 Open Access Article
Open Access ArticleNon-destructive measurement technique for water content in organic solvents based on a thermal approach
Decho Surangsrirat *a,
Vikram Sridharb,
Onsiri Srikunc,
Mananya Puanglamjeaka,
Prab Birdid,
Songphon Dumnina,
Chusak Thanawattanoa and
Kam S. Chanabd
*a,
Vikram Sridharb,
Onsiri Srikunc,
Mananya Puanglamjeaka,
Prab Birdid,
Songphon Dumnina,
Chusak Thanawattanoa and
Kam S. Chanabd
aAssistive Technology and Medical Devices Research Center, National Science and Technology Development Agency, Pathum Thani, Thailand. E-mail: decho.sur@nstda.or.th
bDepartment of Engineering Science, University of Oxford, Oxford, England
cPharmaceutical Ingredient Research Group, The Government Pharmaceutical Organization, Bangkok, Thailand
dProxisense Limited, Oxford, England
First published on 21st February 2022
Abstract
The water content of organic solvents is one of the crucial properties that affect the quality of the products and the efficiency of the manufacturing processes. The established water determination methods such as Karl Fischer titration and gas chromatography require skilled operators, specific reagents, and prolonged measurement time. Thus, they are not suitable for both on-line and in-line applications. In this study, we aim to develop a real-time and low-cost device with reliable accuracy. The proposed device based on a newly developed thermal approach could non-destructively detect the water content in multiple organic solvents at low concentrations with high accuracy and without the use of any specific reagent. Experiments were performed for the determination of water content in organic solvents such as methanol, ethanol, and isopropanol. The results show that the proposed device is feasible for the water content determination in methanol, ethanol, and isopropanol at 0–1% w/w. A Bland–Altman plot to illustrate the differences in measurements between the proposed device and coulometric Karl Fischer titration shows that most of the measurements lie within the limits of agreement where 95% of the differences between the two methods are expected to fall in the range of −0.13% and 0.09%.
1. Introduction
Organic solvents are widely used in the manufacturing processes of various industries. For example, methanol, ethanol, and isopropanol are extensively used in cosmetics, natural products, food and beverage, fuel, dyes, coatings, and pharmaceutical industries.1–4 The water content of the organic solvents is one of the crucial properties that affect the quality of the products and the efficiency of the manufacturing processes. Methanol with an excess amount of water can lead to undesired texture and nonuniformity of the production of paints.5 In the biofuel industry, residual water in ethanol significantly affects the production performance and formation of impurities.6 The water content of isopropanol is crucial to cosmetics quality and stability as water can promote rancidification, the process of oxidation or hydrolysis of fats and oils, resulting in unpleasant tastes, colors, and odors of the products.7 For the pharmaceutical industry, the water content of the product and ingredient is one of the pivotal factors in the production process.8,9 An excessive amount of water in the raw materials can cause the growth of microorganisms in the products which negatively affect the safety of consumers.10 Hence, the water content of the raw materials and the solvents must be controlled precisely at less than 0.5–1%.11,12The Karl Fischer titration (KF) is one of the standard methods for the determination of water content in organic solvents. The method has high sensitivity and accuracy. It is considered as a gold standard for water content measurements according to multiple publications.13–16 ASTM International (ASTM) also published a standard test method for water in organic liquids by coulometric KF.17 However, it is a destructive technique, requires a specific reagent, and takes considerable time for one measurement. Loss on drying (LOD) is also one of the popular methods to determine the moisture content of a sample. It is a simple and easy to operate technique based on the change of weight during the heating process. The drawbacks are the low selectivity due to the evaporation of all the volatiles and can only be used with solid samples.18 Various advanced analytical techniques which required skilled operators such as nuclear magnetic resonance (NMR), near-infrared spectroscopy (NIRS), or gas chromatography-mass spectrometry (GC-MS) are also used for the determination of water content in the research.19–21
Previous works on low-cost, portable, and more convenient methods for the determination of water content in solvents are mostly based on the colorimetric technique. Shahvar et al. developed a method for detecting water content in ethanol based on the color-changing of cobalt(II) chloride.22 The method could detect the water contamination in ethanol in the range of 0.05–2.00% v/v with a recovery of more than 88%. Wang et al. proposed a method for the detection in six organic solvents based on red-emitting carbon dots (RCDs).23 They successfully demonstrated that the method could broadly detect the water content at 10–90% for various organic solvents. Kong et al. used fluorescent Ag nanoclusters (Ag NCs) for detection of the water content in ethanol and dimethyl sulfoxide with good linearity from 20–55%.24
A new technique was developed to detect water content in fuel and oil samples at the University of Oxford. The pulsing thin film gauges are used to measure the thermal product or thermal effusivity of the material. Thermal effusivity is directly proportional to the density, heat capacity, and thermal conductivity of the substance which determines the amount of heat absorbed by the substance. Since each material has a different thermal product, the sensor was able to detect low concentrations of water in fuel and oil samples.25,26 However, there are a few shortcomings such as the laborious manufacturing process, getting the right resistance was difficult due to manual painting, suffered from errors due to thermal expansion of the thin films, noise in electronics due to simultaneous heating and measurement, and poor reliability due to thickness of the platinum films. Therefore, in this study, we learned from the previous technique and explore the possibility of using a newly developed thermal approach sensor for the determination of water content in organic solvents such as methanol, ethanol, and isopropanol. The proposed device could non-destructively detect the water content in such organic solvents at low concentrations with high accuracy and without the use of any specific reagent.
2. Material and methods
2.1 Thermocouple design and development
The proposed technique uses low-cost materials instead of the thin film gauges for the sensing materials. Fig. 1 shows the new sensor and its construction. The sensor consists of K-type thermocouple wires of 1.6 mm diameter electrically connected to a 5 ohms resistance thermistor. The thermocouple wires were flattened and pressed against the sides of the thermistor. A thermally conductive paste called Loctite 9497 was applied to ensure proper thermal contact.The electronic box for sensor control and measurement is shown in Fig. 2a. Fig. 2b shows a sample of the measurement pulse and cooling rate. A high-power pulse with a 5 ms duration was sent through the thermocouple wires. Then the thermistor started to heat up and add energy to the surrounding medium. Once the pulse stopped, the thermistor stopped heating and the electronics started measuring the temperature of the thermistor with those thermocouple wires as it cooled down in the medium. Depending on the thermal product of the medium, the rate of cooling will be different. Thus, the temperature reached after a certain duration, such as 200 ms, will be different. This minuscule difference can be used to detect very low concentrations of impurities in a medium. This proposed sensor and electronics are quite robust, cheaper to manufacture, and have less noise in comparison to the previous technique due to the separation of heating and measurement steps.
 | ||
| Fig. 2 Electronic box for sensor control and measurement (a). Measurement pulse from the proposed device (b). | ||
2.2 Experimental setup
The organic solvents that we used in this study are methanol, ethanol, and isopropanol. Methanol, ethanol, and isopropanol were purchased from Honeywell, Merck, and Colosol, respectively. Deionized water was prepared in-house using Pacific TII 40 (UV). For each experiment, 40 mL of the sample with the water content at 0%, 0.25%, 0.5%, 0.75%, and 1% w/w was used for the measurement. Fig. 3 illustrates the setup and sample testing process. The measurement was performed by fully submerging the sensing element of the sensor into the sample for ten minutes. | ||
| Fig. 3 The testing setup for the proposed device. The measurement was performed by fully submerging the sensing element into the sample. | ||
2.3 Accuracy evaluation
Karl Fischer titration technique was used as a gold standard for the comparison and evaluation of the proposed device. The water content determination was done according to the ASTM E1064 standard methods.17 Linearity evaluation was done based on the coefficient of determination. Each sample was measured five times to ensure the repeatability of the method. Limit of detection (LOD) and limit of quantification (LOQ) were obtained from calculating the standard deviation divided by the slope of the calibration curve at blank concentration then multiplied by three and ten, respectively.3. Results and discussion
3.1 Analytical performance
To measure the analytical performance of the proposed device, firstly, we calculated the linearity between the water content in the solvent and the delta temperature measured from the proposed device. Each sample was measured five times. Fig. 4 depicts the linearity of methanol, ethanol, and isopropanol. A 5-point calibration curve (0%, 0.25%, 0.5%, 0.75%, and 1% w/w) was drawn. The proposed device produced determination coefficients of 0.9875, 0.9651, and 0.9858 for the linear dynamic range of 0% to 1% w/w for methanol, ethanol, and isopropanol, respectively. Limit of detection (LOD) and limit of quantification (LOQ) were obtained from calculating the standard deviation divided by the slope of the calibration curve at blank concentration then multiplied by three and ten, respectively. LODs for methanol, ethanol, and isopropanol were 0.12%, 0.35%, and 0.23%, respectively. LOQs for methanol, ethanol, and isopropanol were 0.35%, 1.06%, and 0.70%, respectively. The device performances were respectable for the water content determination in methanol and isopropanol. However, the performance in ethanol was not as expected. Table 1 shows the data from the measurement of the water content in methanol, ethanol, and isopropanol using the proposed device. The delta temperatures measured by the device decreased as the water content in the solvent increased.| Solution | % Amount of water (w/w) | ||||
|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 0.75 | 1 | |
| Water in methanol | 32.08 ± 0.11 | 31.38 ± 0.25 | 30.80 ± 0.12 | 29.73 ± 0.23 | 29.27 ± 0.04 |
| Water in ethanol | 31.41 ± 0.27 | 30.48 ± 0.16 | 30.00 ± 0.08 | 29.40 ± 0.19 | 29.11 ± 0.10 |
| Water in isopropanol | 32.86 ± 0.13 | 32.57 ± 0.04 | 32.21 ± 0.05 | 31.65 ± 0.09 | 31.19 ± 0.04 |
3.2 Comparison of the proposed device with the ASTM standard method
The accuracy assessment of the proposed device based on the thermal technique was compared with the coulometric Karl Fischer titration, the ASTM E1604 standard method. For statistical comparison, a Bland–Altman plot is shown in Fig. 5 to illustrate the differences in measurements between the two methods. Most of the measurements lie within the limits of agreement. On average, the proposed device measured the amount of water at 0.02% less than the coulometric Karl Fischer titration and 95% of the differences between the two methods are expected to fall in the range of −0.13% and 0.09%.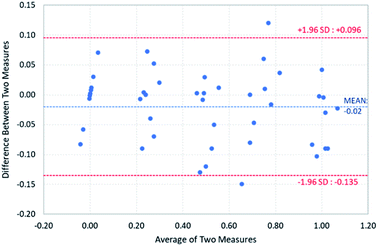 | ||
| Fig. 5 Bland–Altman plot to illustrate the differences in measurements between the proposed device and the coulometric Karl Fischer titration. | ||
Table 2 shows the comparison of the water contents measured from Karl Fischer titration and the proposed device for methanol, ethanol, and isopropanol. The average errors were calculated from the differences between the amounts of water added to the samples and the amounts of water measured by Karl Fischer or the proposed device. Plus sign indicated that the amount of water measured from the equipment was higher than the expected value while the minus sign indicated that the amount of water measured from the equipment was lower than the expected value. For consistency of the measurement, the relative standard deviations of the proposed device were less than one percent and less than KF for most samples. Table 3 shows the spiking recovery results for the proposed device. Each sample was spiked with the known concentration of the water. All the recoveries, apart from the outlier of 0.25% water in ethanol, were in the range of 86% to 109% with relative standard deviations of less than one percent.
| Sample | % Amount of water (w/w) | Average error | ||
|---|---|---|---|---|
| Karl Fischer (% RSD) | Thermocouple (% RSD) | Karl Fischer | Thermocouple | |
| 0.25% water in methanol | 0.28 (1.5) | 0.25 (0.8) | +0.03 | 0.00 |
| 0.5% water in methanol | 0.56 (0.0) | 0.45 (0.4) | +0.06 | −0.05 |
| 0.75% water in methanol | 0.71 (1.4) | 0.82 (0.8) | −0.04 | +0.07 |
| 1% water in methanol | 1.05 (1.7) | 0.98 (0.1) | +0.05 | −0.02 |
| 0.25% water in ethanol | 0.23 (3.7) | 0.33 (0.5) | −0.02 | +0.08 |
| 0.5% water in ethanol | 0.51 (0.0) | 0.54 (0.3) | +0.01 | +0.04 |
| 0.75% water in ethanol | 0.76 (0.5) | 0.80 (0.6) | +0.01 | +0.05 |
| 1% water in ethanol | 1.01 (3.4) | 0.93 (0.3) | +0.01 | −0.07 |
| 0.25% water in isopropanol | 0.26 (4.8) | 0.22 (0.1) | +0.01 | −0.03 |
| 0.5% water in isopropanol | 0.50 (0.8) | 0.43 (0.2) | 0.00 | −0.07 |
| 0.75% water in isopropanol | 0.75 (1.0) | 0.76 (0.3) | 0.00 | +0.01 |
| 1% water in isopropanol | 1.02 (2.0) | 1.03 (0.1) | +0.02 | +0.03 |
| Sample | % Amount spiked (w/w) | % Amount of water measured (w/w) | Recovery (%) |
|---|---|---|---|
| 0.25% water added in methanol | 0.25 | 0.25 | 100 |
| 0.5% water added in methanol | 0.5 | 0.45 | 90 |
| 0.75% water added in methanol | 0.75 | 0.82 | 109 |
| 1% water added in methanol | 1 | 0.98 | 98 |
| 0.25% water added in ethanol | 0.25 | 0.33 | 132 |
| 0.5% water added in ethanol | 0.5 | 0.54 | 108 |
| 0.75% water added in ethanol | 0.75 | 0.80 | 107 |
| 1% water added in ethanol | 1 | 0.93 | 93 |
| 0.25% water added in isopropanol | 0.25 | 0.22 | 88 |
| 0.5% water added in isopropanol | 0.5 | 0.43 | 86 |
| 0.75% water added in isopropanol | 0.75 | 0.76 | 101 |
| 1% water added in isopropanol | 1 | 1.03 | 103 |
4. Conclusion
Non-destructive measurement of water content in methanol, ethanol, and isopropanol was studied using the proposed device equipped with the newly developed thermal sensor. The accuracy and the analytical performance were evaluated at the water contents of 0%, 0.25%, 0.5%, 0.75%, and 1% w/w and compared with those of the coulometric Karl Fischer titration, the ASTM E1604 standard method for water content determination. Major conclusions may be drawn as follows:(1) The proposed device can be used to determine residual water in methanol, ethanol, and isopropanol. A linear regression (least-squares fit method) was plotted between water content (x-axis) and delta temperature (y-axis). The coefficients of determination derived from the regression lines are 0.9875, 0.9651, and 0.9858 for methanol, ethanol, and isopropanol, respectively.
(2) The lowest water contents for quantitative measurement (limit of quantitation, LOQ) are 0.35%, 1.06%, and 0.70% and the lowest water contents for qualitative measurement (limit of detection, LOD) are 0.12%, 0.35%, and 0.23% for methanol, ethanol, and isopropanol respectively.
(3) The Bland–Altman plot was adopted to statistically analyze the differences in measurement between the proposed device and the coulometric Karl Fischer titration. The results show that 95% of the differences are in the range of −0.135% and 0.096%, which are within the limits of agreement.
(4) The standard deviations of all measurements from the proposed devices are less than 1%. For parallel comparison, the proposed device offers superior consistency to the coulometric Karl Fischer titration.
The established water determination methods have both advantages and disadvantages as mentioned earlier. The standard methods such as Karl Fischer titration and gas chromatography require skilled operators, specific reagents, and prolonged measurement time. Thus, they are not suitable for both on-line and in-line applications. We then aim to develop a low-cost and highly accurate device to address the weaknesses of the previous methods. The experimental results show that the proposed device is feasible for the water content determination in methanol, ethanol, and isopropanol at 0–1% w/w. Further experiments on other organic solvents in a wider range could be performed to validate the results. Measurement of the content of the other liquid pharmaceutical products could also be explored. It could pave the way for the development of on-line and in-line measurements to serve process analytical work in the future.
Conflicts of interest
Prab Birdi and Kam Chana work for Proxisense Limited. Proxisense licensed the technology for the proposed device from the University of Oxford for commercialization.Acknowledgements
This research was supported by Health Systems Research Institute (HSRI), Thailand. The experiments were performed at Government Pharmaceutical Organization (GPO) and University of Oxford Thermofluids Institute.References
- V. García, E. Pongrácz, P. S. Phillips and R. L. Keiski, J. Cleaner Prod., 2013, 39, 146–153 CrossRef.
- M. Svanberg, J. Ellis, J. Lundgren and I. Landälv, Renewable Sustainable Energy Rev., 2018, 94, 1217–1228 CrossRef CAS.
- M. A. M. Al-Alwani, A. B. Mohamad, A. A. H. Kadhum and N. A. Ludin, Spectrochim. Acta, Part A, 2015, 138, 130–137 CrossRef CAS PubMed.
- Y. Shen, A. Farajtabar, J. Xu, J. Wang, Y. Xia, H. Zhao and R. Xu, J. Chem. Thermodyn., 2019, 131, 410–419 CrossRef CAS.
- H. H. K. Wu-Hsun Cheng, Methanol Production and use, Marcel Dekker Inc., The United State of America, 1994 Search PubMed.
- J. Martinka, P. Rantuch and I. Wachter, Energies, 2019, 12, 3491 CrossRef CAS.
- A. Svang-Ariyaskul, R. Y. M. Huang, P. L. Douglas, R. Pal, X. Feng, P. Chen and L. Liu, J. Membr. Sci., 2006, 280, 815–823 CrossRef CAS.
- Y. Roggo, V. Pauli, M. Jelsch, L. Pellegatti, F. Elbaz, S. Ensslin, P. Kleinebudde and M. Krumme, J. Pharm. Biomed. Anal., 2020, 179, 112971 CrossRef CAS PubMed.
- K. Ohgi, Y. Hayashi, T. Tsuji, T. Ito, K. H. Leong, S. Usuda, S. Kumada, K. Okada and Y. Onuki, Int. J. Pharm., 2021, 604, 120770 CrossRef CAS PubMed.
- D. J. Željka Marjanović-Balaban, V. Antunović and V. Gojković, J. Hyg. Eng. Des, 2013, 6, 137–141 Search PubMed.
- S. Roy, S. Siddique, S. Majumder, M. I. M. Abdul, S. A. Rahman, D. Lateef, S. Dan and A. Bose, Biomed. Res., 2018, 29, 3336–3343 CrossRef CAS.
- M. C. Chuong, C. J. Kelley, Y. Muhammad, T. D. Caputo, J. M. Gomes, D. Oliveira, A. C. Peixoto, B. S. Pereira, W. Rizg, C. Vazquez, T. M. Zacaron, S. Nguyen and D. A. Williams, J. Anal. Sci. Technol., 2018, 9, 7 CrossRef.
- K.-P. Wang, Y. Lei, J.-P. Chen, Z.-H. Ge, W. Liu, Q. Zhang, S. Chen and Z.-Q. Hu, Dyes Pigm., 2018, 151, 233–237 CrossRef CAS.
- L. Liu, Q. Zhang, H. Duan, C. Li and Y. Lu, Anal. Methods, 2021, 13, 3792–3798 RSC.
- K. Zargoosh, M. Barmaki, A. Abdolmaleki and K. F. Tadavani, J. Iran. Chem. Soc., 2020, 17, 923–933 CrossRef CAS.
- H. Sun, X.-X. Tang, R. Zhang, W.-H. Sun, B.-X. Miao, Y. Zhao and Z.-H. Ni, Dyes Pigm., 2020, 174, 108051 CrossRef CAS.
- ASTM E1064-16, Standard Test Method for Water in Organic Liquids by CoulometricKarl Fischer Titration, ASTM International, 2018 Search PubMed.
- Í. Marques, F. Lucion, C. Bizzi, A. Cichoski, R. Wagner, C. Menezes and J. Barin, J. Food Qual., 2016, 39, 391–397 CrossRef.
- E. Kang, H. R. Park, J. Yoon, H.-Y. Yu, S.-K. Chang, B. Kim, K. Choi and S. Ahn, Microchem. J., 2018, 138, 395–400 CrossRef CAS.
- R. Velvarská, M. Fiedlerová, J. M. Hidalgo Herrador and Z. Tišler, Spectrosc. Lett., 2019, 52, 1–8 CrossRef.
- B.-Q. Xu, C.-Q. Rao, S.-F. Cui, J. Wang, J.-L. Wang and L.-P. Liu, J. Chromatogr. A, 2018, 1570, 109–115 CrossRef CAS PubMed.
- A. Shahvar, D. Shamsaei and M. Saraji, Measurement, 2020, 150, 107068 CrossRef.
- J. Wang, J. Wang, W. Xiao, Z. Geng, D. Tan, L. Wei, J. Li, L. Xue, X. Wang and J. Zhu, Anal. Methods, 2020, 12, 3218–3224 RSC.
- N. Kong, H. Yuan, H. Zhou, Y. Zhao and S. Zhang, Anal. Methods, 2021, 13, 2722–2727 RSC.
- K. Chana and V. Sridhar, presented in part at the 12th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, 2016 Search PubMed.
- V. Sridhar, K. S. Chana and D. Singh, presented in part at the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, 2017 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |


