Open Access Article
Open Access ArticleA new method for simultaneous determination of 14 phenolic acids in agricultural soils by multiwavelength HPLC-PDA analysis
Jia Cheng a,
Chunfu Zhoua,
Yue Xie
a,
Chunfu Zhoua,
Yue Xie *a,
Min Wangb,
Cheng Zhouc,
XiaoShuang Lia,
YaDong Dua and
Fan Lua
*a,
Min Wangb,
Cheng Zhouc,
XiaoShuang Lia,
YaDong Dua and
Fan Lua
aDepartment of Resources and Environment, Anhui Science and Technology University, Fengyang 233100, China. E-mail: xiey@ahstu.edu.cn
bDepartment of Resources and Environment, Hubei University, Wuhan 430062, China
cDepartment of Life and Health Sciences, Anhui Science and Technology University, Fengyang 233100, China
First published on 18th May 2022
Abstract
There are phenolic acids with allelopathy in the rhizosphere soil of plants. At present, the identification and quantification of phenolic acids in different matrix mixtures is usually analysed by high performance liquid chromatography, but the detection of phenolic acids in soil has rarely been studied. As well as, previous studies have evaluated a limited number of target compounds. In this work, we proposed and verified a method for quantitative determination of 14 phenolic acids, including gallic acid, vanillic acid, p-hydroxybenzoic acid, protocatechuic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, chlorogenic acid, benzoic acid, salicylic acid, 2-methoxycinnamic acid, 3-methoxycinnamic acid, and cinnamic acid, which are widely present in rhizosphere soil of plants and have allelopathy. This method used multiwavelength HPLC-PDA analysis for simultaneous determination of these compounds. The detection wavelengths selected 254 nm, 280 nm, 300 nm, and 320 nm. Chromatographic separation of all compounds was achieved using a column of Shim-pack VP-ODS (250 mm × 4.6 mm, 5 μm), kept at 30 °C. Mobile phase A was acetonitrile, B was a 0.5% acetic acid aqueous solution, and the flow rate was 1.0 mL min−1. Under the condition of gradient elution, the mobile phase A was acetonitrile, B was a 0.5% acetic acid aqueous solution, and the flow rate was kept constant at 1.0 mL min−1. The 14 target phenolic acids were completely separated within 45 min. All the calibration curves showed good linearity, and the correlation coefficient was 0.9994–0.9999. With the detection limit varying from 0.003 mg L−1 to 0.239 mg L−1. The recovery rates and the RSD of 14 phenolic acids were 80.54∼107.0% and 1.43–4.35%, respectively. This method has the characteristics of high sensitivity, high accuracy, and high recovery rate. This method is a novel technical means for the simultaneous analysis of compound phenolic acids in soil.
1. Introduction
Allelopathy is common in nature. Plants release their secondary metabolites to their surroundings, which affects their growth and development or other plants around them, leading to mutual exclusion or promotion between plants.1 Allelopathy is a chemical regulation mechanism in the natural environment. It plays an irreplaceable role in the establishment and succession of plant communities and is also one of the important factors leading to continuous cropping obstacles. Many studies have shown that phenolic acids are the most identified and most active allelochemicals in the plant soil environment.2,3 Phenolic acids are a class of organic acids with simple structures containing aromatic phenolic rings, phenolic hydroxyl groups and carboxyl groups. In soil, phenolics can occur in the three following forms: free, reversibly bound, and bound forms. The free phenolic compounds may accumulate in rhizosphere soils, thereby influencing the accumulation and availability of soil nutrients and rates of nutrient cycling in soil, which both ultimately affects plant growth.4 Research shows that phenolic acids at 100 μmol·L−1 concentration can inhibit root length of Chuju, in addition, they may reduce the contents of chlorophyll, soluble protein, and soluble sugar in medicinal Chuju, reduce the activities of the peroxidase (POD), the catalase (CAT), the superoxide dismutase (SOD), and the phenylalanine ammonialyase (PAL) in Chuju, and increase the content of the malonaldehyde (MDA), inducing adverse effects on the growth of Chuju.5 The study of continuous cropping obstacles of Chinese medicinal materials demonstrates that gallic acid, salicylic acid, syringic acid, vanillic acid and protocatechuic acid are the main phenolic acids leading to allelopathy of common genuine medicinal materials such as Panax notoginseng, Rehmannia glutinosa, Panax quinquefolium, Pseudostellaria heterophylla, Salvia miltiorrhiza and Pinellia ternate.6 Li et al.3 found that in the root exudates of Rehmannia glutinosa, a phenolic acid mixture had an inhibitory effect on the growth of beneficial pathogens, while the effect on the growth of harmful pathogens was the opposite, so Rehmannia glutinosa showed strong pathogenicity. In summary, it was necessary to collect, extract, separate and then analyse phenolics in the natural environment to determine their allelopathy. Cucumbers and peanuts are also affected by autotoxic substances such as phenolic acid.7–10Currently, the main detection methods of phenolic acids are folin phenol colorimetry, capillary electrophoresis, infrared absorption spectroscopy, and high-performance liquid chromatography (HPLC).11–17 For folin phenol colorimetry, because of the existence of easily oxidized substances in the sample, the measured phenolic acid content is high; moreover, the soil environment is complex, and there are many interference components. The sensitivity of capillary electrophoresis is low and thus is not suitable for the study of phenolic acids in the soil environment. Infrared absorption spectroscopy is commonly used in qualitative research of substances. If used for quantitative research, complex mathematical models are needed to obtain the content of phenolic acid. Infrared absorption spectroscopy is applied to simultaneous determination of multi-component, which will lead to complex calculation process. In contrast, HPLC has the characteristics of high sensitivity, high accuracy and multicomponent qualitative and quantitative analysis and is commonly used in the detection of phenolic acids. HPLC is commonly used as a separation method in conjunction with various detection methods (e.g. UV, FLD, PDA, etc.). Xie et al.18 determined 9 kinds of phenolic acids in medicinal Chuju samples and planting soil by HPLC gradient elution within 45 min. Their detection limit were 0.01–0.08 mg L−1, which met the requirements of trace detection. González et al.19 established a high-performance liquid chromatography-diode array detector with high selectivity, high sensitivity and high accuracy for the simultaneous determination of six phenolic acids in the water extract of Solanum nigrum and its hydrolysate. The method showed a good linear relationship in the concentration range (r > 0.999), with a recovery of 88.07–109.17% and a matrix effect of less than 5%. In their comments, Stalikas and Kalili et al.20,21 discussed the existing application of HPLC for determining the phenolic compounds of mixtures in different matrices, but their presence in soil was not highlighted and take a long time to be tested. Chen et al.22 used the HPLC-DAD method to detect 10 compounds in 55 min and Arimboor et al.23 determined nine phenolic acids in 60 min. Sun et al.24 used UPLC-ESI-QTOF-MS and HPLC to identify and determine seven phenolic acids in Brazilian green propolis in 105 min.
The abovementioned phenolic acid detection methods generally have problems such as long-time consumption, the use of a variety of mobile phases, and fewer types of phenolic acid detection. Therefore, how to detect multiple compound phenolic acids in soil with high sensitivity, simplicity and rapidity has become a scientific and technical problem that needs to be solved urgently for agricultural enterprises with continuous cropping obstacles caused by phenolic acids. In this study, high-performance liquid chromatography was used to optimize the chromatographic conditions. Under the same mobile phase, a simple, rapid, and simultaneous analysis method for 14 phenolic acids was established, which provided technical support for the detection of phenolic acids.
2. Results and discussion
2.1 Optimization of detection wavelength
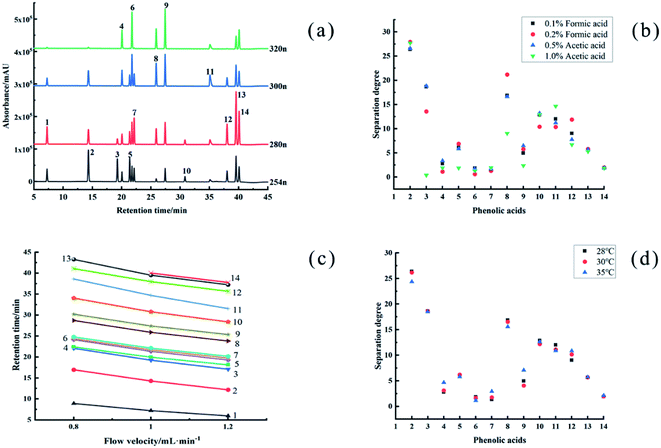
Using to the SPD-M20A PDA diode array detector, the 14 phenolic acids in the single standard solution were scanned at 190–800 nm, and the detection wavelength was determined by the maximum absorption of 14 phenolic acids. As shown in Fig. 1(a), under the set four detection wavelengths, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid and benzoic acid have the maximum absorption intensity at 254 nm, gallic acid, syringic acid, cinnamic acid, 2-methoxycinnamic acid and 3-methoxycinnamic acid have the maximum absorption intensity at 280 nm, p-coumaric acid and salicylic acid have the maximum absorption intensity at 300 nm, and chlorogenic acid, caffeic acid and ferulic acid have the maximum absorption intensity at 320 nm. In summary, the wavelengths of 254, 280, 300 and 320 nm were determined as the detection wavelengths of this method. This is consistent with what is mentioned in the review by stalikas.20 Detection at 280 nm is most generally used for the simultaneous separation of mixtures of phenolic acids although for multiplex monitoring 254, 280 and 320 nm, can be ideal wavelengths.2.2 Optimization of mobile phase composition
The mobile phase is an important factor affecting the separation effect of HPLC. Phenol, phenolic hydroxyl, and carboxyl groups are prone to ionization. Adding an appropriate concentration of acid in aqueous solution has an obvious effect on improving the separation degree and peak shape of the analyte.25,26 However, gradient elution has usually been most common in recognition of the complexity of the phenolics of most samples. Numerous mobile phases have been employed in past studies,2,27,28 but binary systems comprising an aqueous component and a less polar organic solvent such as acetonitrile or methanol remain common. Acid is usually added to both components to maintain a constant acid concentration during gradient runs.29 The commonly used acids for the separation and determination of phenolic acids are formic acid, acetic acid, and phosphoric acid.2,30 In this study, the effects of different proportions of formic acid and acetic acid on the separation and determination of phenolic acids were selected. As shown in Fig. 1(b), the results showed that when using acetonitrile-0.5% acetic acid aqueous solution as the mobile phase, the separation effect of 14 phenolic acids was better, and the peak shape symmetry was good. The separation degrees were all above 1.5, and the tailing factors were between 0.9 and 1.2.It was found that the target component was challenging to separate effectively from the interference peak with isocratic elution, but the gradient elution method can greatly improve the separation effect. The initial parameter setting of gradient elution have been adopted from the chromatographic conditions in Xie et al.18 Under this mobile phase gradient, 14 phenolic acids did not separate, the chromatographic method was concentrated at retention times of 18–20 min, and the resolution was poor. The mobile phase used acetonitrile (A) and ultrapure water with 0.5% (v/v) acetic acid aqueous (B). After that, the ratio of the organic phase to the aqueous phase was adjusted many times, and finally, the gradient elution was determined as follows: 0–5 min, 5% A; 5–35 min, 5–40% A; 35–45 min, 5% A.
2.3 Optimization of flow rate and column temperature
The flow rate had a great influence on the retention time of phenolic acids. As shown in Fig. 1(c). When the flow rate was 0.8 mL min−1, the retention time of each phenolic acid component was significantly delayed, and the peaks were concentrated within 20–25 min with an unsatisfactory separation degree. The peaks of 2-methoxycinnamate did not appear within 45 min. When the flow rate was 1.2 mL min−1, the retention time of each phenolic acid component eluted earlier than that of the 1.0 mL min−1 flow rate; however, the column pressure also increased significantly to 9.6 MPa, which was close to the critical value of 10 MPa. In summary, the optimal flow rate was determined to be 1.0 mL min−1.Analysis temperature affects the selectivity, retention and mobile-phase viscosity in HPLC.21 This study selected 28 °C, 30 °C, 35 °C three temperatures were compared. As shown in Fig. 1(d), when the column temperature was 28 °C, the column pressure increases and the minimum separation degree was 1.316, less than 1.5. When the column temperature reached 35 °C, the separation degree of vanillic acid and caffeic acid was 1.15, and the separation effect was poor, which affected the calculation of phenolic acid content. Therefore, the column temperature selected for the test was 30 °C.
2.4 Analytical method validation
The method was validation following the guidelines from the International Conference on Harmonization (ICH),31 employing assays with standard solutions, blank samples and spiked samples. The HPLC method with diode array detector was validated for linearity, limit of detection and quantification (LOD and LOQ), precision, accuracy, and stability.| Phenolic acid | Concentration (mg L−1) | Retention time (min) | Separation degrees | Linear range (mg L−1) | Regression equation | Correlation coefficient (R2) | LOQ (mg L−1) | LOD (mg L−1) | Precision | Stability | Recovery | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSD (n = 5, %) | RSD (n = 8, %) | Range (%) | RSD (n = 9, %) | |||||||||
| Gallic acid | 3.76 | 7.249 | — | 0.03–18.6 | y = 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 157x − 582.7 157x − 582.7 |
0.9998 | 0.030 | 0.015 | 0.11 | 1.95 | 86.3 | 2.39 |
| Protocatechuic acid | 5.44 | 14.324 | 26.56 | 0.011–27.0 | y = 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683x + 10 683x + 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 618 |
0.9996 | 0.011 | 0.005 | 0.07 | 0.42 | 80.5 | 1.71 |
| p-Hydroxybenzoic acid | 1.96 | 19.274 | 18.82 | 0.015–9.8 | y = 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775x + 7667.8 775x + 7667.8 |
0.9995 | 0.015 | 0.004 | 0.03 | 0.15 | 99.9 | 3.56 |
| Chlorogenic acid | 3.60 | 20.063 | 3.39 | 0.056–17.9 | y = 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514x − 550.7 514x − 550.7 |
0.9999 | 0.056 | 0.028 | 0.27 | 2.95 | 86.9 | 2.09 |
| Vanillic acid | 3.84 | 21.384 | 5.86 | 0.015–19.2 | y = 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233x + 8301.1 233x + 8301.1 |
0.9995 | 0.015 | 0.007 | 0.05 | 0.17 | 99.3 | 3.76 |
| Caffeic acid | 3.60 | 21.771 | 1.71 | 0.028–17.8 | y = 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538x + 3450.9 538x + 3450.9 |
0.9997 | 0.028 | 0.007 | 0.03 | 0.75 | 97.2 | 2.29 |
| Syringic acid | 4.00 | 22.144 | 1.69 | 0.016–19.8 | y = 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056x + 8064.8 056x + 8064.8 |
0.9995 | 0.016 | 0.008 | 0.08 | 0.17 | 98.4 | 1.43 |
| p-Coumaric acid | 1.64 | 25.920 | 16.62 | 0.013–8.2 | y = 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116x + 6055.9 116x + 6055.9 |
0.9995 | 0.013 | 0.003 | 0.03 | 0.14 | 101.0 | 3.74 |
| Ferulic acid | 3.60 | 27.445 | 6.51 | 0.014–18.1 | y = 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916x + 12 916x + 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
0.9995 | 0.014 | 0.007 | 0.03 | 0.25 | 87.4 | 1.56 |
| Benzoic acid | 8.24 | 30.839 | 13.20 | 0.13–41.3 | y = 7711.3x + 2390.5 | 0.9994 | 0.130 | 0.065 | 0.18 | 0.30 | 99.4 | 4.35 |
| Salicylic acid | 7.68 | 35.116 | 11.22 | 0.46–38.3 | y = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 561x − 3187.5 561x − 3187.5 |
0.9995 | 0.460 | 0.239 | 0.09 | 0.43 | 107.0 | 2.59 |
| Cinnamic acid | 1.64 | 38.040 | 7.77 | 0.006–8.3 | y = 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 949x + 7876.4 949x + 7876.4 |
0.9995 | 0.006 | 0.003 | 0.15 | 0.22 | 99.5 | 3.66 |
| 3-Methoxycinnamic acid | 5.04 | 39.572 | 5.69 | 0.010–25.0 | y = 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665x + 20 665x + 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
0.9995 | 0.010 | 0.005 | 0.05 | 0.27 | 99.4 | 3.62 |
| 2-Methoxycinnamic acid | 4.00 | 40.091 | 1.93 | 0.016–20.0 | y = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669x + 11 669x + 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272 |
0.9996 | 0.016 | 0.008 | 0.27 | 0.45 | 97.6 | 3.67 |
A recovery test was used to evaluate the accuracy of the developed method. Known amounts of the 14 phenolic acid standards in triplicate at low, medium, and high concentration levels were added to approximate 1.0 g Chuju soil samples. Then the spiked samples were extracted and quantified in accordance with the methods described. The results shown in Table 1 indicate that our proposed method enjoys an appealing recovery and accuracy performance with recoveries of 14 phenolic acid components of 80.5–107.0% and RSD values of 1.43–4.35%.
2.5 Sample determination
As shown in Table 2, all phenolic acids except gallic acid were detected in the continuous cropping soil, among which benzoic acid had the highest concentration (19.90 μg g−1). Six phenolic acids, gallic acid, chlorogenic acid, salicylic acid, cinnamic acid, 3-methoxycinnamic acid and 2-methoxycinnamic acid, were not detected in the noncontinuous cropping soil. Among the eight phenolic acids detected, p-coumaric acid had the highest content, reaching 1.97 μg g−1. Phenolic acid was detected in soil samples. It can be seen from the determination results of Chuju replant soil, cucumber soil and peanut soil that the content of benzoic acid in the soil was the highest. However, benzoic acid was not detected in this paddy soil. Rice crop disorder studies are less reported and perhaps benzoic acid has a greater effect on plant crop disorder, which is consistent with previous studies.4,18,32| Phenolic acid | Chuju soil (μg g−1) | Chuju replant soil (μg g−1) | Paddy soil (μg g−1) | Peanut soil (μg g−1) | Cucumber soil (μg g−1) |
|---|---|---|---|---|---|
| Gallic acid | ND | ND | 1.26 ± 0.21 | ND | ND |
| Protocatechuic acid | 0.40 ± 0.08 | 1.52 ± 0.09 | ND | 0.21 ± 0.002 | ND |
| p-Hydroxybenzoic acid | 1.08 ± 0.17 | 0.01 ± 0.001 | 0.18 ± 0.03 | 0.24 ± 0.02 | 1.25 ± 0.05 |
| Chlorogenic acid | ND | 0.29 ± 0.0004 | ND | ND | 0.56 ± 0.003 |
| Vanillic acid | 0.99 ± 0.13 | 12.37 ± 0.12 | 0.67 ± 0.02 | 1.50 ± 0.23 | 1.49 ± 0.56 |
| Caffeic acid | 0.17 ± 0.03 | 0.71 ± 0.20 | ND | ND | ND |
| Syringic acid | 0.40 ± 0.08 | 4.35 ± 0.21 | 0.43 ± 0.001 | ND | 0.31 ± 0.02 |
| p-Coumaric acid | 1.97 ± 0.29 | 3.86 ± 0.56 | 2.11 ± 0.73 | 0.14 ± 0.001 | 0.16 ± 0.05 |
| Ferulic acid | 0.58 ± 0.11 | 3.09 ± 0.24 | ND | ND | ND |
| Benzoic acid | 0.82 ± 0.04 | 19.90 ± 1.21 | ND | 6.75 ± 0.24 | 24.41 ± 1.23 |
| Salicylic acid | ND | 14.73 ± 0.81 | ND | ND | 0.44 ± 0.01 |
| Cinnamic acid | ND | 6.33 ± 0.29 | 0.17 ± 0.01 | 0.91 ± 0.12 | 0.02 ± 0.001 |
| 3-Methoxycinnamic acid | ND | 11.66 ± 0.34 | 1.26 ± 0.24 | 0.24 ± 0.02 | 0.25 ± 0.02 |
| 2-Methoxycinnamic acid | ND | 9.02 ± 0.48 | 1.42 ± 0.34 | ND | ND |
3. Experimental
3.1 Instruments and reagents
An LC-20A high-performance liquid chromatography system (Japan, Shimadzu), an electronic balance (one ten-thousandth, Mettler Toledo), TDL-40B centrifuge (Shanghai Anting Science Instrument Factory), and KH-5200B ultrasonic cleaner (Kunshan Hechuang Ultrasonic Instrument Co., Ltd.) were utilized in the experiments.Chromatographically pure methanol, acetonitrile, and acetic acid were purchased from Sigma (Germany). Other reagents were of analytical grade. The water used was Waha purified water. Standard sample of Gallic acid, vanillic acid, p-hydroxybenzoic acid, protocatechuic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, chlorogenic acid, benzoic acid, salicylic acid, 2-methoxycinnamic acid, 3-methoxycinnamic acid, and cinnamic acid with purities of more than 98% were purchased from Shanghai Yuanye Biotechnology Co., Ltd.
Soil samples: Chuju soil and Chuju continuous cropping soil from Chuzhou Jutai Herbal Science and Technology Co., Ltd. Chuju planting base; rice soil is from the rice planting base of Xiaogang Village Institute of Modern Ecological Agriculture, Anhui Science and Technology University; cucumber soil comes from rhizosphere soil of cucumber ‘Jingjinyou 2’ in farm of Anhui Science and Technology University; peanut soil comes from rhizosphere soil of peanut ‘Luhua 8’ in Baoji Experimental Station, Nanjing Soil Research Institute, Chinese Academy of Sciences.
3.2 Preparation of solutions
Blank solvent: 0.5% acetic acid aqueous solution.Preparation of standard solution: each phenolic acid standard was accurately weighed, dissolved with methanol and constant volume, and a single standard stock solution was prepared. The mass concentrations were benzoic acid 1.03 mg mL−1, salicylic acid 0.96 mg mL−1, gallic acid 0.47 mg mL−1, vanillic acid 0.48 mg mL−1, p-hydroxybenzoic acid 0.49 mg mL−1, protocatechuic acid 0.68 mg mL−1, caffeic acid 0.45 mg mL−1, syringic acid 0.50 mg mL−1, p-coumaric acid 0.41 mg mL−1, ferulic acid 0.45 mg mL−1, chlorogenic acid 0.45 mg mL−1, 2-methoxycinnamic acid 0.50 mg mL−1, 3-methoxycinnamic acid 0.63 mg mL−1, cinnamic acid 0.41 mg mL−1. The phenolic acid stock solution was accurately taken, and then diluted 100-fold to single standard solution with blank solvent. A total of 2.0 mL of the single standard stock solution of p-hydroxybenzoic acid, cinnamic acid and p-coumaric acid was accurately measured separately and placed in a 50 mL volumetric flask. Then, 4.0 mL of the single standard stock solution of other phenolic acids was transferred to the same 50 mL volumetric flask and diluted with 0.5% acetic acid aqueous solution to obtain the mixed standard stock solution. The mixed standard stock solution was diluted by 2, 5, 10, 15, 20 and 40 times to prepare mixed standard solutions with six gradient concentrations. The standard solution was filtered by 0.22 μm filter membrane and stored in-20 °C refrigerator.
Preparation of standard curve: six gradient concentrations of mixed reference standard solutions were taken, and the samples were analysed using the optimized chromatographic analysis method in this paper. The peak area (Y, μV·S) was used to plot the mass concentration (X, mg L−1), and the standard curves were plotted to obtain the linear range, linear regression equation and correlation coefficient of 14 phenolic acids.
3.3 Chromatographic conditions
The chromatographic conditions were as follows: An LC-20A high-performance liquid chromatography system (Japan, Shimadzu) was used, which included an LC-20AT infusion unit, SIL-20A automatic sampler, COT-20A column temperature box, and SPD-M20A PDA diode array detector. Shim-pack VP-ODS chromatographic column (250 mm × 4.6 mm, 5 μm); column temperature of 30 °C; detection wavelengths of 254 nm, 280 nm, 300 nm, and 320 nm; flow rate of 1.0 mL min; and injection volume of 20 μL. Mobile phase A was acetonitrile and mobile phase B was 0.5% acetic acid aqueous solution. Gradient elution was conducted as follows: 0–5 min, 5% A; 5–35 min, 5–40% A; 35–45 min, 5% A. All solutions were filtered by a 0.22 μm filter before injection.3.4 Soil phenolic acid extraction
The extraction of soil phenolic acids was carried out according to the method of Lou et al.33 After the soil sample was naturally air dried, ground, and sieved through 60 screens, 2.0 g of the sample was weighed and placed in a centrifuge tube. Then, 25.0 mL of 1 mol L−1 NaOH solution was added, and the sample was shaken on a 160 rpm shaking table for 30 min, followed by ultrasonication for 30 min. Next, the sample was allowed to stand for 24 h, shaken on a 160 rpm shaking table for 30 min, and centrifuged at 3000 rpm for 5 min. After separating the supernatant, the pH of the solution was adjusted to 2.5 with 12 mol L−1 hydrochloric acid. Humic acid precipitated, and the sample was allowed to stand for 2 h. The sample was centrifuged at 3000 rpm for 5 min, the supernatant was separated, and it was stored at 4 °C for analysis. The samples were prepared three times in parallel.3.5 Data processing and statistical analysis
The signals collected from high-performance liquid chromatography software LabSolutions were output in text document format (*.txt), and the original data were plotted in OriginPro 2018 software. This was studied performing statistical analysis by one-way analysis of variance (ANONA) followed by Bonferroni post-hoc test.4. Conclusions
In this paper, a multiwavelength HPLC-PDA multiwavelength method was utilized to determine 14 phenolic acids, including gallic acid, vanillic acid, p-hydroxybenzoic acid, protocatechuic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, chlorogenic acid, benzoic acid, salicylic acid, 2-methoxycinnamic acid, 3-methoxycinnamic acid and cinnamic acid simultaneously and rapidly. The HPLC method was optimized and validated. The method has the characteristics of high sensitivity, high accuracy, high recovery rate and stable sample. The results of this study show that this method can be applied to the rapid simultaneous detection of a variety of phenolic acids in soil under the same mobile phase. It provides a new technical means for the rapid detection of phenolic acids in the production process of medicinal plants, fruits and vegetables, trees, and other industries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Anhui Province (NO. 2008085MC81), Key R&D Program Projects of Anhui Province (NO. 202004a06020003), Graduate Science Research Project of Anhui Science and Technology University (NO. YK202107) and Enterprise Horizontal Project Fund (NO. 2020QX001).Notes and references
- F. A. Macias, F. J. Mejias and J. M. Molinillo, Pest Manage. Sci., 2019, 75, 2413–2436 CrossRef CAS PubMed.
- W. Santos and B. E. A. MagalhAes, An. Acad. Bras. Cienc., 2020, 92, e20190646 CrossRef PubMed.
- Q. Li, Y. Wu, J. Wang, B. Yang, J. Chen, H. Wu, Z. Zhang, C. Lu, W. Lin and L. Wu, Front. Plant Sci., 2020, 11, 787 CrossRef PubMed.
- Z. H. Li, Q. Wang, X. Ruan, C. D. Pan and D. A. Jiang, Molecules, 2010, 15, 8933–8952 CrossRef CAS PubMed.
- Y. Xie, X. Xiao, Y. Zhou, S. Y. Chen, X. L. Li, S. Z. Xing and J. F. Wang, J. Nanjing Agric. Univ., 2012, 35(06), 19–24 CAS.
- H. M. Wu and W. X. Lin, Chin. J. Eco-Agric., 2020, 28(06), 775–793 Search PubMed.
- X.-G. Xie, C.-C. Dai, X.-G. Li, J.-R. Wu, Q.-Q. Wu and X.-X. Wang, BioControl, 2016, 62, 111–123 CrossRef.
- J. Liu, X. Wang, T. Zhang and X. Li, Microbiol. Res., 2017, 205, 118–124 CrossRef CAS PubMed.
- X. Zhou, J. Liu and F. Wu, Plant Soil, 2017, 415, 507–520 CrossRef CAS.
- W. L. Xiao, Z. X. Wang, F. Z. Wu and X. G. Zhou, Environ. Sci. Pollut. Res., 2018, 25, 24093–24100 CrossRef CAS PubMed.
- Y. Xie, C. Zhou, C. Tu, Z. L. Zhang and J. F. Wang, Chin. J. Anal. Chem., 2017, 45(03), 363–368 CAS.
- F. Antognoni, G. Potente, S. Biondi, R. Mandrioli, L. Marincich and K. B. Ruiz, Plants, 2021, 10(6), 1046 CrossRef CAS PubMed.
- R. Marti, M. Valcarcel, J. M. Herrero-Martinez, J. Cebolla-Cornejo and S. Rosello, Food Chem., 2017, 221, 439–446 CrossRef CAS PubMed.
- M. S. Padda and D. H. Picha, J. Food Sci., 2007, 72, C412–C416 CrossRef CAS PubMed.
- M. M. Showkat, A. B. Falck-Ytter and K. O. Straetkvern, Molecules, 2019, 24(18), 3296 CrossRef CAS PubMed.
- W. Cao, Z. Y. Suo, J. R. Song and J. B. Zheng, J. Chem. Eng. Chin. Univ., 2005, 08, 1424–1427 Search PubMed.
- S. Yang, Y. Han, K. Wang, Y. Wang, L. Li, N. Li and X. Xu, RSC Adv., 2021, 11, 33996–34003 RSC.
- Y. Xie, H. Yu, J.-F. Wang, Z.-L. Zhang, S.-Y. Chen, X. Xiao and X.-L. Li, Chin. J. Inorg. Anal. Chem., 2014, 41, 383–388 Search PubMed.
- R. M. González-González, L. Barragán-Mendoza, A. L. Peraza-Campos, R. Muñiz-Valencia, S. G. Ceballos-Magaña and H. Parra-Delgado, Rev. Bras. Farmacogn., 2019, 29, 689–693 CrossRef.
- C. D. Stalikas, J. Sep. Sci., 2007, 30, 3268–3295 CrossRef CAS PubMed.
- K. M. Kalili and A. de Villiers, J. Sep. Sci., 2011, 34, 854–876 CrossRef CAS PubMed.
- Y. Chen, Z. Zhang, Y. Zhang, X. Zhang, Z. Zhang, Y. Liao and B. Zhang, Molecules, 2019, 24(4), 709 CrossRef CAS PubMed.
- R. Arimboor, K. S. Kumar and C. Arumughan, J. Pharm. Biomed. Anal., 2008, 47, 31–38 CrossRef CAS PubMed.
- S. Sun, M. Liu, J. He, K. Li, X. Zhang and G. Yin, Molecules, 2019, 24(9), 1791 CrossRef CAS PubMed.
- A. H. Liu, L. Li, M. Xu, Y. H. Lin, H. Z. Guo and D. A. Guo, J. Pharm. Biomed. Anal., 2006, 41, 48–56 CrossRef CAS PubMed.
- L. Zhong, Z. Yuan, L. Rong, Y. Zhang, G. Xiong, Y. Liu and C. Li, Sci. Rep., 2019, 9, 7745 CrossRef PubMed.
- E. Valkama, J. P. Salminen, J. Koricheva and K. Pihlaja, Ann. Bot., 2003, 91, 643–655 CrossRef CAS PubMed.
- N. Bo, L. Chen, C. Yi, X. Shi and M. J. E. Zhao, Electrophoresis, 2019, 40(21), 2837–2844 CrossRef PubMed.
- D. R. Bedgood, Jr, A. G. Bishop, P. D. Prenzler and K. Robards, Analyst, 2005, 130, 809–823 RSC.
- S. Ijaz, H. M. Shoaib Khan, Z. Anwar, B. Talbot and J. J. Walsh, Biomed. Pharmacother., 2019, 109, 865–875 CrossRef CAS PubMed.
- ICH Harmonised Tripartite Guideline Q2 (R1), European Medicines Agency, 2005, 1(20), 05.
- W. Zhang, L. Y. Lu, L. Y. Hu, W. Cao, K. Sun, Q. B. Sun, A. Siddikee, R. H. Shi and C. C. Dai, Plant Cell Physiol., 2018, 59, 1889–1904 CrossRef CAS PubMed.
- C. Luo, X. Wang, G. Gao, L. Wang, Y. Li and C. Sun, Food Chem., 2013, 141, 2697–2706 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |