Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly sensitive and fast-response ethanol sensing of porous Co3O4 hollow polyhedra via palladium reined spillover effect†
Guotao Yuana,
Yihong Zhonga,
Yufeng Chena,
Qiqi Zhuo*b and
Xuhui Sun *a
*a
aInstitute of Functional Nano and Soft Materials Laboratory (FUNSOM), Soochow University, Suzhou 215123, China. E-mail: zqq88263268@126.com; xhsun@suda.edu.cn
bCollege of Material Science & Engineering, Jiangsu University of Science and Technology, Zhenjiang, China
First published on 28th February 2022
Abstract
Highly sensitive and fast detection of volatile organic compounds (VOCs) in industrial and living environments is an urgent need. The combination of distinctive structure and noble metal modification is an important strategy to achieve high-performance gas sensing materials. In addition, it is urgent to clarify the chemical state and function of noble metals on the surface of the sensing material during the actual sensing process. In this work, Pd modified Co3O4 hollow polyhedral (Pd/Co3O4 HP) is developed through one-step pyrolysis of a Pd doped MOF precursor. At an operating temperature of 150 °C, the Pd/Co3O4 HP gas sensor can achieve 1.6 times higher sensitivity than that of Co3O4 HP along with fast response (12 s) and recovery speed (25 s) for 100 ppm ethanol vapor. Near-ambient pressure X-ray photoelectron spectroscopy (NAPXPS) was used to monitor the dynamic changes in the surface state of Pd/Co3O4 HP. The NAPXPS results reveal that the oxidation and reduction of Pd in the ethanol sensing process are attributed to a spillover effect of oxygen and ethanol, respectively. This work opens up an effective approach to investigate spillover effects in a sensing mechanism of noble metal modified oxide semiconductor sensors.
1. Introduction
Monitoring of volatile organic compounds (VOCs) is becoming ever more important due to stringent environmental regulations and increasing health concerns.1–3 Nowadays, many efforts have been made to improve sensor performance, such as developing high-performance sensing materials and optimizing the sensor structure. Metal oxide semiconductors (MOS) are some of the candidate materials for detection of gas concentration due to their high sensitivity, low cost, good stability and so on.4,5 However, their high operating temperature and still unsatisfactory response/recovery speed hinder their further applications.6In general, porous structured materials are beneficial to gas sensing performance by increasing the surface reactive sites and facilitating the diffusion of target gases.7–9 For example, Chen et al.8 fabricated a mesoporous Co3O4 nanosheet array supported on an N-doped carbon foam for enhanced ethanol sensing application. The working temperature can be decreased to 100 °C and the limit of detection lowers to 0.2 ppm. Metal organic framework (MOF)-derived MOS sensor can provide the less agglomerated nanostructures and larger active site of the sensing materials to obtain higher sensing performance. For examples, Masuda et al.10 successfully synthesized highly porous CuO nanostructures by converting Cu-MOF precursor, which could detect a tiny concentration of acetone gas up to 50 ppb. Zhang et al.11 fabricated porous hollow Co3O4@ZnO cages through ZIF-67@ZIF-8-derived process, which exhibited a high sensitivity of ∼41 with a response/recovery time of only 3/2 s for 33 ppm trimethylamine.
As an additive with excellent catalytic ability, Pd, Pt or Au are good auxiliary materials, in which the electronic sensitization and spillover effect (chemical sensitization) can significantly promote the gas sensing performance. Chen et al.12 fabricated interfacial heterostructures in Pd7.18%W18O49 nanowires by doping different Pd contents, which showed the best sensing response and the fastest response–recovery speeds (5 and 10 s, respectively) at a working temperature of 175 °C. Umarji et al.13 reported a Cr, Pt co-doping SnO2 thin film sensor with enhanced selectivity due to specific catalytic effect and synergistic effect. Moreover, AuPd alloys modified SnO2 sensors were fabricated by Duan et al. and exhibited high response value and selectivity to dimethyl disulfide. The enhanced performance was due to sulfur spillover driven by charge transfer between AuPd alloys and SnO2.14
Despite these great efforts, noble metal-drived spillover effect often is used to clarify the sensing mechanism in metal–support oxide sensors.15,16 Many techniques have been applied to study the noble metal-drived spillover effect in metal–support oxide sensors, including X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS). However, limited to ultrahigh vacuum conditions, it's difficult to identify the surface core-level spectra in the atomic scale, which is beneficial for the clarification of the spillover effect. Near ambient-pressure X-ray photoelectron spectroscopy (NAPXPS) can measure the core level spectra of materials and gas molecules in situ under near ambient pressure conditions. Recently, NAPXPS has been successfully used for the investigation of spillover effect in catalytic reaction.17–19 For instance, Somorjai et al.17 used NAPXPS technique to monitoring Ce 4d core level and found cerium oxide was reduced at lower temperatures for Pt/CeO2 catalyst than for pure mesoporous CeO2. This result identified the spillover effect of atomic hydrogen from Pt to ceria surface. Therefore, further investigation of spillover effect in gas sensor by NAPXPS is still necessary.
In this work, Pd modified Co3O4 hollow polyhedral with a porous structure through one-step pyrolysis of Pd-ZIF-67 composites was synthesized and fabricated as a gas sensor. The as-prepared sensor exhibited high responsibility (∼20.8) and fast response/recovery speed (12 s/25 s) for the selective detection of 100 ppm ethanol vapor at an operating temperature of 150 °C. NAPXPS was used to monitor the dynamic changes in the surface state of Pd/Co3O4 HP to further elucidate the sensing mechanism.
2. Experiments
Materials
Palladium chloride (PdCl2, 98%), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, 98.5%), 2-methylimidazole (C4H6N2, 2MeIm, 99.0%), hydrochloric acid (HCl, 30%) and methanol (CH5OH, 99.5%) were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).Synthesis of Pd-ZIF-67
Firstly, 0.5 mL H2PdCl4 (50 mM) solution and 0.25 g Co(NO3)2·6H2O were dissolved in 50 mL methanol. Then 0.7 g 2-MeIm was dissolved in 50 mL methanol and the former solution was poured in it at 1500 rpm for 5 min. Afterwards the mixed solution was kept at room temperature for 5 h to get the products. Finally, the products were washed with absolute ethanol for three times and dried at 50 °C overnight to obtain Pd-ZIF-67.Synthesis of Pd–Co3O4 HP
Pd/Co3O4 HP were synthesized through one-step pyrolysis of Pd-ZIF-67 composites in the furnace at 500 °C for 2 h in air.Materials characterization
Field-emission scanning electron microscopy (FESEM, Zeiss G500) and transmission electron microscopy (TEM, Thermo Fisher Talos F200X) equipped with energy dispersive X-ray spectroscopy (EDX) were carried out to characterize morphologies and structure of the samples. X-ray diffraction (XRD) patterns were collected on a PANalytical X-ray diffractometer by Cu Kα source.Fabrication of Pd/Co3O4 HP gas sensor
Firstly, the Au interdigital electrodes were deposited on insulating silicon wafer substrate (2 cm × 1 cm). Then, 0.2 mL of Pd/Co3O4 HP dispersion (0.2 g mL−1) was coated on the substrate to fabricate a simple Pd/Co3O4 HP sensor. Before use, the sensor was aged by thermal treatment at 100 °C for 12 h.Gas sensor measurements
The sensing performance of Pd/Co3O4 HP sensor was assessed by a reported sensing system composed of an electrical test system (Keithley-2400) and a home-made gas distribution system. The gas chamber volume was around 1 L and the bias voltage was set at 1 V. Different atmospheres were pumped to the testing chamber at a gas flow rate of 200 sccm. The sensitivity of gas response was defined as the ratio ((Rg − Ra)/Ra) of the resistance in air (Ra) to that in the mix gases (Rg). Response and recovery times were defined as the time required for 90% of the total resistance change upon exposure to gas and air, respectively.Near ambient pressure X-ray photoelectron spectroscopy (NAPXPS) measurement
The synchrotron-based NAPXPS measurements were operated at TLS 24 A. The incident photon energy was adjusted from 530 eV to 730 eV for detecting the electronic state information of elements with the same atomic layer thickness. Au 4f7/2 peak (84 eV) was used for the calibration of the photon energy. Laser irradiation was used to heat the sensors. O 1s and Pd 3d spectrum of Pd/Co3O4 HP sensor were collected at a pass energy of 20 eV under different atmosphere (ultra-high vacuum (UHV), 1 mbar O2, and 1 mbar O2/0.2 mbar ethanol mixed gas, respectively). CasaXPS was used for data processing of NAPXPS spectrum.3. Results and discussion
Synthesis and characterizations
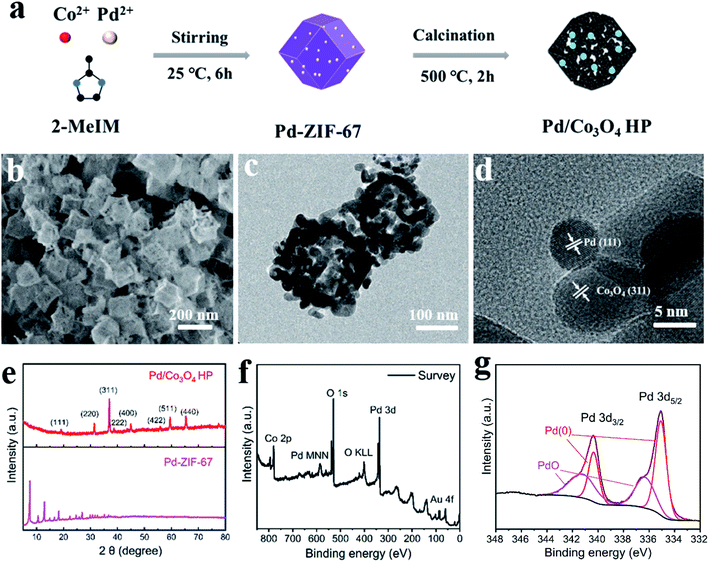
Pd modified Co3O4 hollow polyhedra (Pd/Co3O4 HP) was synthesized by one-step pyrolysis of Pd-ZIF-67 composites, as shown in Fig. 1a. Pd-ZIF-67 nanocomposites were firstly prepared through a coprecipitation method. SEM images (Fig. S1a and b†) indicate the size of around 200 nm and monodispersion of Pd-ZIF-67 composites.The elemental compositions of Pd-ZIF-67 were characterized by STEM. TEM images (Fig. S1c†) and corresponding EDX mappings (Fig. S1d†) verified the doping of Pd on the surface.Subsequently, Pd-ZIF-67 were calcinated at 500 °C in air to form Pd/Co3O4 HP. The SEM image (Fig. 1b) show that as-prepared samples presented porous polyhedron morphology and uniform monodispersion with a size of around 200 nm. The TEM images (Fig. 1c) show that Pd/Co3O4 hollow polyhedrons are composed of small nanoparticles. In addition, the distinct lattice fringes observed in high-resolution TEM (Fig. 1d) are assigned to Pd (111) plane and Co3O4 (311) plane. Typical diffraction peaks in XRD patterns (Fig. 1e) can be well assigned to Co3O4 phase (PDF#42-1467) and ZIF structure, respectively. No Pd peaks were observed in XRD patterns, which could be due to the low content of Pd. XPS was carried out to study the electronic structure of Pd as showed in Fig. 1f and g. The XPS survey verified the composition of Co, O, Pd, C and Au (from Au electrodes) elements. Further fine scanning of Pd 3d and peak fitting results show that there are four characteristic peaks at 335.1 eV, 335.8 eV, 340.4 eV and 341.2 eV. The peaks at 335.1 eV (340.4 eV) and 335.8 eV (341.2 eV) correspond to metallic Pd and PdO, respectively. This result confirms that Pd was successfully modified on the surface of Co3O4 HP and partially oxidized.
Sensing performance
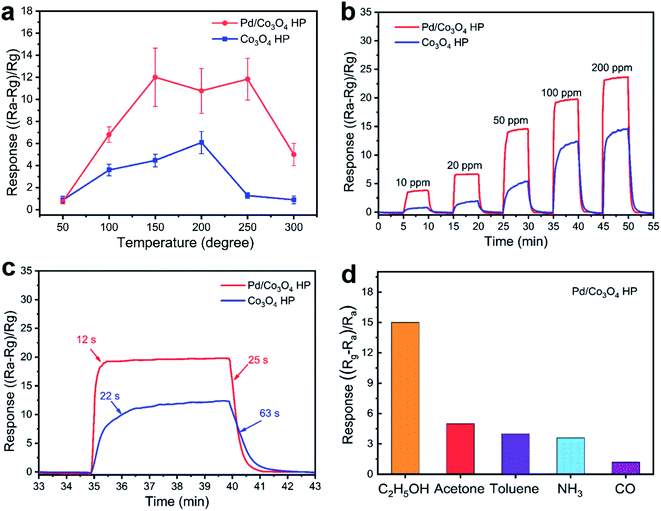
The sensing responses of porous Pd/Co3O4 HP and pure porous Co3O4 HP for ethanol vapor were investigated. According to the sensitivity curves at different operating temperatures (Fig. 2a) and the requirement for lower working temperature, the optimum temperature of 150 °C was proposed. Fig. 2b shows the dynamic transient response of Pd/Co3O4 HP and pure Co3O4 sensors in different atmospheres at 150 °C, respectively. The response of Pd/Co3O4 HP varied from 3.8 to 24.1 when the concentration of ethanol increased from 10 ppm to 200 ppm. The response and recovery curve of Pd/Co3O4 HP and porous Co3O4 sensor upon exposure to 100 ppm ethanol vapor are exhibited in Fig. 2c, highlighting the shorter response times (12 s) and recovery times (25 s) of Pd/Co3O4 HP. To explore the selectivity of Pd/Co3O4 HP sensor, different gases were pumped to the chamber at 150 °C (Fig. 2d). Pd/Co3O4 HP exhibited the highest sensitivity (∼14.8) for 50 ppm ethanol, which can be related to kinetics and thermodynamics of gas adsorption–desorption. To explore the stability of the prepared sensor, the response transient cycles and long-term stability tests were carried out as showed in Fig. S2.† Pd/Co3O4 HP sensor to 100 ppm of ethanol at 150 °C showed a typical p-type sensing behavior. The resistance of sensor increased upon exposure to ethanol and returned to the original value in air in a repeatable way. The response of Pd/Co3O4 HP sensor has been examined for eight weeks as shown in Fig. S2b.† The sensor exhibits a nearly consistent response, which ensures the real application of Pd/Co3O4 HP sensor.Mechanism
Spillover effect involves the dynamic migration of surface adsorbed species between different surface sites and the dynamic changes in surface state. NAPXPS measurements were carried out to investigate dynamic changes in surface state of Pd/Co3O4 HP sensor. Various atmospheres (UHV, O2, and the mixture of O2/ethanol) were pumped into the testing chamber to simulate the working condition of Pd/Co3O4 HP sensor. The temperature was set at 150 °C, which is the optimal working temperature. The kinetic energy of 200 eV was selected in order to obtain sensitive surface information of the same atomic-layer thickness. Specifically, the XPS spectrum of O 1s was collected at the incident light energy of 730 eV and that of Pd 3d spectrum was 530 eV.Fig. 3 shows the XPS spectra of Pd 3d obtained under different atmosphere conditions. The peak around 335.4 eV is considered as metallic Pd,20 while the fitting peak near 336.9 eV is usually considered as PdO.21 Under UHV conditions, it is found that most of Pd on the surface is metallic. A small part of Pd 3d spectra moves slightly to the high binding energy direction due to the Schottky interaction between Pd and oxides.22 When oxygen was introduced, the surface Pd was oxidized rapidly and the proportion of oxidized Pd increased to 64%. Upon the further exposure of ethanol, the oxidized Pd was reduced. It is worth noting that the Pd 3d5/2 characteristic peak position was higher than that of metallic Pd, indicating the interaction between Pd and ethanol vapor. Finally, when the gas was extracted, Pd on the material surface returned to the initial state.
In order to explore the intrinsic changes of oxygen species at different temperatures, O 1s at different temperatures were collected as shown in Fig. 4. Normalized O 1s spectrum (Fig. 4a) are divided into two main components as lattice oxygen and defect oxygen. It is found that the defect oxygen content decreased with the increase of temperature. When the temperature decreased, the defect oxygen content also partially recovered, which may be due to the rearrangement of surface oxygen atoms and the conversion of adsorbed oxygen at a certain temperature.23,24 This phenomenon confirmed that the gas sensing response performance changed with temperature.
Fig. 4b shows O 1s spectrum in different atmospheres. For simplicity, hydroxyl is also distributed in defect oxygen. It is found that the main components in O 1s did not change significantly. The peaks existed at about 529.7 eV were considered to be the lattice oxygen of Pd/Co3O4 HP, and the peaks at the binding energy of 531.8 eV corresponded to the defect oxygen. In the mixture of O2 or O2/ethanol vapor, the peak of gaseous oxygen appeared in the spectrum. The percentages of lattice oxygen and defective oxygen are shown in Fig. 4c. It is found that once ethanol gas was introduced, the proportion of defective oxygen increased significantly, while the percentage of lattice oxygen decreased, suggesting that the surface adsorbed oxygen decreased and the surface was reduced.
By measuring the binding energy of gas molecules on the sample surface and detecting its changes, the work function of sample surface and band structure can be characterized.25 The changes of oxygen gaseous peaks were also observed as shown in Fig. 4d. When ethanol was introduced into the chamber, the gaseous peak of oxygen moved towards high binding energy. This result indicated the change of the energy band structure of Pd/Co3O4 HP sensor contributed to the change of resistance.
According to above results, sensing mechanism model was proposed in Fig. 5. Pd was loaded on the oxide surface by Schottky contact while partial electrons were transferred to the oxide. In air, oxygen firstly adsorbed on the oxide surface and acquired electrons from the conduction band at the same time. Absorbed oxygen can be decomposed into reactive oxygen species and transferred to Pd surface at the operating temperature. This oxygen spillover phenomenon is proved by the oxidation of Pd 3d spectrum and no change in O 1s spectrum under O2 condition. Once ethanol vapor was introduced, ethanol rapidly reacted with reactive oxygen absorbed on the surface of Pd, while Pd was reduced. The electrons were released to the semiconductor, which allowed the sensor resistance to be restored. In this work, the dynamic changes in the surface state of Pd/Co3O4 HP were studied by NAPXPS, which help further explain the spillover sensing mechanism in metal–support oxide sensors.
4. Conclusions
In summary, Pd/Co3O4 HP was fabricated by one-step pyrolysis of Pd-MOF precursor. Due to the combination of unique structure and catalytic sensitization of Pd, Pd/Co3O4 HP sensor achieve 1.6 times higher sensitivity than that of Co3O4 HP along with fast response (12 s) and recovery speed (25 s) for 100 ppm ethanol vapor at an optimal operating temperature of 150 °C. NAPXPS was used to monitor the dynamic changes in the surface state of Pd/Co3O4 HP. It was presented that the phenomenons of oxidation and reduction of Pd in ethanol sensing process were attributed to spillover effect of oxygen and ethanol. This work provides experimental guidance for gas sensors to effectively enhance the ability of gas detection by Pd reined spillover effect, which opens up a unique approach to investigate sensing mechanism of metal–support oxides as high-performance gas sensors.Conflicts of interest
All authors declare that there are no conflicts of interest.Acknowledgements
The work was supported by the National Science and Technology Major Project from Minister of Science and Technology of China (Grant No. 2018AAA0103104), National Nature Science Foundation of China (Grant No. U1932124), the National Key R&D Program of China (Grant No. 2017YFA0205002), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 18KJA535001). This work is also supported by Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the 111 Project, Joint International Research Laboratory of Carbon-Based Functional Materials and Devices. The authors thank Shanghai Synchrotron Radiation Facility (SSRF) beamline 02B; Taiwan Light Source (TLS) beamline 17C1, 20A1, 24A1 and Taiwan Photon Source (TPS) beamline 44 A1 of NSRRC for providing synchrotron beamtime and technical supports.References
- M. Ghazi, S. Janfaza, H. Tahmooressi, N. Tasnim and M. Hoorfar, J. Hazard. Mater., 2021, 127566 Search PubMed.
- M. T. Robinson, J. Tung, M. Heydari Gharahcheshmeh and K. K. Gleason, Adv. Funct. Mater., 2021, 31(22), 2101310 CrossRef CAS.
- T. Yamada, H. Sugiura, H. Mimura, K. Kamiya, T. Osaki and S. Takeuchi, Sci. Adv., 2021, 7(3), eabd2013 CrossRef CAS PubMed.
- S.-Y. Cho, H.-W. Yoo, J. Y. Kim, W.-B. Jung, M. L. Jin, J.-S. Kim, H.-J. Jeon and H.-T. Jung, Nano Lett., 2016, 16(7), 4508–4515 CrossRef CAS PubMed.
- F. S. Fedorov, M. A. Solomatin, M. Uhlemann, S. Oswald, D. A. Kolosov, A. Morozov, A. S. Varezhnikov, M. A. Ivanov, A. K. Grebenko, M. Sommer, O. E. Glukhova, A. G. Nasibulin and V. V. Sysoev, J. Mater. Chem. A, 2020, 8(15), 7214–7228 RSC.
- S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim and T. W. Kim, Nano Energy, 2021, 79, 105369 CrossRef CAS PubMed.
- Z. Li, H. Li, Z. Wu, M. Wang, J. Luo, H. Torun, P. Hu, C. Yang, M. Grundmann, X. Liu and Y. Fu, Mater. Horiz., 2019, 6(3), 470–506 RSC.
- L. Li, C. Zhang, R. Zhang, X. Gao, S. He, M. Liu, X. Li and W. Chen, Sens. Actuators, B, 2017, 244, 664–672 CrossRef CAS.
- H. Tang, L. N. Sacco, S. Vollebregt, H. Ye, X. Fan and G. Zhang, J. Mater. Chem. A, 2020, 8(47), 24943–24976 RSC.
- K. Kim, P. G. Choi, T. Itoh and Y. Masuda, Adv. Mater. Interfaces, 2021, 8(15), 2100283 CrossRef CAS.
- W. Yan, H. Xu, M. Ling, S. Zhou, T. Qiu, Y. Deng, Z. Zhao and E. Zhang, ACS Sens., 2021, 6(7), 2613–2621 CrossRef CAS PubMed.
- P. Li, Z. Zhang, Z. Zhuang, J. Guo, Z. Fang, S. L. Fereja and W. Chen, Anal. Chem., 2021, 93(20), 7465–7472 CrossRef CAS PubMed.
- V. B. Kamble and A. M. Umarji, Sens. Actuators, B, 2016, 236, 208–217 CrossRef CAS.
- B. Liu, K. Li, Y. Luo, L. Gao and G. Duan, Chem. Eng. J., 2021, 420, 129881 CrossRef CAS.
- J. Im, H. Shin, H. Jang, H. Kim and M. Choi, Nat. Commun., 2014, 5(1), 3370 CrossRef PubMed.
- W. Karim, C. Spreafico, A. Kleibert, J. Gobrecht, J. VandeVondele, Y. Ekinci and J. A. van Bokhoven, Nature, 2017, 541(7635), 68–71 CrossRef CAS PubMed.
- S. Alayoglu, K. An, G. Melaet, S. Chen, F. Bernardi, L. W. Wang, A. E. Lindeman, N. Musselwhite, J. Guo, Z. Liu, M. A. Marcus and G. A. Somorjai, J. Phys. Chem. C, 2013, 117(50), 26608–26616 CrossRef CAS.
- Y. Han, H. Zhang, Y. Yu and Z. Liu, ACS Catal., 2021, 11(3), 1464–1484 CrossRef CAS.
- S. Kato, M. Ammann, T. Huthwelker, C. Paun, M. Lampimäki, M.-T. Lee, M. Rothensteiner and J. A. van Bokhoven, Phys. Chem. Chem. Phys., 2015, 17(7), 5078–5083 RSC.
- A. Stanoiu, C. Ghica, S. Somacescu, A. C. Kuncser, A. M. Vlaicu, I. F. Mercioniu, O. G. Florea and C. E. Simion, Sens. Actuators, B, 2020, 308, 127717 CrossRef CAS.
- J. Xiong, J. Yang, X. Chi, K. Wu, L. Song, T. Li, Y. Zhao, H. Huang, P. Chen, J. Wu, L. Chen, M. Fu and D. Ye, Appl. Catal., B, 2021, 292, 120201 CrossRef CAS.
- H. Liu, X. Liu, W. Yang, M. Shen, S. Geng, C. Yu, B. Shen and Y. Yu, J. Mater. Chem. A, 2019, 7(5), 2022–2026 RSC.
- M. Oku and K. Hirokawa, J. Appl. Phys., 1979, 50(10), 6303–6308 CrossRef CAS.
- E. C. Zipf, Planet. Space Sci., 1988, 36(6), 621–628 CrossRef CAS.
- S. Axnanda, M. Scheele, E. Crumlin, B. Mao, R. Chang, S. Rani, M. Faiz, S. Wang, A. P. Alivisatos and Z. Liu, Nano Lett., 2013, 13(12), 6176–6182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra09352e |
| This journal is © The Royal Society of Chemistry 2022 |