Open Access Article
Open Access ArticleEnhanced mechanical quality factor of BiScO3–PbTiO3 piezoelectric ceramics using glass composition
Hongwei Shiab,
Zujian Wang a,
Xiaoming Yanga,
Rongbing Sua,
Xifa Long
a,
Xiaoming Yanga,
Rongbing Sua,
Xifa Long *ab and
Chao He
*ab and
Chao He *ab
*ab
aKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China. E-mail: hechao@fjirsm.ac.cn; lxf@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 14th March 2022
Abstract
Compared with pure Pb-based perovskite ferroelectric materials, Bi(Me)O3–PbTiO3 (Me = Sc3+, In3+, Yb3+) have attracted attention due to their remarkable advantage in their Curie temperature. Among them, BiScO3–PbTiO3 piezoelectric ceramic is a potential piezoelectric material in high-temperature applications for its high Curie temperature and excellent piezoelectric coefficient. However, its shortcomings are high dielectric loss and low mechanical quality factor. Herein, we report the improvement of the mechanical quality factor of 0.36BS–0.64PT ceramics through the addition of glass composition (GeO2). There is a small change in the Curie temperature after GeO2 addition. The piezoelectric coefficient d33 and planar electromechanical coupling factor kp increase first and then decrease, and the mechanical quality factor Qm monotone increases with an increase in GeO2. The 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics have optimal electrical properties with TC of 455 °C, d33 of 385 pC N−1, kp of 58%, and Qm of 90. In addition, the thermal stability of 0.36BS–0.64PT + xGeO2 and 0.36BS–0.64PT ceramics is almost the same. It was concluded that the mechanical quality factor of BS–PT ceramics can be enhanced by the addition of GeO2 with other properties remaining unchanged.
1. Introduction
With the requirement of high-temperature piezoelectric sensors and actuators in aerospace, automobiles, oil and gas exploration fields, high-temperature piezoelectric materials have received unprecedented attention.1–4 The use temperature of piezoelectric materials is about half of the Curie temperature (TC) due to piezoelectric performance degradation at high temperatures.5 The commercial lead–zirconium–titanium (PZT) piezoelectric ceramics have been studied for decades and are widely used in piezoelectric sensors and transducers, but these ceramics cannot be used in environments above 200 °C, while applications in the aerospace and automotive industries require piezoelectric devices to operate at a temperatures of 300 °C or higher.6,7 Therefore, the development of new high-temperature-stable piezoelectric materials is an extremely urgent need.Bismuth-based perovskite solid solutions with the formula of Bi(Me)O3–PbTiO3 (Me = Sc, In, Yb, Mg/Ti, Fe, Ga et al.), which are potential alternatives for high-temperature application, have attracted growing interest due to their ultra-high Curie temperature.8–13 Among them, BiYbO3–PbTiO3, BiInO3–PbTiO3, Bi(Mg1/2Ti1/2)O3–PbTiO3 and BiFeO3–PbTiO3 are not suitable for applications due to their poor piezoelectric properties. BiScO3–PbTiO3 (BS–PT) solid solutions have drawn considerable attention because of their high piezoelectric constant (d33) of 460 pC N−1 and high TC of 450 °C near the morphotropic phase boundary (MPB).14,15 In addition, due to the advantages of high working efficiency, good flexibility, high strength and toughness of flexible ceramic film materials, some researchers have focused on preparing multifunctional materials on flexible substrates. For example, Liu successfully fabricated Mn–In2O3 thin films with excellent mechanical durability and transparent conductive oxide SrVO3 thin films by pulsed laser deposition (PLD) on flexible substrates.16,17 BS–PT solid solutions have small crystal anisotropy and excellent electrical performance, which can be combined with a flexible substrate to improve the piezoelectric response.18
The shortcomings of BS–PT ceramics are high dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ > 3%) and a low mechanical quality factor (Qm < 30),19,20 which limit their application at high temperatures. Previously, a great deal of work has been done to increase the Qm of BS–PT ceramics through chemical methods, such as the addition of Mn, Fe, Pb(Mn1/3Nb2/3)O3, Pb(Mn1/3Sb2/3)O3.4,21–25 The mechanical quality factor can be enhanced by chemical methods, while the piezoelectric performance and Curie temperature are often decreased. For example, the piezoelectric performance of (0.97-x)BiScO3–xPbTiO3–0.03Pb(Mn1/3Nb2/3)O3 ceramics deteriorated severely (d33 < 300 pC N−1).24 The Curie temperature of 0.03Pb(Mn1/3Sb2/3)O3–0.33BS–0.64PT and BiScO3–PbTiO3–Pb(Yb1/2Nb1/2)O3 ceramics is less than 400 °C.23,26 Mn and Fe co-modified BS–PT ceramics show both a lower d33 of 235 pC N−1 and dielectric constant εr of 815 compared with pure BS–PT ceramics.25
δ > 3%) and a low mechanical quality factor (Qm < 30),19,20 which limit their application at high temperatures. Previously, a great deal of work has been done to increase the Qm of BS–PT ceramics through chemical methods, such as the addition of Mn, Fe, Pb(Mn1/3Nb2/3)O3, Pb(Mn1/3Sb2/3)O3.4,21–25 The mechanical quality factor can be enhanced by chemical methods, while the piezoelectric performance and Curie temperature are often decreased. For example, the piezoelectric performance of (0.97-x)BiScO3–xPbTiO3–0.03Pb(Mn1/3Nb2/3)O3 ceramics deteriorated severely (d33 < 300 pC N−1).24 The Curie temperature of 0.03Pb(Mn1/3Sb2/3)O3–0.33BS–0.64PT and BiScO3–PbTiO3–Pb(Yb1/2Nb1/2)O3 ceramics is less than 400 °C.23,26 Mn and Fe co-modified BS–PT ceramics show both a lower d33 of 235 pC N−1 and dielectric constant εr of 815 compared with pure BS–PT ceramics.25
GeO2–glass former is an additive used in glass. This glass former can decrease the dielectric loss and increase insulativity. Ge4+ ions tend to segregate along the grain boundaries, leading to a reduction in the jumping of mobile ions, which in turn causes a decrease in the conductivity of the ceramics.27 Mou et al. used Ge4+ to enhance the piezoelectric performance and adjusted dielectric properties.28 GeO2 can synchronously enhance the Qm and piezoelectric properties in the KNN and BCZT system.29,30 Therefore, we investigated the effect of GeO2 addition on the phase structure, dielectric, piezoelectric, ferroelectric and electromechanical coupling properties of BS–PT ceramics.
2. Experimental procedures
The GeO2 doped 0.36BS–0.64PT piezoelectric ceramics (abbreviated as 0.36BS–0.64PT + xGeO2, x = 0 mol%, 0.25 mol%, 0.5 mol%, 0.75 mol%, 1.0 mol%, 1.5 mol%) were prepared by the conventional solid-state reaction method. Starting materials were Bi2O3 (99.99%), Sb2O3 (99.99%), PbO (99.9%), TiO2 (99.5%), and GeO2 (99.99%). These oxide powders were weighed and mixed according to the chemical formula, adding 1 mol% Bi2O3 and PbO to compensate for evaporation during the sintering process. The mixture was ball milled for 12 h in absolute ethyl alcohol (99.7%) with zirconia balls, then dried and pressed into pellets at 60 MPa. The pellets were placed in a crucible and calcined at 780 °C for 5 h at a heating/cooling rate of 5 °C min−1. Thereafter, the GeO2 of different amounts was added to the calcined powder and ball milled again for another 24 h. After ball milling, the slurry was dried, crushed, and mixed with 5% polyvinyl alcohol binder (PVA). The powder was passed through a 200 mesh sieve and was pressed into pellets of 8 mm in diameter and 1.5 mm in thickness under an actual pressure of 200 MPa. Subsequently, it was heated at 600 °C for 2 h to eliminate the binder, and sintered in a sealed Al2O3 crucible in the range of 1000–1100 °C for 2 h to form ceramic samples.The crystal structure of sintered ceramics was analyzed on an X-ray diffractometer (Rigaku, Miniflex600, Japan) with Cu-Kα radiation. The TEM was performed using a field emission transmission electron microscope (TEM, FEI Tecnai F20, USA). A scanning electron microscope (SEM, SU8010, Hitachi, Japan) was used to investigate the microstructure. The density of the sintered samples was determined by the Archimedes method. The obtained ceramics were polished to a thickness of 0.5 mm, and then coated with silver paste on the surface to form the electrodes. The samples were poled in an oil bath at 120 °C in an electric field of 40 kV cm−1 for 15 min. After standing for 24 h, the electrical properties were tested. The dielectric constant (εr) and dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) were measured on an impedance analyzer (E4990A, Agilent Technologies, USA), and the planar electromechanical coupling factor (kp) and Qm were calculated according to the resonance and anti-resonance method. Ferroelectric hysteresis loop measurements were performed on an aix-ACCT TF2000 ferroelectric analyzer (f = 1 Hz). Piezoelectric coefficients were measured by a quasi-static d33 meter (Institute of Acoustics, Chinese Academy of Sciences, model ZJ-4AN).
δ) were measured on an impedance analyzer (E4990A, Agilent Technologies, USA), and the planar electromechanical coupling factor (kp) and Qm were calculated according to the resonance and anti-resonance method. Ferroelectric hysteresis loop measurements were performed on an aix-ACCT TF2000 ferroelectric analyzer (f = 1 Hz). Piezoelectric coefficients were measured by a quasi-static d33 meter (Institute of Acoustics, Chinese Academy of Sciences, model ZJ-4AN).
3. Results and discussion
3.1 Structure and phase analysis
Fig. 1(a) shows the X-ray diffraction patterns (log scale) of powder 0.36BS–0.64PT + xGeO2 ceramics. The main peaks of all samples matched with the perovskite phase, indicating formed stable perovskite solid solutions. It can be easily seen that the second phase (GeO2) appeared as the doping content of GeO2 increased, indicating that it is difficult for Ge4+ to enter the lattice. The radius of Ge4+ ion (0.53 Å for CN = 6) is almost similar to that of Ti4+ ions (0.605 Å for CN = 6), and it is considered that Ge4+ ions can enter the B site of the perovskite unit cell and substitute Ti4+ ions.31 Fig. 1(b) shows the amplified images of (200)c peaks. It can be easily seen that the phase structure is slightly changed after adding GeO2. The lattice parameters of 0.36BS–0.64PT + xGeO2 ceramics were calculated according to the diffraction patterns, as shown in Fig. 1(c). With the increase in the GeO2 content, the “c” value decreases continuously while the “a” value increases, and the c/a value keeps decreasing from 1.023 to 1.014, which confirms that the Ge4+ ion entered the B site of the perovskite unit cell. | ||
| Fig. 1 (a) X-ray diffraction patterns (log scale) of powder 0.36BS–0.64PT + xGeO2 ceramics. (b) The amplified images of (200)c peaks. (c) Lattice parameters of 0.36BS–0.64PT + xGeO2 ceramics. | ||
Fig. 2 shows the TEM image of 0.36BS–0.64PT + 0.5GeO2 ceramics. One set of lattice fringes with an interplanar spacing of 0.29 nm, corresponding to the (110) plane of the perovskite phase, and another set of lattice fringes with a 0.41 nm interplanar spacing, corresponding to the (100) plane of the perovskite phase, which consistent with the phase structure shown by the XRD results.
Fig. 3 shows the microstructure of 0.36BS–0.64PT + xGeO2 ceramics sintered at 1080 °C for 2 h. These images clearly show the morphology of the ceramic samples with few holes, dense grains and clear grain boundaries. Table 1 lists the density of the 0.36BS–0.64PT + xGeO2 ceramics. The actual density (ρ1) was calculated by the Archimedes method, and the theoretical density (ρ2) was calculated according to XRD data. The relative density (ρr) is 96.17–97.82%, showing a high density. The insets of Fig. 3 show the statistical data of the grain size of 0.36BS–0.64PT + xGeO2 ceramics. With the increase in GeO2, the average grain size decreases from 8.46 μm to 2.54 μm, indicating that GeO2 can inhibit the growth of grains. A similar phenomenon has been reported in other Ge doped ceramics. Fig. 3(e and f) show that a small amount of GeO2 gathers at the grain boundary, which hinders the movement of the grain boundary and restrains the grain growth. The results show that Ge4+ cannot be dissolved into the solid solution.
| x (mol%) | ρ1 (g cm−3) | ρ2 (g cm−3) | ρr (%) |
|---|---|---|---|
| 0.00 | 7.53 | 7.83 | 96.17 |
| 0.25 | 7.58 | 7.82 | 96.93 |
| 0.50 | 7.64 | 7.81 | 97.82 |
| 0.75 | 7.61 | 7.80 | 97.56 |
| 1.00 | 7.58 | 7.79 | 97.30 |
| 1.50 | 7.52 | 7.79 | 96.53 |
3.2 Dielectric properties
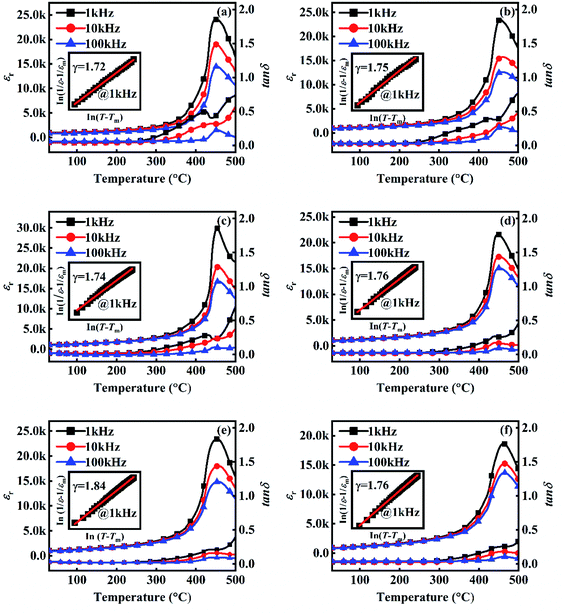
Fig. 4 shows the temperature dependence of εr and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of 0.36BS–0.64PT + xGeO2 ceramics. The temperature of the maximum of dielectric constant (Tm) is considered as the Curie temperature (TC).23 Table 2 summarizes the εr, tan
δ of 0.36BS–0.64PT + xGeO2 ceramics. The temperature of the maximum of dielectric constant (Tm) is considered as the Curie temperature (TC).23 Table 2 summarizes the εr, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and TC of 0.36BS–0.64PT + xGeO2 ceramics. The TC of 0.36BS–0.64PT ceramics with 0, 0.25, 0.5, 0.75,1 and 1.5 mol% GeO2 are 449, 457, 455, 454, 458 and 462 °C, respectively, showing a small change of GeO2. The decrease in c/a indicates that the crystal structure shifts to the rhombohedral phase, and the Curie temperature should be reduced in theory. However, compared with the undoped BS–PT ceramic samples, the introduction of GeO2 slightly increases the Curie temperature. It is indicated that the addition of GeO2 is good for stabilizing the Curie temperature.
δ and TC of 0.36BS–0.64PT + xGeO2 ceramics. The TC of 0.36BS–0.64PT ceramics with 0, 0.25, 0.5, 0.75,1 and 1.5 mol% GeO2 are 449, 457, 455, 454, 458 and 462 °C, respectively, showing a small change of GeO2. The decrease in c/a indicates that the crystal structure shifts to the rhombohedral phase, and the Curie temperature should be reduced in theory. However, compared with the undoped BS–PT ceramic samples, the introduction of GeO2 slightly increases the Curie temperature. It is indicated that the addition of GeO2 is good for stabilizing the Curie temperature.
| x (mol%) | TC (°C) | εr (@1k Hz) | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (%) δ (%) |
EC (kV cm−1) | Pr (μC cm−2) | d33 (pC N−1) | kp | Qm |
|---|---|---|---|---|---|---|---|---|
| 0.00 | 449 | 1130 | 3.31 | 19 | 32 | 355 | 0.49 | 28 |
| 0.25 | 457 | 1150 | 1.47 | 20 | 33 | 370 | 0.53 | 75 |
| 0.50 | 455 | 1280 | 1.18 | 21 | 37 | 385 | 0.58 | 90 |
| 0.75 | 454 | 1240 | 1.44 | 22 | 36 | 335 | 0.55 | 101 |
| 1.00 | 458 | 1200 | 1.71 | 23 | 36 | 305 | 0.54 | 115 |
| 1.50 | 462 | 1070 | 1.97 | 25 | 35 | 270 | 0.52 | 132 |
The values of εr at room temperature increased first and then decreased with the increase in GeO2, as shown in Table 2. The maximum εr observed when x = 0.5 mol% was 1280. An increase in the dielectric constant is related to an increase in density. The addition of GeO2 to 0.36BS–0.64PT ceramics can improve the microstructure of the material. In addition, the GeO2 doping is beneficial to reducing the dielectric loss.
It can be easily seen that all the samples exhibit a diffuse phase transition. The variation of the diffuse phase transition with increase in GeO2 was explored. The diffuse phase transition can be described by the modified Curie–Weiss law:32
ln(1/ε − 1/εm) + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = γ C = γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(T − Tm) ln(T − Tm)
| (1) |
3.3 Ferroelectric and piezoelectric properties
Fig. 5(a) shows the P–E loops and I–E loops of 0.36BS–0.64PT + xGeO2 ceramics measured at room temperature with 1 Hz. According to the P–E hysteresis loops, the variation of the values of remnant polarization (Pr) and coercive field (Ec) was obtained, as shown in Fig. 5(b) and Table 2. The Pr value of the virgin BS–PT ceramics is 32 μC cm−2. The Pr values increase significantly when a small amount of GeO2 is added. 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics have the maximum Pr value (37 μC cm−2). When the GeO2 added is higher than 0.50 mol%, the value of Pr decreases slightly because of the glass phase formed by the addition of GeO2. The value of Ec gradually increases as the GeO2 content increases, indicating the enhanced breakdown strength.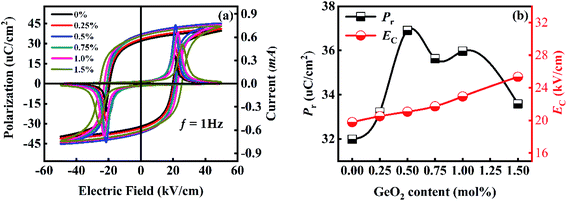 | ||
| Fig. 5 (a) P–E loops and I–E loops, (b) the variation of Pr and EC of 0.36BS–0.64PT + xGeO2 ceramics. | ||
Fig. 6 shows the variation trend of piezoelectric coefficient (d33), planar electromechanical coupling factor (kp), and mechanical quality factor (Qm) of the 0.36BS–0.64PT + xGeO2 ceramics. The detailed data of d33, kp, Qm are listed in Table 2. As shown in Fig. 6, the d33 and kp of 0.36BS–0.64PT + xGeO2 ceramics increased first and then decreased. The maximum value of d33 and kp is 385 pC N−1, 58% at x = 0.50 mol%, which is higher than that of the virgin BS–PT ceramics (355 pC N−1, 49%). The enhanced piezoelectric performance is attributed to the combined effect of the grain size. The piezoelectric performance decreased as the GeO2 content increased beyond 0.50 mol%. The addition of GeO2 can obstruct the movement of the domain and reduce the external contribution to piezoelectricity, resulting in a decrease in d33.29 Interestingly, the Qm value of ceramic increased monotonically with the increase in the GeO2 concentration. It is concluded that the addition of GeO2 can improve the mechanical quality factor, which is consistent with the variation of Ec.
3.4 Thermal stability
Thermal stability is valuable for sensors and actuators in various industrial applications. Fig. 7 shows the temperature dependence of P–E loops of the 0.36BS–0.64PT + 0.5 mol%GeO2 and 0.36BS–0.64PT. All curves were measured under an electric field of 50 kV cm−1 with a frequency of 1 Hz. As the temperature increased, the P–E loops of 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics still maintained a good rectangular loop at 280 °C without obvious leakage conductance. When the temperature rose to 300 °C, the leakage current was too large to form a saturated P–E loop confirmed by the abnormal high value of Pr (see Fig. 7(a)). The variation of the Pr and Ec of 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics are shown in Fig. 7(c). The Ec decreased from 19 kV cm−1 at 100 °C to 10 kV cm−1 at 280 °C, while the Pr remained almost unchanged. The clear leakage conductance appears at 280 °C for 0.36BS–0.64PT ceramics, as shown in Fig. 7(b and d), which is consistent with literature that BS–PT ceramics begin to degrade at 250 °C.34 The temperature stability of piezoelectric devices based on BS–PT ceramics is only 250 °C.35,36 Therefore, it is indicated that the addition of GeO2 is in favor of the enhancement of the thermal stability of BS–PT ceramics. | ||
| Fig. 7 Temperature dependence of (a and b) P–E loops, and (c and d) Pr and Ec for (a and c) 0.36BS–0.64PT + 0.5 mol%GeO2 and (b and d) 0.36BS–0.64PT ceramics. | ||
To further characterize the temperature stability of the samples, the poling samples were annealed at different temperatures for one hour, and their d33 was measured again at room temperature (Fig. 8). It can be easily seen that the d33 of 0.36BS–0.64PT ceramics starts decreasing above 250 °C. Compared with 0.36BS–0.64PT ceramics, the 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics exhibit unchanged d33 above 300 °C. This indicates that GeO2 addition improves the piezoelectric performance of BS–PT ceramics.
4. Conclusions
The phase structure, microstructure, dielectric, ferroelectric, and piezoelectric properties of 0.36BS–0.64PT + xGeO2 ceramics were presented. A small amount of GeO2 can decrease the grain size. With the increase in the GeO2 content, the values of εr, d33, kp increased first and then decreased, while the values of EC, Qm increased monotonically. Interestingly, the Curie temperature of 0.36BS–0.64PT + xGeO2 ceramics changes only slightly with different contents of GeO2. The optimal composition is 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics with TC of 455 °C, d33 of 385 pC N−1, kp of 58%, Qm of 90. Compared with 0.36BS–0.64PT ceramics, the enhanced Qm and stability of 0.36BS–0.64PT + 0.5 mol%GeO2 ceramics is due to the addition of GeO2. This study provides a paradigm for improving the mechanical quality factor of BS–PT-based ceramics.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (11904362, 51902307), the Science and Technology Project of Fujian Province (2020H0038, 2019H0052), the Key Research Program of the Chinese Academy of Sciences (ZDRW-CN-2021-3), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.References
- S. J. Zhang and F. P. Yu, J. Am. Ceram. Soc., 2011, 94, 3153–3170 CrossRef CAS.
- S. Zhang, X. Jiang, M. Lapsley, P. Moses and T. R. Shrout, Appl. Phys. Lett., 2010, 96, 013506 CrossRef.
- D. Damjanovic, Curr. Opin. Solid StateMater. Sci., 1998, 3, 469–473 CrossRef CAS.
- J. Wu, X. Gao, J. Chen, C.-M. Wang, S. Zhang and S. Dong, Acta Phys. Sin., 2018, 67, 207701 CrossRef.
- S. J. Zhang, R. E. Eitel, C. A. Randall, T. R. Shrout and E. F. Alberta, Appl. Phys. Lett., 2005, 86, 262904 CrossRef.
- J. F. Tressler, S. Alkoy and R. E. Newnham, J. Electroceram., 1998, 2, 257–272 CrossRef CAS.
- Y. Jiang, B. Qin, Y. Zhao, Y. Jiang, D. Xiao and J. Zhu, Phys. Status Solidi RRL, 2008, 2, 28–30 CrossRef CAS.
- L. Fan, J. Chen, S. Li, H. Kang, L. Liu, L. Fang and X. Xing, Appl. Phys. Lett., 2013, 102, 022905 CrossRef.
- S. J. Zhang, R. Xia, C. A. Randall, T. R. Shrout, R. R. Duan and R. F. Speyer, J. Mater. Res., 2005, 20, 2067–2071 CrossRef CAS.
- J. R. Cheng, W. Y. Zhu, N. Li and L. E. Cross, Mater. Lett., 2003, 57, 2090–2094 CrossRef CAS.
- R. E. Eitel, C. A. Randall, T. R. Shrout, P. W. Rehrig, W. Hackenberger and S. E. Park, Jpn. J. Appl. Phys., 2001, 40, 5999–6002 CrossRef CAS.
- C. J. Stringer, T. R. Shrout, C. A. Randall and I. M. Reaney, J. Appl. Phys., 2006, 99, 024106 CrossRef.
- W. M. Zhu, H. Y. Guo and Z. G. Ye, Phys. Rev. B, 2008, 78, 014401 CrossRef.
- R. E. Eitel, S. J. Zhang, T. R. Shrout, C. A. Randall and I. Levin, J. Appl. Phys., 2004, 96, 2828–2831 CrossRef CAS.
- R. E. Eitel, C. A. Randall, T. R. Shrout and S. E. Park, Jpn. J. Appl. Phys., 2002, 41, 2099–2104 CrossRef CAS.
- J. Liu, Ceram. Int., 2022, 48, 3390–3396 CrossRef CAS.
- J. Liu, S. Liu and Y. Wu, J. Alloys Compd., 2022, 895, 162725 CrossRef CAS.
- T. S. H Yamazak, W. Sakamoto and T. Yago, J. Ceram. Soc. Jpn., 2010, 118, 631–635 CrossRef.
- S. Zhang, Y. Yu, J. Wu, X. Gao, C. Huang and S. Dong, J. Alloys Compd., 2018, 731, 1140–1145 CrossRef CAS.
- J. Chen, H. Shi, G. Liu, J. Cheng and S. Dong, J. Alloys Compd., 2012, 537, 280–285 CrossRef CAS.
- M. D. Drahus, P. Jakes, E. Erdem, S. Schaab, J. Chen, M. Ozerov, S. Zvyagin and R. A. Eichel, Phys. Rev. B, 2011, 84, 064113 CrossRef.
- S. J. Zhang, E. F. Alberta, R. E. Eitel, C. A. Randall and T. R. Shrout, IEEE Trans. Ultrason. Ferroelectr. Freq. Control., 2005, 52, 2131–2139 Search PubMed.
- B. Deng, Q. Wei, C. He, Z. Wang, X. Yang and X. Long, J. Alloys Compd., 2019, 790, 397–404 CrossRef CAS.
- J. Chen, Y. Dong and J. Cheng, Ceram. Int., 2015, 41, 9828–9833 CrossRef CAS.
- J. Chen, G. Jin, C.-M. Wang, J. Cheng and D. Damjanovic, J. Am. Ceram. Soc., 2014, 97, 3890–3896 CrossRef CAS.
- Z. Lan, J. Liu, S. Ren, X. Jiang, K. Chen, L. Fang, B. Peng, D. Wang and L. Liu, J. Mater. Sci., 2019, 54, 13467–13478 CrossRef CAS.
- P. Kantha, N. Pisitpipathsin, K. Pengpat, S. Eitssayeam, G. Rujijanagul, R. Gua and A. S. Bhalla, Mater. Res. Bull., 2012, 47, 2867–2870 CrossRef CAS.
- F. Mou, A. Xue, Q. Liu, S. Peng, Y. Wang, E. Cai and S. Wang, J. Mater. Sci.: Mater. Electron., 2021, 32, 2432–2440 CrossRef CAS.
- K. Chen, F. Zhang, Y. Jiao, D. Li, J. Tang, F. Gao, L. An and G. Brennecka, J. Am. Ceram. Soc., 2016, 99, 1681–1686 CrossRef CAS.
- F. Zeng, Q. Liu, S. Peng, Y. Wang, E. Cai, A. Xue and S. Zhou, Ceram. Int., 2019, 45, 1416–1419 CrossRef CAS.
- W. M. Zhu and Z. G. Ye, Appl. Phys. Lett., 2006, 89, 232904 CrossRef.
- X. G. Tang, K. H. Chew and H. L. W. Chan, Acta Mater., 2004, 52, 5177–5183 CrossRef CAS.
- T. Shi, L. Xie, L. Gu and J. Zhu, Sci. Rep., 2015, 5, 8606 CrossRef CAS PubMed.
- S. W. Gotmare, S. O. Leontsev and R. E. Eitel, J. Am. Ceram. Soc., 2010, 93, 1965–1969 CAS.
- J. G. Wu, X. Chen, Z. Q. Chu, W. L. Shi, Y. Yu and S. X. Dong, Appl. Phys. Lett., 2016, 109, 173901 CrossRef.
- J. G. Wu, H. D. Shi, T. L. Zhao, Y. Yu and S. X. Dong, Adv. Funct. Mater., 2016, 26, 7186–7194 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |