 Open Access Article
Open Access ArticleMajor contaminants of emerging concern in soils: a perspective on potential health risks
Naga Raju Maddelaab,
Balasubramanian Ramakrishnanc,
Dhatri Kakarlad,
Kadiyala Venkateswarlue and
Mallavarapu Megharaj *f
*f
aDepartamento de Ciencias Biológicas, Facultad de Ciencias de la Salud, Universidad Técnica de Manabí, Portoviejo 130105, Ecuador
bInstituto de Investigación, Universidad Técnica de Manabí, Portoviejo 130105, Ecuador
cDivision of Microbiology, ICAR-Indian Agricultural Research Institute, New Delhi 110012, India
dUniversity of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
eFormerly Department of Microbiology, Sri Krishnadevaraya University, Anantapuramu, 515003, India
fGlobal Centre for Environmental Remediation (GCER), Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE), Faculty of Science, The University of Newcastle, Callaghan, NSW 2308, Australia. E-mail: megh.mallavarapu@newcastle.edu.au
First published on 25th April 2022
Abstract
Soil pollution by the contaminants of emerging concern (CECs) or emerging contaminants deserves attention worldwide because of their toxic health effects and the need for developing regulatory guidelines. Though the global soil burden by certain CECs is in several metric tons, the source-tracking of these contaminants in soil environments is difficult due to heterogeneity of the medium and complexities associated with the interactive mechanisms. Most CECs have higher affinities towards solid matrices for adsorption. The CECs alter not only soil functionalities but also those of plants and animals. Their toxicities are at nmol to μmol levels in cell cultures and test animals. These contaminants have a higher propensity in accumulating mostly in root-based food crops, threatening human health. Poor understanding on the fate of certain CECs in anaerobic environments and their transfer pathways in the food web limits the development of effective bioremediation strategies and restoration of the contaminated soils and endorsement of global regulatory efforts. Despite their proven toxicities to the biotic components, there are no environmental laws or guidelines for certain CECs. Moreover, the information available on the impact of soil pollution with CECs on human health is fragmentary. Therefore, we provide here a comprehensive account on five significantly important CECs, viz., (i) PFAS, (ii) micro/nanoplastics, (iii) additives (biphenyls, phthalates), (iv) novel flame retardants, and (v) nanoparticles. The emphasis is on (a) degree of soil burden of CECs and the consequences, (b) endocrine disruption and immunotoxicity, (c) genotoxicity and carcinogenicity, and (d) soil health guidelines.
1 Introduction
Contaminants of emerging concern (CECs) or emerging contaminants (ECs), a term that has been used for the first time by water quality professionals, describes the pollutants that are being detected increasingly in the waterbodies at low (<ng) levels.1–3 The CECs have miscellaneous applications as pesticides, pharmaceuticals, personal care products, disinfectants, surfactants, household materials, nanomaterials, and illicit drugs.4 Recently, the environmental fate of CECs such as perfluoroalkyl and polyfluoroalkyl substances (PFAS), microplastics (MPs) and nanoplastics (NPs), additives (e.g., biphenyl/phthalates), flame retardants (FRs) like polybrominated biphenyl diethers (PBDEs) and organophosphorus flame retardants (OPFRs), and nanoparticles has gained global attention (Table 1). The term ‘CECs’ includes the newly developed compounds and the chemicals increasingly released into the environments. Most often, PFAS, MPs/NPs, FRs, PCBs, and nanoparticles have subsequent detrimental effects on food safety and health of the ecosystem and humans.4 Though these contaminants pose significant threats to several life forms, they are not included under the regulations of existing environmental laws.2 Unfortunately, the principal sinks of CECs in the environment include agricultural soils, urban surface runoff, and discharges of sewage treatment plants. The research reports on the mobility of CECs in the aquatic systems such as marine environments, surface waters, and wastewaters are plenty.5–10 Such reports are from different regions of Australia, Africa, Europe, Latin America, Asia, and North America.6,7,11–13 However, information on soil pollution by the CECs has gained less attention.14,15 The global soil burden of CECs is of serious concern as their concentrations in soils reach several hundreds to thousands of μg kg−1 soil (Table 2). Because the fate and transformations of CECs in terrestrial environments is not fully understood, the environmental regulations in many countries are less stringent.16| CEC | Source material | Ref. |
|---|---|---|
| Per- and polyfluoroalkyl substances (PFAS) | Food package material, stain- and water-repellent fabrics, non-stick products (e.g., Teflon), polishes, waxes, paints, cleaning products, fire-fighting foams, industrial facilities (e.g., chrome plating, electronic goods, and oil recovery), landfill wastewater treatment plant, and living organisms (e.g., fish, animals, and humans) due to accumulation and persistence over time | 17 |
| Micro-/nano-plastics | Plastic debris | 18 |
| Additives – phthalates | Plastic additives | 19 |
| Additives – biphenyls | Dielectric fluids (used in transformers and capacitors), additives in lubricant fluids, adhesives, building sealants, plasticisers, fire proofing agents, paints, and ink products | 20 and 21 |
| Polybrominated diphenyl ethers (PBDEs) | Electrical and electronic devices, furniture, textiles, plastics, and baby products | 22 |
| Organophosphorus flame retardants (OPFRs) | Polymers used in polyurethane (PU) foam, furniture, baby products, etc. | 23 |
| Nanoparticles | Cosmetics, pharmaceuticals, coatings, paints, and catalytic additives | 24 |
| EC | Country/region | Concentrations | Ref. |
|---|---|---|---|
| PFAS (μg kg1) | China | PFOA: 2.0, PFOS: 20 | 25 |
| Antarctica | PFOA: 1.5, PFOS: 5.4 | 26 | |
| USA | PFOA: 28, PFOS: 126 | 27 | |
| Norway | PFOA: 3.3, PFOS: 162 | 28 | |
| Uganda | PFOA: 0.9, PFOS: 3.0 | 29 | |
| Microplastics (items kg−1) | Chile | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001–34 001–34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
30 |
| Australia | 1271–4191 | ||
| USA | 0–1270 | ||
| Spain | 1271–4191 | ||
| China | 9801–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Additives | Kenya | BPA (μg g−1) 490–154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
31 |
| China | Σ209PCBs (pg g−1) 64.3–4358 | 32 | |
| Turkey | Σ83PCBs (pg g−1) 207.61–5461.95 | 33 | |
| Iowa, USA | ΣPCB (pg g−1) 3–1200 | 34 | |
| Ethiopia | 18ΣPCBs (mg g−1) 17.16 | 35 | |
| Italy | PCB-209 (mg kg−1 day wt) 0.14–0.43 | 36 | |
| FRs (ng g−1) | Nigeria | Σ7PBDE 112–366 (15 cm depth); 26.8–39.7 (500 m depth) | 37 |
| Australia | Σ5NBFR nd-385 | 38 | |
| China | Σ39PBDEs and BDE 2097.02–66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 573 in sediments 573 in sediments |
39 | |
| Italy | PBDE 158.3–9427; BDE209 130.6–9411 in sewage sludge for land application | 40 | |
| Metal nanoparticles in sludge-treated soils | US and Europe | Nano-TiO2 42 μg per kg per year; nano-Ag 662 ng per kg per year | 41 |
| Biosolids treated soils | USA | Titanium containing nanomaterials of 50 and 250 nm | 42 |
Most CECs are not lost from the soil fraction even after a soil wash for one week with water because of their higher affinity with soil matrices as compared to other pollutants.43,44 Teijón et al.45 found from column miscible displacement experiments that Naproxen (a nonsteroidal anti-inflammatory drug) had low sorption to the aquifer matrices, implying higher affinity towards solid particles. Another noteworthy observation was the complexation of CECs due to their different interactive mechanisms within soil matrices. These interactions could have a significant impact on the fate and toxicity of CECs in the soil environments. The adsorption rate and equilibrium capacity of triclosan (TCS) for polyethylene (PE) were 29.3 mg μg−1 h−1 and 1248 μg g−1, respectively, whereas the adsorption rate values of TCS for polystyrene (PS) and soil particles were 0.27 and 0.60 mg μg−1 h−1, respectively.46 Furthermore, the equilibrium capacities (μg g−1) were significantly different for the combinations of TCS and PE (1248), TCS and PS (1033), and TCS and soil particles (961). Similarly, polychlorinated biphenyls (PCBs) were found to interact with microplastics (MPs) such as polyethylene (PE) and polypropylene (PP) with large variabilities. In fact, the reported levels (ng PCBs per g MPs) were in the range of 5–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700,47 2–346 (ref. 48) and 3590–10
700,47 2–346 (ref. 48) and 3590–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125.49 These chemical aspects are the reasons why the concentrations of certain CECs are higher in terrestrial systems than in aquatic systems. For example, the MPs are nearly 4–23 folds more elevated in the terrestrial and freshwater systems than in the aquatic environments, and the accumulation of MPs is high both in rural and urban soils due to human activities.50 What is essential to know is how the soil environmental components interact with the CECs, and whether these interactions generate the complexation. Effectively, the complexation of CECs favours their bioaccumulation in the terrestrial biotic components.51
125.49 These chemical aspects are the reasons why the concentrations of certain CECs are higher in terrestrial systems than in aquatic systems. For example, the MPs are nearly 4–23 folds more elevated in the terrestrial and freshwater systems than in the aquatic environments, and the accumulation of MPs is high both in rural and urban soils due to human activities.50 What is essential to know is how the soil environmental components interact with the CECs, and whether these interactions generate the complexation. Effectively, the complexation of CECs favours their bioaccumulation in the terrestrial biotic components.51
The principal exposure route of humans and other animals to CECs is through the contaminated plant foods. This route is mainly due to the easy uptake and translocation of CECs in plants from the soils.52,53 Among the food crop components, the preference of accumulation of CECs is in the following order: roots > shoot > fruit > grains.15 Also, the CECs have a significant impact on the plant functionalities. For example, MPs exerted adverse effects on the germination rate, shoot height and biomass, and even oxidative damage and genotoxicity in plants.54–56 Several in vitro investigations confirmed the nontarget toxic effects of CECs on different model organisms or cells, such as Dugesia japonica, freshwater mussels, bay mussels, raptors, PA1-cell line, and CYP1A-bla LS-180 cell line.57–62 These results strongly imply that CECs can pose a serious health risk if they find their way into the human body. Nevertheless, the toxic effects of most CECs are not fully understood.63 Despite the apparently high ecological risks, the CECs are not under environmental regulations and policies. The recent reviews on individual CECs pertaining to different aspects such as the assessment of their contamination in the marine environments, their remediation of aqueous environments, human exposure, plant uptake and translocation, their effects as endocrine disruptive chemicals, and their fate in wastewaters have gained increasing attention.1,5,10,12,15,64,65 However, no single source on concise information is readily available in the literature on toxicities and regulatory concerns related to the significantly important CECs in soils. Therefore, the principal objective of the present review is to provide a perspective on the environmental concerns of major CECs such as PFAS, MPs and NPs, additives (biphenyls/phthalates), FRs (PBDEs, novel FRs and OPFRs), and nanoparticles, their potential health effects, and the need for comprehensive regulatory measures.
2 Soil contamination with CECs: from sink to source
The soils are the important global natural resource that deliver critical services to all the life forms and shape the foundation of human civilizations. They are critical to the cycling of all the natural elements as well as the synthetic chemicals which are either applied deliberately for different intended purposes or deposited from atmosphere and aquatic environments. The human societies across the world are challenging the carrying capacity of the Earth, which include the excessive production and utilization of synthetic chemicals. The environmental degradation (breakdown by biotic and abiotic factors) and contamination that includes by the presence of these synthetic chemicals make the access to safe water difficult for more than 900 million humans and innumerable animals and plants.66 The soil contamination by most synthetic chemicals is increasingly becoming the major societal challenge because of the presence of industrial establishments that often increase the risks due to accidents and poor waste management, and overproduction and widespread utilization without realizing the need for environmental regulations. The depositions from atmospheric emissions and transport that can distribute the contaminants widely also make the soil environments as the major sinks. The principle of ‘pollutants have no borders’ has become evident with the introduction of analytical tools with better precision and high-resolution techniques that help detect and quantify the contaminants from the heterogenous media such as soils.The flows of mass, energy, and genetic information and their transformations make the porous soils as the major interface connecting the atmosphere, vegetation, and geosphere of the Earth's critical zone.67 Though adsorption and desorption of CECs are influenced by several soil properties such as pH, redox conditions, temperature, moisture content, organic carbon and clay, and the fate and transformation of CECs are mainly affected by microbial activity.68,69 The soil organic matter is considered to have dissolution sites in rubbery and glassy phases. The diffusion of organic contaminants into the complex phases of soil organic matter leads their sequestration. The hydrophobic and lipophilic characteristics and the recalcitrant chemical structure of certain contaminants modulate CECs resistance to breakdown by the biological, chemical, and photolytic processes. Besides certain factors like pH, electrical conductivity and soil type, prior exposure to soil matrices largely influences the persistence of CECs.70 Though the fate of these contaminants in aerobic conditions in soil has been well addressed, less is known about their fate under anaerobic conditions.71,72 These insights influence the designing of effective remediation strategies for the restoration of CECs-contaminated soils. Since soils become anaerobic under flooding conditions, critical information on the fate of CECs in the anaerobic environment is of utmost importance in soil ecosystem. These conditions will significantly influence the transport pathways of CECs.
The climate change events that include soil warming may have adverse effects on the transport pathways of contaminants. Yang et al.73 and Strååt et al.74 reported that soil warming could lead to increased transportation and transformation of contaminants in soils. The extreme fluctuations in precipitation due to climate change might alter the mobilization, transport and cycling of contaminants in the soil matrix. The transport of contaminants from the soils will increase due to the surface runoff, and erosion due to increased intensity and frequency of rainfall or storms. In addition, the agricultural practices such as tilling and irrigation can accelerate the release of soil particle-bound contaminants and the ensuing uptake by plants.75 Thus, the contaminated soils can become the new source for not only the transport to another medium but also the transformation of contaminants, even facilitating trophic transfers. Plants have potential in absorbing pollutants from the environmental media. It has been estimated that nearly 1261 tons of pollutants are removed by trees from the atmosphere in the central part of Beijing, China in 2002.76 There are similar reports on improvement of atmospheric quality (7–15%) near footpaths by vegetation on greenbelts,77 removal of 88 tons of total atmospheric pollutants (like 12 tons of PM10 and 5 tons of PM2.5) by trees in Strasbourg, France,78 trapping of 7% total air pollutants in Marylebone and London.79 All these insights imply that the plants interact strongly with the pollutants.
The soil burden of contaminants is difficult to quantify as there are no fool-proof chemical extraction procedures and the mechanisms of interactions between the contaminants and soil constituents are complex to describe. The deposition of solid waste and materials, discarded from the industrial, mining, agricultural and livestock, military, and commercial activities alter the chemical and biological properties and degrade the ecosystem services of soils in the long run.80 The Working Group of the International Union of Soil Sciences (IUSS) on Soils in Urban, Industrial, Traffic, Mining and Military Areas suggests that the urban soils with greater anthropogenic influences are vital to the sustainability and resiliency of cities.81 The CECs will create the environmental concerns, more in urban soils than in rural soils. Nevertheless, the contaminants of both conventional and emerging types pose serious scientific and societal challenges to the global soil security, beyond soil health concerns, because of their complicated and cascading effects on the health of individual members and the whole ecosystem. As most of the CECs ultimately reach the soil ecosystem through domestic and industrial wastes (Table 1), they contribute to the overall global soil burden (Table 2). The following five significantly important CECs were chosen for this comprehensive review because they represent diverse class of pollutants with high risks of soil contamination during their industrial production, their entries into the food production chain, human consumption, and health hazards.
3 Per- and polyfluoroalkyl substances (PFAS)
3.1. Soil pollution and pathways of transfer to the food web
Per- and polyfluoroalkyl substances (PFAS) are the principal components of fluoropolymers coatings and other products that resist water, oil, stains, grease, and heat. The most used and detected PFAS are perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). Chemically, PFAS are hydrophobic, fluorine alkylated compounds containing saturated carbon chains and a hydrophilic head (sulfonate or carboxylate) attached at a terminal end. Fluoropolymers form from PFAS by ‘emulsion polymerization’, and these fluoropolymers have high economic potential. The industrial production is maximised by selected companies (e.g., DuPont, 3 M, and W. L. Gore & Associates); the annual market for fluoropolymers as polishes, paints, coatings, and stain repellents is about 100 million to billion USD.82 These fiscal figures reflect the usage and demand for fluoropolymers. The fluoropolymer coatings are commonly involved in certain domestic goods such as clothing, furniture, adhesives, food packings, heat-resistant non-stick cooking surfaces, and electric wire insulation. Because of hydrophobic and heat-stable properties, PAFS are even used in Class B firefighting foam.83 But PFAS are of great concern due to their recalcitrant nature, ability to pass through different environmental media (such as soil and water), and even bioaccumulation.84,85 As PFAS are highly mobile, these pollutants have been detected in Arctic and Antarctic regions.86 The principal sinks of PFAS in the environment are wastewater, PFAS manufacturing and processing facilities, and those facilities producing PFAS-containing materials, airports, and military bases.87,88 Furthermore, PFASs from soil can easily reach the groundwater and atmosphere by leaching and evaporation, respectively.89 Consequently, different regulatory agencies (e.g., EU Water Framework Directive, Stockholm Convention) become concerned about controlling environmental pollution caused by the release of PFAS.90 Unfortunately, available treatment methods are not sufficient to remove PFAS from the wastewater;91 this is factual because poor waste management and wastewater treatment plants (WWTPs) are the major sources of PFAS in Africa.92 Strynar et al.93 estimated that global soil loadings of PFOA and PFOS are 1860 and >7000 metric tons, respectively. However, the recent global soil metadata analysis showed that the maximum concentrations of PFOA and PFOS were about 124 and 162 μg kg−1, respectively.94 Since the root uptake of PFAS is the principal accumulation pathway in several food crops, the soil pollution of PFAS can have a significant threat to food security and human health95 (Fig. 1a and b). Substantial levels of PFAS were detected in foods, e.g., 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 ppt (parts per trillion) of perfluoro-n-pentanoic acid (PFPeA) in chocolate cake with icing, and 134–865 ppt in meat samples (e.g., Frankfurter sausage and tilapia);96 and the levels of PFAS found in the meat samples were significantly higher than the limits set by USEPA (https://www.epa.gov/laws-regulations/regulations). The major human exposure pathways to PFAS followed the order: environmental media (58%) > specialty products (29%) > home products (7%) > personal care products (2%) and clothing (2%) > cleaning products (1%) and food packing (1%);97 where food (40%), water (30%) and soil (9%) occupy the first three places under the environmental media pathways. The toxicity of perfluorinated compounds to soil microorganisms, when analysed by microcalorimetry, was dependent on the functional groups (more due to sulfonic-than carboxylic), carbon chain length, and the soil properties.98 The PFAS-impacted legacy was also observed with changes in the relative abundance of bacterial genera such as Desulfococcus, Gordonia and Acidimicrobium in the groundwater.99 The reports on the accumulation of PFAS in the human body are growing.100–102 Soils become an ultimate reservoir for fluorosurfactants (such as PFOA and PFOS) because of their extensive usage. The contaminated soils can be a long-term source for CECs across the world. The unabated use of PFAS can have a significant impact on food safety and human health.
640 ppt (parts per trillion) of perfluoro-n-pentanoic acid (PFPeA) in chocolate cake with icing, and 134–865 ppt in meat samples (e.g., Frankfurter sausage and tilapia);96 and the levels of PFAS found in the meat samples were significantly higher than the limits set by USEPA (https://www.epa.gov/laws-regulations/regulations). The major human exposure pathways to PFAS followed the order: environmental media (58%) > specialty products (29%) > home products (7%) > personal care products (2%) and clothing (2%) > cleaning products (1%) and food packing (1%);97 where food (40%), water (30%) and soil (9%) occupy the first three places under the environmental media pathways. The toxicity of perfluorinated compounds to soil microorganisms, when analysed by microcalorimetry, was dependent on the functional groups (more due to sulfonic-than carboxylic), carbon chain length, and the soil properties.98 The PFAS-impacted legacy was also observed with changes in the relative abundance of bacterial genera such as Desulfococcus, Gordonia and Acidimicrobium in the groundwater.99 The reports on the accumulation of PFAS in the human body are growing.100–102 Soils become an ultimate reservoir for fluorosurfactants (such as PFOA and PFOS) because of their extensive usage. The contaminated soils can be a long-term source for CECs across the world. The unabated use of PFAS can have a significant impact on food safety and human health.
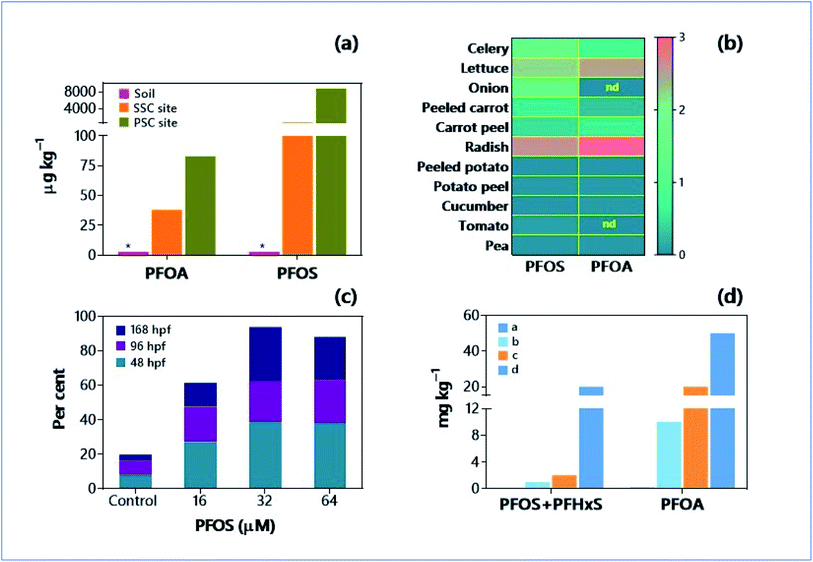 | ||
| Fig. 1 Extent of soil pollution by PFAS and ecotoxicity. (a) PFAS concentrations in background soils. Primary source-contaminated (PSC) soil and secondary source-contaminated (SSC) soil.94 *Maximum concentrations detected for PFOA and PFOS were 123.6 and 162 μg kg−1, respectively; PSC soils were fire-training areas and manufacturing plants; SSC soils were biosolid application sites and irrigation water use areas. (b) Heatmap showing soil to plant transfer factors for PFOS and PFOA.121 (c) Per cent of zebrafish embryos with islet morphological anomalies as affected by PFOS.103 hpf: hours post fertilization. (d) Toxicity reference values (mg kg−1) for PFOS + PFHxS and PFOA in four different soil systems: (a) Residential area with garden/accessible soil; (b) public open space; (c) residential area with minimal opportunities for soil access; (d) industrial/commercial sites.121 | ||
3.2. PFAS toxicity to animals and humans
The animal model experiments show that PFAS has significant endocrine disrupting activity. Exposure of zebrafish embryos (48 and 96 h post-fertilization) to 16 and 32 μM of PFOS resulted in the decreased islet area (P < 0.01 and 0.03, respectively), but the effects of PFOS were neutralized at higher concentration (i.e., 64 μM, P = 0.04) (Fig. 1c).103 Furthermore, elevated islet morphology (13.8–38.5% increase in anomalies), decreased secondary islets (59% reduction at 32 μM), larva size (2% reduction at 32 μM), and pancreas length (7–27% at 96 hours post fertilization, hpf) were observed in the same study. Thus, PFOS significantly impacts the endocrine system by inducing morphological and functional perturbations during pancreatic organogenesis in zebrafish.104,105 In the human pluripotent stem cells, PFASs tend to disrupt the generation of human pancreatic progenitor cells and affect pancreatic and endocrine differentiation.106,107 In an in vitro experiment, pancreatic progenitor cells (i.e., H9 hESCs (H9 human embryonic stem cells)) were exposed to low-, human-relevant doses (5 and 50 nM) of PFOA and PFOS for 13 days. The results revealed that there was a negative effect on the relative gene expression levels of early markers (such as hepatocyte nuclear and endodermal marker genes). Values were found to be in the range of <0.4–1.0 against the control of 1.0.106 PFOA and PFOS affected not only the early stages of pancreas organogenesis but also all other endoderm lineages. PFAS showed significant effects at the transcription level and suppressed the expression of SOX9 (SRY-Box Transcription Factor 9), leading to considerable interferences at the growth and proliferation of pancreatic progenitor cells.107 The PFAS-induced SOX9 upregulation followed by NOTCH signal activation could lead to the failure of endocrine development.107 Thus, the in vitro experiments with human-relevant doses of PFASs have proved their endocrine toxicity. Health risks could be even worse if there is chronic exposure to PFASs because the mean estimated half-lives of PFOS and PFOA in the human body are relatively higher (i.e., 3.4 and 2.7 years, respectively).1083.3. Carcinogenic risks of PFAS
The PFAS exposure has stronger links with carcinogenicity in rats, and occupational exposure in humans resulted in an increased risk of prostate cancer.109,110 Numerous human epidemiological studies suggest that exposure to PFOS and PFOA (occupational, community-level, or populational) heightened the cancer risk. There were 3.3-fold higher rates of prostate cancer mortality in the occupationally exposed workers of the production division in the PFOA industry.111 However, re-analysis of the cohort did not find any link between the exposure and cancer risk. On the contrary, a recent occupational cohort study conducted in Italy found that PFOA exposed individuals demonstrated a 7-fold higher liver cancer mortality and 5-fold higher lymphatic and hematopoietic cancer mortality than non-chemical factory workers.112 Many epidemiologic studies suffer from significant drawbacks such as differences in reporting the results, different approaches and data size. Yet, there is a significant correlation between PFAS exposure and cancer incidences (e.g., kidney, prostate, and testicular) in humans.113,114 PFOA at a concentration of 1.1 to 4.6 mg kg−1 day−1 escalated the pancreatic tumours.115 Two-year feed studies using experimental female rats (i.e., Hsd:Sprague Dewley® SD® rats) revealed that PFOA furthered the incidence of pancreatic acinar cell adenoma hepatocellular carcinoma and adenocarcinomas of the uterus.115 Predictably, maternal exposure to 6 out of 10 measured PFASs during pregnancy showed an increased risk of germ cell tumors (GCTs) in paediatric patients;116 the odds ratio was 19.47 (95% CI: 4.20–90.26) for 1.0 ng L−1 increase in PFHxS. Additionally, children with GCTs had relatively high serum levels of PFAS than the tumor-free paediatric patients.116 Likewise, the utero EtFOSAA (N-ethyl-perfluorooctane sulfonamido acetic acid, precursor to PFOS) and higher maternal, perinatal total cholesterol bestows a significant risk factor breast cancer for daughters.117 Furthermore, the International Agency for Research on Cancer (IARC) classified PFOA, the most studied PFAS, as a possible human carcinogen based on the available limited epidemiologic data. Accordingly, these human epidemiologic data and the animal models' experimental results infer that PFAS has carcinogenic properties and presents a significant maternal link in increasing the cancer risk. Additional investigations are needed to confirm the link of PFAS with the promotion of chronic inflammation, cellular immortalization, and alterations in DNA repair.1103.4. Environmental regulations
PFAS have become a critical soil pollutant because of their environmental persistence and mobility. It is not viable to depend on natural attenuation (degradation without human intervention) and long-term monitoring to restore PFASs-contaminated soil.118 From the soil environments, PFAS easily enter groundwater or drinking water reserves. Based on their chemical nature, PFAS can tend to be at the surface of soil columns or show a high affinity towards soil particles. At the Tindal Royal Australian Air Force (RAAF) base at Katherine, Northern Territory in Australia, the release of toxic PFAS released from firefighting foam during 1995–2005 resulted in the extensive contamination of groundwater to the extent of 4.6 μg L−1 in water supply, which was 66 times higher than the permissible levels (0.07 μg L−1) of drinking water.119 The US Environmental Protection Agency (EPA) has given lifetime health advisory levels for PFAS as just 70 parts per trillion (ppt).118 Nevertheless, the New Jersey Department of Environmental Protection (NJDEP) set much lower values, i.e., 14 and 13 ppt for PFOA and PFOS, respectively. In Delaware (USA), the EPA Region 4's Residential Soil Screening Level for PFOS and PFOA are 6 and 16 mg kg−1, respectively.120 The Australian PFAS National Environmental Management Plan (NEMP) set PFAS toxicity reference values (human health soil screening) for 4 different types of soils (based on land-use), and values are in the range of 0.01–20.0 mg kg−1 and 0.1–50.0 mg kg−1 for PFOS + PFHxS (perfluorohexane sulfonate) and PFOA, respectively (Fig. 1d).121 According to the Health Canada drinking water guidelines, the maximum allowable limits for PFOS and PFOA are 0.2 and 0.6 μg L−1, respectively.122 Nonetheless, information on soil health guidelines regarding PFAS contamination in other states or countries is scantily available. Strict action plans, regulations, risk control rules, and technical guidelines are obligatory on a global scale to prevent soil pollution by PFAS. Smoldering combustion, soil sorption technologies, incineration, soil washing, onsite encapsulation, and immobilisation are some of the possible strategies for restoring PFAS-contaminated soils (https://rembind.com/)https://rembind.com/.118,123 The priority protection and risk control measures and sustainable treatment and remediation strategies should be enforced, for PFAS-contaminated agricultural lands, primarily due to the high-level threat of trophic transfers.4 Micro- and nanoplastics (MNPs)
4.1. Global soil burden and interactions in soil matrices
Micro- and nanoplastics (MNPs; <1.0 mm length, and 0.001–1.0 μm size, respectively) are the present-day emerging contaminants of global concern. The terrestrial pollution by MNPs has been neglected despite several known sources were identified such as plastic mulches and polytunnels in the agroecosystem, sewage sludge, and landfills.124–126 Two other types of plastics, viz., bioplastics and oxo-degradable plastics have also emerged.127 However, these next-generation plastics are not eco-friendly as expected.128 Because a significant portion (79%) of plastic waste is accumulated in the form of landfills and other environmental counterparts, the intensity of pollution by MNPs is more severe in agricultural soils than in the oceanic basins (Fig. 2a).129,130 The analysis of processed food samples evidenced that American children and adults are exposed, by the combination of ingestion and inhalation, to an average of 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331–121
331–121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664 particles per year, implying the severity of pollution and bioaccumulation propensity of MNPs.131 Alarmingly, the agricultural lands are becoming the hotspots for MNPs; different regions and annual loading of MNPs are as follows: Europe (∼63
664 particles per year, implying the severity of pollution and bioaccumulation propensity of MNPs.131 Alarmingly, the agricultural lands are becoming the hotspots for MNPs; different regions and annual loading of MNPs are as follows: Europe (∼63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–430
000–430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons), America (∼44
000 tons), America (∼44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons), and Australia (∼2800–19
000 tons), and Australia (∼2800–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons).132 Therefore, trophic transfer of MNPs becomes one of the possible routes of human exposure to MNPs. Yet, the research on the transfer pathways is in infancy, which is attributed to two principal reasons: (i) there is no validated procedure for sampling, sample preparation, analysis of MNPs, and (ii) there is no information about the sample contamination before the analysis; this information is inevitable as plastics are present everywhere.133 The propensity of plastic debris to interact with soil organic matter and prolonged persistence (up to a few hundred years) are the critical factors in the formation of MNPs.134 Recent studies with model soils revealed that MNPs could interact with PCBs (polychlorinated biphenyls) and heavy metals.135,136 These particles exhibited potential toxicities to soil biota, such as disturbance to gut microbiome in the Enchytraeus crypticus and negative impacts on the functional diversity of soil enzymes.137,138 In the river environments, microplastics serve as the microbial habitat with abundant microbial groups such as Pseudomonas, Arcobacter, Aeromonas, and unclassified Veillonellaceae.139 In the river sediments, microplastics were found to decrease the α-diversity of microorganisms.140 Thus, soils that have become the ultimate sink for plastic debris and rich media even for the formation of MNPs have significant negative impacts on soil biota with considerable threats of trophic transfers.
000 tons).132 Therefore, trophic transfer of MNPs becomes one of the possible routes of human exposure to MNPs. Yet, the research on the transfer pathways is in infancy, which is attributed to two principal reasons: (i) there is no validated procedure for sampling, sample preparation, analysis of MNPs, and (ii) there is no information about the sample contamination before the analysis; this information is inevitable as plastics are present everywhere.133 The propensity of plastic debris to interact with soil organic matter and prolonged persistence (up to a few hundred years) are the critical factors in the formation of MNPs.134 Recent studies with model soils revealed that MNPs could interact with PCBs (polychlorinated biphenyls) and heavy metals.135,136 These particles exhibited potential toxicities to soil biota, such as disturbance to gut microbiome in the Enchytraeus crypticus and negative impacts on the functional diversity of soil enzymes.137,138 In the river environments, microplastics serve as the microbial habitat with abundant microbial groups such as Pseudomonas, Arcobacter, Aeromonas, and unclassified Veillonellaceae.139 In the river sediments, microplastics were found to decrease the α-diversity of microorganisms.140 Thus, soils that have become the ultimate sink for plastic debris and rich media even for the formation of MNPs have significant negative impacts on soil biota with considerable threats of trophic transfers.
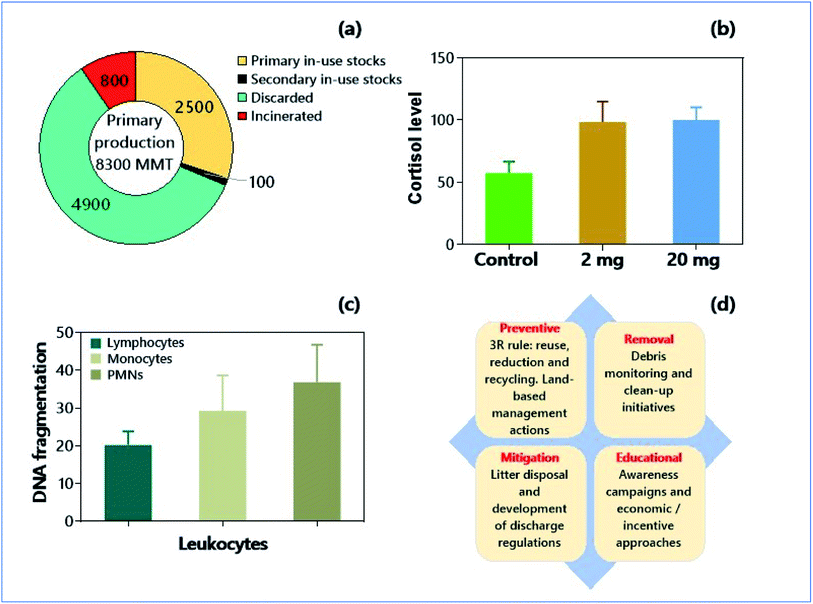 | ||
| Fig. 2 Micro- and nanoplastics as soil pollutants and environmental consequences. (a) Global flow of plastics (polymers resins, synthetic fibers, additives between 1950-2021, in million metric tons, MMT) by different activities such as production, primary and secondary in-use stocks, and disposal (discarded and incinerated).130 (b) Effect of polystyrene nano plastics (PSNPs) on cortisol levels in wild-type strain AB/TL of zebrafish.146 (c) Genotoxicity, in terms of DNA fragmentation, of PSNPs on human blood cells.149 (d) Strategies for the mitigation of micro- and nano-plastics in soil. | ||
4.2. Endocrine disruption and immune toxicity
Immune cells can internalize MNPs by many routes such as phagocytosis, micropinocytosis, and clathrin/caveolae-dependent or -independent internalization, and the interactions between immune cells and MNPs can induce severe immune dysfunctions.141 In an in vitro study, Prietl et al.142 used beads of 20 nm containing polystyrene (PS) at 200 μg mL−1 and reported an induction of oxidative burst and stimulation of L-8 production in human monocytes after 24 h of incubation. The smaller NPs (20 nm) exhibited cellular toxicity by membrane damage, attributed to the internalization of more NPs due to their small size. But the larger NPs induced the cell toxicity by forming ROS (reactive oxygen species) and oxidative burst. Similarly, MPs have also exhibited size-dependent cellular toxicity, where smaller MPs were more toxic to the immune cells than larger MPs.143 On the contrary, no size-specific toxicity was reported in THP-1 macrophages treated with 26 and 100 nm PS beads.144 Notwithstanding, NPs have shown their adverse effects on the immune system in animal models like zebrafish (Fig. 2b).145 There is also convincing evidence for the endocrine-disrupting property of NPs with subsequent behaviour changes in the organisms. Accumulation of polystyrene NPs (PSNPs) in the pancreas of zebrafish resulted in the decreased glucose levels, with consequences related to the aberrant locomotor activity.146 Earlier reports suggested that the accumulation of PSNPs in the body of zebrafish caused decreased rate of depuration from the intestine and pancreas, and alterations in the exocrine and endocrine pancreas functionalities in the later stages of 5 days post fertilization (dpf) embryos.145,147 These observations strongly demonstrate that MNPs have higher potentials for affecting the immune and endocrine system even at nanomolar concentration; the consequences could be even serious upon chronic bioconcentration of MNPs.4.3. Genotoxicities of micro- or nanoplastics (MPs or NPs)
There is compelling evidence of the genotoxic effects of MPs. Polystyrene MPs (PSMPs) manifested significant DNA damage in the human fibroblast Hs27 cell line.148 Similar results have also been observed in monocytes and polymorphonuclear cells (of humans) exposed to PSMPs (Fig. 2c).149 Moreover, chemical modifications have significant effects on the genotoxicity of NPs. The aminated polystyrene nanobeads showed substantial DNA damage in pulmonary epithelial cells and human macrophage cell lines.150 The spherical polystyrene NPs of 100 nm showed genotoxicity in foreskin Hs27 cell lines by inducing chromosome breakage and impaired chromosome segregation machinery.148Conversely, COOH-modified polystyrene NPs neutralize the genotoxicity. Regarding the mechanism of genotoxicity, MPs (e.g., polyethylene-based MPs) may resemble clastogen, which causes mis-repairing of double-strand breaks and/or reducing the DNA repair capacity inducing the DNA strand breaks.151 In addition, MPs and NPs can cause genotoxicity either by damaging the nuclear membrane or by ROS (generated in the cytoplasm) effects on the nucleus.152 Notably, the unrepaired or mis-repaired DNA lesions can cause mutagenic effects. This carcinogenesis is highly possible if the mutations occur in the key genes of genome stability and integrity or cell cycle. For these reasons, both MPs and NPs have higher potentials in inducing carcinogenic effects.152 Yet, additional studies are needed for more insights about the genotoxic nature of MPs in different human cells, especially with MNPs of varying size and types. More importantly, MPs provide suitable adsorption sites other environmental contaminants and endangering environmental security.153 Hence, toxicity studies with mixed pollutants can provide additional insights and give the vital need for the better evaluation of health risks posed by MPs or NPs in the soil environments.
4.4. Regulations in agricultural usage
Though MPs or NPs are present in fertilizers or sewage sludge applied to agricultural soils, these plastics' environmental consequences or transfer pathways in the food webs are scantily documented. Intriguingly, the ban on the usage of microbeads in cosmetics can reduce the load of MPs in sewage sludge, facilitating its application in agricultural lands. A ban of this kind has already been adopted in several countries such as Netherlands, USA, Canada, Australia, and UK.154 Despite the ban on the use of microbeads in the manufacture of cosmetics and personal care products, it is hard to find any agro-environmental-related legislation for MPs or NPs.155 There are suggestions that the restricted use of oxo-degradable plastics can reduce the human health risk and make the recycling and composting process easier (https://epi-global.com/). The regulations need to be stricter on waste legislation and fertilizer usage.154 For example, the objective of ‘The EU strategy for plastics’ is all plastic packaging to be reusable and recyclable by the year 2030. In Germany, the maximum allowable quantity of plastics in fertilizer is 0.1% weight, but <2 mm particles have not been considered.156 The EU fertilizer legislation permits 0.5% (per kg of dry matter) plastics in organic fertilizers. On the other hand, it is hard to find the legislation policies for the limit of MPs or NPs in sewage sludge used for agriculture purpose (Fig. 2d).5 Additives – biphenyls/phthalates
5.1. The abundance of additives as soil pollutants
Most abundant organic contaminants in soils include phthalate esters (PAEs) due to their extensive usage in the preparation of plastic products, pesticides, cosmetics, and personal care products, building materials, automotive parts, medical devices, food packing, toys, teethers, adhesives, paints, floorings, and lubricants.157 Their migration from the source materials because of evaporation, leaching, and abrasion exacerbate the soil pollution.158 The global production of phthalate has become almost doubled between 2006 and 2015 (from 4.7 to 8.0 million metric tons (MMT)).159 More importantly, phthalates are flowing from the soils to crop plants, despite their presence at concentrations far below the recommended soil remediation objective (Fig. 3a and b).160 Often, there are seasonal variations in the deposition of phthalates in the soils. For instance, the concentration of phthalates in topsoils at winter wheat harvest was significantly higher than those at the summer maize harvest.160 Implications are that wet deposition scavenges the phthalates easily from their source materials, subscribing significantly to their transport into soil media. Phthalates can easily reach the agricultural soils by applying agricultural plastic films, irrigation water, sewage sludge, biosolids, and fertilizers.161 Conversely, the e-waste (electronic and electrical waste) contributes to a significant extent of soil pollution by chlorinated chemical class, i.e., polychlorinated biphenyls (PCBs).162 Despite strict regulations and ban imposed, biphenyls are the principal contaminants in the soils and sediments.163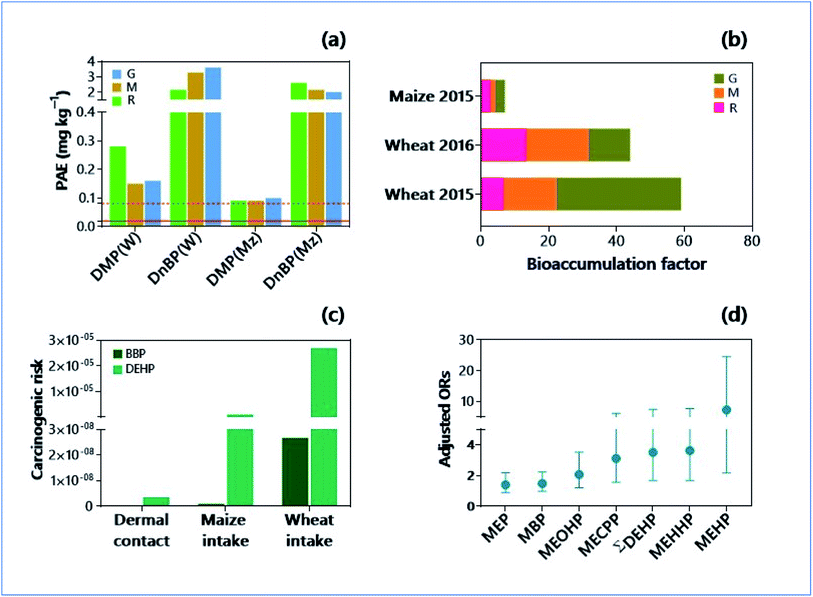 | ||
| Fig. 3 Soil pollution by phthalate esters (PAE), and environmental and health consequences. (a) PAE congeners (DMP: dimethyl phthalate; DnBP: Di-n-butyl phthalate) in soil treated with reclaimed water (R), groundwater (G) and mixture of reclaimed water and ground water (M) at the harvest time of wheat (W) and maize (Mz). Solid and dotted horizontal lines indicate soil allowable concentration of DMP and DnBP in USA, respectively.160 (b) Bioaccumulation factors of DMP and DnBP in soil – cereal grain system under three different water treatments (R, G and M).160 Bioaccumulation factors of PAEs significantly varied in a soil – wheat system among the two periods studied. (c) Human carcinogenic health risk of PAEs [butyl benzyl phthalate, BBP; di(2-ethylhexyl) phthalate, DEHP] subjected to three different exposure pathways.160 Carcinogenic risks significantly varied between the congeners and exposure pathways. (d) Adjusted odd ratios (ORs) of papillary thyroid cancer risk for phthalate metabolites as revealed in a pair-matching case-control study.169 [Monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethylpentyl-5-carboxy) phthalate (MECPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-2-ethylhexyl phthalate (MEHP)]. | ||
5.2. Hormonal and developmental disorders
Phthalate esters and PCBs belong to the EDCs (endocrine disrupting chemicals) as they affect the endocrine system even at low concentrations. Di-2-ethylhexyl phthalate (DEHP), is a phthalate ester congener, decreases the steroidogenesis (at 20 mg kg−1 day−1), deregulates the action of peroxisome proliferator-activated receptors (PPARs), and exhibits agonist effects on endocrine receptors (0.22–0.44 mg kg−1 day−1) in animal models.164 The Sprague-Dawley rats dosed with DEHP (gavage for 30 days) displayed histopathologic changes in the thyroid and liver. Clinical manifestations included diminished thyroid follicular activity diameter and hepatocyte edema.165 Similarly, PCBs have shown agonistic effects on endocrine receptors and anti-androgenic properties at a concentration of 0.2–0.3 ppm and 0.02–0.03 μg kg−1 day−1 concentration, respectively.164 Notably, the combination of PHEs and PCBs exhibited permanent alterations in adult F1 males, and such effects have not been reported in animals dosed with either of the single compounds.166 For instance, testis weight was found to be 10% lower in animals dosed with DEHP/PCB mixture than those of single-ED dosed groups (P ≤ 0.001). Similar results have been previously reported regarding human sperm motility versus interaction effects of DEHP/PCBs.167 Appallingly, the maternal exposure to DEHP/PCBs demonstrated the cumulative toxic effects on hepatic function in both male and female rat offspring.168 These limited studies have focused on investigating mixture effects of PHEs and PCBs, using animal models (mainly mice). Still, the interactive studies of the mixtures of other pollutants on many different soil biotic components are warranted.1665.3. Thyroid carcinogenicity
The pair-matching case-control study demonstrated that the exposure to phthalate congeners increased the risks of papillary thyroid cancer (PTC), where the odds ratio (OR) was 5.3, and the 95% confidence interval (CI) was 1.610–17.83 (Fig. 3c and d).169 In an earlier study by Marotta et al.,170 the exposure to DEHP was found to increase, about 14 times higher the risk of thyroid cancer in persons with thyroid nodules. The multivariable logistic regression model validated the association between the creatinine-corrected urinary phthalate metabolites and thyroid cancer and/or benign nodules in humans.171 Correspondingly, the modelling approach found the link between the risk of malignancy (15 times higher) and exposure to bisphenol AF and DEHP.172 Investigations with different approaches are indispensable to unlock the association between phthalate exposure and thyroid cancer incidence.173 The experiments with thyroid carcinoma cell line (in vitro) and rats (in vivo) evinced the propensity of DEHP to induce cell proliferation and DNA damages in the thyroid.174 Several upstream signals such as thyrotropin receptor (TSHR)-ERK1/2 axis and TSHR-AKT axis were activated upon the exposure of rats treated with DEHP (oral dose at 0–150 mg kg−1 for 90 days from post-natal day 9 in vivo).175 As the estrogen-mimicking and endocrine disruptors, biphenyl congeners were found to be associated with cancers in animals. For example, PCB138 congeners have a significant link in the development of breast cancer (OR = 3.16; 95% CI = 1.14–8.76) in age, race/ethnicity, and BMI adjusted models.176 Bisphenol A has shown an increased thyroid cancer susceptibility in rats pre-treated with N-bis-(2-hydroxypropyl)-nitrosamine, a stimulator of thyroid proliferation.177 Significant evidence on thyroid carcinogenicity conveys the environmental risks of biphenyls/phthalates.5.4. Dispersion in soils and sustainable replacements for phthalates
Both phthalates and bisphenol A are well accumulated in the soil matrices.178 Phthalates can quickly settle and integrate into soil matrices by sorption to the dust particles. Phthalates and bisphenol A can reach the soil environments from the plastic products during landfilling. Despite the convincing evidence of adverse effects (human health risk and environmental pollution) of phthalates and biphenyls, certain consumer products contain them, making it difficult in developing economical and safe replacements. The Lowell Center for sustainable production (University of Massachusetts Lowell) has suggested certain alternative chemicals to phthalates and alternative plastics that do not contain phthalates.179 Citrates, sebacates, adipates, and phosphates are suitable substitutes for phthalates in consumer products (e.g., toys, childcare articles, and medical devices). Additionally, these emerging substitutes can serve as the alternative PVC plasticizers-solvents and fixatives in various products like cosmetics, inks, adhesives, and others. According to the European Union System for the Evaluation of Substances (EUSES), the exposure to edible crops can account for ∼90% of the total human intake exposure for DBP (dibutyl phthalate), mainly due to the residual PAEs in agricultural soils.180 Hence, the additive substitutes also require serious attention to raw material, recyclability, cost savings, and polluting capacities.6 Novel flame retardants (FRs)
6.1. Sources of soil pollution by FRs
Polybrominated diphenyl ethers (PBDEs) are the commonly used flame retardants globally. The most widely used PBDE across the world (83%) is c-decaBDE, and 97% of it constitutes BDE-209 congener.22 Because of their extensive use in plastic polymers and textiles, the BDE-209 congeners are among the predominant PBDEs in agricultural soils.181 In fact, they observed no significant variations in the concentrations of PBDEs in soils of plastic manufacturing plants, waste plastic disposal areas, and the PBDEs production sites. Nevertheless, the agricultural soils contain higher concentrations of PBDEs than mountain soils and rural soils (Fig. 4a).181 This kind of pollutant accretion in agricultural soils poses higher threats through the trophic transfer of PBDEs for food quality. The human intake of PBDE (e.g., BDE47) through food was found to be higher (6 times) than the reference dose of USEPA.182 The organophosphorus esters (OPEs)-based flame retardants have replaced brominated flame retardants (BFRs). Notwithstanding, there are no differences between the environmental risks posed by BFRs and OPEs. The concentrations of OPEs in soils range from 38 to 468 ng g−1 and there were more accumulations of OPEs in the urban soils relative to suburban and rural areas.183,184 Deplorably, tris(2-chloroethyl) phosphate (TCEP), a pervasive flame retardant (FR), is ubiquitous in a range of consumer products such as plasticizers, preservatives, and fragrances.23 Due to the high volatilization propensity of TCEP (volatilization flux of 1100 ng m−3 day−1), it can leach easily from the consumer products and get released into the environment.184 Therefore, TCEP is one of the widespread pollutants in the soil environments.184–186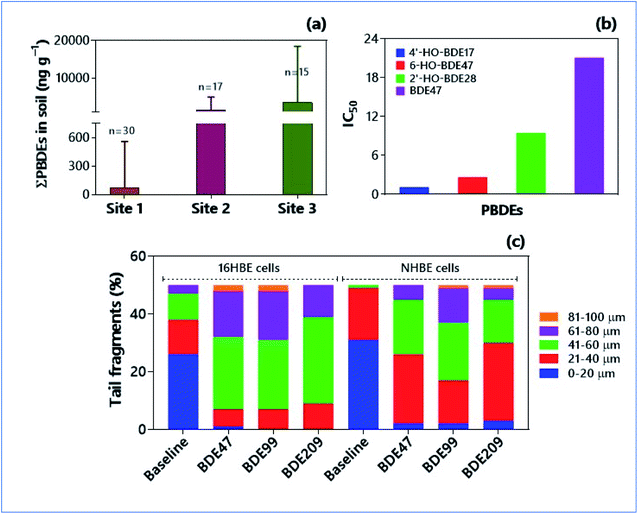 | ||
| Fig. 4 Occurrence of emerging flame retardants in soils and their ecotoxicities. (a) Concentrations of PBDEs in soils collected from three different sampling sites at plastic manufacture plants and surrounding areas in Eastern China.181 (b) Anti-estrogenic potency (IC50, half maximal inhibitory concentration) of BDE47 and its related hydroxylated analogs in transfected green monkey kidney fibroblast (CV-1) cells.189 (c) DNA damage [in terms of tail length (μm)] induced by PBDE congeners in 16HBE and NHBE cells stimulated for 72 h.197 Each sample was analyzed by selecting 50 cells randomly. Tail lengths significantly increased upon treatment with PBDE congeners in cells over control cells. | ||
6.2. Thyroid and reproductive disorders
Brominated flame retardants can disrupt the endocrine system by mimicking the estrogen receptors.187 Both the animal models and human samples analysed provide compelling evidence of endocrine disruption. In the experimental rats, T3 and T4 hormone levels declined with 1000–4000 mg BDE209 per kg body wt.188 The reporter gene assay affirmed the modulation efficiencies of BDE47 (2,2′,4,4′-tetrabromodiphenyl ether) and related hydroxylated analogues on estrogen/thyroid/androgen receptors (ER/TR/AR) (Fig. 4b).189 Interestingly, hydroxylated BDE congeners (e.g., 2′-HO-BDE28, 4′HO-BDE17, 6-HO-BDE47) possessed higher anti-estrogenic potencies (i.e., IC50 = 1.14–9.49 μM) than BDE47 (IC50 = 21.11 μM). The gestation and lactation exposure of rats to BDE47 at the environmentally relevant concentrations severely affected the male reproductive system in offspring; total sperm count has been declined by 20 and 42% over the control at 1.0 and 10.0 mg kg−1 day−1, respectively.190 The fully adjusted, survey-weighted logistic regression model identified a significant association between thyroid disorders in women and serum concentrations of BDE47 (OR 1.48; 95% CI 1.05–2.09), BDE99 (OR 1.78; 95% CI 1.16–2.75), and BDE100 (OR 1.50; 95% CI 0.97–2.31).191 On the other hand, OPE flame retardants exhibit similar endocrine-disrupting properties to PBDEs. In vitro assays and in silico models evinced the adverse effect of ten OPEs on androgen receptor and aryl hydrocarbon receptor activities, transthyretin binding, levels of 17β-estradiol, and IC50 values.1926.3. Carcinogenic risk
The association between cancer risk and PBDE exposure is uncertain. There is extremely limited hypothesis or evidence that proves cancer risk of PBDEs.193 Typically, the serum levels of PBDEs and OH-PBDEs were found to be relatively higher in the thyroid cancer population than in the control group.194 Nonetheless, there is no clarity in the PBDE-induced alterations of thyroid hormone regulations during thyroid cancer. In contrast, inordinately low cancer risk was found in humans through the exposure to PBDEs at the sites engaged in e-waste recycling and open burning of municipal waste.195 In a recent study, no evidence was found between the serum levels of PBDE congeners (e.g., BDE47, BDE100 and BDE153) and cancer risk in women with invasive breast cancer (so-called cases), about 902 in California.196 Nonetheless, the BDE47, BDE99 and BDE209 have strong cytotoxic and genotoxic effects in human bronchial epithelial cells (Fig. 4c),197 suggesting their capacity to induce carcinogenesis in the human respiratory system. Similarly, the genotoxic nature of PBDE congeners have been observed in different experimental models such as human neuroblastoma cells, grass shrimp embryo, and dolphin fibroblast cell cline.198–200 The OPEs are not the exceptions, and they possess carcinogenic activities in humans and animals. The diverse OPE congeners such as EHDPP (2-ethylhexyl diphenyl phosphate), TNBP (tri-N-butyl phosphate), TMPP (2,4,6-trimethoxyphenyl) phosphine, and TPHP (triphenyl phosphate) were significantly (P < 0.05 or 0.01) possess higher risks for breast and cervical cancers in humans.201 Investigations using the rare Chinese minnow demonstrated that the TDCIPP (OPFR) exposure resulted in adverse effects on the cell cycle, DNA replication, Fanconi anaemia pathway, p53 signalling pathway, and various DNA repair pathways.202 However, the exposure to TBOEP or TPHP did not have identical effects.6.4. Regulatory policies for soil pollution abatement
The US EPA has set the screening levels for different congeners for the residential and industrial soils, and these values are significantly higher for industrial soils; the values (mg kg−1) of decaBDE-209, octaBDE, pentaBDE, tetraBDE-47, hexaBDE-153 and pentaBDE-99 were 440, 190, 160, 6.3, 13 and 6.3, and were 3300, 2500, 2300, 82, 162 and 82, for the residential and industrial soils, respectively. However, there are no guidelines by the world health agencies for PBDEs in agricultural soils. Hence, monitoring is inadequate on the entry of PBDEs into crop plants from soils. For the control of OPEs (e.g., TCEP) associated environmental damage, there are several regulatory policies;87 Federal Laws and Regulations (e.g., Toxic Substances Control Act – TSCA Section 6(b), Section 8(a), Section 8(b) and Section 4), State Laws and Regulations (e.g., State Prohibitions, State Drinking Water Standards and Guidelines, Chemicals of High Concern to Children and Proposition 65), and International Laws and Regulations (e.g., Canada Consumer Product Safety Act, Annex XIV of REACH of European Union, Inventory Multi-Tiered assessment and Prioritisation (IMAP – Australia), Japanese Laws (e.g., Chemical Substance Control Law – CSCL; Air Pollution Control Law), Basel Convention) are active in many regions. TCEP can easily reach the soil through biotic and abiotic factors, exhibit phytoaccumulation, and threaten the food chain by accumulating in edible crops (https://www.atsdr.cdc.gov/toxprofiles/tp202-c6.pdf).23,203–205 Hence, policies and regulations on the levels of OPEs should be effective, especially for agricultural lands.7 Nanoparticles
7.1. Newfangled soil pollutants
Nanomaterials are extensively used in different domains (e.g., industries, agriculture, biomedicine, and domestic) since the last two decades.206–208 Nanomaterials' unique features such as a high surface area to volume ratio, higher reactivity, surface potential, tuneable physical/chemical properties, molecular manipulation, and others facilitate their applications.209 All the nanomaterials ultimately reach soils, the primary receptor relative to air and water.210 As pollutants, most engineered nanomaterials (ENMs) reach the soils through uncontrolled emissions through several ways – synthesis, incorporated into the products, the use phase, recycling, and the end-of-life of products (e.g., incineration plant, landfill, wastewater treatment plant).211 According to the US National Nanotechnology Initiative's 2014 draft strategic plans, the two principal routes by which nanoparticles contaminate the soil system are: (i) leaching from nano-coated consumer goods dumped in landfills, and (ii) through treated sewage waste (i.e., biosolids).212 Because of the migration of nanoparticles to the soil via water, most agricultural soils can receive nanoparticles when farmers apply biosolids. However, the threat is quite alarming all over the world. Meanwhile, the counteracting efforts on how nanomaterials are helpful in the remediation of soils polluted by different environmental pollutants are also continuing.213–216 Due consideration of how nanomaterials contaminate soil and subsequent threats to the soil system is needed. Nanoparticles of silver, copper, and zinc are potent anti-microbial substances, and therefore, these nanoparticles can threaten the beneficial microbial communities in soils.210 The metal and metal oxide nanoparticles are more toxic than organic (carbon-based) nanoparticles to the soil microflora; the former nanoparticles exhibit adverse effects on the abundance and diversity of soil microflora even at 1.0 ppm level.217 For a comprehensive understanding of the bioavailability and toxicity of nanoparticles, more investigations are needed because most research has been done with high concentrations of individual nanoparticles.7.2. Concurrent exposure and cellular toxicities
Information on the endocrine disruptive properties of nanoparticles is quite limited, but there are reports on the strong tendency of different nanomaterials that disrupt the normal and physiological activity of the endocrine system. In the 2 h post-fertilized embryos of zebrafish (Danio rerio), titanium dioxide nanoparticles (n-TiO2) did not show any adverse effects on endocrine system. Still, the concurrent exposure of embryos with n-TiO2 + pentachlorophenol (PCP) significantly lowered the contents of T3 by depressing its target genes, i.e., tg (thyroglobulin) and dio2 (deiodinase 2).218 Similarly, bioaccumulation and thyroid disruption by tetrabromobisphenol A in zebrafish larva enhanced in the presence of SiO2 nanoparticles (n-SiO2) (Fig. 5a).219 The presence of n-SiO2 promoted the uptake of BDE-209 by zebrafish embryos due to the formation of n-SiO2-BDE-209 associates.220 The enhanced toxicities of pollutants are made possible in the presence of nanoparticles due to the strong tendency of nanoparticles to interact with the metals and organic contaminants by their large surface areas and adsorptive nature. In the animal models, the metal-based nanomaterials were found to affect the insulin response in follicle-associated epithelial cells, and reproductive and neonatal developments,221 suggesting that nanoparticles can act as endocrine disruptors individually. Due to the insufficient scientific information, European Commission's Scientific Committee for Consumer Safety (SCCS) has recommended ‘an inconclusive nano risk’ associated with copper (nano) and colloidal copper (nano) present in the cosmetics (e.g., leave-on and rinse-off dermal care cosmetics like skin, nail, hair, and scalp) and oral hygiene products.222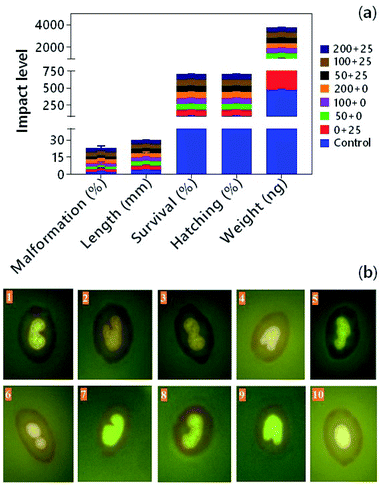 | ||
| Fig. 5 Ecotoxicity of nanoparticles. (a) Effects of single and co-exposure for 120 h of tetrabromobisphenol (TBBPA) and silicon dioxide nanoparticles (n-SiO2) on survival, malformation, hatching, length, and weight of zebrafish larvae (n = 3).219 Values in the legend indicate the concentration of TBBPA (μg L−1) + n-SiO2 (mg L−1). (b) Immunotoxicity of lead (Pb) + titanium dioxide nanoparticles (NpTiO2) on immature erythrocytes (IEs) of fish (Astyanax serratus).226 Alterations (1, 2, 4, 7, 8, 9 = notched; 3, 5, 6 = lobed; 10 = normal nuclei) were detected in IEs by acridine orange after in vivo assay. This figure has been adapted/reproduced from ref Delmond et al. 2019 (ref. 226) with permission from Elsevier, copyright 2019. | ||
7.3. Genotoxic risks
Metal-based nanoparticles are known to induce genotoxicity and carcinogenicity in different experimental systems. Upon 24 h exposure, metallic nickel nanoparticles (0.1–20 μg m−2) caused cytotoxicity and apoptotic induction in the mouse epithelial (JB6) cells.223 The short-term exposure of mussels (Mytilus galloprovincialis) to CuO nanoparticles induced oxidative stress and genotoxicity. Still, it is unknown whether these early events could provoke cancer developments.224 The intraperitoneal injections of TiO2 nanoparticles at 1.5 μg-TiO2-NP g−1 led to an increased micronuclei frequency after 72 h in marine fish species (Trachinotus carolinus).225 But the micronuclei frequency decreased after 72 h exposure in the same experimental animals, when injected at 3.0 μg-TiO2-NP g−1. There were no micronuclei in the fish species (Astyanax serratus) when exposed intraperitoneally for 96 h to NPTiO2 (Fig. 5b).226 The genotoxic effects of nanoparticles depend on the concentration tested, experimental animals, and duration of exposure. Very recently, animal models provide convincing evidence of genotoxic effects of nanoparticles; TiO2 nanoparticles exhibit genotoxic effects in blood and kidney cells of freshwater fish Rhamdia quelen at a dose of 0.01 mL g−1 fish.227 But, the cancer-related genes (e.g., ras, gadd45α) were not affected in mussels upon 21 days exposure to CuO nanoparticles.228 The genotoxicity and carcinogenicity of nanoparticles necessitate the policies and regulations as the preventive measures against the unforeseen, irreversible damage to the complex web of life forms in the soil environments.There are significant differences between the properties of metallic nanoparticles and those synthesized from natural bioresources (i.e., lignin nanoparticles). In animal experiments, large amounts of nanoparticles are used, which are not representatives of actual human contamination. For instance, feed additives containing metal nanoparticles229 and nano veterinary medicines (diagnostics and therapeutics)230 are widely used in the recent times. Also, different metal nanoparticles (e.g., gold, iron, selenium, copper and zinc) are included in equine nutrition while preparing technological additives, sensory additives, zootechnical additives, nutritional additives and coccidiostats or histomonostats.229 It is noteworthy that the nanoparticles have poor absorption either in gut or kidney and are eventually excreted without undergoing in vivo biodegradation.231 Absorption, distribution and excretion levels of TiO2 and ZnO nanoparticles were significantly different in rats when orally administered for 13 weeks (7 days per week),232 which implies that the dissolution rates in acidic gastric fluids vary among the nanoparticles. Thus, animals are exposed to nanoparticles through modern animal management practices, subsequently contributing to the overall soil burden through excreta. Due to their biodegradability, polymeric nanoparticles are widely used in wastewater treatment, synthesis of environmental-friendly chemicals (e.g., fertilizers, herbicides, and pesticides) and in food industry.233 For a substantial information on the soil burden of nanoparticles, it is necessary to identify the sources of metallic nanoparticles and nanoparticles of natural bioresources like polymeric nanoparticles. Though metallic nanoparticles have been significantly used,234 more information is needed on their impact towards the health of humans and environment.235 On the other hand, no detection methods are available to adequately determine the low concentration of nanoparticles. The current research is therefore focussed in developing suitable analytical techniques for detecting even very low concentrations of nanoparticles in the environmental samples.236
7.4. Regulations for agricultural lands
Nanotechnologies have become one of the emerging innovations in agriculture. Several nano-based agrochemicals such as nano-fertilizers, nano-pesticides, and nano-herbicides are transforming the farming practices.237 Ensuing soil burden of nanomaterials, besides non-agricultural pollution threatens the soil health. Biosolids application is one of the commonest practices in sustainable agriculture, which can serve as a way of introducing nanoparticles to agricultural lands.238 This does not mean that the usage of biosolids in agriculture is unacceptable but efficient wastewater treatment facilities are in need. Notably, as of now there is no complete understanding about the long-term in situ field effects of nanoparticles.239 The land-application of nano-based agrochemicals may endanger through several unintended pathways of transfers into different environmental components. There is an urgent need for intensive scientific experimentations to fill the current knowledge gaps, so that human health and food quality are safe. Nanoparticles-laden food stuffs are a new kind of risks. According to the Centre for Food Safety, there are 209 records for food supplements and additives claiming to contain nanomaterials (https://www.centerforfoodsafety.org/nanotechnology-in-food). The threats posed to human health by these nanoparticles-laden food stuffs need critical investigations. Above all, there is a necessity to standardize risk assessment procedures on the use and fate of nanoparticles to formulate and execute the soil health guidelines.2408 Conclusions and future research directions
The CECs have become the contemporary soil pollutants and provide plausible threats to human health and all other ecosystem members. Since most CECs offer new challenges such as genotoxicities and carcinogenic risks, the emergent concerns of these pollutants deserve critical analysis of their soil burden and transfer pathways and even the formulation of policies and regulations.(i) Escalating the global soil burden of PFOA (1860 MT) and PFOS (>7000 MT) favour their entries into the terrestrial food webs, with a higher accumulation of PFAS in the food crops. Human exposure to the soil borne PFAS accounts for 9%, third major source after foodborne (40%) and waterborne (30%) pollutants. The global soil metadata analysis of PFAS (>160 ng per kg soil) and the forecast of their expanding market size (https://www.bluefieldresearch.com/) portend their severe consequences in the ecosystem and human health in the absence of proper regulations on the entry and mobility of PFAS in soils. The PFAS are highly toxic to the animals and cells, and they are carcinogenic (for example, 3.3 times higher rates of prostate cancer in workers exposed at workplaces). The mean half-life of PFAS in the human body is about 2.7–3.4 years. Since the PFAS are under the label of ‘possible human carcinogenic chemicals' by the International Agency for Research on Cancer (IARC), the long-term health effects of these substances deserve attention from the levels of individual organisms to the environmental communities.
(ii) Most intensively cultivated lands are becoming hotspots of micro- and nano plastics (MNPs), and their annual global loading is in the range of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons. Around 80% of plastic waste reaches the soils in landfills, entailing the intensity of their soil burden. The soil matrices function as a platform for forming complex CECs by the interaction of MNPs with PCBs and HMs and produce even more toxic compounds than MNPs alone. The MNPs have strong immune toxicity (e.g., toxic even at 200 μg mL−1 in PS beads to human immune cells) and the potentials for genotoxicity (e.g., 100 nM induced chromosome breakage). Across certain countries, there are flexible legislation policies to minimize the levels of MNPs in sewage sludge used for agriculture purposes. The stringent guidelines will ensure taking appropriate protective measures against the pollution of MNPs in agricultural lands.
000 tons. Around 80% of plastic waste reaches the soils in landfills, entailing the intensity of their soil burden. The soil matrices function as a platform for forming complex CECs by the interaction of MNPs with PCBs and HMs and produce even more toxic compounds than MNPs alone. The MNPs have strong immune toxicity (e.g., toxic even at 200 μg mL−1 in PS beads to human immune cells) and the potentials for genotoxicity (e.g., 100 nM induced chromosome breakage). Across certain countries, there are flexible legislation policies to minimize the levels of MNPs in sewage sludge used for agriculture purposes. The stringent guidelines will ensure taking appropriate protective measures against the pollution of MNPs in agricultural lands.
(iii) The global production of phthalates, which has almost doubled between 2006 and 2015 (4.7 to 8.0 MMT), and rapid movement of these pollutants from the soil matrices to the crop plants contribute significantly to human intake. Phthalates and PCBs can disrupt the endocrine system and possess carcinogenic risks. As there are no sustainable replacements for phthalates and PCBs, their accumulation in soils is becoming a recent global problem. Besides emphasizing the production of sustainable alternatives for phthalates such as citrates, sebacates, adipates, phosphates, the regulations can ascertain the sharing of responsibilities for environmental protection.
(iv) Higher concentrations of PBDEs in agricultural soils than in other soil types such as mountain soils and soils from the rural areas and the stronger propensities for phytoaccumulation necessitate specific guidelines for controlling their transport pathways through soils. The present level of PBDEs that enter humans via food intake is six times higher than the limits set by the USEPA. The anti-estrogenic potencies of both PBDEs and their hydroxylated derivatives, cytotoxic (e.g., impaired cell cycle and p53 signalling pathway) and genotoxic (e.g., impaired DNA replication and DNA repair pathways) characteristics of PBDEs and their association with thyroid disorders suggest the need for understanding the complexities in their effects.
(v) The principal routes of soil contamination by nanoparticles are: (a) leaching from the source materials dumped in the landfills and (b) the application of biosolids. The uncontrolled flow of several pollutants like ENMs, which are composed of different HMs, into the soil matrix imperil different life forms. The nanoparticles can exhibit the ecotoxic effect even at 1 ppm level, and their bioavailability is poorly understood. Generally, the metal-based nanoparticles show strong endocrine disruption-, cytotoxic-, and genotoxic properties. So, these particles are not far from threatening human health. Hu et al.241 argued that MNPs could force global biodiversity changes. There is a strong need for standardized risk assessment procedures to assess the health risks caused by these particles from the polluted soils.
Likewise, the available literature strongly suggests the potential threats of the emerging pollutants in the environment. However, their intended applications through their industrial production, supply chains, and diverse utilization meet human needs and material requirements. There are stronger needs to gather scientific information, which is fundamental to understand the risks, not only of human relevance but also beyond, and to devise methods to manage those risks by the CECs. The biggest challenge is to assess the mixture effects as CECs can interact with other chemicals in the physical world and with biochemicals in living organisms. The life cycle assessment (LCA) of the processes, products, and supply chains of chemical substances is regarded as a necessity.242 The integration of environmental risk assessment in the LCA of CECs is the future challenge.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.References
- J. O. Tijani, O. O. Fatoba, O. O. Babajide and L. F. Petrik, Environ. Chem. Lett., 2016, 14, 27–49 CrossRef CAS.
- CEC, Contaminants of Emerging Concern, 31 December 2020, https://en.wikipedia.org/wiki/Contaminants_of_emerging_concern, accessed January 01, 2021 Search PubMed.
- USEPA, United States Environmental Protection Agency, Contaminants of Emerging Concern including Pharmaceuticals and Personal Care Products, 01 September 2020, https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products, accessed January 01, 2021 Search PubMed.
- F. Pena-Pereira, C. Bendicho, D. M. Pavlović, A. Martín-Esteban, M. Díaz-Álvarez, Y. Pan, J. Cooper, Z. Yang, I. Safarik, K. Pospiskova, M. A. Segundo and E. Psillakis, Anal. Chim. Acta, 2021, 1158, 238108 CrossRef CAS PubMed.
- M. Farré, Curr. Opin. Environ. Sci. Health., 2020, 18, 79–94 CrossRef.
- K. O. K'Oreje, M. Okoth, H. Van Langenhove and K. Demeestere, J. Environ. Manage., 2020, 254, 109752 CrossRef PubMed.
- F. J. Kroon, K. L. E. Berry, D. L. Brinkman, R. Kookana, F. D. L. Leusch, S. D. Melvin, P. A. Neale, A. P. Negri, M. Puotinen, J. J. Tsang, J. P. van de Merwe and M. Williams, Sci. Total Environ., 2020, 719, 135140 CrossRef CAS PubMed.
- O. C. Olatunde, A. T. Kuvarega and D. C. Onwudiwe, Emerging Contam., 2020, 6, 283–302 CrossRef.
- A. I. Shah, M. U. Din Dar, R. A. Bhat, J. P. Singh, K. Singh and S. A. Bhat, Ecol. Eng., 2020, 152, 105882 CrossRef.
- T. K. Kasonga, M. A. A. Coetzee, I. Kamika, V. M. Ngole-Jeme and M. N. Benteke Momba, J. Environ. Manage., 2021, 277, 111485 CrossRef CAS PubMed.
- G. Vandermeersch, H. M. Lourenço, D. Alvarez-Muñoz, S. Cunha, J. Diogène, G. Cano-Sancho, J. J. Sloth, C. Kwadijk, D. Barcelo, W. Allegaert, K. Bekaert, J. O. Fernandes, A. Marques and J. Robbens, Environ. Res., 2015, 143, 29–45 CrossRef CAS PubMed.
- N. H. Tran, M. Reinhard and K. Y.-H. Gin, Water Res., 2018, 133, 182–207 CrossRef CAS PubMed.
- G. Reichert, S. Hilgert, S. Fuchs and J. C. R. Azevedo, Environ. Pollut., 2019, 255, 113140 CrossRef CAS PubMed.
- D. He, Y. Luo, S. Lu, M. Liu, Y. Song and L. Lei, Trends Anal. Chem., 2018, 109, 163–172 CrossRef CAS.
- V. L. R. Pullagurala, S. Rawat, I. O. Adisa, J. A. Hernandez-Viezcas, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Sci. Total Environ., 2018, 636, 1585–1596 CrossRef CAS PubMed.
- A. A. de Souza Machado, W. Kloas, C. Zarfl, S. Hempel and M. C. Rillig, Global Change Biol., 2018, 24, 1405–1416 CrossRef PubMed.
- USEPA, 2018. United States Environmental Protection Agency. PFOA, PFOS and Other PFASs. Basic Information on PFAS - What are PFAS? 06 December 2018. https://www.epa.gov/pfas/basic-information-pfas, accessed 01 January 2021 Search PubMed.
- M. J. B. Amorim and J. J. Scott-Fordsmand, Environ. Pollut., 2021, 271, 116363 CrossRef CAS PubMed.
- S. Benjamin, S. Pradeep, M. Sarath Josh, S. Kumar and E. Masai, J. Hazard. Mater., 2015, 298, 58–72 CrossRef CAS PubMed.
- X. Zhang, M. L. Diamond, M. Robson and S. Harrad, Environ. Sci. Technol., 2011, 45, 3268–3274 CrossRef CAS PubMed.
- K. Breivik, J. M. Armitage, F. Wania, A. J. Sweetman and K. C. Jones, Environ. Sci. Technol., 2016, 50, 798–805 CrossRef CAS PubMed.
- N. R. Maddela, K. Venkateswarlu, D. Kakarla and M. Megharaj, Environ. Pollut., 2020, 266, 115240 CrossRef CAS PubMed.
- N. R. Maddela, K. Venkateswarlu and M. Megharaj, Environ. Sci.: Processes Impacts, 2020, 22, 1809–1827 RSC.
- Pollution, Pollution from Nanomaterials. 19 December 2020, https://en.wikipedia.org/wiki/Pollution_from_nanomaterials, accessed January 01, 2021 Search PubMed.
- Y. Gao, Y. Liang, K. Gao, Y. Wang, C. Wang, J. Fu, Y. Wang, G. Jiang and Y. Jiang, Chemosphere, 2019, 227, 305–314 CrossRef CAS PubMed.
- M. Llorca, M. Farré, M. S. Tavano, B. Alonso, G. Koremblit and D. Barceló, Environ. Pollut., 2012, 163, 158–166 CrossRef CAS PubMed.
- F. Xiao, M. F. Simcik, T. R. Halbach and J. S. Gulliver, Water Res., 2015, 72, 64–74 CrossRef CAS PubMed.
- E. S. Heimstad, T. Nygard, D. Herzke and P. Bohlin-Nizzetto, Norwegian Environment Agency report, M-1402/2019 (NILU Report 19/2019), Kjeller: NILU, https://www.miljodirektoratet.no/globalassets/publikasjoner/m1402/m1402.pdf.
- S. Dalahmeh, S. Tirgani, A. J. Komakech, C. B. Niwagaba and L. Ahrens, Sci. Total Environ., 2018, 631, 660–667 CrossRef PubMed.
- L. Yang, Y. Zhang, S. Kang, Z. Wang and C. Wu, Sci. Total Environ., 2021, 780, 146546 CrossRef CAS PubMed.
- P. A. Odera, Determination of Bisphenol a Bpa in Thermal Papers, Selected Soil Samples and the Effect of Temperature and Humidity on Its Concentration, https://erepository.uonbi.ac.ke/handle/11295/106703, accessed December 06, 2021.
- S. Mao, S. Liu, Y. Zhou, Q. An, X. Zhou, Z. Mao, Y. Wu and W. Liu, Environ. Pollut., 2021, 271, 116171 CrossRef CAS PubMed.
- G. Salihoglu, N. K. Salihoglu, E. Aksoy and Y. Tasdemir, J. Environ. Manage., 2011, 92, 724–732 CrossRef CAS PubMed.
- A. Martinez, N. R. Erdman, Z. L. Rodenburg, P. M. Eastling and K. C. Hornbuckle, Environ. Pollut., 2012, 161, 222–228 CrossRef CAS PubMed.
- S. A. Debela, I. Sheriff, J. Wu, Q. Hua, Y. Zhang and A. K. Dibaba, Sci. African, 2020, 8, e00329 CrossRef.
- A. Di Guardo, E. Terzaghi, G. Raspa, S. Borin, F. Mapelli, B. Chouaia, E. Zanardini, C. Morosini, A. Colombo, E. Fattore, E. Davoli, S. Armiraglio, V. M. Sale, S. Anelli and P. Nastasio, Environ. Pollut., 2017, 223, 367–375 CrossRef CAS PubMed.
- K. Oloruntoba, O. Sindiku, O. Osibanjo, C. Herold and R. Weber, Environ. Pollut., 2021, 277, 116794 CrossRef CAS PubMed.
- T. J. McGrath, P. D. Morrison, A. S. Ball and B. O. Clarke, Emerging Contam., 2017, 3, 23–31 CrossRef.
- Y. Huang, D. Zhang, Y. Yang, X. Zeng and Y. Ran, Environ. Pollut., 2018, 235, 104–112 CrossRef CAS PubMed.
- A. Cincinelli, T. Martellini, L. Misuri, E. Lanciotti, A. Sweetman, S. Laschi and I. Palchetti, Environ. Pollut., 2012, 161, 229–234 CrossRef CAS PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- Y. Yang, Y. Wang, P. Westerhoff, K. Hristovski, V. L. Jin, M.-V. V. Johnson and J. G. Arnold, Sci. Total Environ., 2014, 485–486, 441–449 CrossRef CAS PubMed.
- M. Biel-Maeso, V. Burke, J. Greskowiak, G. Massmann, P. A. Lara-Martín and C. Corada-Fernández, Sci. Total Environ., 2021, 762, 144102 CrossRef CAS PubMed.
- J. A. R. Willemsen and I. C. Bourg, J. Colloid Interface Sci., 2021, 585, 337–346 CrossRef CAS PubMed.
- G. Teijón, L. Candela, J. Šimůnek, K. Tamoh and J. Valdes-Abellán, Soil Sediment Contam., 2014, 23, 736–750 CrossRef.
- X. Chen, X. Gu, L. Bao, S. Ma and Y. Mu, Chemosphere, 2021, 263, 127947 CrossRef CAS PubMed.
- S. Endo, R. Takizawa, K. Okuda, H. Takada, K. Chiba, H. Kanehiro, H. Ogi, R. Yamashita and T. Date, Mar. Pollut. Bull., 2005, 50, 1103–1114 CrossRef CAS PubMed.
- H. Hirai, H. Takada, Y. Ogata, R. Yamashita, K. Mizukawa, M. Saha, C. Kwan, C. Moore, H. Gray and D. Laursen, Mar. Pollut. Bull., 2011, 62, 1683–1692 CrossRef CAS PubMed.
- H. K. Karapanagioti, S. Endo, Y. Ogata and H. Takada, Mar. Pollut. Bull., 2011, 62, 312–317 CrossRef CAS PubMed.
- A. A. Horton, A. Walton, D. J. Spurgeon, E. Lahive and C. Svendsen, Sci. Total Environ., 2017, 586, 127–141 CrossRef CAS PubMed.
- F. K. Mammo, I. D. Amoah, K. M. Gani, L. Pillay, S. K. Ratha, F. Bux and S. Kumari, Sci. Total Environ., 2020, 743, 140518 CrossRef CAS PubMed.
- N. Garvin, W. J. Doucette and J. C. White, Chemosphere, 2015, 130, 98–102 CrossRef CAS PubMed.
- E. L. Miller, S. L. Nason, K. G. Karthikeyan and J. A. Pedersen, Environ. Sci. Technol., 2016, 50, 525–541 CrossRef CAS PubMed.
- B. Boots, C. W. Russell and D. S. Green, Environ. Sci. Technol., 2019, 53, 11496–11506 CrossRef CAS PubMed.
- T. Bosker, L. J. Bouwman, N. R. Brun, P. Behrens and M. G. Vijver, Chemosphere, 2019, 226, 774–781 CrossRef CAS PubMed.
- X. Jiang, H. Chen, Y. Liao, Z. Ye, M. Li and G. Klobučar, Environ. Pollut., 2019, 250, 831–838 CrossRef CAS PubMed.
- S. Önlü and M. T. Saçan, J. Hazard. Mater., 2018, 351, 20–28 CrossRef PubMed.
- S. González-Rubio, A. Ballesteros-Gómez, A. G. Asimakopoulos and V. L. B. Jaspers, Sci. Total Environ., 2020, 143337 Search PubMed.
- Y. Huang, M. Kong, S. Coffin, K. H. Cochran, D. C. Westerman, D. Schlenk, S. D. Richardson, L. Lei and D. D. Dionysiou, Water Res., 2020, 174, 115587 CrossRef CAS PubMed.
- C. A. James, J. Lanksbury, T. Khangaonkar and J. West, Sci. Total Environ., 2020, 709, 136098 CrossRef CAS PubMed.
- D. A. Woolnough, A. Bellamy, S. L. Hummel and M. Annis, J. Great Lake. Res., 2020, 46, 1625–1638 CrossRef.
- P. Bhattacharya, D. Mukherjee, N. Deb, S. Swarnakar and S. Banerjee, Mater. Chem. Phys., 2021, 258, 123920 CrossRef CAS.
- A. Rimer, H. Shaner and M. Moroz, EPA Declines to Set Drinking Water Limits for Perchlorate, 18 June 2020., https://www.environmentallawandpolicy.com/category/emering-contaminants/, accessed January 02, 2021 Search PubMed.
- M. J. Trujillo-Rodríguez, M. Rosende and M. Miró, Trac. Trends Anal. Chem., 2020, 132, 116040 CrossRef.
- Y. Feng, H. H. M. Rijnaarts, D. Yntema, Z. Gong, D. D. Dionysiou, Z. Cao, S. Miao, Y. Chen, Y. Ye and Y. Wang, Water Res., 2020, 186, 116327 CrossRef CAS PubMed.
- C. Nellemann and E. Corcoran, Dead planet, living planet: Biodiversity and ecosystem restoration for sustainable development: A rapid response assessment, UNEP/Earthprint, 2010 Search PubMed.
- S. A. Banwart, N. P. Nikolaidis, Y.-G. Zhu, C. L. Peacock and D. L. Sparks, Annu. Rev. Earth Planet Sci., 2019, 47, 333–359 CrossRef CAS.
- S. Yan, S. B. Subramanian, R. Tyagi, R. Y. Surampalli and T. C. Zhang, Pract. Period. Hazard. Toxic, Radioact. Waste Manag., 2010, 14, 2–20 CrossRef CAS.
- B. Biswas, F. Qi, J. K. Biswas, A. Wijayawardena, M. A. I. Khan and R. Naidu, Soil Syst., 2018, 2, 51 CrossRef CAS.
- Y. Fang, E. Kim and T. J. Strathmann, Environ. Sci. Technol., 2018, 52, 1997–2006 CrossRef CAS PubMed.
- Q. Qin, X. Chen and J. Zhuang, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1379–1408 CrossRef CAS.
- Y. Fang, G. Vanzin, A. M. Cupples and T. J. Strathmann, Sci. Total Environ., 2020, 706, 135327 CrossRef CAS PubMed.
- Z. Yang, W. Fang, X. Lu, G.-P. Sheng, D. E. Graham, L. Liang, S. D. Wullschleger and B. Gu, Environ. Pollut., 2016, 214, 504–509 CrossRef CAS PubMed.
- K. D. Strååt, C.-M. Mörth and E. Undeman, J. Mar. Syst., 2018, 177, 8–20 CrossRef.
- M. L. Flores-Mangual, A. J. Hernández-Maldonado, K. Ortíz-Martínez and N. P. Quiñones, Agrosyst. Geosci. Environ., 2020, 3, e20022 Search PubMed.
- J. Yang, J. McBride, J. Zhou and Z. Sun, Urban For. Urban Green., 2005, 3, 65–78 CrossRef.
- X. Chen, T. Pei, Z. Zhou, M. Teng, L. He, M. Luo and X. Liu, Urban For. Urban Green., 2015, 14, 354–360 CrossRef.
- W. Selmi, C. Weber, E. Rivière, N. Blond, L. Mehdi and D. Nowak, Urban For. Urban Green., 2016, 17, 192–201 CrossRef.
- A. P. Jeanjean, R. Buccolieri, J. Eddy, P. S. Monks and R. J. Leigh, Urban For. Urban Green., 2017, 22, 41–53 CrossRef.
- N. Rodríguez-Eugenio, M. McLaughlin and D. Pennock, Soil pollution: a hidden reality, Rome, FAO, 2018, 142 pp. https://agris.fao.org/agris-search/search.do?recordID=XF2018001459, accessed December 07, 2021 Search PubMed.
- Z. Cheng, G. M. Hettiarachchi and K. H. Kim, J. Environ. Qual., 2021, 50, 2–6 CrossRef CAS PubMed.
- R. Renner, Environ. Sci. Technol., 2006, 40, 12–13 CrossRef PubMed.
- J. A. Laitinen, J. Koponen, J. Koikkalainen and H. Kiviranta, Toxicol. Lett., 2014, 231, 227–232 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, U. Berger, K. Hungerbühler and M. Scheringer, Environ. Int., 2016, 89–90, 235–247 CrossRef CAS PubMed.
- CDC, Center for Disease Control and Prevention, National Biomonitoring Program - Per- and Polyfluorinated Substances (PFAS) Factsheet, April 7, 2017. https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html#:%7E:text=Theper%2Dandpolyfluorinatedsubstances,stains%2Cgrease%2Candwater, accessed January 21, 2021 Search PubMed.
- J. S. Skaar, E. M. Ræder, J. L. Lyche, L. Ahrens and R. Kallenborn, Environ. Sci. Pollut. Res., 2019, 26, 7356–7363 CrossRef CAS PubMed.
- USEPA, Unitedc States Environmental Protection Agency, Final scope of the risk evaluation for tris(2-chloroethyl) phosphate (TCEP). EPA Document# EPA- 740-R-20-009, August 2020, Office of Chemical Safety and Pollution Prevenation, https://www.epa.gov/sites/production/files/2020-09/documents/casrn_115-96-8_tris2-chloroethyl_phosphate_tcep_final_scope.pdf, assessed 15th April, 2021 Search PubMed.
- E. F. Houtz, R. Sutton, J.-S. Park and M. Sedlak, Water Res., 2016, 95, 142–149 CrossRef CAS PubMed.
- C.-H. S. J. Chou and F. Llados, Toxicological Profile for Perfluoroalkyls: Draft, Agency for Toxic Substances and Disease Registry, 2009 Search PubMed.
- M. Fuerhacker, Environ. Sci. Pollut. Res. Int., 2009, 16, 92–97 CrossRef PubMed.
- S. Chen, Y. Zhou, J. Meng and T. Wang, Environ. Pollut., 2018, 242, 2059–2067 CrossRef CAS PubMed.
- P. Ssebugere, M. Sillanpää, H. Matovu, Z. Wang, K.-W. Schramm, S. Omwoma, W. Wanasolo, E. C. Ngeno and S. Odongo, Sci. Total Environ., 2020, 739, 139913 CrossRef CAS PubMed.
- M. J. Strynar, A. B. Lindstrom, S. F. Nakayama, P. P. Egeghy and L. J. Helfant, Chemosphere, 2012, 86, 252–257 CrossRef CAS PubMed.
- M. L. Brusseau, R. H. Anderson and B. Guo, Sci. Total Environ., 2020, 740, 140017 CrossRef CAS PubMed.
- W. Wang, G. Rhodes, J. Ge, X. Yu and H. Li, Chemosphere, 2020, 261, 127584 CrossRef CAS PubMed.
- N. Tom, FDA finds surprisingly high levels of PFAS in certain foods – including chocolate cake, June 03, 2019, http://blogs.edf.org/health/2019/06/03/fda-high-levels-pfas-chocolate-cake/, accessed January 22, 2021 Search PubMed.
- N. M. DeLuca, M. Angrish, A. Wilkins, K. Thayer and E. A. Cohen Hubal, Environ. Int., 2021, 146, 106308 CrossRef CAS PubMed.
- Y. Cai, H. Chen, R. Yuan, F. Wang, Z. Chen and B. Zhou, Sci. Total Environ., 2019, 690, 1162–1169 CrossRef CAS PubMed.
- D. M. O'Carroll, T. C. Jeffries, M. J. Lee, S. T. Le, A. Yeung, S. Wallace, N. Battye, D. J. Patch, M. J. Manefield and K. P. Weber, Sci. Total Environ., 2020, 712, 135994 CrossRef PubMed.
- F. Pérez, M. Nadal, A. Navarro-Ortega, F. Fàbrega, J. L. Domingo, D. Barceló and M. Farré, Environ. Int., 2013, 59, 354–362 CrossRef PubMed.
- J. M. Graber, C. Alexander, R. J. Laumbach, K. Black, P. O. Strickland, P. G. Georgopoulos, E. G. Marshall, D. G. Shendell, D. Alderson, Z. Mi, M. Mascari and C. P. Weisel, J. Exposure Sci. Environ. Epidemiol., 2019, 29, 172–182 CrossRef CAS PubMed.
- H. Jin, S. Lin, W. Dai, L. Feng, T. Li, J. Lou and Q. Zhang, Environ. Int., 2020, 138, 105651 CrossRef CAS PubMed.
- K. E. Sant, H. M. Jacobs, K. A. Borofski, J. B. Moss and A. R. Timme-Laragy, Environ. Pollut., 2017, 220, 807–817 CrossRef CAS PubMed.
- S. E. Brown, K. E. Sant, S. M. Fleischman, O. Venezia, M. A. Roy, L. Zhao and A. R. Timme-Laragy, Birth Defects Res., 2018, 110, 933–948 CrossRef CAS PubMed.
- K. E. Sant, O. L. Venezia, P. P. Sinno and A. R. Timme-Laragy, Toxicol. Sci., 2019, 167, 258–268 CrossRef CAS PubMed.
- S. Liu, N. Yin and F. Faiola, Environ. Sci. Technol. Lett., 2018, 5, 237–242 CrossRef CAS.
- S. Liu, R. Yang, N. Yin and F. Faiola, Chemosphere, 2020, 254, 126709 CrossRef CAS PubMed.
- Y. Li, T. Fletcher, D. Mucs, K. Scott, C. H. Lindh, P. Tallving and K. Jakobsson, Occup. Environ. Med., 2018, 75, 46–51 CrossRef PubMed.
- P. Grandjean and R. Clapp, New Solutions: J. Environ. Occupat. Health Policy: NS, 2015, 25, 47–163 Search PubMed.
- A. M. Temkin, B. A. Hocevar, D. Q. Andrews, O. V. Naidenko and L. M. Kamendulis, Int. J. Environ. Res. Public Health, 2020, 17, 1668 CrossRef CAS PubMed.
- F. D. Gilliland and J. S. Mandel, J. Occup. Med., 1993, 35, 950–954 CrossRef CAS PubMed.
- P. Girardi and E. Merler, Environ. Res., 2019, 179, 108743 CrossRef CAS PubMed.
- K. T. Eriksen, M. Sørensen, J. K. McLaughlin, L. Lipworth, A. Tjønneland, K. Overvad and O. Raaschou-Nielsen, J. Natl. Cancer Inst., 2009, 101, 605–609 CrossRef CAS PubMed.
- V. Barry, A. Winquist and K. Steenland, Environ. Health Perspect., 2013, 121, 1313–1318 CrossRef PubMed.
- N. T. Program, NTP Technical Report on the Toxicology and Carcinogenesis Studies of Perfluorooctanoic Acid (CASRN 335-67-1) Administered in Feed to Sprague Dawley (Hsd: Sprague Dawley® SD®) Rats, https://www.ncbi.nlm.nih.gov/books/NBK560147/, accessed December 06, 2021 Search PubMed.
- H.-W. Lin, H.-X. Feng, L. Chen, X.-J. Yuan and Z. Tan, Nagoya J. Med. Sci., 2020, 82, 323–333 Search PubMed.
- B. A. Cohn, M. A. La Merrill, N. Y. Krigbaum, M. Wang, J.-S. Park, M. Petreas, G. Yeh, R. C. Hovey, L. Zimmermann and P. M. Cirillo, Reprod. Toxicol., 2020, 92, 112–119 CrossRef CAS PubMed.
- R. Darlington, E. Barth and J. McKernan, Mil. Eng., 2018, 110, 58–60 Search PubMed.
- T. Maddocks and N. Notzon, PFAS chemicals: 'Shocked and disgusted' Katherine residents demand action on water contamination, 9 October 2017, https://www.abc.net.au/news/2017-10-10/pfas-chemicals-katherine-residents-shocked-demand-action/9034504, accessed January 24, 2021 Search PubMed.
- Clu-In, Contaminated Site Clean Up Information - Per- and Polyfluoroalkyl Substances (PFASs) Policy and Guidance, 10 December 2020, https://clu-in.org/contaminantfocus/default.focus/sec/Per-_and_Polyfluoroalkyl_Substances_(PFASs)/cat/Policy_and_Guidance/#3 accessed January 24, 2021 Search PubMed.
- HHSSC, Human health soil screening criteria for PFOS, PFHxS and PFOA, State of NSW and Office of Environment and Heritage, May 2019, https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Land-and-soil/human-health-soil-screening-criteria-190208.pdf, accessed January 24, 2021 Search PubMed.
- HC, Health Canada, Water Talk - Perfluoroalkylated substances in drinking water - Perfluoroalkylated substances (PFAS), April 2019, https://www.canada.ca/en/services/health/publications/healthy-living/water-talk-drinking-water-screening-values-perfluoroalkylated-substances.html, accessed January 24, 2021 Search PubMed.
- A. L. Duchesne, J. K. Brown, D. J. Patch, D. Major, K. P. Weber and J. I. Gerhard, Environ. Sci. Technol., 2020, 54, 12631–12640 CrossRef CAS PubMed.
- F. Corradini, P. Meza, R. Eguiluz, F. Casado, E. Huerta-Lwanga and V. Geissen, Sci. Total Environ., 2019, 671, 411–420 CrossRef CAS PubMed.
- H. Gao, C. Yan, Q. Liu, W. Ding, B. Chen and Z. Li, Sci. Total Environ., 2019, 651, 484–492 CrossRef CAS PubMed.
- P. He, L. Chen, L. Shao, H. Zhang and F. Lü, Water Res., 2019, 159, 38–45 CrossRef CAS PubMed.
- V. C. Shruti, F. Pérez-Guevara, P. D. Roy, I. Elizalde-Martínez and G. Kutralam-Muniasamy, Sci. Total Environ., 2020, 739, 140358 CrossRef PubMed.
- J. Brizga, K. Hubacek and K. Feng, One Earth, 2020, 3, 45–53 CrossRef.
- L. Nizzetto, M. Futter and S. Langaas, Environ. Sci. Technol., 2016, 50, 10777–10779 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Environ. Sci. Technol., 2019, 53, 7068–7074 CrossRef CAS PubMed.
- S. Iqbal, J. Xu, S. D. Allen, S. Khan, S. Nadir, M. S. Arif and T. Yasmeen, Chemosphere, 2020, 260, 127578 CrossRef CAS PubMed.
- F. Barbosa, J. A. Adeyemi, M. Z. Bocato, A. Comas and A. Campiglia, Environ. Res., 2020, 182, 109089 CrossRef CAS PubMed.
- M. Bläsing and W. Amelung, Sci. Total Environ., 2018, 612, 422–435 CrossRef PubMed.
- I. Velzeboer, C. Kwadijk and A. A. Koelmans, Environ. Sci. Technol., 2014, 48, 4869–4876 CrossRef CAS PubMed.
- M. Davranche, C. Veclin, A.-C. Pierson-Wickmann, H. El Hadri, B. Grassl, L. Rowenczyk, A. Dia, A. Ter Halle, F. Blancho and S. Reynaud, Environ. Pollut., 2019, 249, 940–948 CrossRef CAS PubMed.
- T. T. Awet, Y. Kohl, F. Meier, S. Straskraba, A. L. Grün, T. Ruf, C. Jost, R. Drexel, E. Tunc and C. Emmerling, Environ. Sci. Eur., 2018, 30, 1–10 CrossRef CAS PubMed.
- B.-K. Zhu, Y.-M. Fang, D. Zhu, P. Christie, X. Ke and Y.-G. Zhu, Environ. Pollut., 2018, 239, 408–415 CrossRef CAS PubMed.
- A. McCormick, T. J. Hoellein, S. A. Mason, J. Schluep and J. J. Kelly, Environ. Sci. Technol., 2014, 48, 11863–11871 CrossRef CAS PubMed.
- C. Li, Y. Gan, J. Dong, J. Fang, H. Chen, Q. Quan and J. Liu, Chemosphere, 2020, 253, 126740 CrossRef CAS PubMed.
- R. Firdessa, T. A. Oelschlaeger and H. Moll, Eur. J. Cell Biol., 2014, 93, 323–337 CrossRef CAS PubMed.
- B. Prietl, C. Meindl, E. Roblegg, T. R. Pieber, G. Lanzer and E. Fröhlich, Cell Biol. Toxicol., 2014, 30, 1–16 CrossRef CAS PubMed.
- J. Hwang, D. Choi, S. Han, J. Choi and J. Hong, Sci. Total Environ., 2019, 684, 657–669 CrossRef CAS PubMed.
- M. Heinlaan, K. Kasemets, V. Aruoja, I. Blinova, O. Bondarenko, A. Lukjanova, A. Khosrovyan, I. Kurvet, M. Pullerits and M. Sihtmäe, Sci. Total Environ., 2020, 707, 136073 CrossRef CAS PubMed.
- N. R. Brun, B. E. V. Koch, M. Varela, W. J. G. M. Peijnenburg, H. P. Spaink and M. G. Vijver, Environ. Sci.: Nano, 2018, 5, 904–916 RSC.
- N. R. Brun, P. van Hage, E. R. Hunting, A.-P. G. Haramis, S. C. Vink, M. G. Vijver, M. J. M. Schaaf and C. Tudorache, Commun. Biol., 2019, 2, 382 CrossRef PubMed.
- N. S. Yee, K. Lorent and M. Pack, Dev. Biol., 2005, 284, 84–101 CrossRef CAS PubMed.
- A. Poma, G. Vecchiotti, S. Colafarina, O. Zarivi, M. Aloisi, L. Arrizza, G. Chichiriccò and P. Di Carlo, Nanomaterials, 2019, 9, 1299 CrossRef CAS PubMed.
- S. Ballesteros, J. Domenech, I. Barguilla, C. Cortés, R. Marcos and A. Hernández, Environ. Sci.: Nano, 2020, 7, 3431–3446 RSC.
- V. Paget, S. Dekali, T. Kortulewski, R. Grall, C. Gamez, K. Blazy, O. Aguerre-Chariol, S. Chevillard, A. Braun and P. Rat, PLoS One, 2015, 10, e0123297 CrossRef PubMed.
- Y. Kim, J. Jeong, S. Lee, I. Choi and J. Choi, J. Hazard. Mater., 2020, 388, 121725 CrossRef CAS PubMed.
- B. Fadeel, A. Pietroiusti and A. A. Shvedova, Adverse effects of engineered nanomaterials: exposure, toxicology, and impact on human health, Academic Press, 2017 Search PubMed.
- D. Pandey, A. Singh, A. Ramanathan and M. Kumar, J. Environ. Manage., 2021, 279, 111557 CrossRef CAS PubMed.
- S. Gionfra, Plastic Pollution In Soil, Institue for European Environmental Policy, May 2018, https://ieep.eu/uploads/articles/attachments/3a12ecc3-7d09-4e41-b67c-b8350b5ae619/Plasticpollutioninsoil.pdf?v=63695425214, accessed February 15, 2021 Search PubMed.
- P. T. Pukclai, Microplastics in Agriculture: Challenges for Regulation, 11 August 2020, https://www.agribusinessglobal.com/plant-health/npk/microplastics-in-agriculture-challenges-for-regulation/#:%7E:text=Microplasticscaninteractwithsoil,sources%2Candalsotheecosystem, accessed February 15, 2021 Search PubMed.
- N. Weithmann, J. N. Möller, M. G. Löder, S. Piehl, C. Laforsch and R. Freitag, Sci. Adv., 2018, 4, eaap8060 CrossRef PubMed.
- Q.-Y. Cai, C.-H. Mo, Q.-T. Wu, A. Katsoyiannis and Q.-Y. Zeng, Sci. Total Environ., 2008, 389, 209–224 CrossRef CAS PubMed.
- P. Gimeno, S. Thomas, C. Bousquet, A. F. Maggio, C. Civade, C. Brenier and P. A. Bonnet, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 949–950, 99–108 CrossRef CAS PubMed.
- Y. Wang, H. Zhu and K. Kannan, Toxics, 2019, 7, 21 CrossRef CAS PubMed.
- Y. Li, G. Huang, H. Gu, Q. Huang, C. Lou, L. Zhang and H. Liu, Water, 2018, 10(8), 999 CrossRef.
- L. Niu, Y. Xu, C. Xu, L. Yun and W. Liu, Environ. Pollut., 2014, 195, 16–23 CrossRef CAS PubMed.
- X. Zhang, F. Li, T. Liu, C. Peng, D. Duan, C. Xu, S. Zhu and J. Shi, ISRN Soil Sci., 2013, 2013, 126391 Search PubMed.
- R. Jing, S. Fusi and B. V. Kjellerup, Front. Environ. Sci., 2018, 6, 79 CrossRef.
- J. B. Graceli, R. S. Dettogni, E. Merlo, O. Niño, C. S. da Costa, J. F. Zanol, E. A. Ríos Morris, L. Miranda-Alves and A. C. Denicol, Mol. Cell. Endocrinol., 2020, 518, 110997 CrossRef CAS PubMed.
- H. Ye, M. Ha, M. Yang, P. Yue, Z. Xie and C. Liu, Sci. Rep., 2017, 7, 40153 CrossRef CAS PubMed.
- N. Fiandanese, V. Borromeo, A. Berrini, B. Fischer, K. Schaedlich, J.-S. Schmidt, C. Secchi and P. Pocar, Reprod. Toxicol., 2016, 65, 123–132 CrossRef CAS PubMed.
- R. Hauser, P. Williams, L. Altshul and A. M. Calafat, Environ. Health Perspect., 2005, 113, 425–430 CrossRef CAS PubMed.
- C. Pereira, K. Mapuskar and C. V. Rao, Acta Histochem., 2007, 109, 29–36 CrossRef CAS PubMed.
- H. Miao, X. Liu, J. Li, L. Zhang, Y. Zhao, S. Liu, S. Ni and Y. Wu, Chemosphere, 2020, 241, 125093 CrossRef CAS PubMed.
- V. Marotta, G. Russo, C. Gambardella, M. Grasso, D. La Sala, M. G. Chiofalo, R. D'Anna, A. Puzziello, G. Docimo and S. Masone, Chemosphere, 2019, 218, 885–894 CrossRef CAS PubMed.
- C. Liu, Y.-L. Deng, T.-Z. Zheng, P. Yang, X.-Q. Jiang, E.-N. Liu, X.-P. Miao, L.-Q. Wang, M. Jiang and Q. Zeng, J. Hazard. Mater., 2020, 383, 121189 CrossRef CAS PubMed.
- S. Benjamin, E. Masai, N. Kamimura, K. Takahashi, R. C. Anderson and P. A. Faisal, J. Hazard. Mater., 2017, 340, 360–383 CrossRef CAS PubMed.
- V. Marotta, P. Malandrino, M. Russo, I. Panariello, F. Ionna, M. G. Chiofalo and L. Pezzullo, Crit. Rev. Oncol. Hematol., 2020, 150, 102950 CrossRef PubMed.
- S. Kim, G.-Y. Park, Y. J. Yoo, J. S. Jeong, K. T. Nam, S.-H. Jee, K.-M. Lim and Y.-S. Lee, Food Chem. Toxicol., 2019, 124, 265–272 CrossRef CAS PubMed.
- M. J. Kim, S. Moon, B.-C. Oh, D. Jung, K. Choi and Y. J. Park, Thyroid, 2019, 29, 183–192 CrossRef CAS PubMed.
- M. Morgan, A. Deoraj, Q. Felty and D. Roy, Mol. Cell. Endocrinol., 2017, 457, 89–102 CrossRef CAS PubMed.
- J. Zhang, X. Zhang, Y. Li, Z. Zhou, C. Wu, Z. Liu, L. Hao, S. Fan, F. Jiang and Y. Xie, Oncotarget, 2017, 8, 69874 CrossRef PubMed.
- USEPA, United States Environmental Protection Agency, Exposure Assessment Tools by Chemical Classes – Other Organics, October 23, 2020, https://www.epa.gov/expobox/exposure-assessment-tools-chemical-classes-other-organics#med-bpa, accessed April 03, 2021 Search PubMed.
- LCSP, The Lowell centre for sustainable production, University of Massachusetts Lowell – Technical Briefing: Phthalates and their alternatives – Health and Environmental Concerns, January 2011, https://www.sustainableproduction.org/downloads/PhthalateAlternatives-January2011.pdf, accessed April 03, 2021 Search PubMed.
- W. Tan, Y. Zhang, X. He, B. Xi, R. Gao, X. Mao, C. Huang, H. Zhang, D. Li, Q. Liang, D. Cui and A. N. Alshawabkeh, Sci. Rep., 2016, 6, 31987 CrossRef CAS PubMed.
- J. Xu, W. Qian, J. Li, X. Zhang, J. He and D. Kong, Environ. Geochem. Health, 2019, 41, 2315–2327 CrossRef CAS PubMed.
- K. Cai, Q. Song, W. Yuan, J. Ruan, H. Duan, Y. Li and J. Li, Environ. Pollut., 2020, 267, 115634 CrossRef CAS PubMed.
- P. Kurt-Karakus, H. Alegria, A. Birgul, E. Gungormus and L. Jantunen, Sci. Total Environ., 2018, 625, 555–565 CrossRef CAS PubMed.
- Y. Wang, Z. Li, F. Tan, Y. Xu, H. Zhao and J. Chen, Environ. Pollut., 2020, 265, 114850 CrossRef CAS PubMed.
- X. Ge, S. Ma, X. Zhang, Y. Yang, G. Li and Y. Yu, Environ. Int., 2020, 139, 105741 CrossRef CAS PubMed.
- J. He, J. Li, L. Ma, N. Wu, Y. Zhang and Z. Niu, Sci. Total Environ., 2019, 697, 133997 CrossRef CAS PubMed.
- NIHNational Institute of Health: 3-D images show flame retardants can mimic estrogens in NIH study, August 19, 2013, https://www.nih.gov/news-events/news-releases/3-d-images-show-flame-retardants-can-mimic-estrogens-nih-study, accessed April 10, 2021 Search PubMed.
- V. Matovic, A. Buha, M. Curcic, Z. Bulat and B. Antonijevic, Toxicol. Lett., 2017, 280, S168 CrossRef.
- H. Liu, W. Hu, H. Sun, O. Shen, X. Wang, M. H. W. Lam, J. P. Giesy, X. Zhang and H. Yu, Mar. Pollut. Bull., 2011, 63, 287–296 CrossRef CAS PubMed.
- X. Li, H. Gao, P. Li, W. Chen, S. Tang, L. Liu, G. Zhou, T. Xia, A. Wang and S. Zhang, Environ. Pollut., 2021, 268, 115773 CrossRef CAS PubMed.
- J. G. Allen, S. Gale, R. T. Zoeller, J. D. Spengler, L. Birnbaum and E. McNeely, Environ. Health, 2016, 15, 60 CrossRef PubMed.
- A. K. Rosenmai, S. B. Winge, M. Möller, J. Lundqvist, E. B. Wedebye, N. G. Nikolov, H. K. Lilith Johansson and A. M. Vinggaard, Chemosphere, 2021, 263, 127703 CrossRef CAS PubMed.
- Y. Zhang, G. L. Guo, X. Han, C. Zhu, B. A. Kilfoy, Y. Zhu, P. Boyle and T. Zheng, Biosci. Hypotheses, 2008, 1, 195–199 CrossRef CAS PubMed.
- S. Liu, G. Zhao, J. Li, H. Zhao, Y. Wang, J. Chen and H. Zhao, Environ. Res., 2017, 159, 1–8 CrossRef CAS PubMed.
- Y. B. Man, B. N. Lopez, H. S. Wang, A. O. W. Leung, K. L. Chow and M. H. Wong, J. Hazard. Mater., 2011, 195, 92–99 CrossRef CAS PubMed.
- S. Hurley, D. Goldberg, J.-S. Park, M. Petreas, L. Bernstein, H. Anton-Culver, S. L. Neuhausen, D. O. Nelson and P. Reynolds, Environ. Int., 2019, 127, 412–419 CrossRef CAS PubMed.
- A. M. Montalbano, G. D. Albano, G. Anzalone, M. Moscato, R. Gagliardo, C. Di Sano, A. Bonanno, S. Ruggieri, F. Cibella and M. Profita, Chemosphere, 2020, 245, 125600 CrossRef CAS PubMed.
- W. He, P. He, A. Wang, T. Xia, B. Xu and X. Chen, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2008, 649, 62–70 CrossRef CAS PubMed.
- H. J. Lee, G. B. Kim and R. F. Lee, Mar. Pollut. Bull., 2012, 64, 2892–2895 CrossRef CAS PubMed.
- I. R. Rajput, S. Yaqoob, S. Yajing, E. Sanganyado and L. Wenhua, Environ. Pollut., 2021, 271, 116131 CrossRef CAS PubMed.
- Y. Liu, Y. Li, S. Dong, L. Han, R. Guo, Y. Fu, S. Zhang and J. Chen, J. Hazard. Mater., 2021, 404, 124020 CrossRef CAS PubMed.
- R. Chen, R. Hou, X. Hong, S. Yan and J. Zha, Environ. Int., 2019, 130, 104914 CrossRef CAS PubMed.
- J. Ding, T. Deng, M. Xu, S. Wang and F. Yang, Environ. Pollut., 2018, 233, 986–991 CrossRef CAS PubMed.
- J. Li, L. Zhao, R. J. Letcher, Y. Zhang, K. Jian, J. Zhang and G. Su, Environ. Int., 2019, 127, 35–51 CrossRef CAS PubMed.
- G. Ren, X. Chu, J. Zhang, K. Zheng, X. Zhou, X. Zeng and Z. Yu, Environ. Pollut., 2019, 246, 374–380 CrossRef CAS PubMed.
- C. Parisi, M. Vigani and E. Rodríguez-Cerezo, Nano Today, 2015, 10, 124–127 CrossRef CAS.
- A. K. Yetisen, H. Qu, A. Manbachi, H. Butt, M. R. Dokmeci, J. P. Hinestroza, M. Skorobogatiy, A. Khademhosseini and S. H. Yun, ACS Nano, 2016, 10, 3042–3068 CrossRef CAS PubMed.
- S. Nafisi and H. I. Maibach, In: K. Sakamoto, H. Lochhead, H. Maibach,ed. Y. Yamashita, Cosmetic Science and Technology: Theoretical Principles and Applications, 2017, p. 337 Search PubMed.
- I. Khan, K. Saeed and I. Khan, Arab. J. Chem., 2019, 12, 908–931 CrossRef CAS.
- M. Simonin and A. Richaume, Environ. Sci. Pollut. Res., 2015, 22, 13710–13723 CrossRef CAS PubMed.
- S. Loureiro, P. S. Tourinho, G. Cornelis, N. W. Van Den Brink, M. Díez-Ortiz, S. Vázquez-Campos, V. Pomar-Portillo, C. Svendsen and C. A. M. Van Gestel, in Soil Pollution, ed. A. C. Duarte, A. Cachada and T. Rocha-Santos, Academic Press, 2018, pp. 161–190 Search PubMed.
- S. Suppan, Nanotechnology Risk to Soil Health: Overview – Nanotechnology and its application to agriculture and food, February 2014, Institute for Agriculture and Trade Policy. https://www.iatp.org/sites/default/files/2014_02_19_Biosolids_Nanomaterials_SS_0.pdf, accessed April 28, 2021 Search PubMed.
- Q. Huang, S. Zhou, L. Lin, Y. Huang, F. Li and Z. Song, Environ. Pollut., 2018, 239, 118–128 CrossRef CAS PubMed.
- Y. Zhu, F. Xu, Q. Liu, M. Chen, X. Liu, Y. Wang, Y. Sun and L. Zhang, Sci. Total Environ., 2019, 662, 414–421 CrossRef CAS PubMed.
- L. Alidokht, I. Anastopoulos, D. Ntarlagiannis, P. Soupios, B. Tawabini, D. Kalderis and A. Khataee, J. Environ. Chem. Eng., 2021, 105533 CrossRef CAS.
- M. Mazarji, T. Minkina, S. Sushkova, S. Mandzhieva, G. N. Bidhendi, A. Barakhov and A. Bhatnagar, J. Environ. Manage., 2021, 284, 112023 CrossRef CAS PubMed.
- EC, European Commission, Science for Environmental Policy – Nanoparticles' Ecological Risks: Effects on Soil Microorganisms, Issue 463, 15 July 2016, https://ec.europa.eu/environment/integration/research/newsalert/pdf/nanoparticles_ecological_risks_effects_on_soil_microorganisms_463na4_en.pdf, accessed April 26, 2021 Search PubMed.
- L. Lei, K. Qiao, Y. Guo, J. Han and B. Zhou, Chemosphere, 2020, 249, 126536 CrossRef CAS PubMed.
- B. Zhu, J. Han, L. Lei, J. Hua, Y. Zuo and B. Zhou, Ecotoxicol. Environ. Saf., 2021, 209, 111845 CrossRef CAS PubMed.
- S.-J. Chao, C. P. Huang, P.-C. Chen, S.-H. Chang and C. Huang, Chemosphere, 2018, 205, 570–578 CrossRef CAS PubMed.
- Ecomundo, Nanomaterials as Endocrine Disruptors, 3 December 2014, https://www.ecomundo.eu/en/blog/nanomaterials-endocrine-disruptors, accessed April, 26, 2021 Search PubMed.
- K. Culliney, Cosmetic ingredient safety: SCCS closes comment period on endocrine disruptor and nano toxicity prelim opinions, 07 January 2021, https://www.cosmeticsdesign-europe.com/Article/2021/01/07/Cosmetic-ingredient-safety-assessments-by-SCCS-being-finalised-for-nano-and-endocrine-disruptor-concerns, accessed April 26, 2021 Search PubMed.
- J. Zhao, L. Bowman, X. Zhang, X. Shi, B. Jiang, V. Castranova and M. Ding, J. Nanobiotechnol., 2009, 7, 1–13 CrossRef PubMed.
- P. Ruiz, A. Katsumiti, J. A. Nieto, J. Bori, A. Jimeno-Romero, P. Reip, I. Arostegui, A. Orbea and M. P. Cajaraville, Mar. Environ. Res., 2015, 111, 107–120 CrossRef CAS PubMed.
- C. P. Vignardi, F. M. Hasue, P. V. Sartório, C. M. Cardoso, A. S. D. Machado, M. J. Passos, T. C. A. Santos, J. M. Nucci, T. L. R. Hewer and I.-S. Watanabe, Aquat. Toxicol., 2015, 158, 218–229 CrossRef CAS PubMed.
- K. A. Delmond, T. Vicari, I. C. Guiloski, A. C. Dagostim, C. L. Voigt, H. C. S. de Assis, W. A. Ramsdorf and M. M. Cestari, Environ. Toxicol. Pharmacol., 2019, 67, 42–52 CrossRef CAS PubMed.
- L. F. Oya-Silva, T. Vicari, G. Rodrigo Disner, J. R. Lirola, T. Klingelfus, H. d. L. S. Gonçalves, T. P. B. Leite, S. L. d. M. Calado, C. L. Voigt, H. C. Silva de Assis and M. M. Cestari, Environ. Toxicol. Pharmacol., 2021, 82, 103551 CrossRef CAS PubMed.
- A. Katsumiti, P. Ruiz, J. Bori, J. A. Nieto, P. Reip, A. Orbea and M. P. Cajaraville, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2012, 163, S50 CrossRef.
- P. R. K. Reddy, D. Yasaswini, P. P. R. Reddy, M. Zeineldin, M. J. Adegbeye and I. Hyder, Vet. World, 2020, 13, 1685 CAS.
- E. K. Hill and J. Li, J. Anim. Sci., 2017, 8, 1–13 Search PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- W. S. Cho, B. C. Kang, J. K. Lee, J. Jeong, J. H. Che and S. H. Seok, Part. Fibre Toxicol., 2013, 10, 9 CrossRef CAS PubMed.
- A. G. Niculescu and A. M. Grumezescu, Nanomaterials, 2022, 12, 186 CrossRef CAS PubMed.
- P. G. Jamkhande, N. W. Ghule, A. H. Bamer and M. G. Kalaskar, J. Drug Deliv. Sci. Technol., 2019, 53, 101174 CrossRef CAS.
- A. M. Schrand, M. F. Rahman, S. M. Hussain, J. J. Schlager, D. A. Smith and A. F. Syed, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 54–568 CrossRef.
- L. E. Marbella and J. E. Millstone, Chem. Mater., 2015, 27, 2721–2739 CrossRef CAS.
- Z. Javed, K. Dashora, M. Mishra, V. D. Fasake and A. Srivastva, Front. Nanosci. Nanotechnol., 2019, 5, 1–9 Search PubMed.
- A. O. Fayiga and U. K. Saha, Annals Environ. Sci. Toxicol., 2017, 2, 059–067 Search PubMed.
- H. Pérez-Hernández, F. Fernández-Luqueño, E. Huerta-Lwanga, J. Mendoza-Vega and D. Álvarez-Solís José, Land Degrad. Dev., 2020, 31, 2213–2230 CrossRef.
- C. Bai and M. Tang, J. Appl. Toxicol., 2020, 40, 37–63 CrossRef CAS PubMed.
- D. Hu, M. Shen, Y. Zhang, H. Li and G. Zeng, Environ. Sci. Pollut. Res., 2019, 26, 19997–20002 CrossRef PubMed.
- J. Kleinekorte, L. Fleitmann, M. Bachmann, A. Kätelhön, A. Barbosa-Póvoa, N. von der Assen and A. Bardow, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 203–233 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |
