Open Access Article
Open Access ArticleExploration of microplastic pollution with particular focus on source identification and spatial patterns in riverine water, sediment and fish of the Swat River, Pakistan†
Luqman Khana,
Sidra Ghias a,
Mazhar Iqbal Zafar
a,
Mazhar Iqbal Zafar *a,
Aiyeshah Alhodaib*b,
Humaria Fatima
*a,
Aiyeshah Alhodaib*b,
Humaria Fatima c,
Tofeeq Ur-Rehmanc,
Amir Waseem
c,
Tofeeq Ur-Rehmanc,
Amir Waseem d and
Haidar Howarie
d and
Haidar Howarie
aDepartment of Environmental Sciences, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan. E-mail: luqmanenviron@gmail.com; sidraaghias@gmail.com; mzafar@qau.edu.pk; Tel: +92-51-90644182
bDepartment of Physics, College of Science, Qassim University, Buraydah 51452, Saudi Arabia. E-mail: ahdieb@qu.edu.sa
cDepartment of Pharmacy, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, 45320, Pakistan. E-mail: hfchughtai@qau.edu.pk; tofeeq.urrehman@qau.edu.pk
dDepartment of Chemistry, Quaid-i-Azam University, Islamabad 45320, Pakistan. E-mail: amir@qau.edu.pk
eDepartment of Physics, Deanship of Educational Services, Qassim University, Buraydah 51452, Saudi Arabia. E-mail: Haidarh1@yahoo.com
First published on 28th March 2022
Abstract
Microplastics (MPs) have been reported as an emerging xenobiotic organic pollutant in freshwater ecosystems and a universal hazard for ecosystems because of the rapid increase in global demand. The present study was conducted to explore MPs' occurrence, abundance and spatial distribution in sediment, water and Schizothorax plagiostomus samples, collected from the Swat River. ATR-FTIR spectroscopy was used for chemical characterization of visually identified MPs by using standard protocols such as digestion using H2O2, density separation using ZnCl2, vacuum filtration with borosilicate glass micro filter papers and digital microscopy using a stereomicroscope connected with a camera. Range of mass abundance of identified MPs in river sediments, river water, tributaries sediment and tributary water was found to be 0.6–2.5 mg kg−1, 0.7–3.8 mg L−1, 0.9–4.5 mg kg−1 and 0.6–1.1 mg L−1 respectively. Meanwhile, in Schizothorax plagiostomus digestive tracts samples, it was 0.6–1.9 mg per fish. Numeric abundance of MPs in all matrices was found to be tributary sediment (202 items per kg) > river water (192 items per L) > river sediment (182 items per kg) > fish (153 items per fish) > tributary water (92 items per L). MPs identified on the basis of morphology in all matrices were found to be fragments > fibers > pellets > films > foams. MPs were dominant in all urban stations while their spatial distribution along with the study site was heterogeneous due to the surroundings such as tourist spots, hydrodynamic conditions, and proximity to urban areas, plastic industries and due to recharge by the highly contaminated tributaries. The MPs identified on the basis of size dimensions show that S1 (0.5–1 mm) in all matrices was highest while S2 (1–5 mm) was the lowest. Primary source MPs identified were fibers, films, fragments and foams particles while secondary sources were pellets. Results of ATR-FTIR showed that PE was the most common plastic type identified in all samples followed by PVC, PET, PP and PS. This is the first study exploring the MPs' occurrence, numeric and mass abundance and spatial distribution in the SR ecosystem. The present study may be a valuable reference for better understanding the MPs' pollution in Pakistan. The findings of the present study can help to identify the potential sources (i.e., primary and secondary) of MPs to improve waste management in the Swat District and model the transport fluxes of these microplastics in other rivers using water quality parameters and basin characteristics.
1. Introduction
Plastic demand has increased over the last five decades as a result of their use in production sectors such as building, packaging, electronics, automobile, household, leisure and sports, agriculture, medical devices, and renewable energy causing harm to aquatic environments.1The plastic particles can be classified by their shape into fragment, pellet, fiber, film and styrofoam and by their size in macroplastics (>25 mm), mesoplastics (between 5 and 25 mm) and microplastics (<5 mm). The nanoplastics (NPs) are usually defined as plastic particles which measure less than 100 nm.2 Plastic pollution has been a subject of special concern in recent years because it cannot only be a source of hazardous chemicals (e.g., additives) added as a result of manufacturing but a sink for hazardous chemicals (e.g., hydrophobic organic contaminants and metals) brought from the surrounding atmosphere during plastic manufacturing.3
In addition to conventional sources of plastic debris (agricultural, industrial, medical, domestic etc.), COVID-19 occurrence rates are significantly increasing the plastic burden through the waste produced by healthcare facilities and personal care products. In this context, the King Abdullah University Hospital in Jordan generated ten folds more medical garbage (650 kg per day, based on 95 COVID-19 patients) than the average generation rate during the hospital's daily operating day.4 Medical waste has also risen exponentially in other parts of the world, including China, Catalonia, and Spain, at the rate of 370% and 350% respectively.5
Plastics are composed from monomers, additives, and other components, with harmful monomers, additives, and chemical byproducts accounting for more than half of all the plastics.3 The presence of floating microplastics in the water may affect the distribution of sunlight that is bioavailable to species, disrupting their normal daily activities. MPs in the ecosystem may establish new environments for microbes and microbial colonization,6 which can have an active impact on their abundance and diffusion.7,8 When organisms living on the surface of floating MPs are transported to new environments, they can serve as alien invasive species.9
Furthermore, MPs in the environmental settings pose a challenge to the respiratory and digestive processes by inhaling and ingesting them. We desperately call for a better understanding of the possible risks of MPs to human health, given the lifelong unavoidable exposure of microplastics. Biodegradable plastics are the right candidates to replace non-biodegradable plastics in this sense. Polyhydroxybutyrate (PHB) that is synthesized by microorganisms from renewable materials (in addition to other biodegradable plastics), has gotten a lot of attention. PHB is now commonly used in biomedical applications.10–12 The literature reports that these biodegradable plastics have a potential to generate secondary nanoplastics under environmental conditions that cause toxicity. These secondary nanoplastics particles may adsorb different chemicals from water (heavy metals, polycyclic aromatic hydrocarbons, pharmaceuticals, polychlorinated biphenyls, and so on), potentially exacerbating nanoplastics' negative effects on living species.13
For the time being, it is promising that a few forward-thinking experts from across the world are attempting to comprehend the effects of the plastic plague that has affected our environment. The phenomenon associated with plastic contamination in the aquatic ecosystem will continue to merit scientific study, given the proliferation of plastics into all spheres of human existence and their growing usage value in the developing world. As a result, a thorough report on this emerging problem is urgently needed to gain a deeper understanding of how to implement cost-effective solutions to the plastic waste for developing countries like Pakistan. Furthermore, we know far less about microplastics in freshwater and terrestrial systems than we do about microplastics in marine environments. Therefore, the present study is carried out to explore the occurrence, abundance spatial distribution and sources of MPs in freshwater riverine sediment and surface water of Swat River (SR).The present research also monitored the accumulation of MPs in Schizothorax plagiostomus digestive tracts. This study will be useful to provide baseline data of MPs pollution that may help future monitoring surveys.
2. Material and methods
2.1 Study area
Swat District located at the northwest corner of Khyber Pakhtunkhwa (KPK) Province of Pakistan lies from 34° 34′ to 35° 55′ North latitudes and 72° 08′ to 72° 50′ East longitudes. Swat River (SR) is among the main Fresh Water Rivers (FWRs) existing in KPK and begins at Kalam with the confluence of Ushu and Utror Rivers. SR drains the district Swat and passes through the entire district Swat for about 160 km up to Chakdara where it joins Panjkora River of district Dir. The combined streams after mixing flow in a south-west direction into the Peshawar plain and joins the Kabul River at Nisatta Charsadda after covering 320 km distance. The total length of the river is 250 km from Kalam to its confluence with Kabul River near Nisatta, Charsadda. SR has an important role in the local economy and is a reason of charm for tourists. The river water is used for irrigation and domestic purposes and is habitat of many fish species and migratory birds. This cold water and sluggish moving river ecosystem is appropriate for Schizothorax plagiostomus fish. SR is exposed to various point and nonpoint pollution sources. Point sources include prohibited fishing (with the use of pesticides and agrochemicals), built up areas and mismanagement of solid waste. Non-point containing domestic and hospital sewage, dumping sites and absence of appropriate sanitation system from local community; an alarming threat for the community and policy makers.14 Study area is divided into two zones: zone one (Z1) (includes urban stations characterized by large human settlements, sewage and plastic industries) and zone two (Z2) includes non-urban stations and dominates agricultural areas (Fig. 1).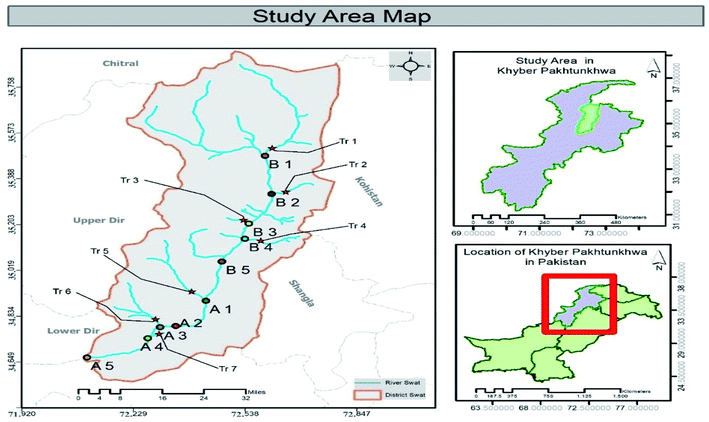 | ||
| Fig. 1 Map of study area showing microplastic sampling stations of Swat River, district Swat. Z1 sampling stations are denoted with A1, A2, A3….An, while, Z2 stations with B1, B2, B3…Bn. The tributaries present in both zones joining the river were represented with Tr1, Tr2, Tr3…Trn (see Table 1). | ||
| Sr. no. | Station name | Station code | Station zones (Z1, Z2) | Coordinates |
|---|---|---|---|---|
| 1 | Kalam | B1 | Z2 | 35°28′′51′N 72°35′′29′E |
| 2 | Mankial | B2 | Z2 | 35°19′′35′N 72°36′′42′E |
| 3 | Bahrain | B3 | Z2 | 35°12′′15′N 72°32′′49′E |
| 4 | Madyan | B4 | Z2 | 35°08′′40′N 72°32′′17′E |
| 5 | Baghderai | B5 | Z2 | 35°03′′14′N 72°28′′28′E |
| 6 | Matta | A1 | Z1 | 34°53′′50′N 72°25′′44′E |
| 7 | Kanju | A2 | Z1 | 34°47′′34′N 72°20′′47′E |
| 8 | Hazara | A3 | Z1 | 34°47′′27′N 72°18′′09′E |
| 9 | Gugdara | A4 | Z1 | 34°44′′47′N 72°16′′19′E |
| 10 | Chakdara | A5 | Z1 | 34°38′′48′N 72°01′′58′E |
2.2 Samples collection and preparation
Sampling stations were selected according to the standard protocol15 by using purposive sampling method starting from upstream of river to downstream. To investigate and highlight the problem of MPs, complete Swat River Basin (SRB) was randomly divided into 10 sampling stations.Sediment samples containing visible organic matter were then digested with 15% H2O2 [(CSA: 231-592-0); Analytical Grade (AG)].19 For this purpose, samples were taken in a 1000 mL Erlenmeyer flask (pre-rinsed with deionized water) placed in fume hood (Model: EscoFrontier; EN14175-3) and then added 200 mL of stabilized H2O2. To stimulate the oxidation process, samples were placed on a hotplate (Model: MSHP-1/02; PCSIR Laboratories, Pakistan) set at a temperature of 50 to 55 °C for 48 hours and covered with aluminum foil. After digestion, for maximum separation of MPs in samples, saturated ZnCl2 [(CAS: 231-592-0; purity, 98%, AG) Sigma Aldrich® Germany] with 1.7 kg L−1 density was used.20 A total 250 mL of saturated ZnCl2 was added in each beaker. The mixture was stirred for 20 minutes to disaggregate and suspend any MPs items. Then samples were kept in a safe place for 24 hours to settle down sediment particles. The supernatant was aliquoted into a beaker and allowed to settle for the next 6 hours and then filtered using a pre-weighted borosilicate glass microfiber filters (Micropore: MGF/C47 mm; San Diego, USA) and a vacuum filtration assembly (Thermo Scientific: Ecolab-glass: 2342, Germany). Filter papers containing samples were then kept in the glass Petri dishes till microscopic examination.
2.3 Observation and identification of microplastics
Filter papers were manually examined for MPs identification, based on colors and shapes and were picked up with stainless steel tweezers, marked and stored in glass bottles. These filter papers were inspected under digital microscope (CX41; Olympus Co. Ltd., Japan) connected with a digital camera (ISH500; U-TV0.5XC-3, Co. Ltd. Japan) at 4× magnification level. MPs were identified based on morphology (fiber, film, fragment, foam and pellet), sizes (S1 and S2) and colors (blue, black, red, yellow, brown, white and transparent) of the items.18 MP items found under microscope based on these parameters were marked, counted and images were taken following the protocols of ref. 23. Finally, chemical characterization of visually identified MPs in each matrix (sediment and water) was evaluated via ATR-FTIR. MPs' samples on the filter papers were scanned for 4000–400 cm−1 frequency range and data was collected at a resolution of 4 cm−1 with a 32 s scanning time. In present study, PE, PP, PS, PVC and PET were identified as the main polymer types.2.4 Experimental control and QA/QC
All solutions, including distilled water, 15% H2O2 and saturated ZnCl2 (1.7 kg L−1) solution, were filtered through a 47 mm Whatman® glass microfiber membrane (GF/B; 1 m pore size) and stored in the glassware wrapped with aluminum foil to avoid MPs' cross-contamination. Filtered distilled water was used to rinse all containers and glasswares. From sample preparation to extraction processes, the blank (control sample) consisted of filtered distilled water of volume 250 mL in capped beakers were placed at 10 separate spots within the laboratory for one week. The water samples were tested in the same way as the blank control solutions. Furthermore, cotton lab coats and polymer-free gloves were worn during the trials to ensure minimal MPs contamination. The laboratory work was done under a laminar-flow hood, and the use of plastic tools or containers were strictly avoided throughout the experiments.3. Results and discussion
3.1 Microscopic analysis
 | ||
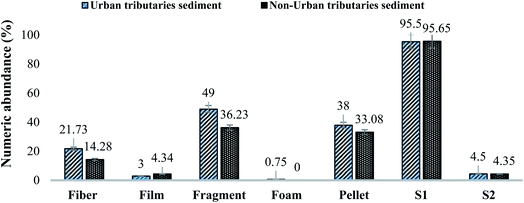
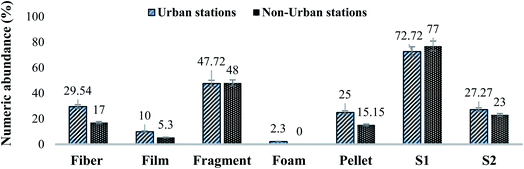
| Fig. 2 Comparison of identified MPs based on morphology and sizes in urban and non-urban sediment samples. Results are displayed as % numeric abundance (items per kg). | ||
 | ||
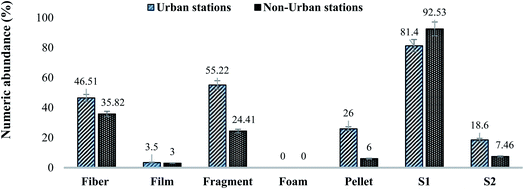
| Fig. 4 Comparison of MPs identified based on morphology and sizes in urban and non-urban water samples. Results are displayed as % numeric abundance (items per L). | ||
 | ||
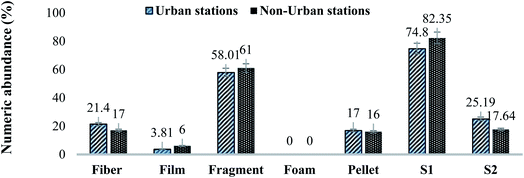
| Fig. 6 Comparison of MPs identified based on morphology and sizes in urban and non-urban Schizothorax plagiostomus samples. Results are displayed as % numeric abundance (items per fish). | ||
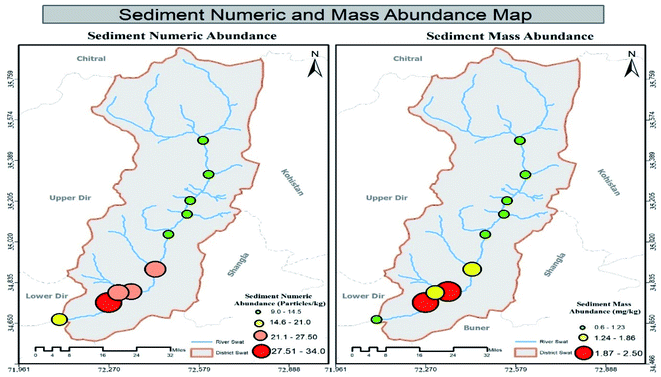
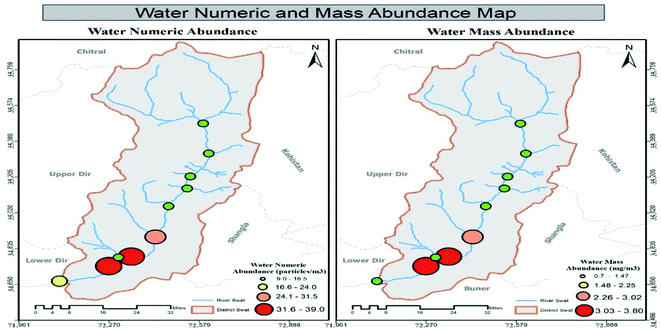
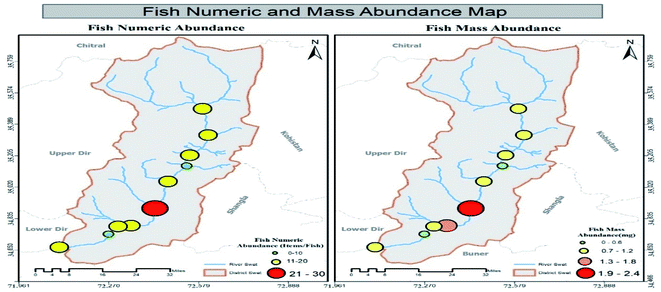
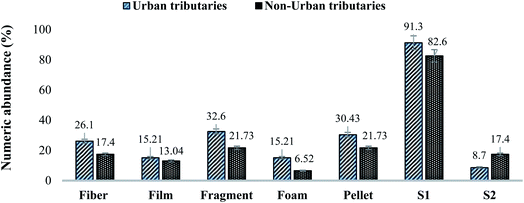
3.2 Mass abundance of MPs in sediment, water and fish specimens in tributaries
Results showed that MPs' mass abundance throughout the study area was inconsistent in all three matrices except in non-urban fish samples in which a consistent decreasing order was observed. Urban station's mass abundance in all three matrices were in decreasing order like water > sediment > fish while in non-urban samples the order was fish > sediment > water, respectively. Station-wise mass abundance results of water, sediment and Schizothorax plagiostomus are listed in Tables SI 8, SI 9 and SI 10† respectively. No uniform trend of increase or decrease of mass abundance was observed in tributary samples. Range of abundance in tributaries water samples and sediments was 0.6–1.1 mg L−1 and 0.9–4.5 mg kg−1 respectively. Mass abundance of urban tributaries in both matrices was in decreasing order of tributaries sediment > tributaries water while in non-urban samples it was found to be tributaries sediment > tributaries water as shown in Tables S11 and S12.†3.3 ATR-FTIR results of environmental matrices (river sediment, river water tributaries sediment and tributaries water)
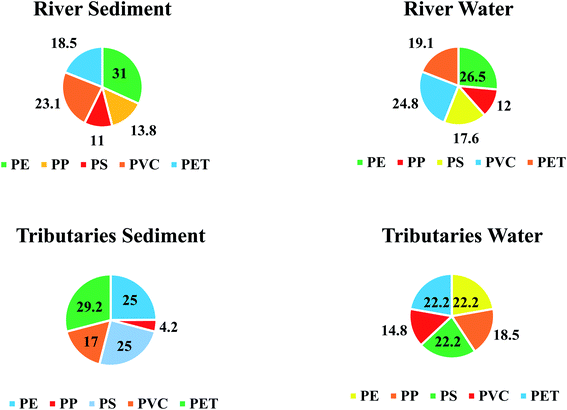
Optically verified MPs items in all matrices were analyzed on ATR-FTIR for their chemical characterization, and a total five type of polymers (PE, PP, PS, PVC and PET) were identified (Fig. 10).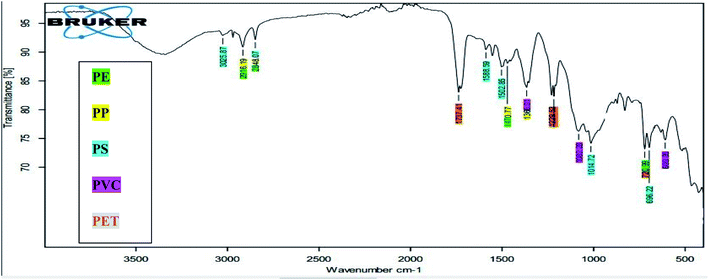 | ||
| Fig. 10 FTIR spectra of identified polymer types in sample Tr4s1. Highlighted peaks are verified from functional groups of each polymer type. | ||
Identified functional groups of the plastics in all environmental samples compared with reference peaks and the identified peaks in the samples are shown in Table SI 13.† PE plastic in present study was confirmed using specific functional groups identified on ATR-FTIR. Absorption band at 2914.94 & 2847.75 cm−1 in present study corresponds to methylene (–CH2) stretches. Strong absorption bands at 1472 & 1462 cm−1 are due to C–H deformation vibration of tertiary carbon and corresponds to the typical methyl (–CH3) bending vibration of PE. Additionally, peaks obtained at 718 and 729.41 cm−1 are due to CH2 rocking vibrations. Due to crystallinity of PE, the peak at 720 cm−1 is split and an additional peak is seen at 730 cm−1 in all polymers.24 These peaks are observed at 730 & 717 cm−1 (ref. 25 and 26) that supported these functional groups for PE. The absorption bands of functional groups identified in literature were, 2915 & 2845 cm−1 (C–H stretch), 1472 & 1462 cm−1 (CH2 bend) and 730 & 717 cm−1 (CH2 rock), respectively.
The peak at 2950.12 cm−1 belongs to the bending bond of methyl (–CH3) functional group of PP. Absorption band 2918 slightly shifted from 2839 and 2915 cm−1 belongs to the stretch –CH2 chemical group. These bands observed at 2916.07 cm−1 shifted to 2836.13 cm−1 (–CH2 symmetric bend) in the present study. Moderate absorption peaks of deformation vibrations of the plane CH3 group arise at 1375.41. This peak in the present study appears at 1375.47 (CH3 bending vibration).
Peaks at the wavelengths of 1490 cm−1 (aromatic ring stretch), 755 cm−1, and 695 cm−1 (aromatic CH out-of-plane bend) are similar to the FTIR PS standard.27 The absorption peak at wavenumber 3024.84 is due to aromatic C–H stretching vibration while absorption peaks at the wave number 2849.18 cm−1 corresponds to the existence of –CH3 stretching frequencies. The peaks over 2800–3100 cm−1 are attributed to C–H stretching vibrations in the main chain.28 The three absorption bands at 1601.19 cm−1, 1492.53 and 1451.93 cm−1, are due to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretching vibration absorption. The absorption at 1025.80 cm−1 in the present study corresponds to the aromatic deformation and skeletal vibrations of C–H of PS MPs. Moreover, the absorption at 698.85 cm−1 in the present study corresponds to C–H out-of-plane bending vibration and indicates that there is only one substituent in the benzene ring. Peaks ranged from 1326.90 and 1248.92 cm−1 in the present study assigned to CH bending of CHCl group that corresponds to CH bending frequency and indicates the presence of PVS polymer. The second band region with absorption band at 1075.25 cm−1 represents C–C bond stretching frequency observed in the present study. The third region called the C–Cl stretching band in the range of 700–600 cm−1 was observed at 612.17 cm−1 in the present study. All these regions were identified in the samples analyzed by ATR-FTIR (Table SI 13†) showed the absorption band ranges and the functional groups for the PET. The stronger absorption band found at 1713.37 cm−1 in the present study is due to the stretching vibration of the C
C ring stretching vibration absorption. The absorption at 1025.80 cm−1 in the present study corresponds to the aromatic deformation and skeletal vibrations of C–H of PS MPs. Moreover, the absorption at 698.85 cm−1 in the present study corresponds to C–H out-of-plane bending vibration and indicates that there is only one substituent in the benzene ring. Peaks ranged from 1326.90 and 1248.92 cm−1 in the present study assigned to CH bending of CHCl group that corresponds to CH bending frequency and indicates the presence of PVS polymer. The second band region with absorption band at 1075.25 cm−1 represents C–C bond stretching frequency observed in the present study. The third region called the C–Cl stretching band in the range of 700–600 cm−1 was observed at 612.17 cm−1 in the present study. All these regions were identified in the samples analyzed by ATR-FTIR (Table SI 13†) showed the absorption band ranges and the functional groups for the PET. The stronger absorption band found at 1713.37 cm−1 in the present study is due to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the ester group. Furthermore, two bands were observed for the C–O stretching bond at 1240.31 cm−1 & 1091.92 cm−1. The absorption band at 721.34 cm−1 corresponds to the ring out-of-plane deformation of the C–H group. Bands of PET in present study are assigned to the ring vibrations except for 730 cm−1 (721.34 cm−1 in present case) as it’s not clear in the literature.25,26,29
O bond of the ester group. Furthermore, two bands were observed for the C–O stretching bond at 1240.31 cm−1 & 1091.92 cm−1. The absorption band at 721.34 cm−1 corresponds to the ring out-of-plane deformation of the C–H group. Bands of PET in present study are assigned to the ring vibrations except for 730 cm−1 (721.34 cm−1 in present case) as it’s not clear in the literature.25,26,29
4 Conclusions
MPs has been listed as an emerging xenobiotic pollutant and universal hazard for ecosystems. In present study the occurrence, abundance and spatial distribution of MPs in freshwater riverine sediment, water and Schizothorax plagiostomus was assessed along the SR system. Optical microscopy results demonstrated that MPs were present in different morphology, sizes and colors in sample matrices. MPs in the urban zone were found to be in a higher percentage as compared to non-urban samples except for tributary water samples. The numeric abundance of MPs was in descending order like tributaries sediment (202 items per kg) > river water (192 items per L) > river sediment (182 items per kg) > fish (153 items per fish) > tributaries water (92 items per L). The study found tributaries as one of the potential sources of MPs in the SR. Results of ATR-FTIR spectroscopy showed that PE (27%) was the most identified plastic type in all matrices while least detected was PP (12.5%). Out of 576 items, 32% were confirmed to be polymers (PE, PP, PS, PVC and PET) while 68% were listed as “Others” as they were not identified by ATR-FTIR. Pellets in present study were found to be the primary source (originated from daily personal care products, industrial preproduction pellets or from fragments that degraded and became small round pellets) of MPs pollution. Other MPs items found in present study were from secondary sources that include fibers, films, fragments and foam items. These items originated from fishing activities, landfilling or dumping sites, agriculture activities and food packaging. Possible sources of PE and PP were found to be fragments originating from littering and fishing tools. For instance MPs were found in all matrices of the study area indicating a potential threat to environmental and human health. The study provides useful and comprehensive information for the evaluation of the MPs' pollution and environmental risk in district Swat. Therefore, it emphasizes the importance of rivers as potential carriage systems of MPs. Monitoring of present study concludes that freshwater fish is extremely vulnerable to MPs pollution and that urbanized areas appear to be a major factor contributing to the pollution of freshwater environments with MPs.Abbreviations
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared |
| FWRs | Freshwater rivers |
| GPS | Global positioning system |
| H2O2 | Hydrogen peroxide |
| KPK | Khyber Pakhtunkhwa |
| L | Liter |
| mL | Milliliters |
| mm | Millimeter |
| MPs | Microplastics |
| PCSIR | Pakistan Council of Scientific and Industrial Research |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| S1 | Size 1 (S1: 0.5–1 mm) |
| S2 | Size 2 (S1: 1–5 mm) |
| SR | Swat river |
| Tr. | Tributaries |
| Z1 | Zone 1 |
| Z2 | Zone 2 |
| ZnCl2 | Zinc chloride |
Author contributions
The role of all authors in present study is as follows: M. I. Z., H. F., T. U.-R. and A. W. designed, conceived, and supervised the complete study. L. Q., S. G. and M. I. Z. experimented, analyzed the data and helped in compiling the results. A. W., H. H. and A. A. assisted in FTIR analysis of all types of microplastics and facilitated in the interpretation of FTIR results. M. I. Z., L. Q. and S. G. wrote the 1st draft of manuscript. M. I. Z., H. F., H. H. and A. A. made a substantial contribution to the interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.Conflicts of interest
The authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence this research work.Acknowledgements
The authors would like to express their high indebtedness to Higher Education Commission (HEC) of Pakistan for providing financial support under NRPU program Grant#: 9139/Federal/NRPU/HEC as well as University Research Funds (URF) for performing present research work in terms of designing, samples collection and their analysis.References
- Plastics – the Facts, an analysis of European plastics production, demand and waste data P. Europe, 2018, https://plasticseurope.org/wp-content/uploads/2021/10/2018-Plastics-the-facts.pdf, accessed 24 December 2021 Search PubMed.
- S. Lambert, C. Sinclair and A. Boxall, Rev. Environ. Contam. Toxicol., 2014, 227, 1–53 CAS.
- D. Lithner, Å. Larsson and G. Dave, Sci. Total Environ., 2011, 409(18), 3309–3324 CrossRef CAS PubMed.
- H. A. Abu-Qdais, M. A. Al-Ghazo and E. M. Alghazo, J. Environ. Sci. Manage., 2020, 6, 1–10 Search PubMed.
- J. J. Klemeš, Y. V. Fan, R. R. Tan and P. Jiang, Renewable Sustainable Energy Rev., 2020, 127 Search PubMed.
- E. R. Zettler, T. J. Mincer and L. A. Amaral-Zettler, Environ. Sci. Technol., 2013, 47, 7137–7146 CrossRef CAS PubMed.
- M. C. Goldstein, M. Rosenberg and L. Cheng, Biol. Lett., 2012, 8, 817–820 CrossRef PubMed.
- A. P. Majer, M. C. Vedolin and A. Turra, Mar. Pollut. Bull., 2012, 64, 1143–1147 CrossRef CAS PubMed.
- M. R. Gregory, Philos. Trans. R. Soc., B, 2009, 364(1526), 2013–2025 CrossRef PubMed.
- A. Getachew and F. Woldesenbet, BMC Res. Notes, 2016, 9(1), 1–9 CrossRef PubMed.
- S. K. Kale, A. G. Deshmukh, M. S. Dudhare and V. B. Patil, J. Biochem. Technol., 2015, 6, 952–961 CAS.
- R. T. Chan, C. J. Garvey, H. Marçal, R. A. Russell, P. J. Holden and L. J. R. Foster, Int. J. Polym. Sci., 2011, 2011, 247–267 Search PubMed.
- A. A. Koelmans, A. Bakir, G. A. Burton and C. R. Janssen, Environ. Sci. Technol., 2016, 50(7), 3315–3326 CrossRef CAS PubMed.
- M. Sinchembe and W. N. Ellery, Afr. J. Aquat. Sci., 2010, 35(3), 227–239 CrossRef.
- I. S. Dewi, A. A. Budiarsa and I. R. Ritonga, DEPIK Jurnal Ilmu-Ilmu Perairan, Pesisirdan Perikanan, 2015, 4(3), 121–131 Search PubMed.
- J. A. Smith, J. L. Hodge, B. H. Kurtz, and J. I. Garver, in Mohawk Watershed Symposium, 2017 Search PubMed.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. John and A. Russell, Science, 2004, 304(5672), 838 CrossRef CAS PubMed.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2010, 46(6), 3060–3075 CrossRef PubMed.
- G. Liebezeit and F. Dubaish, Bull. Environ. Contam. Toxicol., 2012, 89(1), 213–217 CrossRef CAS PubMed.
- H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva and C. Laforsch, Limnol. Oceanogr.: Methods, 2012, 10(7), 524–537 CrossRef CAS.
- A. L. Lusher, M. Mchugh and R. C. Thompson, Mar. Pollut. Bull., 2013, 67(1–2), 94–99 CrossRef CAS PubMed.
- C. G. Avio, S. Gorbi and F. Regoli, Mar. Environ. Res., 2015, 111, 18–26 CrossRef CAS PubMed.
- J. Li, X. Qu, L. Su, W. Zhang, D. Yang, P. Kolandhasamy and H. Shi, Environ. Pollut., 2016, 214, 177–184 CrossRef CAS PubMed.
- J. Charles, Asian J. Chem., 2016, 21(6), 4477 Search PubMed.
- R. Chércoles Asensio, M. San Andrés Moya, J. M. de la Roja and M. Gómez, Anal. Bioanal. Chem., 2009, 395(7), 2081–2096 CrossRef PubMed.
- I. Noda, A. E. Dowrey, J. L. Haynes and C. Marcott, in Physical Properties of Polymers Handbook, ed. J. E. Mark, Springer Science + Business Media LLC, New York, 2007, pp. 395–406 Search PubMed.
- A. Mataji, M. S. Taleshi and E. Balimoghaddas, Arch. Environ. Contam. Toxicol., 2020, 78(1), 86–93 CrossRef CAS PubMed.
- C. D. Dong, C. W. Chen, Y. C. Chen, H. H. Chen, J. S. Lee and C. H. Lin, J. Hazard. Mater., 2020, 385, 121575 CrossRef CAS PubMed.
- A. A. El-Saftawy, A. Elfalaky, M. S. Ragheb and S. G. Zakhary, Radiat. Phys. Chem., 2014, 102, 96–102 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00319h |
| This journal is © The Royal Society of Chemistry 2022 |