–9858 RSC; (c) X. Luo, L. Zhou, H. Lu, G. Deng, Y. Liang, C. Yang and Y. Yang, Palladium-Catalyzed Domino Heck/C−H Activation/Decarboxylation: A Rapid Entry to Fused Isoquinolinediones and Isoquinolinones, Org. Lett., 2019, 21, 9960–9964 CrossRef CAS PubMed; (d) N. Saha, H. Wang, S. Zhang, Y. Du, D. Zhu, Y. Hu, P. Huang and S. Wen, Domino Carbopalladation/C−H Activation as a Quick Access to Polycyclic Frameworks, Org. Lett., 2018, 20, 712–715 CrossRef CAS PubMed; (e) L. Zhou, S. Qiao, F. Zhou, X. Xuchen, G. Deng, Y. Yang and Y. Liang, α-Oxocarboxylic Acids as Three-Carbon Insertion Units for Palladium-Catalyzed Decarboxylative Cascade Synthesis of Diverse Fused Heteropolycycles, Org. Lett., 2021, 23, 2878–2883 CrossRef CAS PubMed; (f) J. Hédouin, V. Carpentier, R. M. Q. Renard, C. Schneider, I. Gillaizeau and C. Hoarau, Regioselective Pd-Catalyzed Carbopalladation/Decarboxylative Allylic Alkynylation of ortho-Iodoallenamides with Alkynyl Carboxylic Acids, J. Org. Chem., 2019, 84, 10535–10545 CrossRef; (g) H. Yoon, D. A. Petrone and M. Lautens, Diastereoselective Palladium-Catalyzed Arylcyanation/Heteroarylcyanation of Enantioenriched N-Allylcarboxamides, Org. Lett., 2014, 16, 6420–6423 CrossRef CAS PubMed; (h) J. Xu, Z. Yang, J. Hua, Y. Lin, M. Bian, Y. Li, C. Liu, W. He, Z. Fang and K. Guo, The continuous-flow electrosynthesis of 4-(sulfonylmethyl)isoquinoline-1,3(2H,4H)-diones from N-alkyl-N-methacryloyl benzamides under metal-free and oxidant-free conditions, Org. Chem. Front., 2020, 7, 3223–3228 RSC.
- D. D. Vachhani, H. H. Butani, N. Sharma, U. C. Bhoya, A. K. Shah and E. V. V. Eycken, Domino Heck/borylation sequence towards indolinone-3-methyl boronic esters: trapping of the σ -alkylpalladium intermediate with boron, Chem. Commun., 2015, 51, 14862–14865 RSC.
- C. Zhang, X. Wu, C. Wang, C. Zhang, J. Qu and Y. Chen, Pd/Cu-Catalyzed Domino Cyclization/Deborylation of Alkene-Tethered Carbamoyl Chloride and 1,1-Diborylmethane, Org. Lett., 2020, 22, 6376–6381 CrossRef CAS PubMed.
- Q. Chen, S. Li, X. Xie, H. Guo, J. Yang and J. Zhang, Pd-Catalyzed Enantioselective Dicarbofunctionalization of Alkene to Access Disubstituted Dihydroisoquinolinone, Org. Lett., 2021, 23, 4099–4103 CrossRef CAS PubMed.
- (a) V. Ramakrishna and N. D. Reddy, Synthesis of zwitterionic palladium complexes and their application as catalysts in cross-coupling reactions of aryl, heteroaryl and benzyl bromides with organoboron reagents in neat water, Dalton Trans., 2017, 46, 8598–8610 RSC; (b) V. Ramakrishna, M. J. Rani and N. D. Reddy, A Zwitterionic Palladium(II) Complex as a Precatalyst for Neat-Water-Mediated Cross-Coupling Reactions of Heteroaryl, Benzyl, and Aryl Acid Chlorides with Organoboron Reagents, Eur. J. Org. Chem., 2017, 48, 7238–7255 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. Crystal data for [Pd(C∧C:)(PPh3)Cl] and 2o CCDC 2087488 and 2087489, 1H and 13C NMR spectra of all new compounds. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2ra00389a |
| This journal is © The Royal Society of Chemistry 2022 |