Open Access Article
Open Access ArticleSynthesis of ultrahigh-molecular-weight ethylene/1-octene copolymers with salalen titanium(IV) complexes activated by methyaluminoxane†
Baokang Zoua,
Yipeng Zhana,
Xiuli Xiea,
TongTong Zhanga,
Runkai Qiua and
Fangming Zhu *ab
*ab
aPCFM, GDHPPC Lab, School of Chemistry, Sun Yat-Sen University, 510275, China. E-mail: ceszfm@mail.sysu.edu.cn; Fax: +86-20-84114033; Tel: +86-20-84113250
bKey Lab for Polymer Composite and Functional Materials of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275, China
First published on 14th April 2022
Abstract
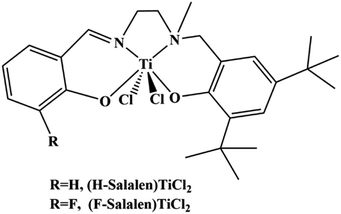
Two salalen titanium(IV) complexes ((H-salalen)TiCl2 and (F-salalen)TiCl2) containing hydrogen and fluorine respectively on the phenolate ring close to the imine were synthesized for the copolymerization of ethylene with 1-octene to prepare poly(ethylene-co-1-octene) in the presence of methylaluminoxane (MAO). The (F-salalen)TiCl2/MAO showed higher catalytic activity and better copolymer characteristics such as a higher molecular weight, narrower molecular weight distribution, and higher 1-octene incorporation than (H-salalen)TiCl2/MAO, which revealed that the electron-withdrawing conjugated effect introduced by fluorine substituents led to improvements on catalytic performance and thermal stability. The influences of copolymerization conditions including temperature, Al/Ti molar ratios and comonomer feed ratios on the copolymerization behavior of (F-salalen)TiCl2/MAO and the copolymer microstructure were investigated in detail. Under the activation of MAO, the (F-salalen)TiCl2 could produce ultrahigh molecular weight poly(ethylene-co-1-octene) with 1-octene incorporation ratios in the range of 0.9–3.1 mol% and exhibit relatively high activity. It could be inferred that long ethylene sequences in the copolymer were segregated by the isolated 1-octene units based on the 13C NMR characterization of the copolymer. Moreover, the thermal properties and crystallization of copolymers were determined by DSC and XRD and correlated to the ethylene sequence length distribution. The reactivity ratios calculated by the triad distribution in 13C NMR revealed the random comonomer distribution in the copolymer chain.
Introduction
Polyethylene including high-density polyethylene (HDPE), linear low-density polyethylene (LLDPE), and low density polyethylene (LDPE) is the most widely used polymer material in the world owing to its excellent comprehensive performance and wide application fields, and its global annual production has exceeded 100 million tons.1 Furthermore, ultrahigh-molecular-weight polyethylene (UHMWPE) with a well-defined linear structure and ultrahigh-weight-average molecular weight (Mw) more than 1.0 × 106 g mol−1 has attracted great attention because of its exceptional wear resistance, impact resistance, chemical stability and lubricity as well as successful application in engineering plastics.2–4 However, the UHMWPE is difficult to process as a result of high chain entanglement density giving rise to poor melt fluidity.5 It is worth noting that the processing performance of UHMWPE can be significantly improved via the introduction of a small amount of long chain branches. Nevertheless, the introduction of long chain branches (carbon atoms ≥ 6) into the UHMWPE molecular chain is difficult to achieve by copolymerization of ethylene with higher α-olefins such as 1-octene and 1-decene.6–8 Therefore, in the past few decades, many catalyst systems with high activity and controllable comonomer incorporation rate have been continuously developed for the copolymerization of ethylene with α-olefin, trying to obtain ultrahigh-molecular-weight polyethylene with improved processing properties.Ziegler–Natta catalysts especially supported Ziegler–Natta catalysts displayed high activity when promoting copolymerization of ethylene with short chain α-olefins. However, copolymers obtained by heterogeneous multi-center Ziegler–Natta systems typically have low commoner content, non-uniform commoner distribution and broad molecular weight distribution, which greatly affects copolymer properties.9,10 Thus single site catalysts include metallocene catalysts and post metallocene catalysts are preferred to produce ethylene/α-olefin copolymers with better mechanical and rheological properties.11–18 In 1992, the Dow Chemical Company (Dow) launched a constrained geometry catalyst (CGC) system viable in commercial operations.19 In contrast with the former metallocene catalyst, Dow's CGC linked mono-cyclopentadienyl group by a bridge to the amide, which gives the catalyst larger coordination space. This structure is beneficial to produce ethylene/1-octene copolymer with controlled 1-octene incorporation and relatively narrow molecular weight distribution to afford polyolefin elastomer (1-octene content from 20 to 30 wt%) and plastomer polyolefin (1-octene content lower than 20 wt%). However, the copolymer obtained by CGC also has a lower molecular weight, which limits the application range of the material to some extent.20 Since then many new CGC and post metallocene catalysts have been designed and developed, especially many group IV transition metal complexes bearing O,N-chelating or N,N-chelating ligands exhibited surprisingly excellent catalytic performance on the copolymerization of ethylene with α-olefin, and made significant improvement on the polymer molecular weight and comonomer content.8,21–25
The salalens are tetradentate [ONN′O]-type ligands can be regard as half-salan/half-salen, raping the metal center in a fac–mer mode with perfect octahedral structure.26,27 Salalen type complexes exhibit amazing catalytic performance on various reactions like ring-opening polymerization, asymmetric oxidations, epoxide-CO2 polymerization and α-olefin homopolymerization.28–39 But to the best of our knowledge, there are few literature has been reported using salalen type complexes on the copolymerization of ethylene with long chain α-olefin. Our research group previously synthesized two types of salalen titanium(IV) complexes (Scheme 1) and successfully applied them to produce ultrahigh-molecular-weight ethylene.40
The fluorinated salalen titanium(IV) complex (F-salalen)TiCl2 performed relatively high catalytic activity and thermal stability on ethylene homopolymerization due to the electron withdrawing conjugated effect, as well as exhibited quasi-living characteristic. Recently, we found that both (H-salalen)TiCl2 and (F-salalen)TiCl2 were able to catalyze copolymerization of ethylene with long chain α-olefin like 1-octene and 1-decene. In particular, ultrahigh-molecular-weight poly(ethylene-co-1-octene) can be obtained by (F-salalen)TiCl2/MAO under certain reaction conditions, which is an exciting result and worthy of further study.
Results and discussion
This work was focused on the copolymerization of ethylene with 1-octene including catalytic behaviour as well as the analysis and characterization of the resultant copolymers. Two salalen titanium complexes were synthesized as comparison according to our previous work. Copolymerization of ethylene with 1-octene were carried out at different temperatures using (H-salalen)TiCl2 and (F-salalen)TiCl2 activated by MAO, and the results were summarized in Table 1.| Entry | Complex | Tp (°C) | Activityb | Mwc ×10−6 | Mw/Mnc | Tmd (°C) | ΔHd (J g−1) | Xcd (%) | 1-Oct. cont.e (mol%) |
|---|---|---|---|---|---|---|---|---|---|
| a Polymerization conditions: catalyst, 20.0 μmol; Al/Ti = 600; 1-octene, 1.0 mol L−1; ethylene pressure, 15 atm; total volume, 70 mL; reaction time, 30 min.b In 103 g polymer (mol Ti)−1 h−1.c g mol−1, determined by HT-GPC in 1,2,4-TCB versus polystyrene standard.d Determined by DSC at a heating rate of 10 °C min−1 and used the second heating curves.e 1-Octene incorporation (mol%) estimated by 13C NMR spectra. | |||||||||
| 1 | (H-Salalen)TiCl2 | 30 | 10.2 | 0.71 | 2.7 | 127.0 | 183.4 | 62.6 | 0.3 |
| 2 | 40 | 34.7 | 0.49 | 3.2 | 123.7 | 114.7 | 39.1 | 1.3 | |
| 3 | 50 | 39.9 | 0.05 | 3.6 | 122.9 | 105.0 | 35.8 | 2.0 | |
| 4 | (F-Salalen)TiCl2 | 30 | 35.6 | 1.89 | 2.1 | 125.0 | 177.3 | 60.5 | 0.9 |
| 5 | 40 | 42.0 | 1.23 | 2.2 | 124.1 | 141.0 | 48.1 | 1.8 | |
| 6 | 50 | 92.3 | 0.87 | 2.6 | 119.4 | 80.6 | 27.5 | 3.1 | |
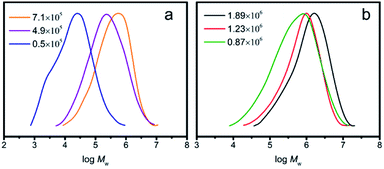
As displayed in Table 1, both salalen type titanium complexes ((F-salalen)TiCl2 and (H-salalen)TiCl2) could catalyze the copolymerization of ethylene with 1-octene under MAO activation to produce poly(ethylene-co-1-octene) with high molecular weight. Note that (F-salalen)TiCl2/MAO showed higher catalytic activity, which was more than twofold that of (H-salalen)TiCl2 at 50 °C. In particular, as depicted by Fig. 1, (F-salalen)TiCl2/MAO were able to afforded poly(ethylene-co-1-octene) with ultrahigh molecular weight of 1.89 × 106 and 1.23 × 106 g mol−1 respectively when polymerization temperature at 30 and 40 °C. In contrast, (H-salalen)TiCl2 can only provide copolymers with molecular weight lower than 7.1 × 105 g mol−1 under the same polymerization conditions. Moreover, the copolymers obtained by (F-salalen)TiCl2/MAO exhibited narrower molecular weight distribution (Mw/Mn ∼ 2.6) and higher 1-octene incorporation than (H-salalen)TiCl2/MAO. These results were in agreement with those obtained from the ethylene homopolymerization based on the electronically flexible property and electron withdrawing conjugated effect consisting of fluorine (F), phenyl ring, imine and titanium(IV) species.41–44 Therefore, there was a reason to believe that the (F-salalen)TiCl2/MAO catalyst revealed stronger thermal stability and better catalytic performance for coordination copolymerization of ethylene with 1-octene than (H-salalen)TiCl2 catalyst.
In order to further investigate the catalytic behaviour of (F-salalen)TiCl2/MAO for the copolymerization of ethylene with 1-octene under different polymerization conditions, the representative polymerization results at various temperatures, Al/Ti molar ratios and monomer feed ratios were listed in Table 2. The catalytic activity of copolymerization of ethylene with 1-octene was higher than ethylene homopolymerization because of the “comonomer effect”. The introduction of 1-octene into polyethylene chain reduced the crystallinity and improved the solubility of polymer, leading to easier exposure and diffusion of active centers. However, the molecular weight of copolymerization products had drop sharply from 2.64 × 106 to 0.87 × 106 g mol−1, and the products were hardly observed in the 1-octene homopolymerization. This probably due to long-chain 1-octene with large steric hindrance were more difficult to coordinate with active species and insert into the polyethylene chain.
| Entry | Al/Ti (mol mol−1) | Tp (°C) | 1-Oct. feed (mol L−1) | Activityb | Mwc ×10−6 | Mw/Mnc | Tmd (°C) | ΔHd (J g−1) | Xcd (%) | 1-Oct. cont.e (mol%) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerization conditions: catalyst, 20.0 μmol; ethylene pressure, 15 atm; total volume, 70 mL; reaction time, 30 min.b 103 g polymer (mol Ti)−1 h−1.c g mol−1, determined by HT-GPC in 1,2,4-TCB versus polystyrene standard.d Determined by DSC at a heating rate of 10 °C min−1 and used the second heating curves.e 1-Octene incorporation (mol%) estimated by 13C NMR spectra.f 1-Octene homopolymerization, ethylene pressure, 0 atm. | ||||||||||
| 1 | 600 | 50 | 0 | 56.5 | 2.64 | 2.4 | 134.6 | 204.5 | 69.8 | 0 |
| 2f | 600 | 50 | 1.2 | n.d | — | — | — | — | ||
| 3 | 600 | 30 | 1.0 | 35.6 | 1.89 | 2.1 | 125.0 | 177.3 | 60.5 | 0.9 |
| 4 | 600 | 40 | 1.0 | 42.0 | 1.23 | 2.2 | 124.1 | 141.0 | 48.1 | 1.8 |
| 5 | 600 | 50 | 1.0 | 92.3 | 0.87 | 2.6 | 119.4 | 80.6 | 27.5 | 3.1 |
| 6 | 600 | 70 | 1.0 | 141.0 | 0.22 | 3.2 | 120.5 | 85.3 | 29.1 | 2.0 |
| 7 | 600 | 90 | 1.0 | 101.2 | 0.10 | 3.9 | 120.9 | 136.9 | 46.7 | 1.9 |
| 8 | 200 | 50 | 1.0 | 63.5 | 1.64 | 1.9 | 123.5 | 101.1 | 34.5 | 2.0 |
| 9 | 400 | 50 | 1.0 | 87.0 | 1.24 | 2.1 | 123.3 | 83.8 | 28.6 | 2.6 |
| 10 | 800 | 50 | 1.0 | 35.0 | 0.57 | 3.4 | 123.5 | 101.4 | 34.6 | 2.2 |
| 11 | 600 | 50 | 0.2 | 79.8 | 1.26 | 2.7 | 128.7 | 172.1 | 58.7 | 1.2 |
| 12 | 600 | 50 | 0.4 | 127.5 | 1.00 | 2.9 | 124.4 | 160.6 | 54.8 | 1.7 |
| 13 | 600 | 50 | 0.8 | 132.5 | 0.89 | 2.6 | 122.3 | 92.2 | 31.5 | 2.8 |
| 14 | 600 | 50 | 2.0 | 64.0 | 0.78 | 2.7 | 117.9 | 65.8 | 22.5 | 6.4 |
To explore the effect of polymerization temperature on copolymers produced by (F-salalen)TiCl2/MAO, different polymerization temperatures involving 30, 40, 50, 70, 90 °C were set (entry 3–7, Table 2) when 1-octene concentration was 1.0 mol L−1. Catalytic activity increased steadily from 35.6 × 103 g polymer (mol Ti)−1 h−1 at 30 °C to 141.0 × 103 g polymer (mol Ti)−1 h−1 at 70 °C and still maintained a moderate high level at 90 °C, implying good thermal stability of (F-salalen)TiCl2. The increase of activity at 30–70 °C came from the increase of the number of active species and the thermal stability was probably due to the electron withdrawing conjugate effect consisting of F, phenyl ring, imine and titanium(IV). Besides, the increase in the number of active species reduced the number-average chain length and thus the molecular weight of copolymers declined. At 30–50 °C, ethylene/1-octene copolymers with ultrahigh-molecular-weight could be prepared in 30 min. While the molecular weight was less than 1.0 × 106 g mol−1 at higher polymerization temperatures, and it decreased to 1.0 × 105 g mol−1 at 90 °C. Besides, molecular weight distribution became broader when temperature increased because the chain transfer rate increased rapidly or appeared other active centers at high temperature.45,46 Appropriate heating may help the insertion of 1-octene according to entries 3–5 in Table 2, but when the temperature rose to 70 °C the content of 1-octene in copolymers started to decline instead.
Influence of Al/Ti molar ratios on the copolymerization of ethylene with 1-octene using (F-salalen)TiCl2/MAO was then investigated. Table 2 showed that the catalytic activity increased with the increase of Al/Ti molar ratios from 200 to 600. On the one hand, less MAO was not enough to remove impurities unfavorable to the catalyst like moisture and oxygen in the polymerization system. On the other hand, only a sufficient amount of cocatalyst could fully activate the catalyst, as MAO could capture the chlorine atoms from the (F-salalen)TiCl2 complex and formed alkyl ligand and vacancy in the central metal to form the active species to promote copolymerization. However, the activity suddenly dropped sharply when the Al/Ti mole ratio reached 800 (Table 2, entry 10). This is probably due to excessive MAO reduced the titanium metal with high oxidation state to low oxidation state, resulting in a decrease in the number of effective active species.47 In addition, lower Al/Ti mol ratio resulted in the formation of higher molecular weight copolymer, for example, the copolymer with ultrahigh molecular weight of 1.64 × 106 g mol−1 and narrow molecular weight distribution (Mw/Mn = 1.9) could be obtained at Al/Ti = 200 (Table 2, entry 8). Correspondingly, the molecular weight started to decrease as well as molecular weight distribution became broader with the increase of Al/Ti molar ratios, which is due to the gradually intensive chain transfer reaction to MAO.
It was obvious from Table 2 that the copolymerization yield, the molecular weight, and the 1-octene incorporation of the copolymers related to the 1-octene concentration in the feed (entry 1, 5, 11–14). When the concentration of 1-octene increased from 0 mol L−1 to 1.0 mol L−1, the catalytic activity continued to increase. But after 1-octene concentration reached a higher value of 2.0 mol L−1, the copolymer yield started to drop again. Molecular weight of copolymers kept decreasing accompanied by the increase of 1-octene concentration. These variations on catalytic activity and molecular weight brought by the introduction of 1-octene were coincided with “comonomer effect” in Ziegler–Natta catalytic system and metallocene catalysts.48 Furthermore, the content of 1-octene in the copolymer increased gradually and reached 6.4% at 2.0 mol L−1.
The 13C NMR spectrum in Fig. 2 showed that the resonance at 30.0 ppm arose from polyethylene sequences was the most abundant peak, which could be assigned to CH2 in polyethylene chain. However, those signals dated from continuous 1-octene block sequence (OOO) or alternating sequence (EOEO) were hardly observed, and the ethylene average sequence length was calculated as 13.8 through 13C NMR triad distribution (Table S2†), suggesting that 1-octene units were isolated by long ethylene segments.
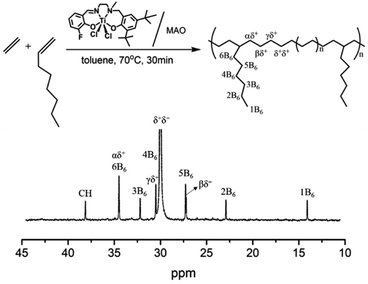 | ||
| Fig. 2 13C NMR spectrum of poly(ethylene-co-1-octene) obtained using the (F-salalen)TiCl2/MAO (entry 6 in Table 2). | ||
The differential scanning calorimeter (DSC) was used to determine the melting temperature of copolymers with different 1-octene incorporation, and the second heating curves were displayed in Fig. 3. Compared with ultrahigh molecular weight polyethylene produced by (F-salalen)TiCl2/MAO, copolymers with introduction of 1-octene exhibited decline in melting temperature. With the increase of 1-octene content in poly(ethylene-co-1-octene), the melting temperatures also showed decrease. When the 1-octene content reached the maximum value of 6.4% at 2.0 mol L−1, the melting temperature was as low as 117.9 °C. These copolymers showed higher melting temperature than many other previously reported poly(ethylene-co-1-octene) with similar comonomer incorporation because of the longer polyethylene segments.49,50 It is worth noting that some shoulders can be seen at lower temperatures for samples with higher 1-octene incorporation (e.g. entry 13). We suggest that the main chain was divided into polyethylene segments with different length due to the heterogeneous distribution of 1-octene. Consequently, the shoulder peaks might originate from the crystallization of some shorter polyethylene segments. This heterogeneous distribution can be proved by DSC-SSA thermogram (Fig. S2†). There were nine narrow peaks appeared at 60–100 °C in the SSA thermogram of entry 13, denoting about nine polyethylene segments with different length, which caused the wide shoulder in DSC heating curve.
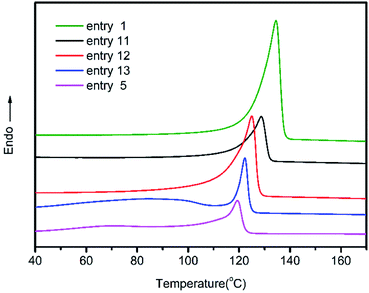 | ||
| Fig. 3 DSC profiles of poly(ethylene-co-1-octene) with different 1-octene incorporation obtained from entry 1, 5, 11–13 in Table 2. | ||
The crystallinity of these copolymers produced by (F-salalen)TiCl2/MAO were also studied. The X-ray diffraction patterns of the resultant ultrahigh-molecular-weight polyethylene and ethylene/1-octene copolymers with different 1-octene content were shown in Fig. 4. It could be seen that there were two obvious peaks at the 2θ values of 21.6° and 24.0° corresponded to 110 and 200 crystal planes of polyethylene orthorhombic crystal form, respectively.50 The crystallinity of copolymers declined from 72.7% to 23.4% (Table S3†) with the increase of 1-octene concentration in the feed, exhibiting a trend consisted with crystallinity results calculated by DSC. The low incorporation of 1-octene (≤6.4%) also means that only a little 1-octene units into polyethylene chain can affect the crystallization of the copolymer significantly. Moreover, a broad peak at 19–20° in the copolymer diffraction pattern appeared and gradually became obvious as the content of 1-octene increases, denoting that the amorphous part of the copolymer was gradually increasing.
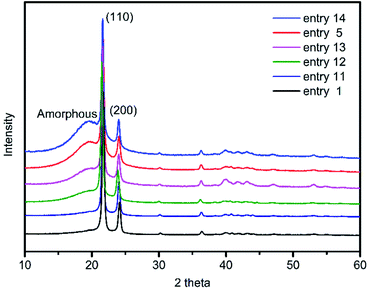 | ||
| Fig. 4 X-ray diffractograms of poly(ethylene-co-1-octene) with different 1-octene incorporation in Table 2, from top to bottom: entry 14, entry 5, entry 13, entry 12, entry 11, entry 1. | ||
In order to further explore the distribution of ethylene and 1-octene in the copolymer chains, the reactivity ratios were calculated by the triad distribution from the 13C NMR spectrum (Table S2†) and provided the values rE = 11.25, rO = 0.15 and rErO = 1.69. The calculation results showed the preference for ethylene incorporation promoted the formation of long polyethylene sequences along the copolymer chain, suggesting that (F-salalen)TiCl2/MAO tends to produce random ethylene/1-octene copolymer.
The melting temperature and crystalline of ethylene/1-octene copolymer are always related to the ethylene sequence length distribution. Using the value of reactivity ratios, the ethylene sequence distribution can be calculated by Flory's theory:51
| Wn = n(1 − p)2pn−1 | (1) |
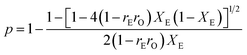 | (2) |
The ethylene mole fraction XE of sample entry 6 in Table 1 and rErO = 1.69 were used in the expression (2). Finally the ethylene sequence distribution of ethylene/1-octene copolymer obtained by (F-salalen)TiCl2/MAO was shown in Fig. 5. The most probable ethylene unit n appeared at 32. To explore the relationship between DSC curve and ethylene sequence length, the following expression (3) and (4) were used:52
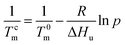 | (3) |
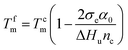 | (4) |
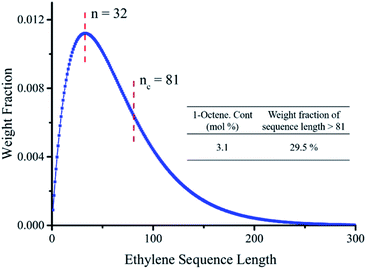 | ||
| Fig. 5 Ethylene sequence length distribution of ethylene/1-octene (entry 14 in Table 2). | ||
Experimental
Materials
All operations involving anhydrous and anoxic should be performed using standard Schlenk line techniques or in a glove box filled with high purity nitrogen. Ethylene (99.9%, GQ-gas) and nitrogen (99.999%, GQ-gas) were used directly without any pretreatment. Toluene (Analytical Reagent, Guangzhou Brand) was refluxed in the presence of sodium or potassium for at least 10 h, using benzophenone as indicator until the mixture turned blue. 1-Octene (98%, Aladdin) was added calcium hydride and stirred overnight and then distilled for use. Methylaluminoxane (MAO) was purchased from AkzoNobel as 10 wt% Al toluene solution. The salalen titanium(IV) complexes used in this work was synthesized as described in the literature,40 and it's structure can be seen in Scheme 1.Copolymerization of ethylene with 1-octene
Copolymerization of ethylene with 1-octene was carried out in a 250 mL autoclave equipped with program temperature controller and mechanical stirring. The autoclave was firstly heated to 150 °C for 3 hours under vacuum in order to remove moisture and oxygen, and then cooled to the reaction temperature. Toluene, 1-octene, and methylaluminoxane (MAO) solution were sequentially injected into the autoclave and stirred for 5 min. The copolymerization was started by charging the salalen titanium(IV) complexes (pre-dissolved in toluene) into the autoclave and then rose the ethylene pressure to 15 atm. The polymerization temperature was kept by the program temperature controller, and the error did not exceed ±2 °C. After the polymerization reached 30 min at the reaction temperature, stopped ethylene feed and injected 5 mL methanol into the autoclave to terminated polymerization. The resulting mixture was poured into acidic ethanol (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol/HCl) and stirred for 12 hours. The copolymers were finally collected after washing with ethanol, filtration and dried under vacuum at 50 °C to a constant weight.
1 ethanol/HCl) and stirred for 12 hours. The copolymers were finally collected after washing with ethanol, filtration and dried under vacuum at 50 °C to a constant weight.
Polymer characterization
Molecular weight (Mw) and molecular weight distribution (Mw/Mn) were measured by using high-temperature chromatograph (GPC) on a PL-GPC 220 type at 150 °C. The instrument was equipped with a refractive index detector and used 1,2,4-trichlorobenzene at a flow rate of 1.0 mL min−1 as the mobile phase, samples were dissolved in 1,2,4-trichlorobenzene as 1 mg mL−1 solution. The calibration curves were made by monodisperse polystyrene with a sample solution concentration of 0.5–1.0 mg mL−1. 13C NMR spectra of the copolymers were obtained with a Bruker Avance III 500 MHz NMR instrument at 120 °C. The samples were dissolved as 100 mg mL−1 in the 10 mm high temperature NMR tubes, using a mixed solvent of deuterated o-dichlorobenzene/o-dichlorobenzene with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The 1-octene contents, ethylene average sequence length and triad sequential distribution of the copolymers were calculated from the 13C NMR spectra. The thermal properties of polymers were determined by differential scanning calorimetry (DSC) on a DSC-4000 from PerkinElmer company under N2 atmosphere. The follow programs were set: firstly weighed 3–5 mg polymers and heated it to 180 °C with a rate of 10 °C min−1, kept at 180 °C for 5 min to remove the thermal history. Then cooled down to 30 °C with a rate of 10 °C min−1, kept at 30 °C for 5 min followed by heating to 180 °C with a rate of 10 °C min−1 again, finally recorded the second heating curve. The crystal structure of samples was determined by X-ray diffraction (XRD) on a RIGAKU D-MAX 2200 at room temperature. Using Cu Kα radiation and the diffraction scans from 10°–80° with a rate of 10° min−1.
1. The 1-octene contents, ethylene average sequence length and triad sequential distribution of the copolymers were calculated from the 13C NMR spectra. The thermal properties of polymers were determined by differential scanning calorimetry (DSC) on a DSC-4000 from PerkinElmer company under N2 atmosphere. The follow programs were set: firstly weighed 3–5 mg polymers and heated it to 180 °C with a rate of 10 °C min−1, kept at 180 °C for 5 min to remove the thermal history. Then cooled down to 30 °C with a rate of 10 °C min−1, kept at 30 °C for 5 min followed by heating to 180 °C with a rate of 10 °C min−1 again, finally recorded the second heating curve. The crystal structure of samples was determined by X-ray diffraction (XRD) on a RIGAKU D-MAX 2200 at room temperature. Using Cu Kα radiation and the diffraction scans from 10°–80° with a rate of 10° min−1.
Conclusions
Two salalen titanium complexes, (H-salalen)TiCl2 and (F-salalen)TiCl2 are firstly used to catalyze the copolymerization of ethylene with 1-octene in the presence of methylaluminoxane (MAO) with moderate activity, in which (F-salalen)TiCl2 shows better catalytic performance and higher thermal stability owing to the electron withdrawing conjugated effect introduced by F atom. Under the optimized polymerization conditions, (F-salalen)TiCl2/MAO can produce ultrahigh-molecular-weight poly(ethylene-co-1-octene) with relatively narrow molecular weight distribution. The 1-octene content of these resultant copolymers was controlled from 0.9 to 6.4 mol% through adjusting the 1-octene feed concentration from 0.2 to 2.0 mol L−1. Based on DSC and XRD characterization, high molecular weight and higher crystallinity lead to relatively high melting temperature (128.7–117.9 °C) of the copolymers, and the results are agreed well with ethylene sequence length distribution. The molecular chain structure of the copolymers is characterized by 13C NMR, suggesting that long ethylene sequences in poly(ethylene-co-1-octene) chains are segregated by isolated 1-octene units. The reactivity ratios (rE = 11.25, rO = 0.15, rErO = 1.69) calculated by the triad sequential distribution indicates that (F-salalen)TiCl2/MAO tends to produce random ethylene/1-octene copolymers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51773228, 51573212).Notes and references
- M. Ronellenfitsch, T. Gehrmann, H. Wadepohl and M. Enders, Macromolecules, 2016, 50, 35–43 CrossRef.
- J. Xia, Y. Zhang, S. Kou and Z. Jian, J. Catal., 2020, 390, 30–36 CrossRef CAS.
- L. Guo, K. Lian, W. Kong, S. Xu, G. Jiang and S. Dai, Organometallics, 2018, 37, 2442–2449 CrossRef CAS.
- D. Romano, N. Tops, E. Andablo-Reyes, S. Ronca and S. Rastogi, Macromolecules, 2014, 47, 4750–4760 CrossRef CAS.
- A. Pandey, Y. Champouret and S. Rastogi, Macromolecules, 2011, 44, 4952–4960 CrossRef CAS.
- Z. Zhou, C. Anklin, R. Cong, X. Qiu and R. Kuemmerle, Macromolecules, 2021, 54, 757–762 CrossRef CAS.
- X. Hu, C. Geng, G. Yang, Q. Fu and H. Bai, RSC Adv., 2015, 5, 54488–54496 RSC.
- S. Ohta, Y. Kasai, T. Toda, K. Nishii and M. Okazaki, Polym. J., 2018, 51, 345–349 CrossRef.
- M. Białek and K. J. P. Czaja, Polymer, 2000, 41, 7899–7904 CrossRef.
- Z. Zhao, T. Mikenas, V. A. Zakharov, M. Nikolaeva, M. Matsko, E. Bessudnova and W. J. P. J. Wu, Polyolefins J., 2019, 6, 117–126 CAS.
- G. Zanchin, F. Bertini, L. Vendier, G. Ricci, C. Lorber and G. Leone, Polym. Chem., 2019, 10, 6200–6216 RSC.
- W. Liu, A. T. Ojo, W.-J. Wang, H. Fan, B.-G. Li and S. Zhu, Polym. Chem., 2015, 6, 3800–3806 RSC.
- X. Ren, F. Guo, H. Fu, Y. Song, Y. Li and Z. Hou, Polym. Chem., 2018, 9, 1223–1233 RSC.
- S. M. M. Mortazavi, H. Arabi, G. Zohuri, S. Ahmadjo, M. Nekoomanesh and M. Ahmadi, Polym. Int., 2010, 59, 1258–1265 CrossRef CAS.
- E. S. Cueny and C. R. Landis, ACS Catal., 2019, 9, 3338–3348 CrossRef CAS.
- K. Kunz, G. Erker, S. Döring, R. Fröhlich and G. Kehr, J. Am. Chem. Soc., 2001, 123, 6181–6182 CrossRef CAS PubMed.
- U. G. Joung, C. J. Wu, S. H. Lee, C. H. Lee, E. J. Lee, W.-S. Han, S. O. Kang and B. Y. J. O. Lee, Organometallics, 2006, 25, 5122–5130 CrossRef CAS.
- J. H. Park, S. H. Do, A. Cyriac, H. Yun and B. Y. Lee, Dalton Trans., 2010, 39, 9994–10002 RSC.
- P. S. Chum and K. W. Swogger, Prog. Polym. Sci., 2008, 33, 797–819 CrossRef CAS.
- K. Soga, T. Uozumi, S. Nakamura, T. Toneri, T. Teranishi, T. Sano, T. Arai and T. Shiono, Macromol. Chem. Phys., 1996, 197, 4237–4251 CrossRef CAS.
- S.-W. Zhang, L.-P. Lu, Y.-Y. Long and Y.-S. Li, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 5298–5306 CrossRef CAS.
- W. Liu, K. Zhang, H. Fan, W.-J. Wang, B.-G. Li and S. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 405–414 CrossRef CAS.
- S. Saadat, J. B. P. Soares, P. J. DesLauriers and J. S. Fodor, Macromol. React. Eng., 2020, 14(2), 1900032 CrossRef CAS.
- S. Guo, H. Fan, Z. Bu, B. G. Li and S. Zhu, Macromol. Rapid Commun., 2015, 36, 286–291 CrossRef CAS PubMed.
- T. J. Williams, J. V. Lamb, J.-C. Buffet, T. Khamnaen and D. O'Hare, RSC Adv., 2021, 11, 5644–5650 RSC.
- K. Matsumoto, B. Saito and T. Katsuki, Chem. Commun., 2007, 3619–3627, 10.1039/b701431g.
- A. Yeori, S. Gendler, S. Groysman, I. Goldberg and M. Kol, Inorg. Chem. Commun., 2004, 7, 280–282 CrossRef CAS.
- A. Cohen, G. W. Coates and M. Kol, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 593–600 CrossRef CAS.
- K. Press, A. Cohen, I. Goldberg, V. Venditto, M. Mazzeo and M. Kol, Angew. Chem., Int. Ed. Engl., 2011, 50, 3529–3532 CrossRef CAS PubMed.
- K. Huang, S. Zhou, D. Zhang, X. Gao, Q. Wang and Y. Lin, J. Organomet. Chem., 2013, 741–742, 83–90 CrossRef CAS.
- A. Berkessel, T. Günther, Q. Wang and J.-M. Neudörfl, Angew. Chem., 2013, 125, 8625–8629 CrossRef.
- Y. Sawada, K. Matsumoto and T. Katsuki, Angew. Chem., Int. Ed. Engl., 2007, 46, 4559–4561 CrossRef CAS PubMed.
- E. P. Talsi, A. A. Bryliakova and K. P. Bryliakov, Chem. Rec., 2016, 16, 924–939 CrossRef CAS PubMed.
- X. Ji, W. Yao, X. Luo, W. Gao and Y. Mu, New J. Chem., 2016, 40, 2071–2078 RSC.
- Y.-L. Duan, Z.-J. Hu, B.-Q. Yang, F.-F. Ding, W. Wang, Y. Huang and Y. Yang, Dalton Trans., 2017, 46, 11259–11270 RSC.
- K. Nie, W. Gu, Y. Yao, Y. Zhang and Q. Shen, Organometallics, 2013, 32, 2608–2617 CrossRef CAS.
- A. Pilone, N. De Maio, K. Press, V. Venditto, D. Pappalardo, M. Mazzeo, C. Pellecchia, M. Kol and M. Lamberti, Dalton Trans., 2015, 44, 2157–2165 RSC.
- X. Zhang, Y. Liu, Y. Chen, Z. Zhang, D. Fan and L. Xingqiang, Inorg. Chem. Commun., 2014, 48, 69–72 CrossRef CAS.
- H. Engler, M. Lansing, C. P. Gordon, J.-M. Neudörfl, M. Schäfer, N. E. Schlörer, C. Copéret and A. Berkessel, ACS Catal., 2021, 11, 3206–3217 CrossRef CAS.
- S. Li, Y. Zhu, H. Liang, X. Xie, Y. Zhan, G. Liang and F. Zhu, RSC Adv., 2019, 9, 41824–41831 RSC.
- G. Xie and C. Qian, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 211–217 CrossRef CAS.
- M. A. M. Capozzi, F. Capitelli, A. Bottoni, M. Calvaresi and C. Cardellicchio, ChemCatChem, 2013, 5, 210–219 CrossRef CAS.
- G. Xie and C. Qian, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 211–217 CrossRef CAS.
- T. Li, W. Song, H. Ai, Q. You, A. Zhang and G. Xie, J. Polym. Res., 2014, 22, 631 CrossRef.
- M. Khoshsefat, S. Ahmadjo, S. Mortazavi, G. Zohuri and J. Soares, New J. Chem., 2018, 42, 8334–8337 RSC.
- M. Khoshsefat, A. Dechal, S. Ahmadjo, S. M. M. Mortazavi, G. Zohuri and J. B. Soares, Eur. Polym. J., 2019, 119, 229–238 CrossRef CAS.
- L. Zhu, H. Yu, L. Wang and Y. Xing, Mater. Today Commun., 2021, 29, 102801 CrossRef CAS.
- M. Yin, Z. Zhang, Y. Xiong, M. Liu, Y. Zhang and P. Mi, Chem. Res. Chin. Univ., 2019, 35, 1089–1094 CrossRef CAS.
- E. Szuromi, J. Klosin and K. A. Abboud, Organometallics, 2011, 30, 4589–4597 CrossRef CAS.
- M. Wannaborworn, P. Praserthdam and B. Jongsomjit, Molecules, 2011, 16, 373–383 CrossRef CAS PubMed.
- M. Atiqullah, S. Adamu and A.-H. M. Emwas, J. Taiwan Inst. Chem. Eng., 2017, 76, 141–155 CrossRef CAS.
- S. Hosoda, Y. Nozue, Y. Kawashima, K. Suita, S. Seno, T. Nagamatsu, K. B. Wagener, B. Inci, F. Zuluaga and G. Rojas, Macromolecules, 2011, 44, 313–319 CrossRef CAS.
- S. Guo, H. Fan, Z. Bu, B.-G. Li and S. Zhu, Polymer, 2015, 80, 109–114 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra00165a |
| This journal is © The Royal Society of Chemistry 2022 |