Open Access Article
Open Access ArticleSynthesis and characterisation of heteroatom-doped reduced graphene oxide/bismuth oxide nanocomposites and their application as photoanodes in DSSCs†
Nonjabulo P. D. Ngidi ,
Edigar Muchuweni‡
,
Edigar Muchuweni‡
 and
Vincent O. Nyamori
and
Vincent O. Nyamori *
*
School of Chemistry and Physics, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban, 4000, South Africa. E-mail: nyamori@ukzn.ac.za; nonjabulongidi@gmail.com; muchuwenie@ukzn.ac.za; Fax: +27-31-2603091; Tel: +27-31-2608256
First published on 18th January 2022
Abstract
Semiconductor materials have been recently employed in photovoltaic devices, particularly dye-sensitized solar cells (DSSCs), to solve numerous global issues, especially the current energy crisis emanating from the depletion and hazardous nature of conventional energy sources, such as fossil fuels and nuclear energy. However, progress for the past years has been mainly limited by poor electron injection and charge carrier recombination experienced by DSSCs at the photoanode. Thus, novel semiconductor materials such as bismuth oxide (Bi2O3) have been investigated as an alternative photoanode material. In this study, Bi2O3 was integrated with nitrogen- or boron-doped reduced graphene oxide (N-rGO or B-rGO, respectively) via a hydrothermal approach at a temperature of 200 °C. Various instrumental techniques were used to investigate the morphology, phase structure, thermal stability, and surface area of the resulting nanocomposites. The incorporation of N-rGO or B-rGO into Bi2O3 influenced the morphology and structure of the nanocomposite, thereby affecting the conductivity and electrochemical properties of the nanocomposite. B-rGO/Bi2O3 exhibited a relatively large surface area (65.5 m2 g−1), lower charge transfer resistance (108.4 Ω), higher charge carrier mobility (0.368 cm2 V−1 s−1), and higher electrical conductivity (6.31 S cm−1) than N-rGO/Bi2O3. This led to the fabrication of B-rGO/Bi2O3 photoanode-based DSSCs with superior photovoltaic performance, as revealed by their relatively high power conversion efficiency (PCE) of 2.97%, which outperformed the devices based on N-rGO/Bi2O3, rGO/Bi2O3, and Bi2O3 photoanodes. Therefore, these results demonstrate the promising potential of heteroatom-doped rGO/Bi2O3-based nanocomposites as photoanode materials of choice for future DSSCs.
1 Introduction
Recent developments in technology have led to a tremendous demand for energy. Thus, renewable energy sources, especially solar energy, which can be converted to electrical energy by photovoltaic devices, particularly dye-sensitized solar cells (DSSCs), have been favoured by many researchers.1–5 The main component of DSSCs is the photoanode, which is responsible for transferring photogenerated electrons from the excited dye molecules to the collecting electrode. Thus, a high electron-transport rate reduces the charge recombination rate and improves the power conversion efficiency (PCE). The development of DSSCs by means of tailoring the properties of semiconductor nanomaterials to suit photoanode applications has attracted a lot of attention in solar energy research. This is because semiconductor nanomaterials have unique physical, chemical, electronic and optical properties. Semiconductor photoanode nanomaterials with a high surface area can facilitate more dye absorption, effective photon harvesting, and fast charge transport, thereby improving the PCE of DSSCs. Various approaches, such as doping,6 surface modification,7 and the use of hybrid semiconducting nanomaterials,8–12 have been proposed to improve the properties of semiconductor materials. Semiconductor nanomaterials such as metal oxides, including titanium dioxide (TiO2),13 zinc oxide (ZnO),14 zirconium dioxide (ZrO2),15 tungsten trioxide (WO3),16 and tin(IV) oxide (SnO2),17 have been extensively explored as photoanode materials in DSSCs.To date, there are few studies based on the application of bismuth oxide (Bi2O3) as a photoanode in DSSCs, yet Bi2O3 has attracted great interest in photocatalysis18 and supercapacitors19 due to its unique crystal structure,20 non-toxicity,21 and wide bandgap, ranging from 2.47 to 3.55 eV.22 Bi2O3 also exhibits a high dielectric permittivity, refractive index, photoconductivity, and oxygen ion conductivity. There are four polymorphs of Bi2O3, namely, body-centred cubic (γ), face-centred cubic (δ), tetragonal (β) and monoclinic (α).23 Among these, α-Bi2O3 and δ-Bi2O3 are thermodynamically stable at ambient conditions, whereas β-Bi2O3 and γ-Bi2O3 are metastable, and are stabilised by the addition of impurities or by controlling the reaction conditions.24 An investigation on the effect of β-Bi2O3 as a photoanode in DSSCs has been reported by Shaikh et al.25 and resulted in a PCE of 0.078%. However, a slight improvement in the PCE to 1.5% was observed when β-Bi2O3 was incorporated with ZnO. Fatima et al.26 reported a PCE of 0.05% for DSSCs with α-Bi2O3-based photoanodes. The low PCE was attributed to the presence of trapping states at the interfaces within the photoanode. Thus, more research on Bi2O3 nanomaterials for photoanode applications in DSSCs is still required.
Recently, metal oxides have been integrated with an allotrope of carbon, especially graphene, and used as positive electrode material in energy storage devices.27,28 Graphene has attracted significant interest due to its high charge carrier mobility, large specific surface area, high mechanical strength, high electrical conductivity, and chemical resilience. Also, graphene is reported to display predominant energy storage performance compared with other carbon nanostructures, such as carbon nanotubes and graphite.29,30 Graphene oxide (GO) is defined as a cutting edge of graphene functionalised with carbonyl and carboxyl groups at the edges; and epoxy and hydroxyl groups in the basal plane.31 The oxygen-based functional groups on the surface of GO are responsible for integrating GO with metal oxides, resulting in GO-based metal oxide nanocomposites.
The main focus in the reported studies of Bi2O3 was to integrate Bi2O3 with reduced graphene oxide (rGO) due to the distinctive mechanical, electrical, and electrochemical properties of rGO.32–34 This is envisaged to help overcome the drawbacks of pristine rGO, such as its usual tendency to agglomerate or restack to form graphite due to π–π stacking and van der Waals forces when the rGO nanosheets are close to each other,35 resulting in poor electrochemical properties. Nitrogen-doped reduced graphene oxide (N-rGO) and boron-doped reduced graphene oxide (B-rGO) have been reported to have improved electrochemical properties than rGO;36 thus, integrating Bi2O3 with N-rGO or B-rGO is expected to yield favourable device performance. However, to the best of our knowledge, no detailed studies have reported the electrochemical properties of heteroatom-doped rGO (N-rGO or B-rGO) integrated with Bi2O3 and their application as photoanodes in DSSCs. Hence, in this study, the effect of nitrogen or boron on the physicochemical and electrochemical properties of heteroatom-doped rGO/Bi2O3 nanocomposites was investigated for the first time by means of various instrumental techniques, so as to enhance the electrochemical properties of Bi2O3, reduce the aggregation of rGO, and subsequently improve the performance of DSSCs.
2 Experimental
2.1 Materials
Bismuth oxide (Bi2O3, 99.99%), 4-nitro-o-phenylenediamine (≥99%), boric anhydride (≥98%), boron standard solution (999.5 mg L−1 ± 20 mg L−1), bismuth standard for ICP traceCert® (1000 mg L−1 Bi in nitric acid), potassium hydroxide (KOH, ≥85%, pellets), Nafion (≤100%), lithium iodide (99.9%), 4-tert-butylpyridine (98%), guanidinium thiocyanate (99%), 1-methyl-3-propylimidazolium iodide (99.99%), acetonitrile (≥99.9%), poly(vinyl acetate) (99.9%), absolute ethanol (99.5%), eosin B (97%) and indium tin oxide (ITO) coated glass slides (15 Ω, 30 × 30 × 0.7 mm) were purchased from Sigma-Aldrich, South Africa.2.2 Synthesis of the nanocomposite
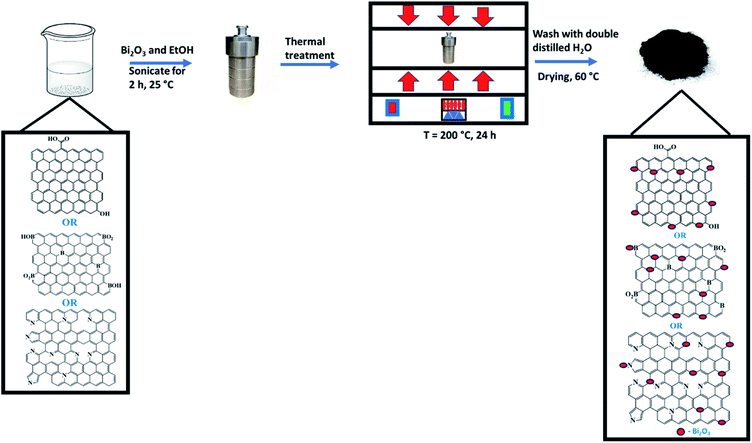
rGO was synthesised via thermal annealing of GO at a temperature of 600 °C under 10% H2 in argon,37 while N-rGO or B-rGO was synthesised by thermal treatment of GO and a precursor of 4-nitro-o-phenylenediamine or boric anhydride, respectively, at a temperature of 600 °C.38,39 Bi2O3 (0.2 g) and rGO, N-rGO, or B-rGO (0.5 g) were dispersed in absolute ethanol (60 mL) and sonicated for 2 h (Scheme 1). After sonication, the mixtures were transferred into a Teflon-lined stainless-steel autoclave and thermally treated at a temperature of 200 °C for 24 h. The nanocomposites were purified by washing them with double-distilled water, followed by drying the samples in an oven for 24 h at 60 °C before characterisation.2.3 Device fabrication
The Bi2O3-based nanocomposite (100 mg) was dispersed in absolute ethanol (0.3 mL), sonicated, and deposited onto an ITO glass of the photoanode by using the Doctor Blade method. The photoanode was then annealed at 300 °C for 30 min. After thermal annealing, 0.3 mM eosin B dye (150 μL) was loaded onto the photoanode. The iodide/triiodide gel state electrolyte was stained onto the dye-coated photoanode, followed by assembling the aluminium-coated cathode in a sandwich-like fashion. The iodide/triiodide gel state electrolyte was prepared by firstly synthesizing the liquid electrolyte. The liquid electrolyte was synthesised by mixing lithium iodide (0.3348 g), 4-tert-butylpyridine (1.6900 g), guanidinium thiocyanate (0.2954 g) and 1-methyl-3-propylimidazolium iodide (0.3173 g) and dissolved in acetonitrile (5 mL). The synthesised liquid electrolyte (1.2484) was mixed with poly(vinyl acetate) (0.6022 g) and stirred using a glass rod until a gel state electrolyte was formed, and further kept in a refrigerator at 0 °C.2.4 Characterisation
Field emission-scanning electron microscopy (FE-SEM, Carl Zeiss Ultra Plus) was used to investigate the surface morphology of the nanocomposites. Transmission electron microscopy (TEM, JEOL JEM, 1010 model) was used to investigate the microstructure of the nanocomposites. The presence of various elements in the nanocomposites was investigated by elemental analysis (Elementar vario EL cube CHNSO elemental analyser), and Bi2O3 was quantified via inductively coupled plasma-optical emission spectrometry (ICP-OES, PerkinElmer Optima 5300 DV). The presence of various functional groups was determined by Fourier transform infrared spectroscopy (PerkinElmer Spectrum 100, FTIR spectrophotometer, with an attenuated total reflectance (ATR) accessory).The phase compositions present in the nanocomposites were evaluated by means of powder X-ray diffraction (XRD, Bruker AXS, Cu Kα radiation source, λ = 0.154 nm). The graphitic nature of the nanocomposites was investigated by a Renishaw inVia Raman microscope at an excitation wavelength of 488 nm. The thermal stability was analysed with a TA Instruments Q series™ thermal analyser DSC/TGA (Q600) instrument. The textural characteristics were determined using a Micromeritics Tristar II 3020 surface area and porosity analyser. The electrical conductivity of the nanocomposites was investigated by a four-point probe (Keithley 2400 source-meter). Hall effect measurements were carried out using an Ecopia Hall effect measurements system, model HMS 3000, equipped with HMS3000 VER 3.15.5 software.
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out on a VersaSTAT3F potentiostat/galvanostat electrochemical workstation, with ZSimpWin software utilised for EIS data analysis. A counter electrode (platinum wire (Pt)), a reference electrode (Ag/AgCl), and a working electrode (mixture of nanocomposite) were used. A mixture of the nanocomposite and a binder (Nafion) were dispersed in absolute ethanol and cast onto the electrode. The potassium hydroxide redox couple was used as the electrolyte. CV was carried out at scan rates of 5, 25, 50, 75, and 100 mV s−1 in the potential range (−1.0 to 1.0 V). The photovoltaic measurements of the fabricated DSSCs with a photoanode active area of 0.96 cm2 were performed with an Oriel Instruments LCS-100 solar simulator accompanied by a Keithley 2420 source meter under one sun illumination (AM 1.5 G, 100 mW cm−2).
3 Results and discussion
The subsequent sections elucidate the physicochemical, electrical conductivity, electrochemical properties, and photovoltaic performance of the nanocomposites.3.1 Elemental analysis
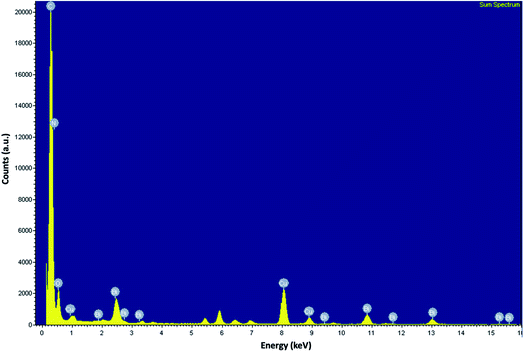
The incorporation of Bi2O3 into rGO or heteroatom-doped rGO is evident from the presence of bismuth peaks in the EDX spectrum (Fig. 1). ESI† – Fig. S1(a–f) also shows the EDX elemental mapping of the N-rGO/Bi2O3 nanocomposite, which confirms that all the constituent elements (carbon, oxygen, nitrogen, and bismuth) were distributed homogeneously in the N-rGO/Bi2O3 nanocomposite. This was also observed in rGO/Bi2O3 and B-rGO/Bi2O3 nanocomposites. The presence of carbon, nitrogen, oxygen, boron, and bismuth in nanocomposites was further confirmed by elemental analysis and ICP-OES (ESI† – Table S1). The Bi2O3 content in rGO/Bi2O3, N-rGO/Bi2O3, and B-rGO/Bi2O3 was found to be 20.8, 28.5, and 25.0%, respectively. N-rGO/Bi2O3 had a nitrogen content of 3.76%, while B-rGO/Bi2O3 had a boron content of 2.56%. ATR-FTIR was further used to investigate the various functional groups present in the nanocomposites.3.2 Surface functional groups
The ATR-FTIR spectrum of Bi2O3 is shown in Fig. 2 (a), where a peak at 850 cm−1 is attributed to the symmetrical stretching of Bi–O–Bi, while a peak at 1401 cm−1 is due to the presence of the metal–oxygen vibration (Bi–O).40 The heteroatom-doped rGO-Bi2O3-based nanocomposites exhibited peaks at around 800–850 cm−1 and 1402 cm−1, associated with the Bi–O–Bi and Bi–O vibrations, respectively. The spectra exhibited a broader peak at around 3000–3400 cm−1 due to the Bi–OH bonds and hydrogen-bonded molecular water species. A weak peak observed at 1665 cm−1 and 1250 cm−1 was assigned to N–H bending and C–N stretching in N-rGO/Bi2O3, respectively. The presence of boron in B-rGO/Bi2O3 was indicated by a peak at around 1180 cm−1, which is due to the B–C stretching vibration. Quantitative analysis and ATR-FTIR spectroscopy confirmed the incorporation of Bi2O3 into heteroatom-doped rGO. Thus, to further study the structures of the nanocomposites, TEM and FE-SEM analyses were conducted.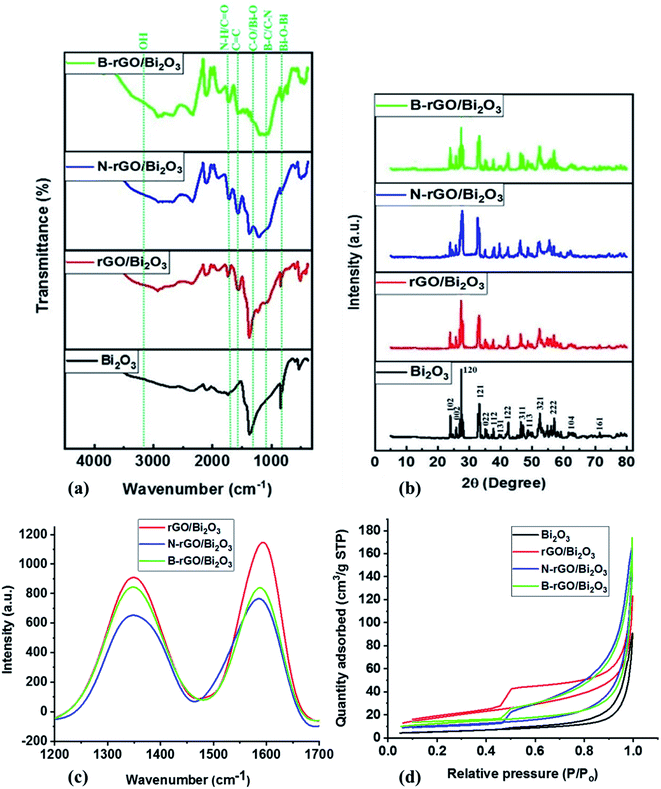 | ||
| Fig. 2 (a) ATR-FTIR spectra, (b) powder X-ray diffractograms, (c) Raman spectra and (d) N2 adsorption–desorption isotherms of the Bi2O3-based nanocomposites. | ||
3.3 Microstructure and surface morphology
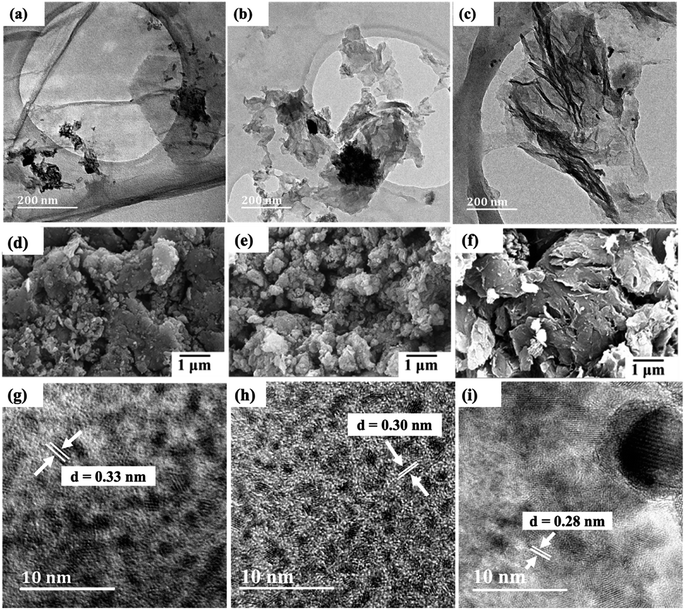
The TEM images in Fig. 3(a)–(c) show wrinkled graphene sheets with Bi2O3 that is uniformly dispersed, indicating the existence of a strong interaction between rGO, N-rGO or B-rGO, and Bi2O3. Moreover, the interface between Bi2O3 and rGO, N-rGO, or B-rGO shows that the Bi2O3 was well anchored on the sheets. This confirms that Bi2O3 was successfully incorporated into heteroatom-doped rGO as indicated in the EDX spectrum (Fig. 1). Fig. 3(d) and (e) show SEM images, where agglomeration of Bi2O3 is observed in the graphene sheets. N-rGO/Bi2O3 exhibited a high degree of agglomeration due to the high Bi2O3 content.Consistent with SEM and TEM results, the HRTEM analysis also indicated the successful formation of Bi2O3-based nanocomposites. Fig. 3(g) to (i) revealed a decrease in interlayer spacing of the Bi2O3-based nanocomposites compared with rGO, N-rGO, or B-rGO (ESI† – Fig. S2) due to the distortion introduced by the inclusion of Bi2O3 and the reduction of oxygen functional groups, such as carboxyl, epoxy and hydroxyl groups.
3.4 Phase composition and defects on the graphitic structure
Fig. 2(b) shows the diffractograms of pure Bi2O3 and the Bi2O3-based nanocomposites. The P-XRD pattern of pure Bi2O3 shows five intense peaks at 2θ values of 24.7°, 26.8°, 33.5°, 42.0°, and 52.5°, which correspond to the (102), (120), (121), (122), and (321) planes, respectively, confirming the formation of an α phase. These characteristic peaks are confirmed by the reported standard JCPDS file number 76-1730, which is associated with the monoclinic phase with space group P21/c.41 When comparing the diffractograms of Bi2O3 and heteroatom-doped rGO-Bi2O3-based nanocomposites, the diffraction peak representing the carbon species expected at 25° was not observed in all nanocomposites because the diffraction peak was obscured by the stronger diffraction peak of α-Bi2O3, and due to the low content of rGO, which was below the detection limit.34,42The sharp diffraction peak at 26.8° indicates that Bi2O3 is in a crystalline state, but this peak was reduced after integrating heteroatom-doped rGO with Bi2O3. Bi2O3 exhibited a relatively high 2θ peak intensity due to the presence of larger nanoparticles with few grain boundaries and low defect density. Theoretically, larger particles have sharp diffraction peaks, while the diffraction peak width increases as the particle sizes are reduced.43 The most intense diffraction peak (120) was fitted with a Lorentzian distribution function to determine the full-width at half-maximum (FWHM), and the value obtained was employed to calculate the crystallite size of the nanocomposites with Scherrer's formula. The crystallite sizes of Bi2O3, rGO/Bi2O3, N-rGO/Bi2O3, and B-rGO/Bi2O3 were 29.2, 20.9, 19.5, and 16.8 nm, respectively. The boron content in B-rGO/Bi2O3 caused an increase in structural strain, thus leading to enhanced surface defects. An interlayer spacing of 0.33 nm was obtained for Bi2O3, which was comparable to previously reported values.44,45 The decrease in interlayer spacing after integrating Bi2O3 with heteroatom-doped rGO was caused by lattice distortions, attributed to the incorporation of boron or nitrogen and the decrease in oxygen content during the reduction process.
The crystallite size and graphitic nature of the nanocomposites were further investigated by Raman spectroscopy. The crystallite size (La) was calculated using eqn (1) reported by Mallet-Ladeira et al.:46
| HWHM = 71 − 5.2La | (1) |
| Sample | P-XRD | Raman | |||
|---|---|---|---|---|---|
| 2θ/degree | Crystallite size/nm | Interlayer spacing/nm | ID/IG | Crystallite size/nm | |
| Bi2O3 | 26.8 | 29.2 | 0.33 | — | 34.4 |
| rGO/Bi2O3 | 27.1 | 20.9 | 0.32 | 1.50 | 29.6 |
| N-rGO/Bi2O3 | 27.9 | 19.5 | 0.30 | 1.79 | 28.1 |
| B-rGO/Bi2O3 | 28.0 | 16.8 | 0.30 | 1.82 | 27.3 |
The graphitic nature of rGO, heteroatom-doped rGO, and Bi2O3-based nanocomposites was further investigated by Raman spectroscopy. The Raman spectrum of rGO (ESI† – Fig. S3 and Table S2) reveals a D-band at 1350 cm−1 and a G-band at 1594 cm−1, whereby the peak area ratio (ID/IG) shows the disorder and the GO structure's graphitic symmetry, respectively. After nitrogen- or boron-doping, the G-bands revealed a minimal shift in the wavenumber. This shift indicated that incorporating nitrogen or boron atoms into the GO lattice prompted an increase in the disordered structure relative to rGO. According to Nanda et al.,47 the shift in the G-band of graphene represents the vibrational mode of sp2-hybridized carbon atoms. Thus, the change in the G-band position suggests the change in the number of layers in graphene. The G-band shift towards a higher wavenumber indicates a decrease in the number of graphene layers.
Fig. 2(c) shows that the intensity of the G-band of rGO/Bi2O3 is highest, corresponding to the sp2 structure of the graphite sheet.48 The low intensities of the D- and G-band of N-rGO/Bi2O3 suggest a strong interaction between N-rGO and Bi2O3. This has also been evidenced by the presence of the highest Bi2O3 content in N-rGO/Bi2O3 (ESI† – Table S1) compared with rGO/Bi2O3 and B-rGO/Bi2O3. Thus, Raman analysis demonstrated the chemical bonding between Bi2O3 and rGO, N-rGO, or B-rGO sheets, in agreement with elemental analysis.
The ID/IG ratio of rGO was lower (0.71 – ESI† (Table S2)) due to the reduction of the oxygen functionalities and the recovery of sp2-hybridized C–C bonds. However, the ID/IG ratios of N-rGO and B-rGO were higher than for rGO due to structural defects introduced by nitrogen or boron atoms implanted at the radicalized graphene sites. B-rGO displayed an ID/IG ratio of 1.18, which was higher than that of N-rGO (1.09), revealing the presence of a higher degree of disorder in B-rGO than N-rGO. Bi2O3-based nanocomposites showed higher ID/IG ratios than rGO, N-rGO and B-rGO, demonstrating that these nanocomposites possess more defects, with B-rGO/Bi2O3 exhibiting the highest ID/IG ratio (1.82).
3.5 Surface area and porosity
The specific surface area and pore size of the Bi2O3-based nanocomposites were investigated by the nitrogen adsorption/desorption method (Table 2). The B-rGO/Bi2O3 nanocomposite was found to have the largest specific surface area of 65.5 m2 g−1. The relatively low surface area of 53.7 m2 g−1 for rGO/Bi2O3 was attributed to the blockage of the rGO lattice by the Bi2O3 particles. The larger surface area of nanomaterials is beneficial for enhancing dye loading in the photoanode of DSSCs. Therefore, nanomaterials with a small surface area result in low dye loading, thereby reducing the short-circuit current density (Jsc) value and the PCE of DSSCs.49 Thus, to enhance the PCE of DSSCs, the photoanode nanomaterials ought to have a larger surface area. All the nanocomposites exhibited pore sizes below 23 nm, which belong to mesoporous material. Such mesoporous materials with pore sizes between 2 and 50 nm have been widely reported to improve the infiltration of materials in electrolytes.50,51| Sample | Surface area/m2 g−1 | Pore size/nm |
|---|---|---|
| Bi2O3 | 4.6 | 33.52 |
| rGO/Bi2O3 | 53.7 | 20.37 |
| N-rGO/Bi2O3 | 58.2 | 22.15 |
| B-rGO/Bi2O3 | 65.5 | 17.80 |
Fig. 2(d) exhibited type IV adsorption–desorption isotherms with an H3 hysteresis loop in the range of 0.45 − 1.0 P/P0. The H3 hysteresis loop indicates the presence of narrower pores, channel-like pores, and a pore network-linking effect.52 This again suggests that the synthesised nanocomposites are mesoporous materials. The relative pressure tends to increase (P/P0 > 0.85), and the shape of the adsorption isotherm rises, indicating an increase in the amount of N2-adsorption and the presence of capillary condensation in the mesoporous material. This is attributed to the rGO nanomaterial having a larger pore size and being capable of adsorbing a large amount of N2.
3.6 Thermal stability
The TGA analysis in Fig. 4(a) shows the thermal stability studies represented as TGA thermograms, and ESI† (Table S3) shows the decomposition temperatures and residual content of the nanocomposites. All the nanocomposites exhibited few weight losses around the decomposition temperature of 100 °C, which was attributed to the removal of moisture adsorbed in the interlayers of the nanocomposites. Furthermore, the weight loss that occurred at 200–400 °C is due to the loss of amorphous carbon and other functional groups with the release of COx species. Such decrease in weight in the 200–400 °C range correlates with TEM and SEM analysis (Fig. 3), inferring the presence of amorphous materials. The thermograms indicated that the order of the nanocomposites' tolerance to heat is B-rGO/Bi2O3 < N-rGO/Bi2O3 < rGO/Bi2O3, i.e., with decomposition temperatures of 437, 490, and 518 °C, respectively. The thermal stability trends agree with the graphitic nature (lower crystallinity) (Raman analysis – Table 1) of the nanocomposites. B-rGO/Bi2O3, with the highest fraction of layered graphene, exhibited the lowest thermal stability of 437 °C and the lowest residual content of Bi2O3 of 38%. The highest amount of residue (57%) for N-rGO/Bi2O3 could be attributed to some remnant Bi2O3 that remains entrapped in the nanocomposite. The elemental analysis also indicates a high Bi2O3 content in N-rGO/Bi2O3 (ESI† – Table S1).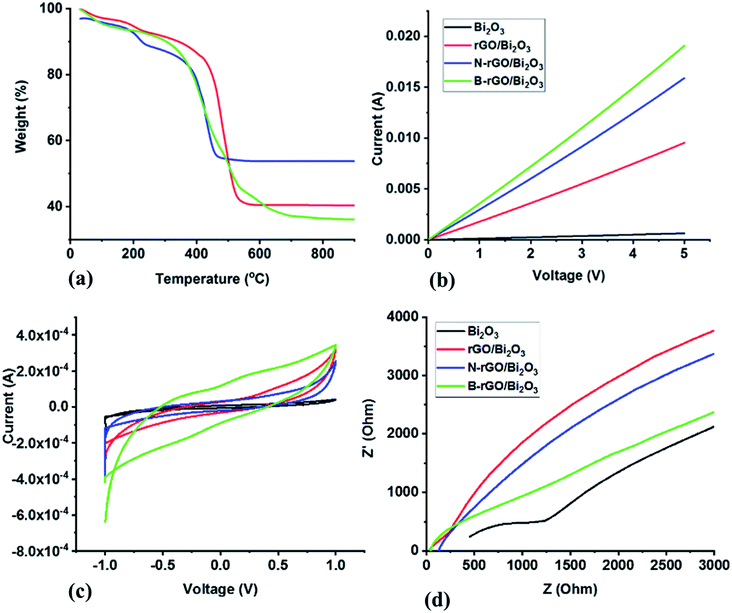 | ||
| Fig. 4 (a) Thermal stability studies represented as TGA thermograms, (b) current–voltage characteristics, (c) cyclic voltammograms and (d) Nyquist plots of the Bi2O3-based nanocomposites. | ||
3.7 Electrical properties
Four-probe and Hall measurements were used to investigate the electrical conductivity and charge carrier mobility, respectively. Hall effect studies showed that Bi2O3, rGO/Bi2O3, and N-rGO/Bi2O3 have n-type charge carriers, while B-rGO/Bi2O3 has p-type charge carriers. Such n- and p-type charge carrier characteristics are due to the change in the electronic structure of the nanocomposite. This suggests that N-rGO-Bi2O3 is a good material for electron transport, while B-rGO/Bi2O3 is a good hole transporter. The I–V characteristics (Fig. 4(b) and Table 3) revealed that the maximum conductivity (6.31 S cm−1) was obtained from B-rGO/Bi2O3 with an improved charge carrier mobility (0.368 cm2 V−1 s−1). The improvement in charge carrier mobility is attributed to the decrease in the scattering probability of the charge carriers. The size of boron ions is smaller than that of bismuth ions (the ionic radius of B3+ is 0.23 Å and Bi3+ is 1.17 Å). Thus, there is less scattering of electrons, thereby increasing the charge carrier mobility. B-rGO/Bi2O3 had significantly improved electrical conductivity, and this is attributed to its relatively large surface area and high charge carrier mobility.| Sample | Conductivity/S cm−1 | Carrier mobility/cm2 V−1 s−1 |
|---|---|---|
| Bi2O3 | 2.10 × 10−2 | 0.080 |
| rGO/Bi2O3 | 3.09 | 0.267 |
| N-rGO/Bi2O3 | 3.84 | 0.295 |
| B-rGO/Bi2O3 | 6.31 | 0.368 |
3.8 Electrochemical properties
The presence of oxygen-functional groups in Bi2O3 decreases its electrical conductivity and deteriorates in the aqueous electrolyte. During the integration of Bi2O3 with rGO or heteroatom-doped rGO, the oxygen-functional groups were reduced during thermal treatment and the high-pressure environment of the hydrothermal system. Therefore, reducing oxygen-functional groups increased the degree of wetting between the electrode and the electrolyte, thus resulting in an enhanced electrical conductivity, consequently improving the capacitance. A similar observation has been reported by Yang et al.,54 who reported an increase in the capacitance of highly orientated Bi2O3/rGO nanocomposites. The electrochemical activity and electrochemical reversibility are also enhanced due to the introduction of defects and heteroatom-containing groups at the electrode surface, which accelerates the charge transfer rate across the electrode. This also indicates that Bi2O3-based nanocomposites have ideal capacitor characteristics. The reversible charge–discharge characteristics in Bi2O3-based nanocomposites can probably be attributed to the electrical conductivity of the randomly distributed β-Bi2O3 that is among the α- and δ-phases.55 Thus, the nanocomposites were further investigated with EIS.
| Sample | Rct/Ω |
|---|---|
| Bi2O3 | 201.1 |
| rGO/Bi2O3 | 169.8 |
| N-rGO/Bi2O3 | 125.7 |
| B-rGO/Bi2O3 | 108.4 |
The series resistance is different for different electrodes used in Fig. 4(d) due to the presence of various oxygen-functional groups in the Bi2O3-based nanocomposites. This could also be attributed to the constriction phenomenon of the thick porous film, which had a film thickness of 2 μm that might not be thick enough for electrodes to allow better current collection. Similar observations have been previously reported by Tezyk et al.59
The Rct values (Table 4) were calculated from the EIS data. The Rct values of the Bi2O3-based nanocomposites were lower than that of Bi2O3 due to the free charge transfer in the nanocomposites. The relatively small Rct values for the nanocomposites indicate that the modification of Bi2O3 with heteroatom-doped rGO drastically reduces the electrode–electrolyte interfacial resistance. The introduction of heteroatom-doped rGO into Bi2O3 lowered the Rct value, which indicates the efficient separation of photogenerated electron–hole pairs and faster interfacial charge transfer. Therefore, heteroatom-doped rGO acts as the conducting channels inside the Bi2O3 matrix. These results affirm that the effective incorporation of heteroatom-doped rGO sheets improves the electron transport and electrical conductivity of the nanocomposites, resulting in a significant enhancement in electrochemical performance.
3.9 Photovoltaic performance of the fabricated DSSCs
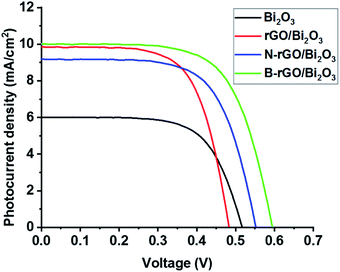
DSSCs were fabricated using pure Bi2O3, rGO/Bi2O3, N-rGO/Bi2O3, and B-rGO/Bi2O3 as photoanodes; and the open-circuit voltage (Voc), Jsc, fill factor (FF) and PCE were determined. The photovoltaic properties of DSSCs are listed in Table 5, and the J–V curves are shown in Fig. 5. Bi2O3-based DSSCs exhibited a higher Voc than rGO/Bi2O3-based DSSCs due to back electron transfer (from Bi2O3 to the redox couple in the electrolyte), which is associated with electrons located in a shallow conduction band edge, thus resulting in a short lifetime. The low PCE in the Bi2O3-based DSSC is due to poor dye absorption and back recombination at the photoanode–electrolyte interface. A similar observation was reported by Fatima et al.,26 who obtained a PCE of 0.05% for the Bi2O3-based DSSC. Poor dye absorption causes a decrease in light absorption, which reduces the photogeneration of charge carriers, and hence, lowers the current density, resulting in poor device performance. Thus, optimisation by surface modification of Bi2O3 has been used to increase dye absorption and reduce back recombination, thereby improving the performance of DSSCs.| Photoanode | Voc/V | Jsc/mA cm−2 | FF/% | PCE |
|---|---|---|---|---|
| Bi2O3 | 0.53 | 6.0 | 20.63 | 0.42 ± 0.04 |
| rGO/Bi2O3 | 0.48 | 9.8 | 43.9 | 1.68 ± 0.01 |
| N-rGO/Bi2O3 | 0.55 | 9.2 | 47.4 | 1.97 ± 0.02 |
| B-rGO/Bi2O3 | 0.59 | 10.0 | 50.2 | 2.79 ± 0.01 |
When Bi2O3-based nanocomposites were used as a photoanode, the DSSCs exhibited a higher PCE than the Bi2O3-based DSSCs. From the EIS analysis and electrical properties of Bi2O3-based nanocomposites, it can be suggested that the improvement in PCE is attributed to the low charge transfer resistance and high electrical conductivity of these nanocomposites. When carbon atoms in the graphene lattice are substituted by nitrogen or boron atoms, the conductivity of N-rGO/Bi2O3 or B-rGO/Bi2O3 increases remarkably, and the excited electrons are transferred faster in the delocalized π structure of N-rGO/Bi2O3-based photoanodes or B-rGO/Bi2O3-based photoanodes than rGO/Bi2O3-based photoanodes.
B-rGO/Bi2O3-based DSSCs exhibited the highest PCE due to enhanced transportation and collection of photogenerated charge carriers. The high PCE is attributed to the hole (h+) leaving the valence band of B-rGO and quickly moving to the valence band of Bi2O3, which facilitates electron and hole (e−/h+) separation. B-rGO has been reported to have a lower bandgap;60 thus, the lower bandgap of B-rGO is responsible for providing a photovoltaic effect in Bi2O3 with a high bandgap,61 enhancing charge separation and extending the energy of photoexcitation in DSSCs. Moreover, the electron mobility rate in B-rGO/Bi2O3 is higher than in rGO/Bi2O3 and N-rGO/Bi2O3, enabling the efficient transportation of electrons and reducing charge recombination. Less charge recombination means more electron density and a shift of the Fermi level, resulting in an increased Voc. This is consistent with EIS data, in which the B-rGO/Bi2O3 nanocomposite exhibited the lowest Rct value. This results in fast electron collection and low charge carrier recombination, leading to the fabrication of a B-rGO/Bi2O3-based DSSC with the highest PCE of 2.79%.
The introduction of rGO or heteroatom-doped rGO into Bi2O3 did not only improve the PCE, but also enhanced the Jsc and Voc values. In the case of the rGO/Bi2O3-based DSSC and N-rGO/Bi2O3-based DSSC, low Voc values of 0.48 and 0.55 V, respectively (Table 5 and Fig. 5), were attributed to faster dye regeneration, which reduces the lifetime of the oxidised dye molecule to suppress charge recombination, thus reducing the Voc. The presence of a low Bi2O3 content in rGO/Bi2O3 increased the Jsc but lowered the Voc, which substantially reduced the PCE of DSSCs. Although N-rGO/Bi2O3 showed a higher electrical conductivity and charge carrier mobility than rGO/Bi2O3, its performance in DSSCs as a photoanode resulted in a low Jsc due to charge recombination, which is attributed to various bonding configurations of nitrogen (pyrrolic-N, pyridinic-N, and graphitic-N) present on the GO surface.
The Jsc is simultaneously affected by electron transfer efficiency, light scattering, and dye absorption. Thus, the improvement of Jsc is due to the increase in the absorption of dye molecules in DSSCs. The large surface area of the nanocomposite is beneficial for enhancing dye loading in the photoanode of DSSCs. Therefore, the dye molecules are capable of harvesting more light energy for the effective photogeneration of charge carriers. B-rGO/Bi2O3 had the largest surface area for efficient dye-loading, which increased the Jsc and promoted a higher PCE in DSSCs.
4 Conclusion
In summary, heteroatom-doped rGO/Bi2O3-based nanocomposites were successfully synthesised and applied as photoanodes in DSSCs. P-XRD analysis showed that pristine Bi2O3 is in the monoclinic (α) form, and this polymorph was maintained even after introducing heteroatom-doped rGO. The heteroatom-doped rGO/Bi2O3-based nanocomposites exhibited better catalytic activity than pure Bi2O3. Favourable properties, such as large surface area, small pore size, low charge transfer resistance, high charge carrier mobility, and high electrical conductivity, were observed in the heteroatom-doped rGO/Bi2O3-based nanocomposites. This demonstrated the suitability of the prepared Bi2O3-based nanocomposites as potential photoanode materials for DSSCs. The highest PCE of 2.79% was achieved for the B-rGO/Bi2O3-based DSSC, which was attributed to the enhanced channels for electron transfer and electrolyte diffusion. Therefore, the synthesised heteroatom-doped rGO/Bi2O3-based nanocomposites are ideal materials for the photoanode in DSSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the National Research Foundation (NRF, Grant numbers – 101357, 116505 and 103979), Moses Kotane Institute, Eskom Tertiary Education Support Programme (TESP), UKZN Nanotechnology Platform, and the University of KwaZulu-Natal for financial support and facilities.References
- G. Boschloo, Front. Chem., 2019, 7, 1–9 CrossRef PubMed.
- Q. Huaulmé, V. M. Mwalukuku, D. Joly, J. Liotier, Y. Kervella, P. Maldivi, S. Narbey, F. Oswald, A. J. Riquelme, J. A. Anta and R. Demadrille, Nat. Energy, 2020, 5, 468–477 CrossRef.
- G. George, R. S. Yendaluru and A. Mary Ealias, Energy Sources, Part A, 2020, 1, 1–15 CrossRef.
- J. Castro-Gutiérrez, A. Celzard and V. Fierro, Front. Mater., 2020, 7, 1–25 CrossRef.
- Y. Ma, D. Chen, Z. Fang, Y. Zheng, W. Li, S. Xu, X. Lu, G. Shao, Q. Liu and W. Yang, Proc. Natl. Acad. Sci. U.S.A., 2021, 118, e2105610118–e2105610218 CrossRef CAS PubMed.
- R. Krishnapriya, C. Nizamudeen, B. Saini, M. S. Mozumder, R. K. Sharma and A. H. I. Mourad, Sci. Rep., 2021, 11, 16265–16277 CrossRef CAS PubMed.
- H. Ozawa, Y. Okuyama and H. Arakawa, Dalton Trans., 2012, 41, 5137–5139 RSC.
- M. U. Rahman, M. Wei, F. Xie and M. Khan, Catalysts, 2019, 9, 273–274 CrossRef CAS.
- P. M. Pataniya, D. Late and C. K. Sumesh, ACS Appl. Energy Mater., 2021, 4, 755–762 CrossRef CAS.
- P. M. Pataniya, V. Patel and C. K. Sumesh, ACS Appl. Energy Mater., 2021, 4, 7891–7899 CrossRef CAS.
- S. Yousaf, S. Zulfiqar, M. I. Din, P. O. Agboola, M. F. Aly Aboud, M. F. Warsi and I. Shakir, J. Mater. Res. Technol., 2021, 12, 999–1009 CrossRef CAS.
- M. Adeel, M. Saeed, I. Khan, M. Muneer and N. Akram, ACS Omega, 2021, 6, 1426–1435 CrossRef CAS PubMed.
- T. Solaiyammal and P. Murugakoothan, Mater. Sci. Energy Technol., 2019, 2, 171–180 Search PubMed.
- R. Chauhan, A. Kumar, G. G. Umarji, U. P. Mulik and D. P. Amalnerkar, J. Solid State Electrochem., 2015, 19, 161–168 CrossRef CAS.
- M. Waghmare, N. Beedri, A. Ubale and H. Pathan, Eng. Sci., 2019, 6, 36–43 Search PubMed.
- X. Chen, J. Ye, S. Ouyang, T. Kako, Z. Li and Z. Zou, ACS Nano, 2011, 5, 4310–4318 CrossRef CAS PubMed.
- A. Birkel, Y.-G. Lee, D. Koll, X. Van Meerbeek, S. Frank, M. J. Choi, Y. S. Kang, K. Char and W. Tremel, Energy Environ. Sci., 2012, 5, 5392–5400 RSC.
- K. Yang, R. Li, C. Zhu and J. Pei, J. Mater. Res., 2021, 36, 2936–2949 CrossRef CAS.
- C. Li, P. He, F. Dong, H. Liu, L. Jia, D. Liu, L. Du, H. Liu, S. Wang and Y. Zhang, Mater. Lett., 2019, 245, 29–32 CrossRef CAS.
- Y. Qiu, H. Fan, X. Chang, H. Dang, Q. Luo and Z. Cheng, Appl. Surf. Sci., 2018, 434, 16–20 CrossRef CAS.
- J. M. Bothwell, S. W. Krabbe and R. S. Mohan, Chem. Soc. Rev., 2011, 40, 4649–4707 RSC.
- Y. Qiu, M. Yang, H. Fan, Y. Zuo, Y. Shao, Y. Xu, X. Yang and S. Yang, CrystEngComm, 2011, 13, 1843–1850 RSC.
- M. Ciszewski, A. Mianowski, P. Szatkowski, G. Nawrat and J. Adamek, Ionics, 2015, 21, 557–563 CrossRef CAS.
- M. Schlesinger, S. Schulze, M. Hietschold and M. Mehring, Dalton Trans., 2013, 42, 1047–1056 RSC.
- S. Shaikh, G. Rahman, R. S. Mane and O.-S. Joo, Electrochim. Acta, 2013, 111, 593–600 CrossRef CAS.
- M. J. Jabeen Fatima, C. V. Niveditha and S. Sindhu, RSC Adv., 2015, 5, 78299–78305 RSC.
- P. Fernández-Ibáñez, M. I. Polo-López, S. Malato, S. Wadhwa, J. W. J. Hamilton, P. S. M. Dunlop, R. D’Sa, E. Magee, K. O'Shea, D. D. Dionysiou and J. A. Byrne, Chem. Eng. J., 2015, 261, 36–44 CrossRef.
- E. Kusiak-Nejman, A. Wanag, Ł. Kowalczyk, J. Kapica-Kozar, C. Colbeau-Justin, M. G. Mendez Medrano and A. W. Morawski, Catal. Today, 2017, 287, 189–195 CrossRef CAS.
- J. P. Mensing, C. Poochai, S. Kerdpocha, C. Sriprachuabwong, A. Wisitsoraat and A. Tuantranont, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8, 033001–033008 Search PubMed.
- M. I. A. Abdel Maksoud, R. A. Fahim, A. E. Shalan, M. Abd Elkodous, S. O. Olojede, A. I. Osman, C. Farrell, A. H. Al-Muhtaseb, A. S. Awed, A. H. Ashour and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 375–439 CrossRef CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- Z. Bo, Z. Wen, H. Kim, G. Lu, K. Yu and J. Chen, Carbon, 2012, 50, 4379–4387 CrossRef CAS.
- F. M. Ascencio Aguirre and R. Herrera Becerra, Appl. Phys. A, 2015, 119, 909–915 CrossRef CAS.
- X. Liu, L. Pan, T. Lv, Z. Sun and C. Q. Sun, J. Colloid Interface Sci., 2013, 408, 145–150 CrossRef CAS PubMed.
- T. Kuila, S. Bose, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Prog. Mater. Sci., 2012, 57, 1061–1105 CrossRef CAS.
- D.-Y. Yeom, W. Jeon, N. D. K. Tu, S. Y. Yeo, S.-S. Lee, B. J. Sung, H. Chang, J. A. Lim and H. Kim, Sci. Rep., 2015, 5, 9817–9836 CrossRef CAS PubMed.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- N. P. D. Ngidi, M. A. Ollengo and V. O. Nyamori, Materials, 2019, 12, 3376–3401 CrossRef CAS PubMed.
- N. P. D. Ngidi, M. A. Ollengo and V. O. Nyamori, New J. Chem., 2020, 44, 16864–16876 RSC.
- Y. Astuti, B. M. Listyani, L. Suyati and A. Darmawan, Indones. J. Chem., 2021, 21, 108–117 CrossRef CAS.
- M. Malligavathy and D. P. Padiyan, Adv. Mater. Processes, 2017, 2, 51–55 CrossRef.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- Z. A. Zulkifli, K. A. Razak and W. N. W. A. Rahman, AIP Conf. Proc., 2017, 1901, 020011–020016 CrossRef.
- M. Jalalah, M. Faisal, H. Bouzid, J.-G. Park, S. A. Al-Sayari and A. A. Ismail, J. Ind. Eng. Chem., 2015, 30, 183–189 CrossRef CAS.
- E. Pargoletti, S. Mostoni, G. Rassu, V. Pifferi, D. Meroni, L. Falciola, E. Davoli, M. Marelli and G. Cappelletti, Environ. Sci. Pollut. Res., 2017, 24, 8287–8296 CrossRef CAS PubMed.
- P. Mallet-Ladeira, P. Puech, C. Toulouse, M. Cazayous, N. Ratel-Ramond, P. Weisbecker, G. L. Vignoles and M. Monthioux, Carbon, 2014, 80, 629–639 CrossRef CAS.
- S. S. Nanda, M. J. Kim, K. S. Yeom, S. S. A. An, H. Ju and D. K. Yi, TrAC, Trends Anal. Chem., 2016, 80, 125–131 CrossRef CAS.
- W.-D. Yang and L. Yu-Jiang, J. Electr. Eng., 2019, 70, 101–106 Search PubMed.
- K. Song, I. Jang, D. Song, Y. S. Kang and S.-G. Oh, Sol. Energy, 2014, 105, 218–224 CrossRef CAS.
- G. Gryglewicz, J. Machnikowski, E. Lorenc-Grabowska, G. Lota and E. Frackowiak, Electrochim. Acta, 2005, 50, 1197–1206 CrossRef CAS.
- S. Liu, Y. Wang and Z. Ma, Int. J. Electrochem. Sci., 2018, 13, 12256–12265 CrossRef CAS.
- K. S. Sing and R. T. Williams, Adsorp. Sci. Technol., 2004, 22, 773–782 CrossRef CAS.
- H. Shen, Y. Zhang, X. Song, Y. Liu, H. Wang, H. Duan and X. Kong, J. Alloys Compd., 2019, 770, 926–933 CrossRef CAS.
- W.-D. Yang and Y.-J. Lin, Int. J. Electrochem. Sci., 2020, 15, 1915–1929 CrossRef CAS.
- X. Yang, X. Lian, S. Liu, G. Wang, C. Jiang, J. Tian, J. Chen and R. Wang, J. Phys. D: Appl. Phys., 2012, 46, 035103–035110 CrossRef.
- T. Zhan, X. Wang, X. Li, Y. Song and W. Hou, Sens. Actuators, B, 2016, 228, 101–108 CrossRef CAS.
- Z. Wei, Y. Wang and J. Zhang, Sci. Rep., 2018, 8, 6929–6937 CrossRef PubMed.
- X. Bai, X. Zhang, Z. Hua, W. Ma, Z. Dai, X. Huang and H. Gu, J. Alloys Compd., 2014, 599, 10–18 CrossRef CAS.
- V. Tezyk, C. Rossignol, N. Sergent, E. Djurado, J. Laurencin and E. Siebert, Electrochim. Acta, 2019, 304, 312–322 CrossRef CAS.
- M. Junaid, M. H. M. Khir, G. Witjaksono, N. Tansu, M. S. M. Saheed, P. Kumar, Z. Ullah, A. Yar and F. Usman, Molecules, 2020, 25, 3646–3665 CrossRef CAS PubMed.
- J. Li, B. Z. Wu and Z. X. Zhou, Micro Nano Lett., 2018, 13, 1443–1446 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08888b |
| ‡ On leave from Bindura University of Science Education, Department of Engineering and Physics, Private Bag 1020, Bindura, Zimbabwe. |
| This journal is © The Royal Society of Chemistry 2022 |