Open Access Article
Open Access ArticleOriented exchange-coupled L10-FePt/Co core-shell nanoparticles with variable Co thickness†
Xin Liu ,
Shulan Zuo,
Hui Wang,
Tianli Zhang,
Ying Dong
,
Shulan Zuo,
Hui Wang,
Tianli Zhang,
Ying Dong and
Chengbao Jiang*
and
Chengbao Jiang*
School of Materials Science and Engineering, Beihang University, Beijing 100191, P. R. China. E-mail: jiangcb@buaa.edu.cn
First published on 8th March 2022
Abstract
Exchange-coupled core–shell nanoparticles are expected to be the new generation of permanent magnets, where the orientation of the hard magnetic phase is supposed to play a key role in improving their magnetic performance. In this work, L10-FePt/Co core–shell nanoparticles with Co thickness ranging from 0.6 to 2.2 nm have been synthesized by a seed-mediated growth method. The exchange coupling effect between the hard core and soft shell led to a 60% improvement of the maximum magnetic energy product ((BH)max), compared with the pure L10-FePt core. By tuning the amount of precursor, nanoparticles with different Co shell thicknesses were synthesized. Furthermore, the L10-FePt/Co core–shell nanoparticles were dispersed in epoxy resin and oriented under an external magnetic field. The (BH)max of the anisotropic nanocomposite magnet with a Co thickness of 1 nm is 7.1 MGOe, enhanced by 117% compared with the isotropic L10-FePt magnet, which paves the way for the development of high-performance permanent magnets for energy conversion applications.
Introduction
Over the past few decades, the growing demands for motors applied in electric vehicles and wind-driven generators have impelled the improvement of the maximum magnetic energy product ((BH)max) of permanent magnets.1–4 Conventional high-performance permanent magnets such as Nd2Fe14B and Sm2Co17 are increasingly constrained by the scarcity of rare earths.5–7 Consequently, there is a need to exploit an alternative magnet.8–10 The exchange-coupled nano-magnets with the advantages of both hard and soft magnetic phases have the potential to achieve high (BH)max with little or even no rare earth.11–13 Since Skmoski and Coey predicted that the (BH)max of exchange-coupled magnets could reach 120 MGOe in 1993,14 considerable efforts have been made to improve (BH)max of exchange coupled magnets. However, the practical (BH)max is far below the theoretical prediction up to now. For achieving performance breakthroughs, the orientation of the hard magnetic phase is considered to be an effective method.Hard magnetic L10-FePt nanoparticles prepared by the wet chemical method15–18 have large magnetocrystalline anisotropy, small superparamagnetic critical size, controllable shape, catalytic activity, and can be aligned by a magnetic field.19,20 They are often used as building blocks of exchange-coupled magnets such as FePt/Fe3Pt,21,22 FePt/Co,23–25 FePt/Fe,26 FePt/FeCo,27,28 and FePt/Fe3O4.15,23,29,30 Exchange-coupled FePt/Co core–shell nanoparticles with controllable Co thickness was prepared by Liu et al. for the first time,23 where the introduction of the soft phase increased the saturation magnetization (Ms) of the nanoparticles. However, compared with L10-FePt nanoparticles, due to the exchange coupling effect, the hardness of L10-FePt/Co core–shell nanoparticles is reduced, which makes the orientation of nanoparticles difficult.31 Our previous work shows that L10-FePt/Co nanoparticles can be aligned under a magnetic field, but the magnetic shielding effect caused by the agglomeration of nanoparticles hindered the process of orientation.25
In this work, Co was chosen for its high Ms to construct the exchange-coupled core–shell nanoparticles with L10-FePt. The (BH)max of L10-FePt nanoparticles can be increased to a certain extent by Co coating. But the introduction of soft magnetic phase makes the orientation of nanoparticles difficult. Therefore, we need to find a critical point where the orientation of the nanoparticles is not so difficult, but also allow a certain increase in magnetic remanence, and finally the maximum (BH)max was obtained. This needs to find the best thickness value by adjusting the thickness of Co. In this work, different thicknesses of Co shell were coated on the surface of the L10-FePt nanoparticles by seed-mediated growth method.25 Furthermore, the anisotropic magnets were prepared by fixing the L10-FePt/Co core–shell nanoparticles in uncured epoxy resin under an external magnetic field, where the agglomeration problem was solved by the method proposed in our last work.19 The nanoparticles with different Co thicknesses were prepared to study the orientation of exchange-coupled nanoparticles. The (BH)max of 7.1 GMOe is obtained in the anisotropic L10-FePt/Co magnet with a Co thickness of 1 nm, topping out at 217% of the isotropy magnet.
Experimental
Materials
Platinum acetylacetonate [Pt(acac)2, purity 97%], magnesium acetylacetonate [Mg(acac)2·2H2O, purity 98%], cobalt acetylacetonate [Co(acac)2, purity 97%], oleylamine (OAm, 70%) and oleic acid (OA, 90%) was purchased from Sigma Aldrich. Pentacarbonyl iron [Fe(CO)5 purity 99%], nitric acid(HNO3, concentration 68%), methylbenzene(purity 99%) and absolute ethyl alcohol was purchased from Beijing Chemical Works. All chemicals were used as received.Preparation of L10-FePt/Co core–shell nanoparticles
Orientation
1 mg L10-FePt or L10-FePt/Co core–shell nanoparticles were dispersed in 0.5 ml epoxy. After stirring for 1 h, 0.5 ml curing agent was added to the mixture. Before the epoxy solidifying, the composite was transferred into a permanent magnet's cavity with a magnetic field of 2 T. The anisotropic L10-FePt/Co-based exchange-coupled nano-magnet was obtained after the epoxy solidified, as shown in Fig. S1.†Characterization
Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) imaging of nanoparticles were performed on a JEOL 2100F with an accelerating voltage of 200 kV. TEM samples were prepared by dropping toluene dissolved nanoparticles on the corban grids. The magnetic properties of nano-magnets were evaluated using a physical property measurement system (PPMS, Quantum Design Co., Ltd, USA).Results and discussion
L10-FePt nanoparticles were synthesized by a wet chemical method. The decomposition of Fe(CO)5 and the reduction of Pt(acac)2 led to the formation of superparamagnetic fcc-FePt nanoparticles. The ferromagnetic fct-FePt nanoparticles were obtained by annealing these fcc-FePt nanoparticles at 700 °C.32,34 To prevent the nanoparticles from oxidation, a shell of MgO was coated on the fcc-FePt nanoparticle (see Fig. S2†). Fct-FePt/MgO nanoparticles were obtained after annealing and were then acid-dipped to remove MgO and obtain fct-FePt nanoparticles. As can be seen from Fig. 1(a) and (b), fct-FePt nanoparticles are irregular spherical shapes and have an average size of about 11 nm. It should be pointed out that the size of nanoparticles is smaller than the single-domain critical size of FePt alloys (about 340 nm). The HRTEM image of fct-FePt nanoparticles in Fig. 1(b) shows complete and transparent lattice fringes with the fringe spacing of 0.22 nm, matching the standard lattice spacing 0.22 nm of (111) planes for L10-FePt. The XRD patterns, as shown in Fig. S3,† also suggest the synthesis of L10-FePt. These facts indicate that the prepared nanoparticles are single crystal and single domain L10-FePt nanoparticles.L10-FePt/Co core–shell nanoparticles were synthesized by the seed-mediated method. The precursor Co(acac)2 was added and acted as the Co source. The thickness of the Co layer was determined by the addition amount of Co(acac)2. Fig. 1(c)–(f) shows the TEM and HRTEM photographs of fct-FePt/Co core–shell nanoparticles with different Co shell thicknesses. The elemental mapping of L10-FePt/Co in Fig. S4† suggests that the Co layer is coated on the L10-FePt core. The core–shell structures are revealed by contrast difference between the center and the edge of nanoparticles in Fig. 1(c) and (e). The marked fringes spacing of the shells in Fig. 1(d) and (f) are 0.21 nm, corresponding to the interplanar spacing of fcc-Co (111) planes, while the core of 0.22 nm is corresponding to L10-FePt (111). Also, straight fringes indicate that the Co shell is epitaxial on the core surface.
The amount of Co(acac)2 was taken as a variable to prepare different L10-FePt/Co core–shell nanoparticles. Four samples with the amount of Co(acac)2 of 3 mg, 5 mg, 11 mg, and 12.5 mg were labelled as S3, S5, S11, and S12.5, respectively. In contrast, the L10-FePt nanoparticles were denoted as S0. TEM images of sample S5 and S12.5 are shown in Fig. 1(c)–(f), where the thicknesses of Co shells were measured to be 1.4 nm and 2 nm. The Co shell thickness was a function of the amount of Co(acac)2, and the calculated results are listed in Table 1.
| Sample | Co(acac)2 added, mg | Thickness n, nm | MCo/M, % |
|---|---|---|---|
| S0 | 0 | 0 | 0 |
| S3 | 3 | 0.6 | 9 |
| S5 | 5 | 1 | 17 |
| S11 | 11 | 2 | 30 |
| S12.5 | 12.5 | 2.2 | 33 |
L10-FePt/Co nanoparticle-based exchange-coupled magnets were prepared by fixing the core–shell nanoparticles in epoxy resin, where nanoparticles were fully dispersed in epoxy to avoid performance deterioration caused by agglomeration. The hysteresis loops of the samples measured at 300 K are shown in Fig. 2. L10-FePt nanoparticles without any Co coated layer exhibit typical hard magnetic performance (Fig. 2(a)), where the coercivity Hc reach up to 23.9 kOe and the saturation magnetization Ms is 32.1 emu g−1.
 | ||
| Fig. 2 Hysteresis loops measured at 300 K of samples with different Co(acac)2. (a–e) Isotropic samples. (f–j) Oriented samples. | ||
For the core–shell nanoparticles, the Hc reduces with the increase of Co thickness while the Ms is improved, as shown in Fig. 2(a)–(e), where the hysteresis loop changes from a typical hard type (Fig. 2(a)) to a soft type (Fig. 2(e)). Although the magnetic properties have changed, the hysteresis loops are still smooth and continuous, which could account for that the nanoparticles behave as a single-phase characteristic, demonstrating the good coupling between the L10-FePt cores and Co shells.35 The (BH)max of S3 with a thin Co shell is 4.9 GMOe, 48% higher than that of sample S0 of 3.3 GMOe. However, magnetic properties of L10-FePt/Co core–shell nanoparticles deteriorate when the Co shells are too thick. For example, the (BH)max decreases from 4.6 GMOe of S5 to 2.4 GMOe of S12.5.
Anisotropic exchange-coupled L10-FePt/Co nano-magnets were prepared by orienting nanoparticles under an external magnetic field, in which OAm and OA were added to increase the dispersibility. After the epoxy solidified, the easy axes of nanoparticles would be aligned to the magnetic field direction as schematically shown in Fig. S1.†
The hysteresis curves of anisotropic nano-magnets were shown in Fig. 2(f)–(j). The orientated samples made by nanoparticles with different Co thicknesses were correspondingly labelled as  ,
,  ,
,  ,
,  ,
, 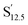 . The coloured curves and grey curves in Fig. 2 were obtained by loading the measuring magnetic field parallel to and perpendicular to the easy axes of nanoparticles, respectively. The distinction of the curves in Fig. 2(f) intuitively demonstrated that the magnet has a significant anisotropy, a sign of successful orientation. When the nanoparticles were coated with a Co shell (sample
. The coloured curves and grey curves in Fig. 2 were obtained by loading the measuring magnetic field parallel to and perpendicular to the easy axes of nanoparticles, respectively. The distinction of the curves in Fig. 2(f) intuitively demonstrated that the magnet has a significant anisotropy, a sign of successful orientation. When the nanoparticles were coated with a Co shell (sample  ), the Hc declined slightly, and the Ms increased, as shown in Fig. 2(g). Meanwhile, the distinction between the two curves was still evident, indicating the good orientation of L10-FePt/Co core–shell nanoparticles. Nanoparticles with thicker Co shells exhibited gradually decreasing Hc and increasing Ms. These two curves gradually coincide as the Co shells getting thicker, indicating the magnets tend to be isotropic.
), the Hc declined slightly, and the Ms increased, as shown in Fig. 2(g). Meanwhile, the distinction between the two curves was still evident, indicating the good orientation of L10-FePt/Co core–shell nanoparticles. Nanoparticles with thicker Co shells exhibited gradually decreasing Hc and increasing Ms. These two curves gradually coincide as the Co shells getting thicker, indicating the magnets tend to be isotropic.
Fig. 3 plots the magnetic properties of exchange-coupled L10-FePt/Co nano-magnets. The degree of orientation (V1/V2) of uniaxial permanent magnetic nanoparticles is closely related to the remanence ratio (Mr/Ms) as expressed by the equation V1/V2 = 2Mr/Ms − 1. As shown in Fig. 3(c), the aligned samples exhibit bigger Mr/Ms than the random ones due to the high degree of orientation. However, Mr/Ms declines dramatically with increasing Co shell thickness because the orientation becomes difficult as the Co shell is thick. As shown in Fig. 3(d), the (BH)max of both kinds of samples increases first and then decreases with Co layer thickness. It is well known that (BH)max is determined by two factors, namely Ms and Hc. On the one hand, when the Co shell is thin, the increase of Ms is dominant, which leads to an increase in (BH)max. On the other hand, Hc goes down rapidly with the increase of Co thickness, leading to decreased (BH)max. Furthermore, the (BH)max of the orientated samples is significantly higher than that of the random sample. The (BH)max of the oriented sample with a Co layer of 1 nm  reaches 7.16 GMOe, more than twice as high as 3.3 GMOe of the sample without orientation and Co shells. The (BH)max of sample
reaches 7.16 GMOe, more than twice as high as 3.3 GMOe of the sample without orientation and Co shells. The (BH)max of sample 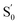 ,
,  ,
,  and
and  are 5.88, 6.95, 5.45 and 2.2 GMOe, respectively, which suggests that sample
are 5.88, 6.95, 5.45 and 2.2 GMOe, respectively, which suggests that sample  has the largest (BH)max. This is because the increase of Co thickness can increase the (BH)max, but after a certain point, further increase in Co thickness will lead to orientation difficulties, and the increase of Co itself will seriously deteriorate the coercivity, resulting in a decrease in (BH)max. Therefore, when the Co layer thickness is 1 nm, the performance of the sample exceeds 0.6 nm, 2 nm and 2.2 nm.
has the largest (BH)max. This is because the increase of Co thickness can increase the (BH)max, but after a certain point, further increase in Co thickness will lead to orientation difficulties, and the increase of Co itself will seriously deteriorate the coercivity, resulting in a decrease in (BH)max. Therefore, when the Co layer thickness is 1 nm, the performance of the sample exceeds 0.6 nm, 2 nm and 2.2 nm.
 | ||
| Fig. 3 Plotted curves of Hc, Ms, Mr/Ms, and (BH)max of exchange-coupled nano-magnets with different Co thickness. | ||
Based on the theoretical model of exchange-coupled magnets proposed by Skomski and Coey,14 the theoretical effective magnetic anisotropy constants Keff of the isotropic samples could be expressed by the following formula,
 | (1) |
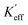 of the unoriented samples could be calculated by the following formula,36
of the unoriented samples could be calculated by the following formula,36
 | (2) |
Here, the ε0 is the permeability of the vacuum. To compare the Keff and  , Keff/K0 and
, Keff/K0 and  are plotted in Fig. 4, where K0 and
are plotted in Fig. 4, where K0 and  are the theoretical and experimental effective magnetic anisotropy constants of the pure L10-FePt nano-magnet, respectively. The Keff/K0 linearly decreases with the increase of the Co shell thickness, while the variation of the experimental
are the theoretical and experimental effective magnetic anisotropy constants of the pure L10-FePt nano-magnet, respectively. The Keff/K0 linearly decreases with the increase of the Co shell thickness, while the variation of the experimental 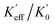 can be divided into two stages. At stage I, when the Co shell thickness is less than 1 nm, the practical
can be divided into two stages. At stage I, when the Co shell thickness is less than 1 nm, the practical  falls more slowly than the theoretical Keff/K0. This can be interpreted as the soft Co shell was hardened by the hard L10-FePt core through an intense exchange coupling effect. At stage II, the slop of
falls more slowly than the theoretical Keff/K0. This can be interpreted as the soft Co shell was hardened by the hard L10-FePt core through an intense exchange coupling effect. At stage II, the slop of  curve was equal to or less than Keff/K0, illustrating the anisotropy of samples decreases more quickly than the theoretical prediction because the newly added Co shells can no longer be hardened by the hard L10-FePt core.
curve was equal to or less than Keff/K0, illustrating the anisotropy of samples decreases more quickly than the theoretical prediction because the newly added Co shells can no longer be hardened by the hard L10-FePt core.
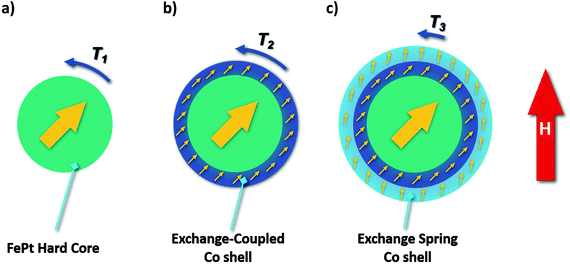
Fig. 5 schematically shows one model of the phenomenon above. When the Co shell is very thin, strong magnetic hardening occurs, resulting in a slight decrease in the anisotropy. As the Co thickness grows, the Co shells transform into two layers with different coupling modes with the core. When the Co thickness exceeds 1 nm, the outer sphere becomes spring-exchange-coupled (Fig. 5(c)) instead of hard-exchange-coupled with the core (Fig. 5(b)), where hard-exchange-coupling and spring-exchange-coupling refers to the uniform rotation of the magnetic moment and the rotation of the magnetic moment in the soft phase that drives part of the hard phase to rotate, respectively.24 The orientation pattern of the samples will change with the increase of Co thickness as applying an external magnetic field. Coherent rotation is known as the motion mode of single-domain nanoparticles in a magnetic field (Fig. 5(a)). A thin Co shell does not affect this rotation mode, and the Co shell can be considered to be co-phased with the core due to the pinned effect of exchange-coupling (Fig. 5(b)). Hence, the torque of orientation T1 and T2 should be the same. A thicker layer of Co shell leads to an exchange-spring effect between the outer and core layers (Fig. 5(c)). The constraint on the outer layers is reduced, and the magnetic moment of the outer layer rotates in advance so that the intersection angle between the magnetic moment and the magnetic field becomes small under the external magnetic field. Therefore, the torque T3 is smaller than T1 and T2, which is consistent with the above conclusion that the thicker the Co shell, the more difficult the orientation.
Conclusions
In summary, magnetic L10-FePt nanoparticles were synthesized by a wet chemical method. Co shells with thickness ranging from 0.6 to 2.2 nm were successfully coated on the L10-FePt nanoparticles. Due to the exchange-coupled effect, the Hc monotonically decreased while the Ms increased with increasing Co thickness. When the Co shell was 1 nm thick, the (BH)max of the anisotropic magnets was increased by more than 80% with increased remanence and the substantially unchanged Hc. The Co thickness affects the proportion of the soft phase and hence the anisotropy constant. A thin Co shell tinily reduces the anisotropic constant, while a thicker Co, more than 1 nm, rapidly lowers the anisotropic constant. Meanwhile, when the Co shell becomes thick, the outer layer is no longer stringently controlled by the core. Hence, the magnetic moment in the outer shell is easily deflected by the external magnetic field, and the orientation was not complete. The anisotropic exchange-coupled nanoparticles built by a hard core and a soft shell are of much concern to the high-performance permanent magnets. This work focuses on the orientation of the hard-soft exchange-coupled nanoparticles, which is essential to prepare the next generation of high-performance permanent magnets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financial supported by the National Nature Science Foundation of China under Grant No. 52121001.References
- F. Liu, Y. Hou and S. Gao, Chem. Soc. Rev., 2014, 43, 8098–8113 RSC.
- O. Gutfleisch, M. A. Willard, E. Brueck, C. H. Chen, S. G. Sankar and J. P. Liu, Cheminform, 2011, 42, 1–22 CrossRef.
- K. T. Chau, C. C. Chan and C. Liu, IEEE Trans. Ind. Electron., 2008, 55, 2246–2257 Search PubMed.
- A. M. El-Refaie, IEEE Trans. Ind. Electron., 2010, 57, 107–121 Search PubMed.
- J. M. D. Coey, Scr. Mater., 2012, 67, 524–529 CrossRef CAS.
- R. Skomski, P. Manchanda, P. K. Kumar, B. Balamurugan and D. J. Sellmyer, IEEE Trans. Magn., 2013, 49, 3215 CAS.
- D. Li, Y. Li, D. S. Pan, Z. D. Zhang and C. J. Choi, J. Magn. Magn. Mater., 2019, 469, 535–544 CrossRef CAS.
- A. Kirkeminde, J. Shen, M. G. Gong, J. Cui and S. Q. Ren, Chem. Mater., 2015, 27, 4677–4681 CrossRef CAS.
- B. Balamurugan, B. Das, W. Y. Zhang, R. Skomski and D. J. Sellmyer, J. Phys.: Condens. Matter, 2014, 26, 064204 CrossRef CAS PubMed.
- O. Gutfleisch, M. A. Willard, E. Bruck, C. H. Chen, S. G. Sankar and J. P. Liu, Adv. Mater., 2011, 23, 821–842 CrossRef CAS PubMed.
- A. D. Volodchenkov, Y. Kodera and J. E. Garay, J. Mater. Chem. C, 2016, 4, 5593–5601 RSC.
- B. Balamurugan, B. Das, V. R. Shah, R. Skomski, X. Z. Li and D. J. Sellmyer, Appl. Phys. Lett., 2012, 101, 5 CrossRef.
- E. Lottini, A. Lopez-Ortega, G. Bertoni, S. Turner, M. Meledina, G. Van Tendeloo, C. D. Fernandez and C. Sangregorio, Chem. Mater., 2016, 28, 4214–4222 CrossRef CAS.
- R. Skomski and J. M. Coey, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 15812–15816 CrossRef CAS PubMed.
- A. Figuerola, A. Fiore, R. D. Corato, A. Falqui, C. Giannini, E. Micotti, A. Lascialfari, M. Corti, R. Cingolani, T. Pellegrino, P. D. Cozzoli and L. Manna, J. Am. Chem. Soc., 2008, 130, 1477–1487 CrossRef CAS PubMed.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 CrossRef CAS PubMed.
- K. E. Elkins, T. S. Vedantam, J. P. Liu, H. Zeng, S. H. Sun, Y. Ding and Z. L. Wang, Nano Lett., 2003, 3, 1647–1649 CrossRef CAS.
- Y. Yu, L. He, J. Xu, J. Li and Y. Hou, Nano Res., 2021, 1–6 Search PubMed.
- X. Liu, H. Wang, S. Zuo, T. Zhang, Y. Dong, D. Li and C. Jiang, Nanoscale, 2020, 12, 7843–7848 RSC.
- Y. Tamada, S. Yamamoto, S. Nasu and T. Ono, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 214428 CrossRef.
- F. Casoli, P. Lupo, L. Nasi, R. Cabassi, S. Fabbrici, F. Bolzoni, P. Ranzieri and F. Albertini, J. Appl. Phys., 2015, 117, 3588–3317 CrossRef.
- H. Zeng, J. Li, J. P. Liu, Z. L. Wang and S. H. Sun, Nature, 2002, 420, 395–398 CrossRef CAS PubMed.
- F. Liu, J. Zhu, W. Yang, Y. Dong, Y. Hou, C. Zhang, H. Yin and S. Sun, Angew. Chem., Int. Ed. Engl., 2014, 53, 2176–2180 CrossRef CAS PubMed.
- D. J. Carnevale, M. Shatruk and G. F. Strouse, Chem. Mater., 2016, 28, 5480–5487 CrossRef CAS.
- D. Li, H. Wang, Z. Ma, X. Liu, Y. Dong, Z. Liu, T. Zhang and C. Jiang, Nanoscale, 2018, 10, 4061–4067 RSC.
- W. Yang, W. Lei, Y. Yu, W. Zhu, T. A. George, X. Z. Li, D. J. Sellmyer and S. Sun, J. Mater. Chem. C, 2015, 3, 7075–7080 RSC.
- M. Pousthomis, C. Garnero, C. G. Marcelot, T. Blon, S. Cayez, C. Cassignol, V. A. Du, M. Krispin, R. Arenal, K. Soulantica, G. Viau and L. M. Lacroix, J. Magn. Magn. Mater., 2017, 424, 304–313 CrossRef CAS.
- W. Zhang, W. B. Yang, R. U. Chandrasena, V. B. Ozdol, J. Ciston, M. Kornecki, S. Raju, R. Brennan, A. X. Gray and S. Q. Ren, Chem. Commun., 2018, 54, 11005–11008 RSC.
- S. J. A. Figueroa, S. J. Stewart, T. Rueda, A. Hernando and P. de la Presa, J. Phys. Chem. C, 2011, 115, 5500–5508 CrossRef CAS.
- Y. Chai, F. Feng, Q. Li, C. Yu, X. Feng, P. Lu, X. Yu, M. Ge, X. Wang and L. Yao, J. Am. Chem. Soc., 2019, 141, 3366–3370 CrossRef CAS PubMed.
- A. López-Ortega, M. Estrader, G. Salazar-Alvarez, A. G. Roca and J. Nogués, Phys. Rep., 2015, 553, 1–32 CrossRef.
- Q. Li, L. Wu, G. Wu, D. Su, H. Lv, S. Zhang, W. Zhu, A. Casimir, H. Zhu, A. Mendoza-Garcia and S. Sun, Nano Lett., 2015, 15, 2468–2473 CrossRef CAS PubMed.
- J. Kim, C. Rong, Y. Lee, J. Ping Liu and S. Sun, Chem. Mater., 2008, 7242–7245 CrossRef CAS.
- J. Kim, C. Rong, J. P. Liu and S. Sun, Adv. Mater., 2009, 21, 906–909 CrossRef CAS.
- S. H. Moon, S. H. Noh, J. H. Lee, T. H. Shin, Y. Lim and J. Cheon, Nano Lett., 2017, 17, 800–804 CrossRef CAS PubMed.
- J. Arcas, A. Hernando, J. M. Barandiarán, C. Prados and A. Neuweiler, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 5193–5196 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra09304e |
| This journal is © The Royal Society of Chemistry 2022 |