Open Access Article
Open Access ArticleA type II heterojunction α-Fe2O3/g-C3N4 for the heterogeneous photo-Fenton degradation of phenol†
Fuxiang Geab,
Xuehua Lia,
Mian Wuc,
Hui Dingc and
Xiaobing Li *a
*a
aNational Engineering Research Center of Coal Preparation and Purification, China University of Mining and Technology, Xuzhou 221000, Jiangsu, China. E-mail: lixh@cumt.edu.cn
bKaifeng Ecological and Environmental Monitoring Center, Henan Province, China
cSchool of Chemical Engineering &Technology, China University of Mining and Technology, Xuzhou, Jiangsu, China
First published on 16th March 2022
Abstract
The heterogeneous photo-Fenton reaction is an effective method of chemical oxidation to remove phenol in wastewater with environmental friendliness and sustainability. Herein, the composite α-Fe2O3/g-C3N4, as a catalyst of the heterogeneous photo-Fenton reaction, has been synthesized by hydrothermal-calcination method using the abundant and low-cost FeCl3·6H2O and g-C3N4 as raw materials. The influence of the annealing temperature during calcination was also investigated. The UV-Vis diffuse reflectance spectra of samples show that the composite α-Fe2O3/g-C3N4 possesses the widest light response range. Furthermore, the transient photocurrent response curves demonstrated the strongest intensity of α-Fe2O3/g-C3N4. The annealed α-Fe2O3/g-C3N4 is indicative of the highest degradation efficiency in all samples due to the improvement of the charge transfer ability caused by the tight heterojunction structure. The results of the scavenger trapping experiments show that the hydroxyl radical was the main active species in degradation. Based on experimental results, a type II heterojunction should be built in the composite α-Fe2O3/g-C3N4, driving the photoelectrons transfer and migration by internal electronic field. This work provides a facile and new method to synthesize α-Fe2O3/g-C3N4 as an effective heterogeneous photo-Fenton catalyst for environmental remediation.
1 Introduction
Recently, the electronic, automotive and construction industries have driven a rapid growth of bisphenol A, phenolic resin, and raw phenol. However, a large amount of phenol wastewater is inevitably produced in these processes.1 Phenol has a benzene ring structure with high stability, leading to difficulty in its degradation in a natural environment.2,3 Phenol can deactivate cellular protein by food chain intake, skin and mucosa. It also can convert into a phenoxy radical in molecular structure due to the loss of electronic behavior, which will result in mutations of DNA or RNA and cancer in chromosome sites.4 Therefore, how to degrade phenol has received much attention in the field of organic wastewater remediation. Many treatments have been established, including biological, physical and chemical methods. Biological methods face bacteria breeding difficulty and long processing cycles.5,6 Physical methods require desorption processing and retreatment. Moreover, the removal effect is poor for low concentration conditions. Based on oxidation, the chemical method has attracted increasing attention due to its simple operation, high removal efficiencies, and no secondary pollution.7,8As a typical chemical method, the Fenton reaction forms hydroxyl radicals (˙OH) via ferrous ions activating hydrogen peroxide, which can non-selectively remove organic pollutants owing to the high oxidation–reduction potential of +2.80 V.9 However, the acidic aqueous-based medium (pH = 2.5–4) and huge ferric hydroxide sludge dramatically increase the cost of degradation. The heterogeneous Fenton reaction, using the immobilized solid iron species (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe) instead of the iron ion, not only prevents the generation of sludge, but also widens the pH range of application.10 Unfortunately, the solid iron has a lower rate of regeneration of
Fe) instead of the iron ion, not only prevents the generation of sludge, but also widens the pH range of application.10 Unfortunately, the solid iron has a lower rate of regeneration of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) than the iron ion. How to accelerate the regeneration of
Fe(II) than the iron ion. How to accelerate the regeneration of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) has also become a crucial issue for the heterogeneous Fenton reaction.11 The heterogeneous photo-Fenton reaction, using a photo-induced electron to accelerate the regeneration of
Fe(II) has also become a crucial issue for the heterogeneous Fenton reaction.11 The heterogeneous photo-Fenton reaction, using a photo-induced electron to accelerate the regeneration of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II), has attracted increasing attention owing to its environmental friendliness and sustainability.12 Alpha-Fe2O3, as a n-type semiconductor, can absorb visible light owing to its inherent band gap of 2.2 eV. It is also a common candidate for the composite photocatalyst of the heterogeneous photo-Fenton reaction as a solid iron source.13–15 Moreover, it has shown great potential due to its natural abundance, low-cost, high thermal and chemical stability. However, α-Fe2O3 suffers from a high recombination rate of photoinduced electron–hole pairs and narrow visible light response range. Thus, constructing a composite with a suitable band energy gap is a mainstream approach to widen the light absorption region and reduce the charge recombination.16–20 Graphitic carbon nitride (g-C3N4), as a two-dimensional layered semiconductor, has drawn particular attention in photocatalysis due to its element abundance, facile preparation, great visible light activity, high thermal and outstanding chemical stabilities, and excellent electronic structure.21 Recently, the composite photocatalysts combining α-Fe2O3 and g-C3N4 have been successfully fabricated and showed great performance in water splitting,22 carbon dioxide reduction,23 photochemical reaction,24 and heavy metal ion reduction.25
Fe(II), has attracted increasing attention owing to its environmental friendliness and sustainability.12 Alpha-Fe2O3, as a n-type semiconductor, can absorb visible light owing to its inherent band gap of 2.2 eV. It is also a common candidate for the composite photocatalyst of the heterogeneous photo-Fenton reaction as a solid iron source.13–15 Moreover, it has shown great potential due to its natural abundance, low-cost, high thermal and chemical stability. However, α-Fe2O3 suffers from a high recombination rate of photoinduced electron–hole pairs and narrow visible light response range. Thus, constructing a composite with a suitable band energy gap is a mainstream approach to widen the light absorption region and reduce the charge recombination.16–20 Graphitic carbon nitride (g-C3N4), as a two-dimensional layered semiconductor, has drawn particular attention in photocatalysis due to its element abundance, facile preparation, great visible light activity, high thermal and outstanding chemical stabilities, and excellent electronic structure.21 Recently, the composite photocatalysts combining α-Fe2O3 and g-C3N4 have been successfully fabricated and showed great performance in water splitting,22 carbon dioxide reduction,23 photochemical reaction,24 and heavy metal ion reduction.25
Although the combination of α-Fe2O3 and g-C3N4 have been applied in various fields, the use of the composite α-Fe2O3/g-C3N4 as a heterogeneous photo-Fenton catalyst has been rarely reported to the best of our knowledge. Moreover, there was no composite of α-Fe2O3/g-C3N4 prepared by hydrothermal-calcination steps to enhance the crystallinity of the nanostructure and further improve the transfer of photo-induced electron–holes. Finally, there is no consensus on whether the composite α-Fe2O3/g-C3N4 belongs to the Z-scheme or type II heterojunction.22,26 Based on the above discussions, we introduce a method involving a hydrothermal step first, followed by calcination (hydrothermal-calcination steps), to synthesize a novel photocatalyst for the heterogeneous photo-Fenton degradation of phenol. The calcination step in this combined method aimed to improve the crystallinity, which facilitates the transfer of photo-induced charges. According to characterization measurements, the obtained α-Fe2O3/g-C3N4 has a wide visible-light responsive feature, tight heterojunction structure, and superior electron transfer ability, which finally lead to the higher degradation of phenol. The photoelectrochemical and scavenger trapping experimental results indicate that a type II heterojunction had been built to drive the photo-induced charge transfer and migration. Under the effect of this internal electronic field, the excited electrons from both components accumulated in the CB of α-Fe2O3 and further reduced ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) of the active site to
Fe(III) of the active site to ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II), which accelerated the
Fe(II), which accelerated the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III)/
Fe(III)/![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) cycles and finally improved the generation of ˙OH and the degradation efficiency. This work provides a facile new avenue to synthesize the α-Fe2O3/g-C3N4 composite, and uses it as an effective heterogeneous photo-Fenton catalyst for environmental remediation.
Fe(II) cycles and finally improved the generation of ˙OH and the degradation efficiency. This work provides a facile new avenue to synthesize the α-Fe2O3/g-C3N4 composite, and uses it as an effective heterogeneous photo-Fenton catalyst for environmental remediation.
2 Experimental section
2.1 Synthesis
The g-C3N4 was prepared by pyrolysis approach. 5 g melamine was heated in a tube furnace from room temperature to 520 °C at a rate of 20 °C min−1. After maintaining the temperature for 2 h, the sample was cooled down to room temperature. Finally, the as-prepared yellow solid was milled into the g-C3N4 powder.The α-Fe2O3/g-C3N4 sample was fabricated by hydrothermal-calcination steps. In a typical procedure, 0.2 g of g-C3N4 and 0.6 g FeCl3·6H2O were dissolved in 50 mL water, and the solution was heated for 6 h at a temperature of 180 °C in the autoclave. Then, the solid composites were separated from the solution by centrifuging at 3000 rpm for 20 min. After cleaning three times by distilled water, the solid was calcined at 520 °C for 2 h with nitrogen protection to obtain the α-Fe2O3/g-C3N4 sample.
2.2 Characterization
X-ray diffraction (XRD) was performed on a Panalytical X'Pert PRO system using Cu-Kα radiation. X-ray photoelectron spectroscopy (XPS) analysis was carried out on an X-ray photoelectron spectrometer (PHI 5000 Versa Probe, UIVAC-PHI, Japan). Fourier transform infrared spectroscopy (FTIR) measurements were conducted on a Nicolet 5700 spectrophotometer (Nicolet, USA) in the range of 400–4000 cm−1. Ultraviolet-visible diffuse reflectance spectra (UV-Vis DRS) were measured on a Cary 300 spectrophotometer (Varian, USA). Fluorescence spectra were obtained using a Fluoromax-4 fluorescence spectrophotometer (Horiba, Japan) with different excitation wavelengths.The transient photocurrent response curves and electrochemical impedance spectroscopy (EIS) curves were measured by electrochemical workstation (Chenhua CHI 760E). The Ag/AgCl electrode and Pt wire were used as the reference electrode and counter electrode, respectively. The working electrode was prepared via the following methods. 10 mg of photocatalyst was added to 1 mL of 0.5% Nafion solution, which was subsequently treated by ultrasonication for 10 min. The dispersed solution was dropped onto a fluorine-doped tin oxide glass (with a size of 1 × 1 cm2) and dried at 50 °C for 4 h in a low vacuum atmosphere. Then, the obtained electrode was inserted in 0.1 mol L−1 Na2SO4 solution as the electrolyte. Under the irradiation of a 350 W xenon lamp, the photocurrent response curves were measured at a bias potential of 0.1 V and with given irradiation intervals of 30 s. EIS was performed on an applied voltage of 0 V with an amplitude of 5 mV over a frequency range between 10−2 and 105 Hz.
2.3 Heterogeneous photo-Fenton degradation and radical oxygen species analysis
The heterogeneous photo-Fenton degradation experiments were conducted in an assembled photochemical system, in which a beaker with a volume of 100 mL is placed under a 350 W Xenon lamp with a distance of 10 cm. The lamp surface is 12.5 cm away from the 100 mL scale of the beaker. Before degradation, the lamp was warmed up for 2 min to a rated output intensity of 350 W. Circulating water was introduced to cool the rising temperature in the solution from irradiation.In a typical degradation procedure, 10 mg α-Fe2O3/g-C3N4 was added to 100 mL phenol solution with a concentration of 50 mg L−1, and the solution was then stirred for 30 min in the dark to reach adsorption equilibrium. After 45 mM H2O2 was added, the solution was irradiated by the xenon lamp. Then, a 3 mL aliquot of the solution was taken out at certain time intervals, and the solid catalysts were separated by centrifugation. Then, the concentration of phenol remaining in solution was determined by UV-Vis spectroscopy at the maximum absorbance peak of 510 nm. The degradation efficiency formula is defined as:
 | (1) |
3 Results and discussions
3.1 Characterization of the heterogeneous photocatalyst
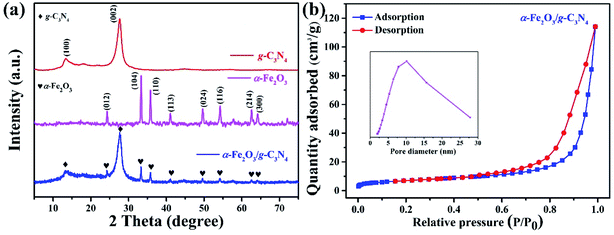
The crystal structures of g-C3N4, α-Fe2O3 and α-Fe2O3/g-C3N4 were investigated by XRD technique and the results are shown in Fig. 1(a). The g-C3N4 sample shows a characteristic diffraction peak at 13.2°, which is indexed to the (100) diffraction plane and corresponds to the in-plane tri-s-thiazine repeating motifs. Another prominent peak is located at 27.5°, which is assigned to the (002) plane of the interlayer stacking of the conjugated aromatic systems.27 The characteristic diffraction peaks of iron oxide are present at 2θ = 24.3°, 33.3°, 35.9°, 41.0°, 49.6°, 54.3°, 62.6° and 64.1°, which correspond to the (012), (104), (110), (113), (024), (116), (214) and (300) diffraction planes of α-Fe2O3,28 indicating that the iron oxide is hematite. The composite α-Fe2O3/g-C3N4 shows all of the characteristic diffraction peaks of g-C3N4 and α-Fe2O3, suggesting the success of the combination.The specific surface area and porosity are of great importance for the absorption of contaminants and transmission of radical oxygen species, respectively. The nitrogen adsorption–desorption isotherm and the pore size distribution curve of α-Fe2O3/g-C3N4 were obtained and are shown in Fig. 1(b), including the BET surface area, total pore volume and average pore size. The isotherm showed that α-Fe2O3/g-C3N4 presents a typical IV pattern in the range of 0.5–0.98 relative pressure, indicating the presence of a mesoporous structure in the size of 2–50 nm. According to the IUPAC classification, the hysteresis loop of the isotherm was type H3,24 which indicates that narrow slit-shaped pores are associated with the structure of the plate-like g-C3N4 and α-Fe2O3 nanoparticles. The surface area and average pore diameter of α-Fe2O3/g-C3N4 were calculated to be 28.75 m2 g−1 and 10.2 nm, respectively.
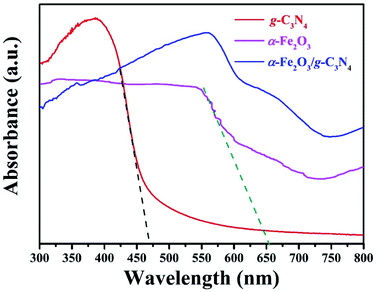
The ability to harvest visible light plays an important role in improving the photocatalytic activity (degradation in current work) for photocatalysts because visible light accounts for nearly half of the solar spectrum. The UV-Vis diffuse reflectance spectra of g-C3N4, α-Fe2O3 and α-Fe2O3/g-C3N4 were measured and are shown in Fig. 2. For pure g-C3N4, the characteristic spectrum shows a fundamental absorption edge at 470 nm, corresponding to a band gap energy of 2.7 eV, which is in great agreement with the previous reports.27 The as-prepared α-Fe2O3 has an absorption edge at approximately 630 nm ascribed to the intrinsic band gap. Both components have the ability to absorb visible light. Compared with α-Fe2O3 or g-C3N4, α-Fe2O3/g-C3N4 showed a significant increase in visible light absorption due to the synergy of both materials.
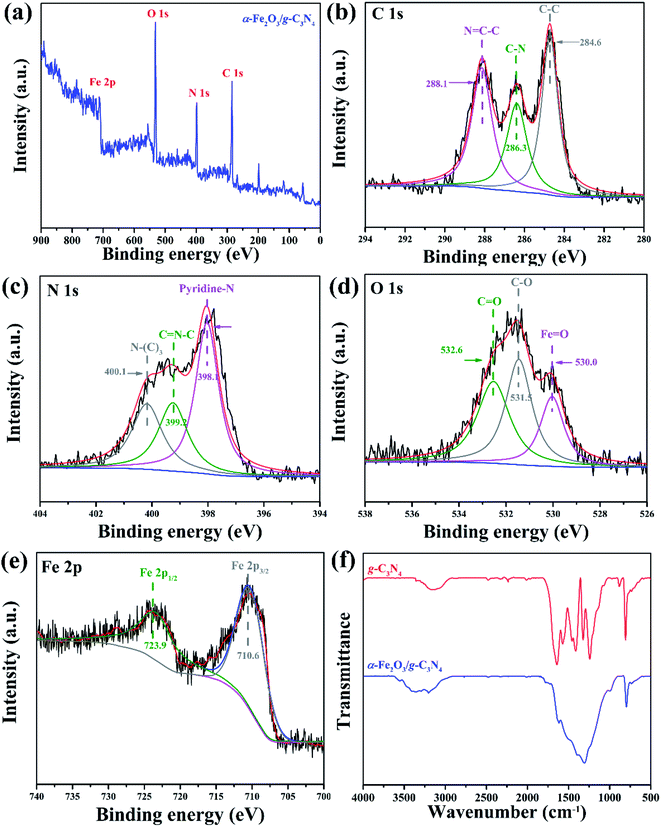
XPS has been carried out to deeply evaluate the binding energy of the elements and surface groups in the composite catalyst. As shown in Fig. 3(a), the survey spectra of α-Fe2O3/g-C3N4 show several characteristic peaks at around 284.8, 399.0, 532.0 and 723 eV, corresponding to C 1s, N 1s, O 1s and Fe 2p, respectively, and indicate the existence of these elements. The high-resolution C 1s spectrum of α-Fe2O3/g-C3N4 shows three peaks located at 284.6, 286.3 eV and 288.1 eV, which are assigned to the C–C bonding of the sp2-hybridized carbon, C–N bonding groups and sp2-hybridized bonding of the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, respectively (Fig. 3(b)).29,30 The N 1s spectrum of α-Fe2O3/g-C3N4 also could be deconvoluted into three characteristic peaks at around 398.1, 399.2 and 400.1 eV, which were attributed to pyridinic nitrogen (C–N
N bonds, respectively (Fig. 3(b)).29,30 The N 1s spectrum of α-Fe2O3/g-C3N4 also could be deconvoluted into three characteristic peaks at around 398.1, 399.2 and 400.1 eV, which were attributed to pyridinic nitrogen (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), pyrrolic nitrogen (C–N–C) and quaternary nitrogen (N–(C)3), respectively (Fig. 3(c)).31,32 The O 1s peak has three deconvolution peaks at 531.9, 532.8 and 530.7 eV in Fig. 3(d), corresponding to the bonds of C–O, C
C), pyrrolic nitrogen (C–N–C) and quaternary nitrogen (N–(C)3), respectively (Fig. 3(c)).31,32 The O 1s peak has three deconvolution peaks at 531.9, 532.8 and 530.7 eV in Fig. 3(d), corresponding to the bonds of C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Fe–O–C, respectively. The existence of Fe–C–O indicated the strong interaction between the α-Fe2O3 moiety and g-C3N4 moiety, which might serve as an electron pathway to facilitate electron transfer and migration between interfaces.33 In the high-resolution spectra of Fe 2p (Fig. 3(e)), the composite has two obvious peaks at 710.6 and 724.5 eV, corresponding to Fe 2p3/2 and Fe 2p1/2, respectively. In addition, both peaks are fully consistent with the typical values of Fe in α-Fe2O3,34 suggesting the formation of α-Fe2O3.
O and Fe–O–C, respectively. The existence of Fe–C–O indicated the strong interaction between the α-Fe2O3 moiety and g-C3N4 moiety, which might serve as an electron pathway to facilitate electron transfer and migration between interfaces.33 In the high-resolution spectra of Fe 2p (Fig. 3(e)), the composite has two obvious peaks at 710.6 and 724.5 eV, corresponding to Fe 2p3/2 and Fe 2p1/2, respectively. In addition, both peaks are fully consistent with the typical values of Fe in α-Fe2O3,34 suggesting the formation of α-Fe2O3.
 | ||
| Fig. 3 (a) XPS survey spectrum of α-Fe2O3/g-C3N4. The high-resolution binding energy spectra of (b) C 1s, (c) N 1s, (d) O 1s, and (e) Fe 2p. The (f) FTIR spectra of g-C3N4, and α-Fe2O3/g-C3N4. | ||
FTIR spectra were measured to verify the functional groups of g-C3N4 and α-Fe2O3/g-C3N4, which are shown in Fig. 3(f). For pure g-C3N4, the sharp peak at 806 cm−1 originates from the breathing mode of the triazine unit. The α-Fe2O3/g-C3N4 composite shows the same peak at 806 cm−1 with lower intensity, indicating that the triazine unit is still the main structure after the formation of the composite. Several peaks at 1242, 1323, 1407 and 1454 cm−1 in the range of 1200–1500 cm−1 are ascribed to the typical stretching modes of the C–N heterocycles of g-C3N4, and the peak at 1647 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration.35 These peaks merged into a broad peak in the α-Fe2O3/g-C3N4 composite due to the covering influence of α-Fe2O3 on the surface of g-C3N4. Both g-C3N4 and α-Fe2O3/g-C3N4 have a broad peak located in the range of 3300–2900 cm−1, which is assigned to the N–H stretching vibration. These results illustrate that both composites maintain the same polycondensation structure as pure g-C3N4.
N stretching vibration.35 These peaks merged into a broad peak in the α-Fe2O3/g-C3N4 composite due to the covering influence of α-Fe2O3 on the surface of g-C3N4. Both g-C3N4 and α-Fe2O3/g-C3N4 have a broad peak located in the range of 3300–2900 cm−1, which is assigned to the N–H stretching vibration. These results illustrate that both composites maintain the same polycondensation structure as pure g-C3N4.
3.2 Degradation tests
The performance of the as-prepared α-Fe2O3/g-C3N4 was evaluated by testing the degradation efficiency of phenol (concentration 100 mg L−1). First, the influence of the components in the composite on degradation efficiency was investigated, and the results are shown in Fig. 4(a). As a semiconductor and solid iron source, α-Fe2O3 not only can absorb visible light, but also can decompose H2O2 into ˙OH radicals by transferring an electron to H2O2 through its active site. The bare α-Fe2O3 shows a poor degradation efficiency of about 55% in 70 min. Although α-Fe2O3 was able to degrade phenol, the removal efficiency was limited by the low light absorption ability and high electron–hole recombination. The strategy of combining with other semiconductors is a mainstream method to alleviate these limitations. After combining with g-C3N4, the composite α-Fe2O3/g-C3N4 showed a significant improvement in the degradation efficiency up to 90% under the same conditions. The introduction of g-C3N4 improves the absorption ability in the visible range (as shown in Fig. 2), and was developed to achieve the spatial separation of the electron and holes between different components and prevent the charge recombination through electron–hole trapping.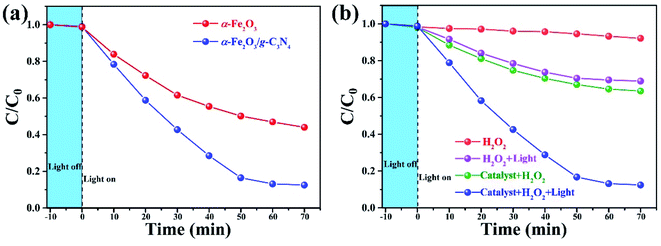 | ||
| Fig. 4 Phenol degradation profile under different conditions (a) and with different photocatalysts (b). | ||
The results of phenol degradation by photocatalysts, heterogeneous Fenton reaction and heterogeneous photo-Fenton reaction are shown in Fig. 4 (b). The phenol was not degraded by H2O2 in the dark, suggesting that H2O2 does not decompose into ˙OH without the catalyst and light irradiation. When only adding catalysts in the same condition of darkness (heterogeneous Fenton reaction), the degradation efficiency of phenol increased up to 33%, indicating that α-Fe2O3/g-C3N4 catalytically decomposed H2O2 into ˙OH as the iron source. After irradiation by a xenon lamp for 70 min, about 30% phenol in the solution was degraded in the presence of H2O2 and without a catalyst (photo-Fenton reaction), which was attributed to the decomposition of H2O2 into ˙OH by an ultraviolet ray of simulated light.35 Although both methods slightly increased the degradation efficiency of phenol, the degradation efficiency was still moderate. Under heterogeneous photo-Fenton conditions (heterogeneous photocatalysts, H2O2 and light irradiation), the degradation efficiency of phenol was significantly boosted up to 90%. It is worth noting that the total degradation efficiency is not a simple superposition of the efficiency of each part. This result is indicative of a synergistic effect between the heterogeneous Fenton reaction and photo-Fenton reaction, which will be further investigated in the mechanism analysis section.
The degradation performance of α-Fe2O3/g-C3N4 without the annealing step and under a different annealing temperature was also investigated to provide better insights, which is shown in Fig. S1 (ESI†). It was found that the degradation efficiency of α-Fe2O3/g-C3N4 showed a significant increase after calcination, indicating that calcination has a promoting effect on the degradation process due to the improvement of the photo-induced charge transfer. Moreover, as the annealing temperatures increase from 360 °C to 520 °C, the degradation only slightly increased about 24%. Nevertheless, when the annealing temperature was up to 560 °C, the obtained efficiency decreased from 92 to 60%, which be attributed to the decomposition of some g-C3N4 caused by excessive temperature.
A series of experiments were carried out to obtain the optimal degradation conditions, including photocatalyst amounts, H2O2 dosage, pH value and initial contaminant concentration, as shown in Fig. 5. It is generally known that adding some quantity of catalysts directly affects the H2O2 decomposition. Fig. 5(a) compares the degradation performance when various catalyst concentrations were added to the solutions. The degradation efficiencies of phenol increased with increasing catalyst concentration from 0.05 to 0.15 mg L−1. This is because of the boost of ˙OH from the decomposition of H2O2 catalyzed by α-Fe2O3/g-C3N4. However, when the concentration increased from 0.15 to 0.25 mg L−1, the degradation efficiency slightly decreased. This is attributed to the excessive iron species consuming some ˙OH radicals. The influence of the H2O2 dosage shown in Fig. 5(b). It could be easily found that the degradation efficiencies remained at the high level of about 90% in 70 min, when the dosage increased from 90 to 180 mmol L−1. The efficiencies would decrease when the dosage was below or above this range. This was because of the lockage of H2O2 leading to less ˙OH radicals or the scavenging effect of excessive H2O2 reducing the availability of ˙OH, respectively. At different pH values of 2.5–7.5, as shown in Fig. 5(c), the degradation efficiencies gradually decreased with increasing pH values. However, the α-Fe2O3/g-C3N4 catalyst shows good performance in the range of 2.5–5.5, wherein at least 81% phenol was degraded in 70 min. The decrease of efficiency in an alkaline environment is associated with the self-decompositions of H2O2 molecules and weak oxidation potential of ˙OH radicals at higher pH (E0 = +2.8 V and +2.0 V at pH = 0 and 14, respectively).34 The initial concentration of phenol is another important factor that affects the degradation, as shown in Fig. 5(d). The degradation performance decreased with increasing contaminant concentration. When the initial concentration increased from 50 mg L−1 to 100 mg L−1, the degradation efficiency was maintained at 90% in 70 min. Subsequently, the efficiency decreased with an increase of the phenol concentration, which can be attributed to the limitation of reaction sites on the surface of the photocatalyst.
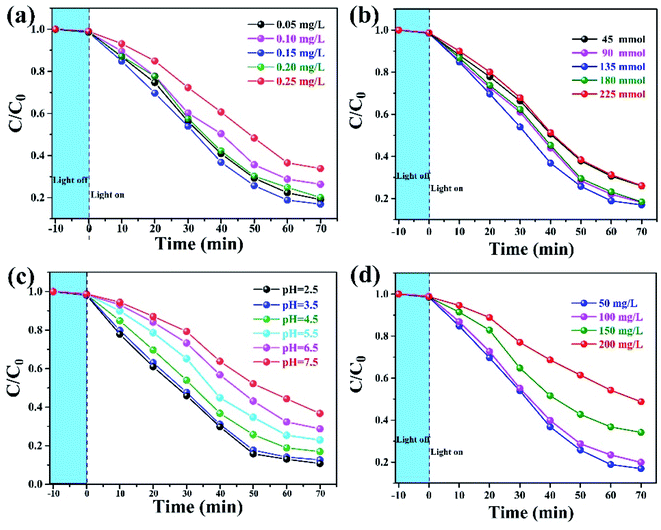 | ||
| Fig. 5 Effects of the removal experimental conditions on the degradation efficiency, (a) photocatalyst amount, (b) H2O2 dosage, (c) pH value, and (d) initial contaminant concentration. | ||
The reusability of composite α-Fe2O3/g-C3N4 is a crucial factor for its practical and industrial applications. To explore this performance, recycling experiments were carried out. As illustrated in Fig. S2 (ESI†), there was a slight decrease of about 8% in the degradation efficiencies after five cycles, indicating that the composite retained good reusability after undergoing five reaction cycles. The slight decrease in the catalytic performance is unavoidable, which is because of the difficulty in the full recovery of the spent catalyst and pore blockage on the catalyst surface caused by pollutants and residual intermediates.
3.3 Possible mechanism of the heterogeneous photo-Fenton reaction
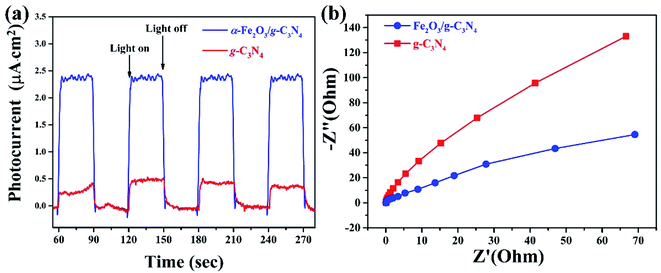
The photoelectrochemical technique was used to investigate the separation and transfer behaviors of the photo-induced electron–hole pairs in the α-Fe2O3/g-C3N4 catalyst, including the transient photocurrent response and electrochemical impedance spectroscopy (EIS). As shown in the transient photocurrent response curves of Fig. 6(a), g-C3N4 and α-Fe2O3/g-C3N4 have stable photocurrent cycles under on-off light irradiation cycles. The α-Fe2O3/g-C3N4 composite shows a photocurrent density of 5.68 μA cm−2, which is about 5 times than that of g-C3N4. The well-engineered bandgap arrangement of α-Fe2O3/g-C3N4 is responsible for the higher performance by facilitating both charge separation and transfer through an internal electric field. During the formation of α-Fe2O3 on the surface of g-C3N4, some key chemical bonds like Fe–O–C were formed at the same time, and were also revealed by the XPS and FTIR results. These bonds act as a bridge or channel-linked g-C3N4 with α-Fe2O3, enhancing the interfacial contact of adjacent semiconductors and accelerating the transfer or migration of photo-induced charges between different semiconductors.35 These improvements were further demonstrated by the EIS spectra of g-C3N4 and α-Fe2O3/g-C3N4 in Fig. 6(b). The α-Fe2O3/g-C3N4 composite shows a much smaller arc radius than pure g-C3N4, indicating the lowest charge transfer resistance during the photo-excited process. The formed chemical bonds between the semiconductors could reduce the transfer resistance of the photo-induced charges. The photoelectrochemical results showed that α-Fe2O3/g-C3N4 has more separation efficiency and faster interfacial charge transfer behaviors than g-C3N4.In addition, the type of active species in degradation is crucial to elucidate the mechanism of the heterogeneous photo-Fenton reaction. Thus, a series of quenchers (IPA and BQ) were used to scavenge possible species in degradation, such as the hydroxyl radical (˙OH) and superoxide radical (˙O2−), and the results are shown in Fig. 7(a). A significant inhibition phenomenon is observed after the ˙OH scavenger (IPA) is added to the reaction system. The degradation efficiency decreased from 90% to 12% in 70 min. In contrast, a slight inhibition of BQ as a scavenger for the superoxide radical was detected from 90 to 86%. These results indicate that the hydroxyl radical, rather than the superoxide radical, is the main active species in this degradation.
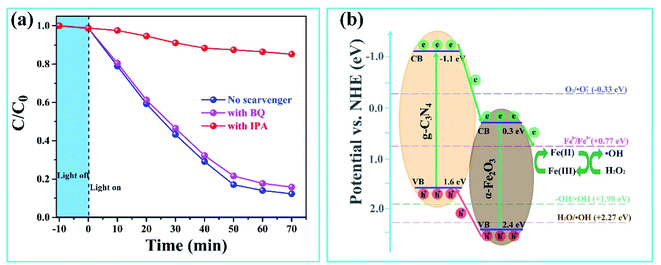 | ||
| Fig. 7 (a) Degradation of phenol over α-Fe2O3/g-C3N4 with the addition of different radical scavengers. (b) The proposed heterogeneous photo-Fenton mechanism of α-Fe2O3/g-C3N4. | ||
Based on the above trapping experiments, a photo-induced charge transfer and generation of ˙OH was proposed for α-Fe2O3/g-C3N4 as the heterogeneous photo-Fenton catalyst, which is shown in Fig. 7(b). Both g-C3N4 and α-Fe2O3 generated electrons and holes under visible irradiation due to the intrinsic energy band structure, in which an electron jumps up to the conduction band (CB) and a hole is left in the valence band (VB). Many previous reports demonstrate that the CB and VB of g-C3N4 are located at −1.10 eV and +1.6 eV (vs. NHE),36,37 while the CB and VB of α-Fe2O3 are located at +0.3 eV and +2.4 eV (vs. NHE),38,39 respectively. According to these energy level structures, two potential alignments of the band energy were proposed for the mechanism of the electron–hole pair transfer and migration in the α-Fe2O3/g-C3N4 composite, including the type II or Z-scheme heterojunction. If a Z-scheme alignment would be built, the electron in CB of α-Fe2O3 will transfer to and further recombine with the holes in VB of g-C3N4. This would lead to an enhanced driving force for charge transfer because of the lower CB position of g-C3N4 and higher VB location of α-Fe2O3. However, the electron accumulated in CB of g-C3N4 will transfer to O2 molecules, and inevitably generate a lot of ˙O2− species in the degradation reaction. This is due to it being more negative CB than the redox potential of O2/˙O2− (−0.33 eV). This hypothesis contradicts the results of the scavenger trapping experiment, in which only a few ˙O2− species were detected in the reaction.
Hence, a mechanism based on a type II heterojunction should be reasonable. As shown in Fig. 7(b), the excited holes in the VB of α-Fe2O3 transfer to the VB of g-C3N4, while the electrons in the CB of g-C3N4 will transfer to and accumulate in the CB of α-Fe2O3. Compared to the dissolved oxygen molecule in solution with a potential barrier at the solid–liquid interface, the photo-induced electron in the CB of α-Fe2O3 was more inclined to transfer to the CB of g-C3N4 due to the tight solid–solid interface. The semiconductor of α-Fe2O3 not only generated electrons under irradiation, but also obtained the photoelectron from the CB of g-C3N4. The accumulated electrons reduced ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) of the iron site to
Fe(III) of the iron site to ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) in α-Fe2O3. This is because the CB of α-Fe2O3 (+0.30 eV) is more negative than the standard potential of Fe3+/Fe2+ (+0.77 eV).37 Hence, these photo-induced electrons from the excitation of α-Fe2O3 and g-C3N4 accelerated the
Fe(II) in α-Fe2O3. This is because the CB of α-Fe2O3 (+0.30 eV) is more negative than the standard potential of Fe3+/Fe2+ (+0.77 eV).37 Hence, these photo-induced electrons from the excitation of α-Fe2O3 and g-C3N4 accelerated the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III)/
Fe(III)/![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) recycling in the component of α-Fe2O3. The obtained
Fe(II) recycling in the component of α-Fe2O3. The obtained ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) would further decompose H2O2 into ˙OH, as discussed in many previous heterogeneous photo-Fenton reports, wherein ˙OH takes a dominant role in the degradation of organic contaminants. In addition, due to the redox potential of –OH/˙OH (+1.98 eV) and H2O/˙OH (+2.27 eV) being more positive than the VB value of g-C3N4 (+1.60 eV), the photo-induced holes accumulated on the VB of g-C3N4 were not sufficiently positive to form the ˙OH radical.40,41 This indicated that the ˙OH radicals in the degradation were mainly produced by a heterogeneous photo-Fenton reaction.
Fe(II) would further decompose H2O2 into ˙OH, as discussed in many previous heterogeneous photo-Fenton reports, wherein ˙OH takes a dominant role in the degradation of organic contaminants. In addition, due to the redox potential of –OH/˙OH (+1.98 eV) and H2O/˙OH (+2.27 eV) being more positive than the VB value of g-C3N4 (+1.60 eV), the photo-induced holes accumulated on the VB of g-C3N4 were not sufficiently positive to form the ˙OH radical.40,41 This indicated that the ˙OH radicals in the degradation were mainly produced by a heterogeneous photo-Fenton reaction.
4 Conclusions
Heterogeneous photo-Fenton degradation offers a green and sustainable approach to remove phenol in wastewater by using solar energy. As typical semiconductors for photocatalysis, α-Fe2O3 and g-C3N4 have shown great potential due to their natural abundance, low-cost, high thermal and chemical stability. Herein, a heterogeneous catalyst of α-Fe2O3/g-C3N4 for the photo-Fenton reaction was synthesized by hydrothermal-calcination method. The results from the UV-Vis DRS and transient photocurrent response curves demonstrated that the α-Fe2O3/g-C3N4 composite possesses the optimal performance in photocatalysis. The calcination process played an important role in improving the transfer of photo-induced charges by tightening the heterojunction structure. Both improvement of light absorption and photoelectron transfer led to the best degradation efficiency of α-Fe2O3/g-C3N4. To better understand the mechanism of action, scavenger trapping experiments were carried out to measure the active species in degradation. The results show that the hydroxyl radical was the main active species. Based on these experimental results, a type II heterojunction should be built in the α-Fe2O3/g-C3N4 composite driving the photoelectrons transfer and migration by internal electronic field. Additionally, a reusability study of α-Fe2O3/g-C3N4 showed only a slight decrease of the degradation efficiency (about 8%) after five cycles. Overall, α-Fe2O3/g-C3N4 proved to be an effective heterogeneous photo-Fenton catalyst for environmental remediation.Conflicts of interest
All authors declare that there are no competing interests.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 52004279), Jiangsu Province Key Research and Development Project (BE2018635) and Six Talents Peaks Project in Jiangsu Province (JNHB-077).References
- E. Brillas and S. Garcia-Segura, Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule, Sep. Purif. Technol., 2020, 237, 116337 CrossRef CAS.
- L. Yu, J. Chen, Z. Liang, W. Xu, L. Chen and D. Ye, Degradation of phenol using Fe3O4-GO nanocomposite as a heterogeneous photo-Fenton catalyst, Sep. Purif. Technol., 2016, 171, 80–87 CrossRef CAS.
- T. W. Leal, L. A. Lourenco, H. d. L. Brandao, A. da Silva, S. M. A. G. U. de Souza and A. A. U. de Souza, Low-cost iron-doped catalyst for phenol degradation by heterogeneous Fenton, J. Hazard. Mater., 2018, 359, 96–103 CrossRef CAS PubMed.
- A. M. Salazar-Arias, G. I. Giraldo-Gomez and N. R. Sanabria-Gonzalez, Degradation of phenol using mill scale as a Fenton-type catalyst, Water Environ. J., 2019, 34, 183–191 CrossRef.
- C. Justino, A. G. Marques, K. R. Duarte, A. C. Duarte, R. Pereira, T. Rocha-Santos and A. C. Freitas, Degradation of phenols in olive oil mill wastewater by biological, enzymatic, and photo-Fenton oxidation, Environ. Sci. Pollut. Res., 2010, 17(3), 650–656 CrossRef CAS.
- M. Tyagi, N. Kumari and S. Jagadevan, A holistic Fenton oxidation-biodegradation system for treatment of phenol from coke oven wastewater: Optimization, toxicity analysis and phylogenetic analysis, J. Water Process. Eng., 2020, 37, 101475 CrossRef.
- Y. Yavuz, A. S. Koparal and U. B. Ogutveren, Phenol removal through chemical oxidation using Fenton reagent, Chem. Eng. Technol., 2007, 30(5), 583–586 CrossRef CAS.
- N. Inchaurrondo, C. P. Ramos, G. Zerjav, J. Font, A. Pintar and P. Haure, Modified diatomites for Fenton-like oxidation of phenol, Microporous Mesoporous Mater., 2017, 239, 396–408 CrossRef CAS.
- L. Clarizia, D. Russo, I. Di Somma, R. Marotta and R. Andreozzi, Homogeneous photo-Fenton processes at near neutral pH: A review, Appl. Catal., B, 2017, 209, 358–371 CrossRef CAS.
- P. V. Nidheesh, Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review, RSC Adv., 2015, 5(51), 40552–40577 RSC.
- L. Lyu and C. Hu, Heterogeneous Fenton Catalytic Water Treatment Technology and Mechanism, Prog. Chem., 2017, 29(9), 981–999 CAS.
- A. N. Soon and B. H. Hameed, Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process, Desalination, 2011, 269, 1–16 CrossRef CAS.
- J. Liu, B. Wang, Z. Li, Z. Wu, K. Zhu, J. Zhuang, Q. Xi, Y. Hou, J. Chen, M. Cong, J. Li, G. Qian and Z. Lin, Photo-Fenton reaction and H2O2 enhanced photocatalytic activity of alpha-Fe2O3 nanoparticles obtained by a simple decomposition route, J. Alloys Compd., 2019, 771, 398–405 CrossRef CAS.
- L. Guo, F. Chen, X. Fan, W. Cai and J. Zhang, S-doped alpha-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol, Appl. Catal., B, 2010, 96, 162–168 CrossRef CAS.
- Y. Liu, W. Jin, Y. Zhao, G. Zhang and W. Zhang, Enhanced catalytic degradation of methylene blue by alpha-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions, Appl. Catal., B, 2017, 206, 642–652 CrossRef CAS.
- M. K. Ahmed, A. E. Shalan, M. Afifi, M. M. El-Desoky and S. Lanceros-Méndez, Silver-Doped Cadmium Selenide/Graphene Oxide-Filled Cellulose Acetate Nanocomposites for Photocatalytic Degradation of Malachite Green toward Wastewater Treatment, ACS Omega, 2021, 6(36), 23129–23138 CrossRef CAS PubMed.
- S. A. Mousa, A. E. Shalan, H. H. Hassan, A. A. Ebnawaled and S. A. Khairy, Enhanced the photocatalytic degradation of titanium dioxide nanoparticles synthesized by different plant extracts for wastewater treatment, J. Mol. Struct., 2022, 1250, 131912 CrossRef CAS.
- M. Batool, M. F. Nazar, A. Awan, M. B. Tahir, A. Rahdar, A. E. Shalan, S. Lanceros-Méndez and M. N. Zafar, Bismuth-based heterojunction nanocomposites for photocatalysis and heavy metal detection applications, Nano-Struct. Nano-Objects, 2021, 27, 100762 CrossRef CAS.
- A. E. Shalan, M. Afifi, M. M. El-Desoky and M. K. Ahmed, Electrospun nanofibrous membranes of cellulose acetate containing hydroxyapatite co-doped with Ag/Fe: morphological features, antibacterial activity and degradation of methylene blue in aqueous solution, New J. Chem., 2021, 45(20), 9212–9220 RSC.
- M. F. Sanad, A. E. Shalan, M. A. Ahmed and M. F. A. Messih, The controlled synthesis and DFT investigation of novel (0D)–(3D) ZnS/SiO2 heterostructures for photocatalytic applications, RSC Adv., 2021, 11(36), 22352–22364, 10.1039/D1RA02284A.
- J. Q. Wen, J. Xie, X. B. Chen and X. Li, A review on g-C3N4-based photocatalysts, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- X. She, J. Wu, H. Xu, J. Zhong, Y. Wang, Y. Song, K. Nie, Y. Liu, Y. Yang, M.-T. F. Rodrigues, R. Vajtai, J. Lou, D. Du, H. Li and P. M. Ajayan, High Efficiency Photocatalytic Water Splitting Using 2D alpha-Fe2O3/g-C3N4 Z-Scheme Catalysts, Adv. Energy Mater., 2017, 7(17), 1700025 CrossRef.
- Z. Jiang, W. Wan, H. Li, S. Yuan, H. Zhao and P. K. Wong, A Hierarchical Z-Scheme alpha-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction, Adv. Mater., 2018, 30(10), 1706108 CrossRef PubMed.
- S. Liu, S. Wang, Y. Jiang, Z. Zhao, G. Jiang and Z. Sun, Synthesis of Fe2O3 loaded porous g-C3N4 photocatalyst for photocatalytic reduction of dinitrogen to ammonia, Chem. Eng. J., 2019, 373, 572–579 CrossRef CAS.
- S. Balu, Y.-L. Chen, R. C. Juang, T. C. K. Yang and J. C. Juan, Morphology-Controlled Synthesis of alpha-Fe2O3 Nanocrystals Impregnated on g-C3N4-SO3H with Ultrafast Charge Separation for Photoreduction of Cr (VI) Under Visible Light, Environ. Pollut., 2020, 267, 115491 CrossRef CAS PubMed.
- L. Kong, J. Yan, P. Li and S. F. Liu, Fe2O3/C-C3N4-Based Tight Heterojunction for Boosting Visible Light-Driven Photocatalytic Water Oxidation, ACS Sustainable Chem. Eng., 2018, 6(8), 10436–10444 CrossRef CAS.
- S. W. Cao, J. X. Low, J. G. Yu and M. Jaroniec, Polymeric Photocatalysts Based on Graphitic Carbon Nitride, Adv. Mater., 2015, 27(13), 2150–2176 CrossRef CAS PubMed.
- Q. Liu, T. Chen, Y. Guo, Z. Zhang and X. Fang, Grafting Fe(III) species on carbon nanodots/Fe-doped g-C3N4 via interfacial charge transfer effect for highly improved photocatalytic performance, Appl. Catal., B, 2017, 205, 173–181 CrossRef CAS.
- Y. Deng, L. Tang, C. Feng, G. Zeng, J. Wang, Y. Lu, Y. Liu, J. Yu, S. Chen and Y. Zhou, Construction of Plasmonic Ag and Nitrogen-Doped Graphene Quantum Dots Codecorated Ultrathin Graphitic Carbon Nitride Nanosheet Composites with Enhanced Photocatalytic Activity: Full-Spectrum Response Ability and Mechanism Insight, ACS Appl. Mater. Interfaces, 2017, 9(49), 42816–42828 CrossRef CAS PubMed.
- J. Ju and W. Chen, Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media, Biosens. Bioelectron., 2014, 58, 219–225 CrossRef CAS PubMed.
- S. S. Yi, J. M. Yan and Q. Jiang, Carbon quantum dot sensitized integrated Fe2O3@g-C3N4 core-shell nanoarray photoanode towards highly efficient water oxidation, J. Mater. Chem. A, 2018, 6(21), 9839–9845 RSC.
- X. Shi, X. Li, G. Liu, M. Du, J. Zhang, G. Liu and Q. Lu, NaCl-assisted synthesis of Fe2+ self-doped Fe2O3/C3N4 nanosheets as efficient Fenton catalyst, J. Mater. Sci., 2020, 55(23), 10035–10046 CrossRef CAS.
- Y. Wu, J. Ward-Bond, D. Li, S. Zhang, J. Shi and Z. Jiang, g-C3N4@alpha-Fe2O3/C Photocatalysts: Synergistically Intensified Charge Generation and Charge Transfer for NADH Regeneration, ACS Catal., 2018, 8(7), 5664–5674 CrossRef CAS.
- M. Ahmad, X. Quan, S. Chen and H. T. Yu, Tuning Lewis acidity of MIL-88B-Fe with mix-valence coordinatively unsaturated iron centers on ultrathin Ti3C2 nanosheets for efficient photo Fenton reaction, Appl. Catal., B, 2020, 264, 118534 CrossRef.
- S. Zhong, Y. Xi, Q. Chen, J. Chen and S. Bai, Bridge engineering in photocatalysis and photoelectrocatalysis, Nanoscale, 2020, 12(10), 5764–5791 RSC.
- J. Wang, X. Zuo, W. Cai, J. Sun, X. Ge and H. Zhao, Facile fabrication of direct solid-state Z-scheme g-C3N4/Fe2O3 heterojunction: a cost-effective photocatalyst with high efficiency for the degradation of aqueous organic pollutants, Dalton Trans., 2018, 47(43), 15382–15390 RSC.
- T. Guo, K. Wang, G. Zhang and X. Wu, A novel alpha-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance, Appl. Surf. Sci., 2019, 469, 331–339 CrossRef CAS.
- Y. Chen, H. Li, Q. Ma, J. Wang, Q. Che, G. Wang, Y. Li and P. Yang, Facile synthesis of various alpha-Fe2O3 micro/nanostructures: Highlighting on the enhanced catalysis activities by formation of bowl-like alpha-Fe2O3/Au composites, Mater. Res. Bull., 2018, 103, 285–293 CrossRef CAS.
- P. Huang, S. Xu, M. Zhang, W. Zhong, Z. Xiao and Y. Luo, Modulation doping of absorbent cotton derived carbon dots for quantum dot-sensitized solar cells, Phys. Chem. Chem. Phys., 2019, 21(47), 26133–26145 RSC.
- S. Babar, N. Gayade, H. Shinde, P. Mahajan, K. H. Lee, N. Mane, A. Deshmukh, K. Garadkar and V. Bhuse, Evolution of Waste Iron Rust into Magnetically Separable g-C3N4-Fe2O3 Photocatalyst: An Efficient and Economical Waste Management Approach, ACS Appl. Nano Mater., 2018, 1(9), 4682–4694 CrossRef CAS.
- Y. Shen, Q. Han, J. Hu, W. Gao, L. Wang, L. Yang, C. Gao, Q. Shen, C. Wu, X. Wang, X. Zhou, Y. Zhou and Z. Zou, Artificial Trees for Artificial Photosynthesis: Construction of Dendrite-Structured alpha-Fe2O3/g-C3N4 Z-Scheme System for Efficient CO2 Reduction into Solar Fuels, ACS Appl. Energy Mater., 2020, 3(7), 6561–6572 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra09282k |
| This journal is © The Royal Society of Chemistry 2022 |