Open Access Article
Open Access ArticleA comparative analysis of biopolymer production by microbial and bioelectrochemical technologies†
Brenda Alvarez Chavez ab,
Vijaya Raghavana and
Boris Tartakovsky
ab,
Vijaya Raghavana and
Boris Tartakovsky *ab
*ab
aMcGill University, Bioresource Engineering Department, 21111 Lakeshore Rd., Ste-Anne-de-Bellevue, QC H9X 3V9, Canada. E-mail: Boris.Tartakovsky@cnrc-nrc.gc.ca
bNational Research Council of Canada, 6100 Royalmount Ave, Montreal, QC H4P 2R2, Canada
First published on 1st June 2022
Abstract
Production of biopolymers from renewable carbon sources provides a path towards a circular economy. This review compares several existing and emerging approaches for polyhydroxyalkanoate (PHA) production from soluble organic and gaseous carbon sources and considers technologies based on pure and mixed microbial cultures. While bioplastics are most often produced from soluble sources of organic carbon, the use of carbon dioxide (CO2) as the carbon source for PHA production is emerging as a sustainable approach that combines CO2 sequestration with the production of a value-added product. Techno-economic analysis suggests that the emerging approach of CO2 conversion to carboxylic acids by microbial electrosynthesis followed by microbial PHA production could lead to a novel cost-efficient technology for production of green biopolymers.
1. Introduction
Bio-based materials produced from renewable sources of organic carbon instead of petroleum hydrocarbons can play an important role in reducing consumption of fossil fuels and moving our society towards a circular economy. Polyhydroxyalkanoates (PHAs), which are produced for storage of carbon and energy by a large number of microorganisms within the bacterial and archaea domains, are often used for bioplastic production. The insoluble PHA granules inside the microorganisms can make up to 90% of the dry weight of the cell mass.1 More than 150 types of PHAs have been identified. The most common form of PHA is polyhydroxybutyrate (PHB). Depending on the composition and properties of the PHA, applications can range from use in biodegradable packaging, to use as chemical additives, to usage in the fields of medicine, agriculture, wastewater treatment, and cosmetics.2–4In spite of multiple benefits of using biopolymers, their commercialization continues to be problematic due to high biopolymer production costs compared to polymers produced from conventional feedstock. Indeed, the price of polypropylene and polyethylene is about US $1.25–2.53 per kg,5 while that for PHAs has been reported to be up to 16 times higher than the major petroleum-derived polymers.6 According to a study of the global PHA market for 2018,7 the average PHA price was US $8.0 per kg. This price varied according to the target application and quality of the PHAs. In the same study, the average price of PHAs destined for packaging and food services was calculated to be US $7.6 per kg with an estimated market size of US $25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, while for biomedical applications the average price was US $11.9 per kg with a market size of US $15
000, while for biomedical applications the average price was US $11.9 per kg with a market size of US $15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. In 2019, the global PHA market was estimated to be US $57
000. In 2019, the global PHA market was estimated to be US $57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a projected compound annual growth rate of 11.2%.7
000 with a projected compound annual growth rate of 11.2%.7
Integration of biopolymers into the global market can be facilitated through a thorough cost analysis and identification of technologies capable of reducing production costs, while minimizing environmental impact. Energy consumption, PHA yield, and the efficiency of the downstream processing are the most important parameters determining the cost of production.8 Carbon sources used in pure culture microbial fermentations contribute significantly to overall environmental impact and production costs. Therefore, the use of mixed microbial communities capable of PHA production from liquid and gaseous waste streams, such as food wastes,9 agricultural wastes,10,11 landfill gas, carbon dioxide (CO2), wastewater,12 polystyrene waste,13 and glycerol14,15 is seen as a sustainable approach for bioplastics production. Biopolymers produced from CO2 are of particular interest, as this approach also provides a sustainable method for utilization of CO2 captured from industrial off-gases and from air.16
In this review, PHA production technologies are described based on the type of carbon source (liquid or gaseous) used. Also, single-stage and two-stage production processes are considered. Typically, a single-stage production can be accomplished if a well-defined liquid carbon source is used, either with pure or mixed microbial cultures. If more complex carbon sources such as agro-industrial wastes or flue gases are used, the production of PHAs must be carried out in two stages, where the conversion of complex carbon sources into simple sugars or carboxylic acids is followed by a stage of PHA production. Finally, a novel approach of biopolymers production from CO2 and electrons in a microbial electrosynthesis (MES) system is reviewed. An overview of these technologies is followed by the review of techno-economic assessments evaluating the costs of PHA production, including PHA production from CO2 through MES. By combining a detailed review of PHA production technologies with a review of published techno-economic assessments, this study helps to select promising cost-efficient technologies for producing biopolymers from renewable carbon sources, including waste biomass and CO2.
2. PHA production technologies
Microorganisms promote their survival by production of PHAs to store carbon and energy. Typically PHA production is induced by stress caused by a lack of nutrients.17 Often microbial growth can be followed by PHA production under nutrient-limited conditions. Microbial production of PHAs has been achieved from multiple sources of carbon, including well-defined substrates such as glucose, agro-industrial wastes containing complex carbon sources, gas mixtures (e.g., syngas, biogas) and, more recently, CO2. In the following discussion we will use PHA as a term to include PHB, unless the article we are citing focuses on PHB.2.1 PHA production from liquid carbon sources
A number of Gram-positive and Gram-negative bacterial strains are capable of producing PHAs, although most of the PHA producing bacteria are Gram-negative. Table 1 summarizes PHA production from well-defined dissolved (liquid) carbon sources using different fermentation systems. Most of the biopolymer production studies cited in this table are batch processes, which are easier to implement in a laboratory, but also represent disadvantages such as variability in the quality of the product and downtime for the preparation of the bioreactor equipment for the next batch. To resolve this limitation, Atlić et al.18 evaluated PHB production using a mineral medium with glucose in a multi-reactor system consisting of five continuous stirred tank reactors, which approximates a continuous tubular plug flow reactor. The first reactor was used for balanced bacterial growth using Cupriavidus necator (also known as Ralstonia eutropha), while PHB accumulation was achieved in the subsequent reactors under nitrogen-limited conditions. This approach demonstrated a specific volumetric productivity of 1.97 g (L h)−1 and a polymer content of 77% w w−1.| Carbon source | Limiting nutrient | Culture | Fermentation system | PHA polymer | Cell dry weight, g L−1 | PHA content,% | PHA productivity g (L h)−1 | PHA yield g g−1 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Units: the amount of PHA produced (g SCOD) per the amount of active biomass (g) per hour.b Storage yield is defined as the amount of PHA produced in g COD per amount of carbon source consumed (g COD).c Notations: SCOD – soluble chemical oxygen demand; SBR – sequencing batch reactor; CSTR continuously stirred tank reactor; GAO – glycogen accumulating organisms; PAO – polyphosphate-accumulating organisms.d Estimated based on available information.e Estimated in gPHA (gVSS h)−1. | |||||||||
| Glucose | Nitrogen | C. necator DSM 545 | Fed-batch | PHB | 81 | 77 ± 7.5 | 1.97 ± 0.56 | — | 18 |
| Acetic acid | Nitrogen | C. necator DSM 545 | Fed-batch | PHB, PHBV | 60–65 | PHB – 72 PHBV – 74 | PHB – 0.37; PHBV – 0.41 | — | 19 |
| Glucose | Glucose | Bacillus cereus SPV | Fed-batch | PHB | 3 | 38 | — | — | 20 |
| Sucrose | None | Alcaligenes latus DSM1124 | Fed-batch pH-stat | PHB | 143 | 50 | 3.97 | 0.3 | 22 |
| Sucrose | Nitrogen | Alcaligenes latus DSM1123 | Fed-batch | PHB | 112 | 88 | 4.94 | — | 23 |
| Sucrose | Sucrose, nitrogen | Alcaligenes latus DSM1124 | Fed-batch | PHB | 39 | 75 | 0.6 | — | 21 |
| Glucose | Nitrogen | A. eutrophus NCIMB 11 599 | Fed-batch | PHB | 164 | 76 | 2.42 | 0.17 | 76 |
| Condensed corn medium | Nitrogen | C. necator H16 ATCC 17699 | Batch | PHB | 6–15 | 29–41 | 0.02–0.04 | — | 11 |
| Acetate | Acetate, nitrogen | Sludge-GAOc enriched culture | SBR | P(3HB/3HV) | 41% DW | 41 | — | 0.3–0.4b | 32 |
| Paper mill effluent | Phosphorus | GAOc enriched culture | SBR | P(3HB/3HV/3HMV) 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
— | 42 | 0.093a,e | 0.34b | 33 |
| Acetate | None | PAOc enriched culture | SBR | PHB | 29% | 50 | 0.2 g (g h)−1 | 0.6 (mol mol−1) | 36 |
| Wastewater | Substrate | Activated sludge | SBR | P(3HB/3HV) 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | 53 | 0.23a,d | 0.9b | 34 |
| Sugar cane molasses | Nitrogen | Mixed culture | Fed-batch, CSTR | P(3HB/3HV) 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
— | 56 | 0.37 | 0.9b | 35 |
| Fermented cheese whey | Nitrogen | P(3HB/3HV) 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
— | 65 | 0.56 | 0.7b | |||
| Molasses and cheese whey | Nitrogen | P(3HB/3HV) 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
— | 40 | 0.15 | 0.6b | |||
| Wastewater | Nitrogen, phosphorus | Sludge | SBR | — | — | 8–18 | — | — | 80 |
PHB production from acetate and valerate using a pure culture of C. necator was evaluated by Garcia-Gonzalez et al.19 After 118 h of fermentation, 60 g L−1 cell dry matter (CDM) with 72% PHB content was obtained, demonstrating the feasibility of producing PHB from acetate. In another study, PHB production by Bacillus cereus SPV from glucose in batch and fed-batch bioreactors was evaluated.20 The two main differences between the batch and fed-batch fermentations were the time required for maximum PHB accumulation, which was reduced from 48 to 32 h in the fed-batch fermentation, and in which the final PHB yield increased from 29% DCW to 38%. In a recent study of Gahlawat et al.21 PHB productivity was improved from 0.17 g (L h)−1 in the batch process to 0.6 g (L h)−1 in the fed-batch process. Also, the PHB content increased from 51 to 75%. Attempts to increase PHA production include the work of Chakraborty et al.,11 which used a condensed corn medium (by-product of ethanol production from corn) for cultivation of C. necator at high cell density. Furthermore, this work suggests that butyric and propanoic acids provided the best results in terms of PHA production and optimal levels of these volatile fatty acids (VFAs) were determined. Overall, these studies demonstrated the advantages of using continuous or, at least, fed-batch bioprocesses for reducing cultivation times and maximizing production rates.
The effect of different conditions of nutrient deficiency on some PHB producing cultures have been also explored. For example, it is known that Alcaligenes latus produces PHBs even under nutrient-sufficient conditions. Nevertheless, the process of recovering PHBs is more expensive under these conditions and the resulting PHB content was less than 50%.22 A study conducted by Wang and Lee23 demonstrated the effect of nitrogen limitation on the production of PHBs by A. latus in batch cultures using sucrose as a carbon source. Nitrogen limitation was applied after 12 h with a sucrose concentration between 5 and 20 g L−1. After 8 h of nitrogen limitation, the cell concentration, PHB concentration and PHB content reached 111.7 g (dry cell weight) L−1, 98.7 g L−1 and 88%, respectively, resulting in a productivity of 4.94 g PHB per (L h).
In these studies, C. necator stands out for its unique physiology. This facultative chemolithoautotrophic microorganism is capable of producing PHBs in the range of 60 to 90% of CDM (cell dry matter) from a broad range of carbon sources. The biosynthesis of PHB in this bacterium can switch between heterotrophic and autotrophic modes for growth and production of PHBs, respectively.24 In the heterotrophic growth mode, this microorganism can use organic compounds such as sugars, organic acids, VFAs and vegetable oils, while under autotrophic conditions it uses H2 and CO2 as energy and carbon sources, respectively, where CO2 is fixed by the Calvin–Benson–Bassham cycle.24
Production of PHAs using mixed microbial consortia (MMC) is a more attractive practical approach since it can reduce operating costs. With MMC, non-sterile conditions are used and the microorganisms are adapted to various carbon sources, including waste effluents. Furthermore, the microorganisms capable of accumulating biopolymers are selected by the operational conditions, i.e. the ecosystem is designed instead of the strains.9 The production of PHA by mixed microbial cultures occurs under transient conditions of carbon or oxygen availability, known respectively as dynamic aerobic feeding and anaerobic/aerobic process.25 There are two main groups of bacteria responsible for the accumulation of PHAs under these conditions, polyphosphate (PAOs) and glycogen accumulating organisms (GAOs). Under anaerobic conditions, carbon substrates are consumed, PAOs release phosphate, gaining energy for the PHA accumulation process, while GAOs gain energy only from the glycolysis of glycogen. Under aerobic/anoxic conditions, both microbial groups use the stored PHA for growth, maintenance and replenishment of the glycogen reserve.26
Depending on the type of substrate used as feedstock, the production of PHA using mixed cultures can takes place in either one or two stages, as illustrated in Fig. 1. In the single-stage process, the growth of PHA-accumulating organisms (under aerobic or anaerobic/aerobic conditions) and the ensuing accumulation of PHAs occurs in the same bioreactor (Fig. 1A). This approach has been mainly applied when organic acids are used as feedstock.27 The two-stage process shown in Fig. 1B often uses waste-based raw materials, many of which are carbohydrate-rich, and must include a pre-fermentation stage. An additional stage is required to transform the carbohydrates into VFAs, which can then be used for production and accumulation of PHA.28 This is primarily because mixed cultures subjected to feast and famine conditions are often incapable of storing biopolymers from sugar-based compounds.29 The culture selection step is performed to enrich for microorganisms that are not only capable of storing the polymers but also have a high storage capacity. Selection of cultures with complex substrates can be performed by implementing anaerobic/aerobic (AN/AE) and aerobic dynamic feeding (ADF) regimes.
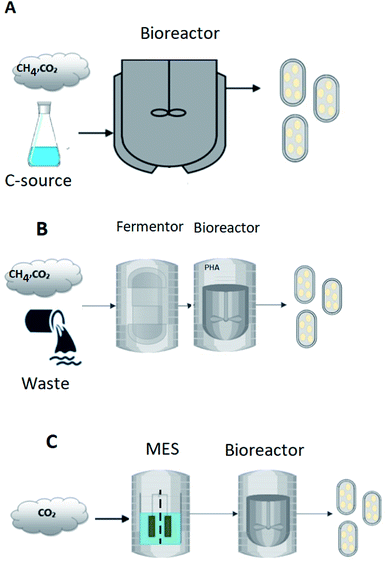 | ||
| Fig. 1 PHA production from liquid and gaseous sources of carbon in (A) single stage fermentation, (B) two stage fermentation, and (C) two stage microbial electrosynthesis process. | ||
The source of carbon for PHA production plays a crucial role, as it can represent more than half the overall production costs.30,31 For this reason, many studies have focused on finding inexpensive sources of carbon, such as agro-industrial wastes, including whey, lignocellulosic materials, glycerol (obtained from biodiesel production) and other organic waste compounds. Table 1 includes studies that describe production of PHAs from different carbon sources using mixed cultures. In one such study,32 acetate was used to produce PHBs, poly-3-hydroxyvalerate (PHV) and poly-3-hydroxy-2-methyl-valerate (PHMV), using glycogen accumulating organisms (GAOs). The culture selection stage was carried out for the enrichment of the GAO culture by alternating anaerobic and aerobic conditions with acetate as feedstock. Bengtsson et al.33 investigated the feasibility of using paper mill effluent to produce PHAs (PHB, PHV and PHMV) using GAO-enriched activated sludge. A two-phase process comprising acidic fermentation and culture enrichment/batch accumulation of PHAs based on the anaerobic/aerobic cycle was used. It was observed that the highest PHA content was obtained by applying aerobic conditions, in which PHA and glycogen were produced, followed by anaerobic conditions, in which stored glycogen was used for further production and accumulation of PHA. The highest PHA content achieved was 42%, and total yield in the two-stage process was 0.10 kg of PHA per kg of soluble chemical oxygen demand (COD). In another study,34 a mixed microbial consortium was used to produce PHAs using primary solids fermenter liquor from municipal wastewater as feedstock under AN/AE and aerobic conditions. This resulted in 10 to 25% w w−1 PHA content. It was observed that when the batch aerobic reactor was seeded from the anaerobic/aerobic reactor and fed fermenter liquor, approximately 53% PHAs w w−1 was obtained. In another study,35 cheese whey (CW) and sugar cane molasses (SCM) were used as carbon sources in a two-phase PHA production process using mixed microbial cultures. Acetate and butyrate were the main products from CW, while valerate was the dominant intermediate compound from SCM. Maximum PHA contents of 56% and 65% were obtained with fermentation on SCM and CW, respectively, in the second stage. Also, PHB production from synthetic wastewater, mainly composed of acetate, by a PAO-enriched activated sludge showed a PHB content of up to 29%.36
2.2 PHA production from gaseous carbon sources
Production of biopolymers from gaseous carbon sources such as CH4 and CO2 is gaining attention since these are greenhouse gases (GHG). In particular, several recent studies were focused on the use of CO2 for the production of PHAs. CO2 represents about 81% of total greenhouse gas emissions,37 so it is broadly available and does not compete with the food supply chain. Production of biopolymers has been also explored using syngas and exhaust gases containing carbon monoxide (CO). Table 2 summarizes several studies on the production of biopolymers from gaseous substrates. As can be seen from this table, studies for converting gaseous substrates to biopolymers were conducted using either pure or mixed microbial cultures. In pure culture experiments, the model microorganism is once again C. necator, since it is metabolically versatile and capable of changing between heterotrophic growth using organic compounds and autotrophic growth using CO2 as carbon source, H2 as energy source, and O2 as electron acceptor. There are two main cultivation methods to directly use CO2 for the production of PHBs by C. necator: autotroph–autotroph, which uses a gaseous mixture of CO2, H2 and O2 for both cell mass growth and PHB accumulation, and heterotroph–autotroph, which uses an organic substrate such as glucose for cell growth followed by autotrophic PHB production. The presence of O2 in the gas mixture creates significant difficulties for the practical implementation of this approach, as care must be taken to avoid explosion.| Carbon source | Limiting nutrient | Culture | Fermentation and time | PHA | Cell dry weight g L−1 | PHA concentration g L−1 | PHA content,% | PHA productivity g (L h)−1 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Value estimated based on available information. | |||||||||
Glucose, biorefinery off-gas (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 84.0 CO2 = 84.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.2) 13.2) |
Nitrogen | C. necator, DSM 545 | Autotrophic/heterotrophic | PHB | 21–38 | 15.3–24 | 63–73 | CO2–biogas: 0.23, CO2–bioEtOH: 0.11 | 16 |
Gas mixture (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 3.6 N2 = 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6 7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.3 12.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76.5) 76.5) |
Nitrogen and/or phosporus | C. necator, ATCC 17699, strain 1F2 | Flask, autotrophic | P(3HB), PHBV | 0.31–0.52 | 0.02–0.27 | Up to 70 | 0.00013–0.0018a | 38 |
Fructose, gas mixture (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 10 H2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), valeric acid 60), valeric acid |
Nitrogen | C. necator, B-5786 | Heterotrophic/autotrophic, two-stage batch, 60–70 h | PHBV | 18 | 15 | PHA: 76%; P3HB: 37%, P3HV: 63% | 0.2 | 78 |
Glucose, gas mixture (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 84.0 CO2 = 84.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.2) 13.2) |
Nitrogen | C. necator, DSM 545 | Heterotrophic/autotrophic, 68 h | PHBV | 32 | 24.7 | 78 | 0.87 | 79 |
Gas mixture (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 77 CO2 = 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11), valeric acid 11), valeric acid |
Nitrogen | C. necator, ATCC 17697 | Autotrophic, 100 h | P(3HB), PHBV | 12 | P(3HB): 0.81, P(3HV): 0.25 | 63 | 0.012–0.005 | 39 |
An alternative method for PHB production from CO2 is to use autotrophic–heterotrophic–heterotrophic cultures, which imply co-culturing or using two different microorganisms in different stages. For example, anaerobic acetogenic bacteria can be used to convert H2 and CO2 to acetate followed by acetate utilization for biomass growth and PHB production in the presence of O2.19 The same study showed that the autotrophic–autotrophic cultivation with CO2 as feedstock could theoretically consume 2.84 ton-CO2 per (ton-PHB) and 0.96 kg H2 per (kg PHB). The autotrophic–heterotrophic–heterotrophic cultivation consumes the same amount of CO2 per ton of PHB, but requires about 55% less H2 (0.42 kg H2 per (kg PHB)). In the heterotrophic–autotrophic cultivation process where CO2 is used with glucose, only 1.58 tons of CO2 per (ton of PHB) are consumed. In contrast, in a heterotrophic cultivation using glucose or similar compounds instead of CO2, 2.81 tons of CO2 per (ton of PHB) are emitted. While the autotrophic–autotrophic cultivation results in the highest amount of CO2 removed, the heterotrophic growth is generally faster, thus reducing the overall cultivation time.
The impact of nitrogen limitation on P(3HB) production was studied by Miyahara et al.38 This work used gas mixture with low hydrogen content (3.6H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6O2
7.6O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.3CO2
12.3CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76.5N2) for autotrophic fermentation of a C. necator culture. Here, a nitrogen deficient culture medium yielded the highest polymer content of 70% w·w−1. Garcia-Gonzalez et al.16 investigated the impact of using CO2-rich waste gases on the production of PHBs in two-phase fermentation system using glucose as a substrate for heterotrophic growth followed by autotrophic production of biopolymers from industrial waste gases. Bacterial performance was not affected by the use of CO2-rich exhaust gases, reaching final PHB content and productivity of up to 73% and 0.227 g (L h)−1, respectively. Park et al.39 determined that a 1% CO2 concentration in a gas mixture of H2
76.5N2) for autotrophic fermentation of a C. necator culture. Here, a nitrogen deficient culture medium yielded the highest polymer content of 70% w·w−1. Garcia-Gonzalez et al.16 investigated the impact of using CO2-rich waste gases on the production of PHBs in two-phase fermentation system using glucose as a substrate for heterotrophic growth followed by autotrophic production of biopolymers from industrial waste gases. Bacterial performance was not affected by the use of CO2-rich exhaust gases, reaching final PHB content and productivity of up to 73% and 0.227 g (L h)−1, respectively. Park et al.39 determined that a 1% CO2 concentration in a gas mixture of H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2
O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 7
N2 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91% (v/v) was optimal for C. necator growth and PHB accumulation.
91% (v/v) was optimal for C. necator growth and PHB accumulation.
The use of CH4 for PHB production has also been explored with a mixed methanotrophic consortium. The methanotrophs can produce PHBs even under non-sterile conditions, thus reducing operating costs.40 The methanotrophs responsible for the biodegradation of CH4 (type II methanotrophs), need specific conditions to divert the flow of carbon associated with the assimilation of CH4 to synthesize intracellular PHB. A recent study41 evaluated the effects of temperature and phosphorus on the rate of CH4 consumption and the potential for PHB accumulation of different methanotroph-enriched inocula. Higher rates of CH4 consumption for growth were obtained under non-limiting concentrations of phosphorus at temperatures ranging from 25 to 37 °C. Subsequent PHB production occurred under phosphorus-limited conditions, with the highest PHB content (13.6 ± 5.6%) obtained with the Sphagnum – derived inoculum at 25 °C. Luangthongkam et al.42 and Myung et al.43 have demonstrated the feasibility of PHB and PHBV production using mixed methanotrophic cultures dominated by Methylosinus sp. and Methylocystis sp., respectively.
2.3 PHA production from CO2 through microbial electrosynthesis
The recently introduced concept of CO2 reduction in a microbial electrosynthesis (MES) cell utilizes electroactive microorganisms capable of either direct electron uptake from the cathode and production of short chain fatty acids (SCFAs) and/or CH4, or bioelectrochemical production of H2, which is then used for microbial CO2 reduction.44–49 A typical MES configuration consists of anodic and cathodic chambers separated by a proton exchange membrane (PEM), although membraneless MES systems have also been developed.50,51 The bioelectrosynthesis is supported by an applied voltage, typically at a level above the threshold for water electrolysis, which results in water splitting at the anode. Notably, the bioelectrochemical system can be also operated with applied voltages below the onset of water electrolysis if a carbon source is provided to the anode as a source of electrons for anodophilic electroactive microorganisms.47 However, such microbial electrolysis cell (MEC) shows significantly lower current density and feature CO2 release at the anode.50 In MES cells, acetogenic microorganisms can reduce CO2 using the H2 produced by the electroactive microorganisms, thus a microbial consortium is formed.52 Typically, the indirect metabolic pathways of acetogens result in acetate as the predominant product, although the formation of other organic compounds such as propionate, butyrate, ethanol, isopropanol, caproate, and caprylate has been reported.51Recently, the use of a consortium of electroactive and acetogenic microorganisms growing in the MES cell cathode was explored for its ability to produce VFAs from CO2,53 which can be subsequently used for PHA production,52 as shown in Fig. 1C. The use of MES to produce VFAs has certain advantages over a more conventional approach of organic wastes fermentation, including a more consistent composition of produced VFAs and better process control. Indeed, organic waste fermentation products depend on many variables such as temperature, pH, inoculum, type of feed, etc.52 Moreover, hydrolysis of complex organic molecules is notoriously slow, while higher VFA production can be expected in the MES using a specialized microbial community growing on a single carbon source (CO2).
The electroactive microorganisms found in MEC and MES cells are generally chemolithoautotrophic.44 These microorganisms can either form a biofilm or be planktonic. It has been observed that pure cultures were more efficient at CO2 bioelectrochemical conversion to acetate, typically higher than 80%, while it was around 60% for a mixed community54 due to formation of other products such as CH4. Nevertheless, preference has been given to the use of mixed cultures since sterile conditions are difficult to maintain for industrial-scale operations. For this reason, research has focused on enriching mixed consortia by bioaugmentation, which could lead to higher product yields.
Table 3 summarizes studies on using MES to convert CO2 to organic compounds. Nevin et al.55 conducted one of the first studies to demonstrate the feasibility of CO2 reduction to acetate and 2-oxobutyrate in a MES. In this work, Sporomusa ovata was used and the cathode potential was maintained at −0.4 V vs. Ag/AgCl reference electrode. Tremblay et al.56 demonstrated increased CO2 conversion by microbial electrosynthesis through adaptive evolution of S. ovata, which was shown to grow more rapidly autotrophically with methanol as the sole substrate, leading to 6.5 higher rate of acetate production from CO2. Furthermore, Marshall et al.57 demonstrated the potential of mixed cultures to produce CH4, acetate, and H2 at a granular graphite cathode and high rate of acetate production (1330 g m−2 d−1) from CO2 was achieved at pH 6.7 by Jourdin et al.58 using macroporous vitreous carbon cathode. Bajracharya et al.54 compared CO2 reduction at different electrode potentials using a mixed culture and a pure culture of Clostridium ljungdahlii. The reactor with a pure culture achieved higher production of acetate, CH4 and H2 at −1.1 V (vs. Ag/AgCl electrode). Batlle-Vilanova et al.59 followed the same approach in their study on CO2 reduction in a tubular bioelectrochemical system. The cathode was inoculated with mixed microbial culture taken from a syngas fermentation reactor dominated by Clostridium spp. Production of butyrate and acetate was studied under CO2 limited conditions and high partial pressure of H2, which favored butyrate production.
| Microbial culture | Cathode potential (vs. SHE) | Cathode | Products synthesized | Production rate (mM m−2 d−1) | References |
|---|---|---|---|---|---|
| Sporomusa ovata | −0.4 | Graphite | Acetate, 2-oxobutyrate | 0.2 | 55 |
| S.ovata met-T18-2 | −0.69 | Graphite | Acetate | 133.5 | 56 |
| 866.7 | |||||
| S. ovata | −0.4 | Carbon cloth | Acetate | 30.0 | 81 |
| Chitosan treated carbon cloth | 229.0 | ||||
| Cyanuric chloride treated carbon cloth | 205.0 | ||||
| Nickel treated carbon cloth | 136.0 | ||||
| CNT–cotton treated carbon cloth | 102.0 | ||||
| Clostridium ljungdahlii | −0.69 | Carbon felt | Acetate, ethanol, H2 | — | 54 |
| Mixed culture | H2, acetate, CH4 | — | |||
| Mixed culture | −0.8 | Carbon cloth | Acetate | 34.7 | 59 |
| Butyrate | 87.5 | ||||
| Pond sediments and wastewater treatment plant sludge | −0.8 | Nanoweb 3D RVC | Acetate | 59 | 82 |
| Mixed culture from septic tank | −1 | Carbon felt | Acetate | 50.2 | 49 |
| Butyrate | 39.8 | ||||
| Propionate | 27.1 |
Another recent study49 determined the optimal potential required to synthesize organic compounds from the CO2 present in biogas by aerobic sludge in the cathode chamber. Several cathode potentials from −0.6 V to −1.0 V vs. a standard hydrogen electrode (SHE), were tested to evaluate their effect on the MES performance. It was observed that as the applied potential increased, the yields of acetate and butyrate also increased. Consequently, production of organic compounds from CO2 was achieved with a low energy consumption of 9.15 W h at an applied potential of −0.7 V vs. SHE.
VFAs produced in a MES can be used for bioplastics production in the second production step, as proposed by Sciarria et al.52 In the first stage of this work, acetate and butyrate were produced from CO2 in a MES. Then the VFAs were concentrated and fed to a mixed microbial culture in the second bioreactor to produce PHBs. The MES was operated in batch mode and the cathode was initially inoculated with an enriched carboxydotrophic mixed microbial culture dominated by Clostridium spp. The CO2 fixation efficiency was 73%. In the PHB production step, a maximum PHB concentration of 74% was obtained. The system-wide efficiency calculated in terms of carbon conversion was 0.41 kg of carbon in PHB per 1 kg of initial carbon as CO2.
Interestingly, Srikanth et al.60 studied the use of a biocathode for PHA production in a microbial fuel cell (MFC) under oxygen-limited conditions. In this work, both electrode compartments (the anaerobic anode and aerobic cathode) were fed with a glucose-based solution resulting in current generation at the anode and heterotrophic accumulation of PHAs of up to 19% of dry cell weight at the cathode after 48 h of cultivation. PHA production required oxygen-limiting (micro-aerobic) conditions at the cathode, although low oxygen concentration also limited current generation. While this MFC setup required glucose supply to the anode to provide a source of electrons and featured low current density due to low oxygen concentration at the cathode, it can be hypothesized that PHA production can be achieved at a VFA-producing MES cathode provided with a limited supply of oxygen. Accordingly, such MES would be able to combine the steps of VFA and PHA production in the MES cathode, thus resulting in a single – step system for biopolymers production from CO2 and electrons.
3. Cost comparison
Currently, most industrial scale bioplastics production is carried out using one-step production from well-defined carbon sources. The largest producers are Danimer Scientific (USA), producing PHA from canola oil (a production capacity of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year); Shenzhen Ecomann Biotechnology Co. Ltd (China), producing PHA from sugars (a production capacity of 5000 tons per year); TianAn Biological Materials Co. Ltd. (China) producing PHB from dextrose (a production capacity of 2000 t per year); Kaneka Corporation (Japan) producing PHB from plant oils, and several other companies.7,61 The following review of techno-economic assessments (TEA) is aimed at comparing these technologies with newly emerging approaches for PHA production from alternative carbon sources described in the previous chapter. As explained below, to enable such comparison the calculation methods were unified and applied to the same production capacity.
000 t per year); Shenzhen Ecomann Biotechnology Co. Ltd (China), producing PHA from sugars (a production capacity of 5000 tons per year); TianAn Biological Materials Co. Ltd. (China) producing PHB from dextrose (a production capacity of 2000 t per year); Kaneka Corporation (Japan) producing PHB from plant oils, and several other companies.7,61 The following review of techno-economic assessments (TEA) is aimed at comparing these technologies with newly emerging approaches for PHA production from alternative carbon sources described in the previous chapter. As explained below, to enable such comparison the calculation methods were unified and applied to the same production capacity.
3.1 Methodology
To compare the bioplastics production technologies reviewed in the previous chapters, the technologies were divided into three groups and then compared based on already published TEA studies. The following groups were considered. The first group of PHA-producing processes is single-stage reactors using well-defined carbon sources, which combines the growth of PHA accumulating microorganisms followed by the accumulation of PHA in the same bioreactor (Fig. 1A). The second group of processes includes PHA production from complex carbon sources such as wastewater or agricultural biomass, which requires a two-stage process, including the pre-treatment of the feedstock by an acidogenic fermentation followed by the growth of PHA producers and PHA accumulation in the second bioreactor (Fig. 1B). Finally, the third group considers PHA production from CO2 using MES technology, typically in a two-stage process of CO2 conversion to VFAs followed by the step of PHA production from the VFAs (Fig. 1C). Detailed description of the calculation methods for each process group is provided in ESI.† These calculations were derived from the published TEA studies reviewed in the following discussion.3.2 PHA production from well-defined carbon sources
Table 4 summarizes analyses of PHA production costs in a single stage process using three different well-defined carbon sources: glycerol, glucose, and CH4. All costs are provided in USD. As mentioned above, Leong et al.62 performed a cost analysis of the production of PHB from glycerol using C. necator H16 and the methodology developed in this study was used for cost analyses of PHA production from glucose and CH4. Taking into consideration the volume of the proposed bioreactor and the time required to produce the bioplastics, an overall yield of 0.32 kg of PHB per kg of glycerol and a PHB production cost of $6.72 per kg were estimated. Here, the cost of the carbon source represented about 30% of the total operating costs. In another relevant study conducted by Choi and Lee,64 the results of using different carbon sources and four bacterial strains were compared with respect to the production of PHBs. Overall, the lowest production costs were obtained when using a recombinant Escherichia coli culture and glucose as the carbon source. Two polymer recovery techniques were also evaluated. The authors concluded that the method of recovery using surfactant-hypochlorite digestion is the most cost-efficient. The analysis was conducted for an annual production capacity of 2850 ton with an overall yield of 0.29 kg of PHB per kg of glucose, resulting in a production cost of $6.14 per kg of PHB. When the production capacity was scaled up, the costs dropped to $5.11 per kg of PHB. Adjustment of this cost to 2020 values resulted in $7.87 per kg (Table 4).| Parameters | Study | ||
|---|---|---|---|
| Reference | Leong et al., 2017 (ref. 62) | Choi and Lee, 1997 (ref. 64) | Levett et al., 2016 (ref. 65) |
| PHB recovery method | Surfactant and sodium hypochlorite digestion | Surfactant and sodium hypochlorite digestion | Acetone–water solvent |
| Pure strain | C. necator H16 | Recombinant E. coli | Thermophilic methanotrophs |
| Carbon source | Glycerol | Glucose | Methane |
| Productivity, g (L h)−1 | 4.00 | 2.18 | 2.70 |
| Global yield, kg PHB per (kg substrate) | 0.32 | 0.29 | 0.54 |
| Fed-batch fermentation time, h | 42 | 39 | 24 |
| Volume per run, m3 | 305.30 | 584.46 | 434.54 |
| Target PHB production, ton per (year) | 9000.00 | 9000.00 | 9000.00 |
| Carbon source cost, $ per (kg) | $0.53 | $0.77 | $0.26 |
| Total carbon source, ton per (year) | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982.48 982.48 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758.62 758.62 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246 246 |
| Total direct fixed capital, $ | $178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 342 |
$103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 699 699![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 386 |
$61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 614 614![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
| Total annual operating cost, $ | $60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488 488![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 |
$70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792 792![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016 016 |
$71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267 267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
| PHB production cost, $ per (kg) | $6.72 | $7.87 | $7.92 |
Another TEA study was performed by Levett et al.65 for PHB production from CH4 at a scale of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year. A culture of thermophilic methanotrophs and the acetone–water solvent extraction method for PHB purification were used. Using a carbon source considered to be a waste, costs related to raw material was reduced to 20% of annual operating costs, resulted in a cost of $4.32 per (kg PHB) produced. When the production capacity was adjusted to 9000 ton per (year), the production cost was estimated to be $7.92 per (kg PHB) (Table 4).
000 tons per year. A culture of thermophilic methanotrophs and the acetone–water solvent extraction method for PHB purification were used. Using a carbon source considered to be a waste, costs related to raw material was reduced to 20% of annual operating costs, resulted in a cost of $4.32 per (kg PHB) produced. When the production capacity was adjusted to 9000 ton per (year), the production cost was estimated to be $7.92 per (kg PHB) (Table 4).
A comparison of these three studies shows that when using well defined carbon sources, the overall yield of PHA production is in a range of 0.3–0.5 kg per kilogram of substrate. The key parameters that determine the size of equipment needed and therefore impact the capital and operating costs are the productivity and the fermentation time. All three cases had similar operating costs, which resulted in PHB production costs of $6.7–7.9 per kg.
3.3 PHA production from complex carbon sources
Production of bioplastics using complex carbon sources, such as organic wastes, requires a two-stage process. Table 5 lists three TEA studies on the production of PHAs using different carbon sources. In the first study, Fernandez-Dacosta et al.12 analyzed techno-economic and environmental aspects of PHB production from wastewater. Three recovery methods were also evaluated in this study: two of them were based on chemical treatment with surfactants combined with either alkali or hypochlorite and the third one was based on solvent extraction combined with dichloromethane. The data reported for the purification method using surfactant and sodium hypochlorite digestion was used as the basis for comparison of the three methods. The authors based their evaluation on the production of 1500 ton PHB per year by a mixed microbial culture using wastewater from either paper mills or the food industry. The overall yield obtained in this analysis was 2.2 kg of PHB per m3 of wastewater. The first stage of the production process was acidogenic fermentation to obtain VFAs from the wastewater, followed by PHB production from the VFAs using a feast/famine regime in the second reactor. The costs associated with wastewater treatment were considered as a credit to offset the costs of bioplastics production. Final costs were calculated to be $1.29 US per (kg PHB) when considering these credits, and $1.77 US per (kg PHB) without the credits. It must be mentioned that such low costs were due to relatively high PHB production rates and low estimations for operating costs as compared to costs reported in Table 4. Table 5 also shows costs for the three methods normalized for an annual PHB production of 9000 tons.| Parameters | Mixed microbial culture using municipal wastes | Pure culture using CO and H2 | |
|---|---|---|---|
| Fernández-Dacosta et al., 2015 (ref. 12) | Mudliar et al., 2008 (ref. 66) | Choi et al., 2010 (ref. 68) | |
| PHB recovery method | Surfactant and sodium hypochlorite digestion | Alkali-surfactant | Surfactant and sodium hypochlorite digestion |
| Culture | Mixed microbial culture | Activated sludge | Rhodospirillum rubrum |
| Carbon source | Wastewater paper mill or food industry | Wastewater | Switchgrass biomass |
| Product formed in step 1 | VFA | VFA | Syngas (CO and H2) |
| Global yield | 2.20 kg PHB per m3 | 1.42 kg PHB per m3 | 0.17 kg PHB per (kg switchgrass) |
| Target PHB production, ton per year | 9000.00 | 9000.00 | 9000.00 |
| Total feedstock | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684.92 m3 684.92 m3 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.09 m3 732.09 m3 |
518![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522.88 ton 522.88 ton |
| Feedstock per run | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411.54 m3 411.54 m3 |
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411.37 m3 411.37 m3 |
1576.05 ton |
| Cost carbon source | — | — | $20.58 |
| Total production step 1, ton per run | 1038.97 | 1852.69 | 3764.88 |
| Total direct fixed capital, US $ | — | $43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 833![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472.13 472.13 |
$119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 718![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664.20 664.20 |
| Credits obtained | Wastewater treatment credits | Wastewater treatment credits | Hydrogen production and sale credits |
| Operating and maintenance cost – no credits, US $ | $15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 909![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022.98 022.98 |
$39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792.79 792.79 |
$84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003.25 003.25 |
| Operating and maintenance cost – with credits, US $ | $11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 597 597![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231.71 231.71 |
— | $2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234 234![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292.95 292.95 |
| PHB production cost – without credits, US $ per kg | $1.77 | $4.38 | $9.35 |
| PHB production cost – with credits, US $ per kg | $1.29 | — | $0.25 |
The study conducted by Mudliar et al.66 considered a two-stage PHB production process from organic wastes with a processing capacity of 100 m3 d−1 and a PHB production capacity of 46.20 ton per year. Since not all values required for TEA calculations were provided by the authors, some values were assumed from the publication of Fernandez-Dacosta et al.12 A PHB production cost of $11.8 per kg was estimated following the methodology outlined in ESI† based on a PHB yield of 44%. The work suggests that by increasing this PHB yield to 70% (based on the results published by Tamis et al.67 with this type of carbon source), the costs would be reduced to $5.38 per kg. When normalized to a production volume of 9000 tons per year, the estimated PHB production cost was $4.38 per kg (Table 5). These results suggest that the production of bioplastics using wastewater as substrate could be feasible, although a number of practical hurdles, such as the planning and construction of a production plant with a very high-volume requirement per batch, around 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 m3, remain to be solved.
500 m3, remain to be solved.
Cost estimations were also obtained for PHA production using agricultural waste biomass as a carbon source. Choi et al.68 conducted a TEA to investigate the feasibility of simultaneous production of H2 and PHBs using switchgrass as feedstock in a two-stage process biorefinery. First, the biomass was converted by thermochemical methods into syngas. Then, the syngas was fermented by a Rhodospirillum rubrum culture to produce PHBs and H2. A total daily biorefinery production capacity of 12 tons PHB and 50 tons of H2 was considered for the TEA. The H2 production resulted in a credit of $2 per (kg H2). The cost of PHB production was estimated to be $9.35 per kg. This cost substantially decreased to $0.25 per kg when H2 production credit was taken into account. Overall, when comparing the estimated costs for PHA production from well-defined and complex carbon sources it is important to note that the production costs could be similar, but these estimations are not yet supported by existing large scale production systems.
3.4 PHA production in a MES
As discussed previously, the production of biopolymers from CO2 in a MES can be achieved in a two-stage process in which VFAs (mainly acetate) are produced in the MES and then used in the second stage in a conventional bioreactor to produce PHA (Fig. 1C). A single step PHA production can be also considered, where CO2 reduction to VFAs and PHA production are accomplished by a mixed-culture at the MES cathode.16 CO2 conversion in a MES is a biological method of carbon sequestration, therefore following other TEA studies listed above, a CO2 removal credit could be applied in the calculations. International regulatory initiatives have stated that carbon credits should be considered in the range of $40–80 per (ton CO2) by 2020 and $50–100 per (ton CO2) by 2030 to meet the temperature reduction targets of the Paris Agreement.69 Therefore, a value of $60 per (ton CO2) was used in the following calculations.Table 6 summarizes the studies of three scenarios for biopolymer (PHB) production in a MES. The first two scenarios consider the two-stage process and assumes PHB production rates adapted from studies, which used either glycerol62 or acetate19 as carbon sources. The third scenario considers PHB production in a single stage by heterotrophic–autotrophic fermentation using CO2 as a carbon source. This scenario was evaluated based on the data reported by Garcia-Gonzalez et al.16
| PHB production from CO2 | Scenario 1 (2 step) | Scenario 2 (2 step) | Scenario 3 (single step) |
|---|---|---|---|
| Step 1: | Production of acetic acid from CO2 by MES Christodoulou 2016 (ref. 70) | Production of PHB from acetic acid using pure strains Garcia-Gonzalez & De Wever, 2017 (ref. 16) | |
| Acetic acid production, ton per (year) | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983.00 983.00 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091.83 091.83 |
— |
| Yield, kg acetic acid per (kg CO2) | 0.68 | 0.68 | 0.47 |
| Productivity, g (L h)−1 | 4.0 | 0.41 | 0.23 |
| Amount of CO2 required, ton per (year) | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 887.43 887.43 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484 484![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844.02 844.02 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 887.43 887.43 |
| Total direct fixed capital – without credits, US $ | $22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 874![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424.99 424.99 |
$7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032 032![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 364.45 364.45 |
N/A |
| Total annual operating cost – without credits, US $ | $18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165.51 165.51 |
$6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491 491![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605.29 605.29 |
N/A |
| Acetic acid production cost – without credits, US $ per (kg) | $0.67 | $0.15 | N/A |
| Acetic acid production cost – with CO2 credits, US $ per (kg) | $0.57 | $0.06 | N/A |
| Step 2: production of PHB from acetic acid using pure strains | Leong et al., 2017 (ref. 62) | Leong et al., 2017 (ref. 62); Garcia-Gonzalez 2018 (ref. 19) | N/A |
| PHB recovery method | Surfactant and sodium hypochlorite digestion | Surfactant and sodium hypochlorite digestion | Surfactant and sodium hypochlorite digestion |
| Culture | C. necator H16 | C. necator H16 | C. necator, DSM 545 |
| Carbon source | Acetate | Acetate | CO2 |
| Global yield, kg PHB per (kg substrate) | 0.32 | 0.21 | 0.47 |
| Target PHB production, ton per (year) | 9000.00 | 9000.00 | 9000.00 |
| Volume per run, m3 | 321.44 | 3121.72 | 5649.76 |
| Total carbon source quantity, ton per (year) | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481.05 481.05 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 091.83 091.83 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963 963![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045.91 045.91 |
| Total direct fixed capital – without credits, US $ | $178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913 913![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631.74 631.74 |
$1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 674 674![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989.85 989.85 |
$3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 685 685![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727.92 727.92 |
| Total annual operating cost – without credits, US $ | $65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199.25 199.25 |
$376![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961.87 961.87 |
$706![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 277 277![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586.13 586.13 |
| PHB total production cost – without credits, US $ per kg | $7.24 | $41.79 | $6.26–$78.48 |
| PHB total production cost – with CO2 credits, US $ per kg | $6.82 | $41.18 | $5.71–$78.37 |
For Scenarios 1 and 2, the first stage in the production process was evaluated based on the TEA analysis carried out by Christodoulou and Velasquez-Orta70 for the conversion of CO2 to acetate. This TEA study modeled a MES plant with a capacity of producing 100 ton acetate per year based on a production rate of 11.4 kg h−1 and a global yield of 0.68 kg of acetate produced per kg of CO2. The cost of acetate production was estimated to be $1.88 per kg. The second stage corresponds to the production of PHB from acetate. Considering a broad range of reported PHB production rates on well-defined carbon sources such as glycerol and acetate, the rate of PHB production from glycerol provided by Leong et al.62 (Table 4) was used for this calculation. In this scenario, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983 tons of acetate per year are required to achieve the target annual production of 100 ton of PHB. The calculations reported by Christodoulou and Velasquez-Orta70 were scaled to match this production capacity and a production cost of $0.67 per kg of acetate was estimated. By applying the CO2 conversion credits, this cost was further reduced to $0.57 per (kg). The second step was the fermentation using a pure culture of C. necator for the production of PHB from the acetate with an overall assumed yield of 0.21 kg PHB per (kg acetate) and a productivity of 4.0 g (L h)−1. Consequently, PHB production costs of $7.24 per kg without considering CO2 credits and $6.82 per kg with such credits were calculated.
983 tons of acetate per year are required to achieve the target annual production of 100 ton of PHB. The calculations reported by Christodoulou and Velasquez-Orta70 were scaled to match this production capacity and a production cost of $0.67 per kg of acetate was estimated. By applying the CO2 conversion credits, this cost was further reduced to $0.57 per (kg). The second step was the fermentation using a pure culture of C. necator for the production of PHB from the acetate with an overall assumed yield of 0.21 kg PHB per (kg acetate) and a productivity of 4.0 g (L h)−1. Consequently, PHB production costs of $7.24 per kg without considering CO2 credits and $6.82 per kg with such credits were calculated.
Scenario 2 assumed the same two-stage technology, but with a much lower rate of PHB production. This assumption was based on the results reported in the study of Garcia-Gonzalez et al.,19 which estimated an overall yield of 0.21 kg of PHB per kg of acetate and an estimated productivity of 0.4 g (L h)−1. Consequently, the required amount of acetate was significantly higher. Annually, 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926
926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092 tons of acetate would be needed to achieve the target production of 100 tons PHB. The estimated cost of acetate production was lower at $0.15 per kg due to the increased production volume. The production cost decreased to $0.06 per kg when CO2 credits were considered. Nevertheless, the overall cost of PHB production was substantially higher due to the low rate of PHB production reported by Garcia-Gonzalez et al.19 Accordingly, PHB production cost of $41.18 per kg and $41.79 per kg with and without CO2 credits, respectively were estimated. The lack of experimental results corresponding to PHA production from VFAs in a MES leads to a broad range of cost estimations for the two scenarios in Table 6.
092 tons of acetate would be needed to achieve the target production of 100 tons PHB. The estimated cost of acetate production was lower at $0.15 per kg due to the increased production volume. The production cost decreased to $0.06 per kg when CO2 credits were considered. Nevertheless, the overall cost of PHB production was substantially higher due to the low rate of PHB production reported by Garcia-Gonzalez et al.19 Accordingly, PHB production cost of $41.18 per kg and $41.79 per kg with and without CO2 credits, respectively were estimated. The lack of experimental results corresponding to PHA production from VFAs in a MES leads to a broad range of cost estimations for the two scenarios in Table 6.
From these calculations, it can be seen that the productivity and global yield are the two parameters significantly affecting PHA production costs in Scenarios 1 and 2. Low productivity and yield lead to large bioreactor volumes, about 10 times higher in Scenario 2 as compared to Scenario 1. Because it is possible to produce a range of carboxylic acids and CH4 (ref. 71) from CO2 through MES technology, significant productivity improvements might be expected. Indeed, recent advances in optimizing operating conditions and developing new cathode materials resulted in CH4 and VFA production rates that were significantly greater than previously reported.58,72,73 Furthermore, a broader range of carboxylic acids can be produced by changing operating conditions,74 which would potentially result in improved productivity and yields during the PHA production phase. Clearly, more experimental work is needed to optimize both phases of this novel CO2 conversion process.
Calculations for hypothetical single step Scenario 3 to estimate costs used results obtained by Garcia-Gonzalez et al.16 In this study, heterotrophic biomass growth on glucose was followed by autotrophic production of biopolymers in the same reactor using a gas mixture of H2, O2, and CO2 in a ratio of 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.2, respectively. A productivity of 0.227 g PHB per (L h) and an overall yield of 0.47 g PHB per g CO2 were reported. Production of PHBs using this methodology leads to a mitigation potential of 1.58 ton CO2 per (ton PHB). The productivity of 4 g PHB per (L h) used by Leong et al., 2017 (ref. 62) was used for the calculation of expected costs. Also, it was assumed that PHB production rather than CO2 conversion to VFAs is the rate-limiting step of these biotransformations. The resulting calculations for Scenario 3 are given in Table 6. A broad range of production costs from $5.7 to $78.4 per kg was obtained, depending on the value used for productivity. Once again, such broad range is attributed to a lack of experimental results and is expected to be narrowed with the emergence of new experimental studies. Further discussion of the productivity impact on PHA production costs is provided below.
13.2, respectively. A productivity of 0.227 g PHB per (L h) and an overall yield of 0.47 g PHB per g CO2 were reported. Production of PHBs using this methodology leads to a mitigation potential of 1.58 ton CO2 per (ton PHB). The productivity of 4 g PHB per (L h) used by Leong et al., 2017 (ref. 62) was used for the calculation of expected costs. Also, it was assumed that PHB production rather than CO2 conversion to VFAs is the rate-limiting step of these biotransformations. The resulting calculations for Scenario 3 are given in Table 6. A broad range of production costs from $5.7 to $78.4 per kg was obtained, depending on the value used for productivity. Once again, such broad range is attributed to a lack of experimental results and is expected to be narrowed with the emergence of new experimental studies. Further discussion of the productivity impact on PHA production costs is provided below.
It should be emphasized that while the estimations presented in Tables 4 and 5 are based on published experimental results and TEA calculations, calculations for Scenario 3 in Table 6 represent a hypothetical process and only provide preliminary estimations. Once experimental results are available, a thorough TEA should be carried out to update cost estimations.
3.5 Cost comparison
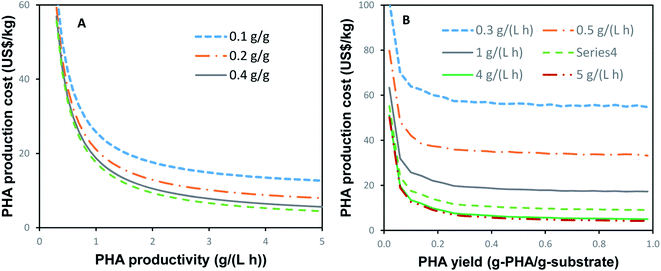
As can be seen from the estimations presented above, the costs of PHA production are strongly dependent on the rate of PHA productivity and the PHA yield. The effects of these parameters on the PHA production costs are illustrated in Fig. 2. The PHA costs shown in Fig. 2 were calculated for a single-stage process with a production capacity of 9000 tons per year and assuming a carbon source cost of $0.5 per kg, which would be similar to the cost of well-defined carbon sources such as glucose, sucrose, and acetate.Fig. 2A suggests that a cost-efficient PHA production requires a productivity of 0.8–1.0 g (L h)−1 or higher. Lower productivity values result in a steep increase in PHA production costs, especially at PHA productivities below 0.3 g (L h)−1. PHA yield is another significant factor (Fig. 2B). To obtain competitive costs of PHA production, yields higher than 0.2 g (g)−1 are required. To achieve PHA production costs below the $10 per kg, productivity and yields of greater than 2 g (L h)−1 and 0.4 g g−1, respectively, are required.
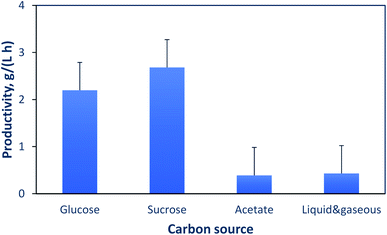
An important factor that significantly affects the productivity is the type of carbon source used. Fig. 3 shows the impact of different liquid and gaseous carbon sources on the productivity. The information presented in this figure is from the studies cited in Tables 4 and 5. For liquid carbon sources such as glucose, several studies reported similar productivities and global yields, i.e., 2.2 g (L h)−1 and 0.2 kg PHA per (kg substrate), respectively.64,75,76 With sucrose as a carbon source, experimental results from different studies gave an average productivity of 2.7 g (L h)−1.21–23 With acetate as a carbon source, a lower productivity of 0.4 (L h)−1 was obtained based on the results of Garcia-Gonzalez et al.19 The heterotrophic/autotrophic fermentation, which uses a liquid carbon source for growth and then CO2 for PHA production is also included in this comparison. Two studies report similar productivities when using glucose (0.23 g (L h)−1)77 or fructose (0.19 g (L h)−1)78 as a carbon source for growth, while a recent study reported a higher productivity of 0.87 g (L h−1) when using glucose and a gas mixture.79 This comparison suggests that to decrease the cost of PHA production from gaseous carbon sources, such as CO2, formation of intermediates other than acetate is desirable, as it might lead to increased rate of PHA formation.
4. Conclusion
This review provided an overview of experimental and TEA studies of bioplastics production from various carbon sources. Such comparison of results in available literature showed that the technical and economic feasibility of bioplastics production depends on multiple factors including carbon source, process design, operating conditions, etc. However, the most prominent factors affecting production costs are PHA yield, productivity, and the type of carbon source selected for PHA production. In fact, carbon source selection appears to be the most important, as it affects both the productivity and the overall PHA yield per unit of carbon source consumed.By comparing different TEA studies, it was shown that by using well-established carbon sources, such as glucose and glycerol and assuming a production capacity of 9000 tons per year, PHA production costs fall within the range of $6.9–$7.5 per kg. Also, several TEA studies suggest that when using more complex carbon sources such as wastewater or agricultural biomass, in which an additional carbon source preparation step is necessary, the unit cost of PHA production can range from $5.2 to $11.0 per kg. This range of costs is similar to that obtained when using well-established carbon sources, since feedstock costs are considered to be zero, and credits can be applied to reduce production costs.
Interestingly, our estimation of production costs using the emerging approach of bioplastics production through microbial electrosynthesis from CO2 suggests that this approach could provide a feasible alternative to traditional carbon sources, such as glucose. Although a broad range of production costs was obtained ($5.71–$78) due to the uncertainties of this novel process, future studies are expected to result in significant improvements in the observed process yield and productivity. A detailed TEA study is needed to evaluate the feasibility of direct CO2 conversion to bioplastics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Dr Darwin Lyew (McGill University) for thoughtful comments.References
- J. M. G. Alcantara, F. Distante, G. Storti, D. Moscatelli, M. Morbidelli and M. Sponchioni, Biotechnol. Adv., 2020, 42, 107582 CrossRef PubMed.
- G. Y. A. Tan, C. L. Chen, L. Li, L. Ge, L. Wang, I. M. N. Razaad, Y. Li, L. Zhao, Y. Mo and J. Y. Wang, Polymers, 2014, 6, 706–754 CrossRef.
- R. Rai, T. Keshavarz, J. A. Roether, A. R. Boccaccini and I. Roy, Mater. Sci. Eng., R, 2011, 72, 29–47 CrossRef.
- G. de Roo, M. B. Kellerhals, Q. Ren, B. Witholt and B. Kessler, Biotechnol. Bioeng., 2002, 77, 717–722 CrossRef CAS PubMed.
- P. Technology, Prices Up for All Commodity Resins, https://www.ptonline.com/blog/post/prices-up-for-all-commodity-resins, accessed, 5 October 2020 Search PubMed.
- L. R. Castilho, D. A. Mitchell and D. M. Freire, Bioresour. Technol., 2009, 100, 5996–6009 CrossRef CAS PubMed.
- Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Length), Production Method (Sugar Fermentation, Vegetable Oil Fermentation, Methane Fermentation), Application, and Region – Global Forecast to 2024, https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html, accessed, 06 October 2020 Search PubMed.
- C. S. K. Reddy, R. Ghai, Rashmi and V. C. Kalia, Bioresour. Technol., 2003, 87, 137–146 CrossRef CAS PubMed.
- C. S. S. Oliveira, C. E. Silva, G. Carvalho and M. A. Reis, New Biotechnol., 2017, 37, 69–79 CrossRef CAS PubMed.
- B. B. Salgaonkar and J. M. Braganca, Bioengineering, 2017, 4, 50 CrossRef PubMed.
- P. Chakraborty, W. Gibbons and K. Muthukumarappan, J. Appl. Microbiol., 2009, 106, 1996–2005 CrossRef CAS PubMed.
- C. Fernández-Dacosta, J. A. Posada, R. Kleerebezem, M. C. Cuellar and A. Ramirez, Bioresour. Technol., 2015, 185, 368–377 CrossRef PubMed.
- B. Johnston, I. Radecka, D. Hill, E. Chiellini, V. I. Ilieva, W. Sikorska, M. Musiol, M. Zieba, A. A. Marek, D. Keddie, B. Mendrek, S. Darbar, G. Adamus and M. Kowalczuk, Polymers, 2018, 10(9), 957 CrossRef PubMed.
- J. M. Naranjo, J. A. Posada, J. C. Higuita and C. A. Cardona, Bioresour. Technol., 2013, 133, 38–44 CrossRef CAS PubMed.
- J. A. Posada, J. M. Naranjo, J. A. Lopez, J. C. Higuita and C. A. Cardona, Process Biochem., 2011, 46, 310–317 CrossRef CAS.
- L. Garcia-Gonzalez and H. De Wever, FEMS Microbiol. Lett., 2017, 364, fnx196 CrossRef PubMed.
- L. J. Vandi, C. M. Chan, A. Werker, D. Richardson, B. Laycock and S. Pratt, Polymers, 2018, 10(7), 751 CrossRef PubMed.
- A. Atlić, M. Koller, D. Scherzer, C. Kutschera, E. Grillo-Fernandes, P. Horvat, E. Chiellini and G. Braunegg, Appl. Microbiol. Biotechnol., 2011, 91, 295–304 CrossRef PubMed.
- L. Garcia-Gonzalez and H. De Wever, Appl. Sci., 2018, 8(9), 1416 CrossRef.
- S. P. Valappil, S. K. Misra, A. R. Boccaccini, I. Keshavarz, C. Bucke and I. Roy, J. Biotechnol., 2007, 132, 251–258 CrossRef CAS PubMed.
- G. Gahlawat and A. K. Srivastava, Appl. Biochem. Biotechnol., 2017, 183, 530–542 CrossRef CAS PubMed.
- T. Yamane, M. Fukunaga and Y. W. Lee, Biotechnol. Bioeng., 1996, 50, 197–202 CrossRef CAS PubMed.
- F. L. Wang and S. Y. Lee, Appl. Environ. Microbiol., 1997, 63, 3703–3706 CrossRef CAS PubMed.
- R. Shimizu, K. Chou, I. Orita, Y. Suzuki, S. Nakamura and T. Fukui, BMC Microbiol., 2013, 13, 169 CrossRef CAS PubMed.
- L. S. Serafim, P. C. Lemos, M. G. E. Albuquerque and M. A. M. Reis, Appl. Microbiol. Biotechnol., 2008, 81, 615–628 CrossRef CAS PubMed.
- H. Pereira, P. C. Lemos, M. A. Reis, J. P. Crespo, M. J. Carrondo and H. Santos, Water Res., 1996, 30, 2128–2138 CrossRef CAS.
- M. G. E. Albuquerque, M. Eiroa, C. Torres, B. R. Nunes and M. A. M. Reis, J. Biotechnol., 2007, 130, 411–421 CrossRef CAS PubMed.
- D. Dionisi, M. Beccari, S. Di Gregorio, M. Majone, M. P. Papini and G. Vallini, J. Chem. Technol. Biotechnol., 2005, 80, 1306–1318 CrossRef CAS.
- F. Carta, J. J. Beun, M. C. M. Van Loosdrecht and J. J. Heijnen, Water Res., 2001, 35, 2693–2701 CrossRef CAS PubMed.
- J. Choi and S. Y. Lee, Appl. Microbiol. Biotechnol., 1999, 51, 13–21 CrossRef CAS.
- R. Sirohi, J. P. Pandey, V. K. Gaur, E. Gnansounou and R. Sindhu, Bioresour. Technol., 2020, 123536 CrossRef CAS PubMed.
- Y. Dai, Z. G. Yuan, K. Jack and J. Keller, J. Biotechnol., 2007, 129, 489–497 CrossRef CAS PubMed.
- S. Bengtsson, A. Werker and T. Welander, Water Sci. Technol., 2008, 58, 323–330 CrossRef CAS PubMed.
- E. R. Coats, F. J. Loge, M. P. Wolcott, K. Englund and A. G. McDonald, Water Environ. Res., 2007, 79, 2396–2403 CrossRef CAS PubMed.
- A. F. Duque, C. S. S. Oliveira, I. T. D. Carmo, A. R. Gouveia, F. Pardelha, A. M. Ramos and M. A. M. Reis, New Biotechnol., 2014, 31, 276–288 CrossRef CAS PubMed.
- M. Rodgers and G. Wu, Bioresour. Technol., 2010, 101, 1049–1053 CrossRef CAS PubMed.
- U. S. E. P. A. EPA, Greenhouse Gas Emissions, https://www.epa.gov/ghgemissions/overview-greenhouse-gases, accessed, 01 November 2020, 2018 Search PubMed.
- Y. Miyahara, M. Yamamoto, R. Thorbecke, S. Mizuno and T. Tsuge, Biotechnol. Lett., 2020, 42, 1655–1662 CrossRef CAS PubMed.
- I. Park, E. H. Jho and K. Nam, J. Polym. Environ., 2014, 22, 244–251 CrossRef CAS.
- K. D. Wendlandt, U. Stottmeister, J. Helm, B. Soltmann, M. Jechorek and M. Beck, Eng. Life Sci., 2010, 10, 87–102 CAS.
- R. Perez, J. Casal, R. Munoz and R. Lebrero, Sci. Total Environ., 2019, 688, 684–690 CrossRef CAS PubMed.
- P. Luangthongkam, P. J. Strong, S. N. S. Mahamud, P. Evans, P. Jensen, G. Tyson, B. Laycock, P. A. Lant and S. Pratt, Bioresour. Bioprocess., 2019, 6, 50 CrossRef.
- J. Myung, W. M. Galega, J. D. Van Nostrand, T. Yuan, J. Z. Zhou and C. S. Criddle, Bioresour. Technol., 2015, 198, 811–818 CrossRef CAS PubMed.
- B. Bian, S. Bajracharya, J. Xu, D. Pant and P. E. Saikaly, Bioresour. Technol., 2020, 302, 122863 CrossRef CAS PubMed.
- S. Das, S. Das, I. Das and M. M. Ghangrekar, Mater. Sci. Energy Technol., 2019, 2, 687–696 Search PubMed.
- P. Dessi, L. Rovira-Alsina, C. Sanchez, G. K. Dinesh, W. Tong, P. Chatterjee, M. Tedesco, P. Farras, H. M. V. Hamelers and S. Puig, Biotechnol. Adv., 2021, 46, 107675 CrossRef CAS PubMed.
- T. Hua, S. Li, F. Li, Q. Zhou and B. S. Ondon, J. Chem. Technol. Biotechnol., 2019, 94, 1697–1711 CrossRef CAS.
- A. Prevoteau, J. M. Carvajal-Arroyo, R. Ganique and K. Rabaey, Curr. Opin. Biotechnol., 2020, 62, 48–57 CrossRef CAS PubMed.
- S. Das, I. Das and M. M. Ghangrekar, J. Environ. Chem. Eng., 2020, 8(4), 104028 CrossRef CAS.
- S. A. Patil, S. Gildemyn, D. Pant, K. Zengler, B. E. Logan and K. Rabaey, Biotechnol. Adv., 2015, 33, 736–744 CrossRef CAS PubMed.
- S. Das, L. Diels, D. Pant, S. A. Patil and M. M. Ghangrekar, J. Electrochem. Soc., 2020, 167(15), 155510 CrossRef CAS.
- T. P. Sciarria, P. Batlle-Vilanova, B. Colombo, B. Scaglia, M. D. Balaguer, J. Colprim, S. Puig and F. Adani, Green Chem., 2018, 20, 4058–4066 RSC.
- K. Rabaey and R. A. Rozendal, Nat. Rev. Microbiol., 2010, 8, 706–716 CrossRef CAS PubMed.
- S. Bajracharya, A. ter Heijne, X. D. Benetton, K. Vanbroekhoven, C. J. N. Buisman, D. Strik and D. Pant, Bioresour. Technol., 2015, 195, 14–24 CrossRef CAS PubMed.
- K. P. Nevin, T. L. Woodard, A. E. Franks, Z. M. Summers and D. R. Lovley, Mbio, 2010, 1(2), e00103-10 CrossRef PubMed.
- P. L. Tremblay, D. Hoglund, A. Koza, I. Bonde and T. Zhang, Sci. Rep., 2015, 5(1), 1–11 Search PubMed.
- C. W. Marshall, D. E. Ross, E. B. Fichot, R. S. Norman and H. D. May, Appl. Environ. Microbiol., 2012, 78, 8412–8420 CrossRef CAS PubMed.
- L. Jourdin, S. Freguia, V. Flexer and J. Keller, Environ. Sci. Technol, 2014, 50, 1982–1989 CrossRef PubMed.
- P. Batlle-Vilanova, R. Ganigue, S. Ramio-Pujol, L. Baneras, G. Jimenez, M. Hidalgo, M. Dolors Balaguer, J. Colprim and S. Puig, Bioelectrochemistry, 2017, 117, 57–64 CrossRef CAS PubMed.
- S. Srikanth, M. V. Reddy and S. V. Mohan, Bioresour. Technol., 2012, 125, 291–299 CrossRef CAS PubMed.
- C. Kourmentza, J. Placido, N. Venetsaneas, A. Burniol-Figols, C. Varrone, H. N. Gavala and M. A. M. Reis, Bioengineering, 2017, 4, 55 CrossRef PubMed.
- Y. K. Leong, P. L. Show, J. C. W. Lan, H. S. Loh, H. L. Lam and T. C. Ling, Clean Technol. Environ. Policy, 2017, 19, 1941–1953 CrossRef CAS.
- U. S. B. o. L. Statistics, PPI industry group data for Total manufacturing industries, not seasonally adjusted, https://data.bls.gov/cgi-bin/surveymost, accessed, 12 March 2021 Search PubMed.
- J. I. Choi and S. Y. Lee, Bioprocess Eng., 1997, 17, 335–342 CrossRef CAS.
- I. Levett, G. Birkett, N. Davies, A. Bell, A. Langford, B. Laycock, P. Lant and S. Pratt, J. Environ. Chem. Eng., 2016, 4, 3724–3733 CrossRef CAS.
- S. Mudliar, A. Vaidya, M. S. Kumar, S. Dahikar and T. Chakrabarti, Clean Technol. Environ. Policy, 2008, 10, 255 CrossRef CAS.
- J. Tamis, K. Luzkov, Y. Jiang, M. C. M. van Loosdrecht and R. Kleerebezem, J. Biotechnol., 2014, 192, 161–169 CrossRef CAS PubMed.
- D. Choi, D. C. Chipman, S. C. Bents and R. C. Brown, Appl. Biochem. Biotechnol., 2010, 160, 1032–1046 CrossRef CAS PubMed.
- J. E. Stiglitz, N. Stern, M. Duan, O. Edenhofer, G. Giraud, G. M. Heal, E. L. La Rovere, A. Morris, E. Moyer and M. Pangestu, Report of the High-Level Commission on Carbon Prices, International Bank for Reconstruction and Development and International Development Association/The World Bank, 2017 Search PubMed.
- X. Christodoulou and S. B. Velasquez-Orta, Environ. Sci. Technol., 2016, 50, 11234–11242 CrossRef CAS PubMed.
- P. Dessì, L. Rovira-Alsina, C. Sánchez, G. K. Dinesh, W. Tong, P. Chatterjee, M. Tedesco, P. Farràs, H. M. Hamelers and S. Puig, Biotechnol. Adv., 2020, 107675 Search PubMed.
- F. Geppert, D. Liu, E. Weidner and A. ter Heijne, Int. J. Hydrog. Energy, 2019, 44, 21464–21469 CrossRef CAS.
- D. Liu, M. Roca-Puigros, F. Geppert, L. Caizan-Juanarena, S. P. Ayudthaya, C. Buisman and A. ter Heijne, Front. Bioeng. Biotechnol., 2018, 6, 78 CrossRef PubMed.
- P. Dessi, C. ánchez, S. Mills, F. Giuseppe Cocco, M. Isipato, U. Z. Ijaz, G. Collins and P. N. L. Lens, Bioelectrochemistry, 2021, 137, 107686 CrossRef CAS PubMed.
- A. Atlić, M. Koller, D. Scherzer, C. Kutschera, E. Grillo-Fernandes, P. Horvat, E. Chiellini and G. Braunegg, Appl. Microbiol. Biotechnol., 2011, 91, 295–304 CrossRef PubMed.
- B. S. Kim, S. C. Lee, S. Y. Lee, H. N. Chang, Y. K. Chang and S. I. Woo, Biotechnol. Bioeng., 1994, 43, 892–898 CrossRef CAS PubMed.
- L. Garcia-Gonzalez and H. De Wever, FEMS Microbiol. Lett., 2017, 364, 20 CrossRef PubMed.
- T. G. Volova and G. S. Kalacheva, Microbiology, 2005, 74, 54–59 CAS.
- S. Ghysels, M. S. I. Mozumder, H. De Wever, E. I. P. Volcke and L. Garcia-Gonzalez, Bioresour. Technol., 2018, 249, 858–868 CrossRef CAS PubMed.
- C. Chan, A. Guisasola and J. A. Baeza, J. Clean. Prod., 2020, 242, 118495 CrossRef CAS.
- T. Zhang, H. R. Nie, T. S. Bain, H. Y. Lu, M. M. Cui, O. L. Snoeyenbos-West, A. E. Franks, K. P. Nevin, T. P. Russell and D. R. Lovley, Energy Environ. Sci., 2013, 6, 217–224 RSC.
- L. Jourdin, S. Freguia, B. C. Donose, J. Chen, G. G. Wallace, J. Keller and V. Flexer, J. Mater. Chem. A, 2014, 2, 13093–13102 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d1ra08796g |
| This journal is © The Royal Society of Chemistry 2022 |