Open Access Article
Open Access ArticleBoosting nitrogen fertilization by a slow releasing nitrate-intercalated biocompatible layered double hydroxide–hydrogel composite loaded with Azospirillum brasilense†
Rimjim Gogoia,
Arup Borgohainab,
Madhusmita Baruaha,
Tanmoy Karakb and
Jiban Saikia *a
*a
aDepartment of Chemistry, Dibrugarh University, Dibrugarh 786004, Assam, India. E-mail: jibansaikia@dibru.ac.in
bUpper Assam Advisory Centre, Tea Research Association, Dikom 786101, Assam, India
First published on 28th February 2022
Abstract
Indiscriminate use of chemical fertilizers leads to soil environmental disbalance and therefore, preparation and application of environment-friendly slow-release multifunctional fertilizers are of paramount importance for sustainable crop production in the present scenario. In this study, we propose a slow-release multifunctional composite nitrogen (N) fertilizer, which possesses the ability to supply plant accessible N in the form of ammonium (NH4+) and nitrate (NO3−) to improve nitrate assimilation coupled with zinc (Zn, a major micronutrient for plants in the soil) after its degradation. For this purpose, NO3−-intercalated zinc–aluminum (Zn–Al) layered double hydroxide (LDH) was synthesized using a co-precipitation protocol. The prepared LDH was added as 25.45% of total polymer weight to a sodium carboxymethyl cellulose/hydroxyethyl cellulose citric acid (NaCMC/HEC-CA) biodegradable hydrogel. A. brasilense, commonly used nitrogen-fixing bacteria in soils, was added to the LDH–hydrogel composite along with LDH alone to augment the availability of NH4+ and NO3−. Adjusting the pH under acidic (pH 5.25) and neutral (pH 7) conditions, the release pattern of NO3− from LDH and the composite was monitored for 30 days at normal temperature. The pH was selected based on the soil analysis data of North East India. The LDH-composite released 90% (w/w) and 85.45% (w/w) of intercalated NO3− at pH 5.25 and 7.00 respectively in 30 days. However, 100% (w/w) and 87% (w/w) of intercalated NO3− at pH 5.25 and 7.00 respectively were released in 30 days when only LDH was applied, which indicated the lower performance of LDH alone in comparison to the LDH-composite for the nitrate holding pattern. The pH of the bacteria-loaded system was observed to be acidic (pH = 5–6) during the study of nitrate assimilation and Zn2+ release. A. brasilense improved nitrate assimilation and increased the NH4+ ion concentration in the studied system. A significant increase in Zn2+ release was observed from day 5 in the presence of A. brasilense in the LDH-composite compared with that in the absence of A. brasilense. In conclusion, the prepared LDH–hydrogel–A. brasilense composite fertilizer system increases the availability of plant accessible N form (both NO3− and NH4+) and can potentially improve soil fertility with the addition of Zn and bacteria to the soil in the extended course.
1. Introduction
The concept of slow-release fertilizers emerged for the limitation of crises erupting from various dimensions of applying conventional chemical fertilizers including nutrient burn and ecological contamination, following leaching of nutrient ions.1 Llive et al.2 and Gungula et al.3 have recently reported that the slow-release fertilizers prevent nutrient loss as well as control the uptake of nutrients by plants to increase the fertilizer use efficiency. An excellent review by Vejan et al.4 highlighted that slow-release fertilizers reduce nutrient ion runoff to water streams and rich nutrient use results in reduced environmental pollution, offering sustainability in agriculture and ecological hazard control compared to conventional fertilizers.Recently, layered double hydroxide (LDH) materials have received tremendous attention as a very suitable agent for the formulation of controlled-release fertilizers.5,6 Their structure comprises a ‘two-dimensional lamellar structure’ having dipositive and tripositive ions at the center of octahedra, which form an infinite sheet by joining edge to edge.7–9 Hydrated anions such as NO3− and PO43− are present in the interlayer gallery region of the positively charged sheets for charge compensation.9,10 The general formula of LDH materials is [MII(1−x)MIIIx(OH)2] [An−]x/n·zH2O, where MII and MIII are dipositive and tripositive metal ions. An− is the intercalated charge compensating anion and x represents the molar ratio M3+/(M3+ + M2+).7 Due to their exceptional anion exchange property, versatility in intercalating a broad class of anions and non-toxic character, LDH materials and their polymer composites have been effectively used as slow-release agents in the agricultural and biomedical applications.11–13
Acidic soil is very much prone to plant nutrient dynamics, and under such conditions, LDH materials in the soil supply need-based nutrients due to their structural stability. The rupture of the lamellar structure of the LDH material and faster nutrient release caused by a lower soil pH are prevented to certain degree by the alkaline buffering of hydroxyl groups liberating from the LDH layered structures.14 When the soil acidity predominates the alkaline buffering, the LDH material gradually loses its lamellar structure and the original slow release character. To overcome this, a composite formulation of the LDH material with cellulose and alginate-based biodegradable hydrogel materials had been documented by several researchers.11,14–18 Carboxymethyl cellulose (CMC), hydroxyethyl cellulose (HEC) and alginate-based biodegradable hydrogels have already established their property of nutrient uptake and delivery, along with excellent water holding capacity, non-toxicity, and renewability.19–22 However, their versatile applicability is hindered by the low mechanical strength of the materials. The composite formulation of the LDH material with biodegradable hydrogels presents a mutual complementary system, where the LDH material induces mechanical stability in the composite, while the hydrogel polymer provides structural barrier with soil acidity, along with water retention property. Several works improvising this concept have been reported.19–22. [Zn–Al–NO3−]–LDH encapsulated with biodegradable alginate beads for enhanced nitrogen fertilization,23 composites of norfloxacin-intercalated Mg–Al LDH with carboxymethyl cellulose, cross-linked by citric acid for biomedical applications,24 carboxymethyl cellulose-based nanocomposite hydrogel beads with Cu–Al LDH for bacterial infection treatment18 and several other layered nanocomposites are reported to have shown controlled release property.25–28
Furthermore, soil existing microbes convert the released nutrients into simpler plant-useable forms.29 Plants take up N in the NO3− or NH4+ form depending upon the various constraints related to the plant environment. Some plants prefer a mixture of NO3− and NH4+ forms as a source of N from the growing medium.30,31 Nitrogen taken up as NH4+ directly does not need any further conversion post intake and can be directly used in amino acid production, termed as ammonium assimilation. NO3− after being absorbed from the soil follows two paths. The first involves immediate reduction of NO3− in the roots and the second involves NO3− reduction in leaves after the root-to-shoot transportation via the xylem. The assimilation of N taken up as NO3− proceeds via the reduction of NO3− into NO2− by a nitrate reductase enzyme, followed by the reduction of NO2− into NH4+ by a nitrite reductase enzyme. The enzyme nitrate reductase is a homodimer possessing flavin adenine dinucleotide (FAD), haem, and a molybdenum cofactor (MoCo).32 The nitrite reductase enzyme contains sirohaem and a FeS cluster. The reduction of NO3− into NH4+ requires 8 moles of electrons per mole of NO3−, proving to be an energy-consuming process for plants. Thus, during the reduction of NO3− in the roots, the microbes existing therein, possessing the required enzymes, can offer assistance in nitrate assimilation.30,31
Apart from the mentioned benefits of using LDH and its composite materials for the loading and release of different essential nutrient ions from the LDH gallery regions, the LDH material could also serve as a source for additional nutritional elements for the plants. Hatami et al.33 reported increased availability of zinc in [Zn–Al–PO4]–LDH-applied soil samples. Lopez-Rayo et al.34 also reported increased zinc uptake by plants on the application of Zn-doped Mg–Al–LDH materials to plants. This was attributed to the partial dissolution of Zn from the LDH sheet structure due to various soil stimuli, which may be soil acidity or action of different microorganisms present already in the rhizosphere.
From all the above-mentioned information, we herein report the synthesis of a multifunctional N fertilizer system. The Zn–Al–LDH material was used as a basal source for the intercalation of NO3− ions. To restrict the faster release of NO3− from LDH and to induce water retention properties, the CMC/HEC hydrogel was incorporated into the system. Further to this system, a soil existing bacterial species, Azospirillum brasilense, was added to enhance nitrate assimilation and N2 fixation (NH4+ source) from the atmosphere. Eventually, the degradation of the LDH material was also anticipated, and the release of plant essential Zn from LDH in the presence of the bacteria was carried out. The purpose of this study was to synthesize this highly efficient and multifunctional composite system and analyze its efficacy for providing plant essential nutrients N (NO3− and NH4+), Zn (Zn2+), and the bacteria for improving the overall soil fertility regime.
2. Materials and methods
2.1 Reagents
Zinc nitrate hexahydrate [Zn(NO3)2·6H2O; CAS Number: 1019618-6], aluminium nitrate nonahydrate [Al(NO3)3·9H2O; CAS Number: 7784-27-2], dipotassium hydrogen phosphate [K2HPO4; CAS Number: 7758-11-4], and magnesium sulphate [MgSO4; CAS Number: 7487-88-9] were purchased from Merck KGaA, Darmstadt, Germany. Sodium molybdate and manganese sulphate were purchased from FINAR, Ahmedabad, Gujrat, India. Sodium nitrate was obtained from MERCK, Maharashtra, India. Sodium hydroxide and citric acid were obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. Carboxymethyl cellulose sodium salt (high viscosity) with an average molecular weight of ∼900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and hydroxyethyl cellulose (high viscosity) with an average molecular weight of ∼380
000 Da and hydroxyethyl cellulose (high viscosity) with an average molecular weight of ∼380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, and ferrous sulphate were purchased from Research Lab Fine Chem Industries Mumbai, India. Malic acid and bromothymol blue were obtained from CDH, New Delhi, India. Sodium chloride and potassium hydroxide were purchased from RANKEM, New Delhi, India. For all synthesis purposes, ultra-pure water or Milli-Q (18.2 MΩ cm) water was used throughout the experiment.
000 Da, and ferrous sulphate were purchased from Research Lab Fine Chem Industries Mumbai, India. Malic acid and bromothymol blue were obtained from CDH, New Delhi, India. Sodium chloride and potassium hydroxide were purchased from RANKEM, New Delhi, India. For all synthesis purposes, ultra-pure water or Milli-Q (18.2 MΩ cm) water was used throughout the experiment.
2.2 Synthesis of [Zn/Al–NO3]–LDH, CMC/HEC–CA hydrogels, and LDH–hydrogel composites
[Zn–Al–NO3]–LDH (ZLDH) was synthesized by a co-precipitation method.8,13,35 A 30 mL solution of Zn(NO3)2·6H2O and Al(NO3)3·9H2O in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was added to 30 mL of 1 M solution of NaNO3 in Milli-Q water, with constant addition of a 2 M NaOH solution to control the pH and precipitate LDH, keeping the temperature at 75 °C. The nitrogen atmosphere was maintained throughout to minimize CO32− intercalation. The solution was aged for 24 hours in a nitrogen atmosphere and the same temperature condition. The white precipitate of LDH formed was separated by high-speed centrifugation and repeatedly washed with Milli-Q water. The product was then allowed to dry in a vacuum desiccator for 2–3 days and stored for further analyses.
1 ratio was added to 30 mL of 1 M solution of NaNO3 in Milli-Q water, with constant addition of a 2 M NaOH solution to control the pH and precipitate LDH, keeping the temperature at 75 °C. The nitrogen atmosphere was maintained throughout to minimize CO32− intercalation. The solution was aged for 24 hours in a nitrogen atmosphere and the same temperature condition. The white precipitate of LDH formed was separated by high-speed centrifugation and repeatedly washed with Milli-Q water. The product was then allowed to dry in a vacuum desiccator for 2–3 days and stored for further analyses.
The CMC/HEC-CA (CMCH)-based hydrogel from the sodium salt of carboxymethyl cellulose (NaCMC) and hydroxyethyl cellulose (HEC), cross-linked with citric acid in Milli-Q water was synthesized as reported.20 The sodium salt of carboxymethyl cellulose (NaCMC) and hydroxyethyl cellulose (HEC) was mixed in Milli-Q water in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with constant stirring. After attaining a clear solution, 3% (w/w polymer) citric acid cross-linker was added. The resulting gel-like mixture was transferred to a Petri dish and dried in a hot air oven at 70 °C temperature. The LDH–hydrogel composite was prepared by adding the synthesized ZLDH sample as 25.45% of total polymer weight to the solution mixture containing NaCMC (1.5 g) and HEC (0.5 g), along with the cross-linker citric acid (0.06 g), as described by Nia et al.18 and Demetri et al.20 The resulting mixture was stirred, followed by ultra-sonication to obtain a homogeneous solution. The solution was then transferred to a Petri dish and heated in a hot air oven at 45–50 °C for 12 hours. The sample obtained was named C-3 and stored for further analyses.
1 ratio with constant stirring. After attaining a clear solution, 3% (w/w polymer) citric acid cross-linker was added. The resulting gel-like mixture was transferred to a Petri dish and dried in a hot air oven at 70 °C temperature. The LDH–hydrogel composite was prepared by adding the synthesized ZLDH sample as 25.45% of total polymer weight to the solution mixture containing NaCMC (1.5 g) and HEC (0.5 g), along with the cross-linker citric acid (0.06 g), as described by Nia et al.18 and Demetri et al.20 The resulting mixture was stirred, followed by ultra-sonication to obtain a homogeneous solution. The solution was then transferred to a Petri dish and heated in a hot air oven at 45–50 °C for 12 hours. The sample obtained was named C-3 and stored for further analyses.
2.3 Preparation of Azospirillum culture media
Nitrogen-free malate media were prepared for Azospirillum brasilense by mixing malic acid (5.0 g), K2HPO4 (0.5 g), FeSO4 (0.05 g), MnSO4 (0.01 g), MgSO4 (0.1 g), NaCl (0.02 g), CaCl2 (0.01 g) and Na2MoO4 (0.002 g) in 1.0 L deionized water. To this, 2 mL of 0.5% alcoholic solution of bromothymol blue and 4.0 g KOH were added. The pH of the solution was kept around 6–7. The solution was autoclaved at 120 °C and 15 lbs. pressure for 15 minutes and put under a UV light inside a laminar airflow cabin for complete sterilization. This sterile culture media solution was then inoculated with A. brasilense bacteria. The cultured bacterial broth solution was then incubated at 30 °C for 72 hours.362.4 Characterization of [Zn–Al–NO3]–LDH, CMC/HEC–CA hydrogels, and LDH–hydrogel composite
Fourier transform infrared (FTIR) spectra of the samples were recorded using an FTIR spectrometer (Agilent Model no: Cary 630, USA; SL. no. MY20192018) in the range of 400–4000 cm−1. Thermogravimetric (TGA) analyses of the samples were carried out using a PerkinElmer JS-1 TGA/DSC thermogravimetric analyzer, operating in a nitrogen environment in the temperature range of 25–900 °C at a heating rate of 10 °C min−1. The PXRD patterns of the samples were obtained using a Bruker D8 Advance X-ray diffractometer by irradiating with monochromatic Cu Kα radiation (λ = 1.54056 Å). The surface morphology of the samples was analyzed using a JEOL JSM-IT300 Scanning Electron Microscope. The concentration of Zn present in test samples was determined using an atomic absorption spectrometer (AAS) (Agilent, Model no. AA240, Malaysia). For Zn estimation, two standard reference materials (SRMs), namely, tomato leaves (SRM-1573a) and Montana soil (SRM-2710a) were used for quality assurance. The estimated values of Zn were in excellent agreement with the certified values in SRM1573a and SRM-2710a. All the samples were analyzed with three replications.2.5 Species detection test for inoculated A. brasilense bacterial species
The growth of A. brasilense was first tested by streaking from the incubated culture into an agar plate. The constituents of agar medium are the same as the constituents of the culture media for A. brasilense with the addition of agar–agar powder for solidification of the media. Malic acid (5.0 g), K2HPO4 (0.5 g), FeSO4 (0.05 g), MnSO4 (0.01 g), MgSO4 (0.1 g), NaCl (0.02 g), CaCl2 (0.01 g), Na2MoO4 (0.002 g), 4.0 g KOH and 2 mL of 0.5% alcoholic solution of bromothymol blue were added to 1.0 L deionized water. To this solution, 13 g of agar–agar powder was added and then the solution was autoclaved and then poured on Petri plates. On cooling, the media solidified and the agar plates were ready to use.The catalase test was performed to confirm the presence of the species. For this, the pre-cultured bacterial broth was streaked onto an agar plate and incubated for 24 hours at 30 °C. To the plate, 3–4 drops of 3% H2O2 solution were allowed to drip. The formation of bubbles shows the presence of A. brasilense species.37
2.6 Investigation of water retention property
The water retention property of the synthesized ZLDH and the composite sample C-3 was investigated by teabag method at pH 5.25 and 7.0 at 25 °C for 24 hours. The swollen weight was recorded. Following this, the weight of the sample was recorded for the next six consecutive days to observe the water retention ability.To observe the recyclability in swelling and water retention capacity, the C-3 sample was put for four consecutive cycles of swelling and drying at pH 5.25. Accurately, 0.3553 g of the C-3 sample was weighed and allowed to swell at pH 5.25 for 24 hours. The swollen weight of the sample for consecutive days was recorded and the cycle was repeated with the remaining dry sample after weighing at a temperature varying from 25 to 35 °C. The data for four consecutive cycles were recorded in triplicates.
2.7 Nitrate release study
The percentage loading and slow nitrate release studies for ZLDH and composite C-3 were performed by a spectrophotometric method38,39 using a SHIMADZU UV-VIS Spectrophotometer within the range of 200–800 nm. KNO3 was taken as the standard for the calibration curve. Milli-Q water was used as a blank throughout the experiments. Each observation was made in triplicates.For a comparative study of nitrate release from ZLDH in distilled water, Milli-Q water, and carbonated water, 350 mg of the ZLDH sample was added to 250 mL each of distilled water, Milli-Q water, and 0.08% Na2CO3 solution, and the solutions were stirred. From each solution at specific time intervals, test solutions were withdrawn and centrifuged and the volume of the parent solutions was made up. Required volume was taken from the supernatant for spectroscopic analyses. Linearly, for comparative studies, 350 mg of C-3 sample was taken in 250 mL distilled water and stirred. Test solutions were taken out and analyzed as mentioned above. To compare the nitrate release rate from ZLDH and C-3 samples with conventional nitrate fertilizer KNO3, 1.4 mM solution KNO3 was prepared, test solutions were withdrawn at similar time intervals and spectrophotometric analyses were carried out.
2.8 A. brasilense
3. Results
3.1 FTIR analysis
The FTIR spectra (Fig. 1a) of ZLDH exhibited a broad band around 3400–3600 cm−1 due to the O–H stretching vibration of H2O molecules and physically adsorbed hydroxyl groups in the LDH layers. Bands appearing at 1642 cm−1 and 1385 cm−1 are assigned to the bending mode of H2O molecules and the characteristic stretching vibration for intercalated NO3− ions in the LDH layers. CMCH (Fig. 1b) shows its characteristic absorption peak at 1715 cm−1 due to ester bond formation, resulting after the loss of water molecules and a strong characteristic absorption peak at 1600 cm−1. The band at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 cm−1 is due to the C–O–C stretching vibration. A broad peak around 3346 cm−1 is due to the O–H stretching vibration, and the peak at 2927 cm−1 is due to the C–H stretching vibration. C-3 (Fig. 1c) exhibited a broad peak around 3436 cm−1 due to the O–H stretching vibration of physically adsorbed water molecules and hydroxyl groups present in the basal layers. The peak around 1385 cm−1 shows NO3− ion intercalation in the LDH layers. The peak at 2927 cm−1 was obtained due to C–H stretching, which comes from the hydrogel part.
65 cm−1 is due to the C–O–C stretching vibration. A broad peak around 3346 cm−1 is due to the O–H stretching vibration, and the peak at 2927 cm−1 is due to the C–H stretching vibration. C-3 (Fig. 1c) exhibited a broad peak around 3436 cm−1 due to the O–H stretching vibration of physically adsorbed water molecules and hydroxyl groups present in the basal layers. The peak around 1385 cm−1 shows NO3− ion intercalation in the LDH layers. The peak at 2927 cm−1 was obtained due to C–H stretching, which comes from the hydrogel part.
3.2 PXRD analysis
The PXRD patterns of ZLDH (Fig. 2a) showed sharp and symmetric characteristic peaks corresponding to the 003, 006 planes, characteristic of clay minerals having a layered structure. The same peak appears in the spectra of the C-3 (Fig. 2b) composite sample at a 2θ value of 12.39°, with a basal spacing of 7.1442 Å, but with a lower intensity.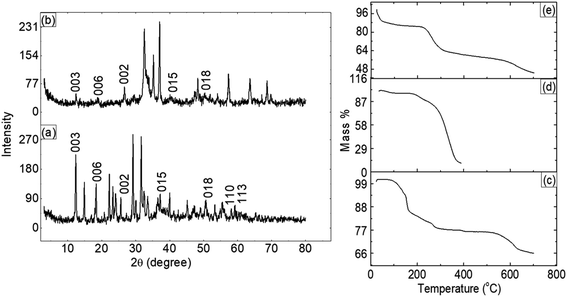 | ||
| Fig. 2 PXRD patterns for (a) ZLDH and (b) C-3. TGA patterns for (c) ZLDH, (d) CMCH and (e) C-3 samples. | ||
3.3 TGA analysis
In the TGA thermogram (Fig. 2c) for ZLDH, the first break was observed in the temperature range of 110–147 °C, due to the elimination of physically adsorbed water molecules from the layered surface. The second break was obtained in the range of 170–274 °C, whereas the third break was observed in the range of 570–640 °C. From the TGA thermogram of CMCH (Fig. 2d), it is observed that the first break was obtained near 100 °C due to the loss of water molecules. Around 300 °C, the sample decomposes. In case of composite sample C-3 (Fig. 2e), the first weight loss was obtained in the temperature range of 20–110 °C. The second break was obtained around 235–296 °C and the third break was observed around 632 °C.3.4 Surface morphology analysis
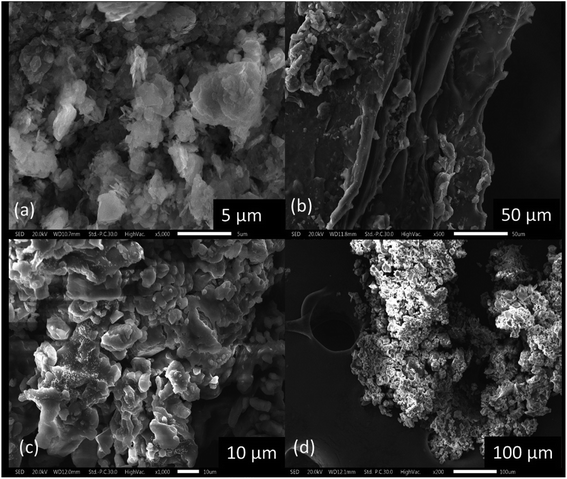
In the SEM images (Fig. 3a) of ZLDH, an aggregated mass of irregularly shaped particles, characteristic of LDH materials was obtained. LDH surfaces are observed as agglomerated and flake-like with a crystalline outer structure. The SEM micrograph of CMCH (Fig. 3b) shows ripped portions due to cross-linking. The composite C-3 (Fig. 3c and d) shows the aggregated mass, but not as flakes.3.5 Study of the water retention property
The water retention and release profiles were studied at pH 7 and 5.25 for the samples as mentioned in Section 2.6.The swelling behavior of the different samples in the respective pH media is expressed by the percentage swelling as follows:
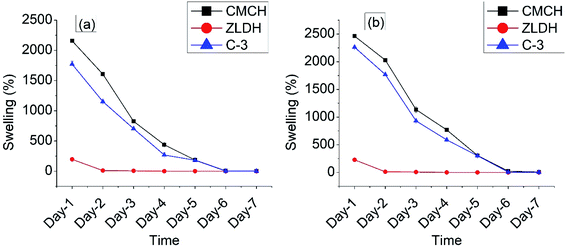
The water swelling and retaining capacity of the CMCH sample was observed to be highest at both the pH values (Fig. 4). The C-3 sample exhibited decent water swelling and retention behavior in both pH media. The swollen weight and the water-retaining capacity of the CMCH and the composite C-3 were observed to be highest in a neutral medium (Fig. 4a, b and 5a). At an acidic pH value, water molecules were released from the samples at a faster rate.
The recyclability in swelling and water-retaining capacity of the C-3 sample were studied for four consecutive cycles, and it was observed that the sample indeed exhibited profound water retention behavior till the fourth cycle, extending to the fourth week (Fig. 5b). However, the original mass of the sample could not be recovered. With each cycle, mass loss of the sample was observed, and a comparative degree of swelling was observed to get reduced as well.
3.6 Release studies
The NO3–N content in the synthesized ZLDH and C-3 was estimated by the Kjeldahl method.43,44 The percentage loading of NO3− in the ZLDH and C-3 sample was observed to be 11.83 (w/w) and 5.39 (w/w) respectively. Following this, 350 mg of the ZLDH and C-3 sample taken for experiment contained 41.405 mg and 18.86 mg of intercalated nitrate. The nitrate release studies were carried out in different media and the nitrate release percentage for the samples is expressed as follows:From the nitrate release studies of ZLDH (Fig. 6a) in Milli-Q water, distilled water, and carbonated water at pH 7 and temperature 25–30 °C, the nitrate release rate was observed to be highest in the case of carbonated water. The LDH material released almost 98% of intercalated NO3− in 3 days in abundance of carbonate ions. In distilled water, around the same time, almost 91% and in Milli-Q water, almost 86% of the intercalated NO3− anions were released. For a duration of 30 days, ZLDH retained 1.218% (0.504 mg) in carbonated water, 8.227% (3.407 mg) in distilled water and 13.995% (5.767 mg) of the intercalated nitrate in Milli-Q water.
Comparative nitrate release rate for ZLDH, composite C-3 and KNO3 solutions in a neutral pH medium exhibited slower nitrate release from ZLDH and C-3 composites. The KNO3 solution released 99% of its NO3− content in merely 5 minutes (Fig. 6b). The LDH material retained ∼12% (5.148 mg) of the loaded NO3− ions up to 7 days, whereas the C-3 composite sample retained ∼20% (3.860 mg) of the intercalated NO3− ions in the identical duration. The comparative nitrate release profile (Fig. 6c and d) for the synthesized ZLDH and C-3 composite samples at pH 7 and pH 5.25 showed the nitrate release rate to be faster at acidic pH for both the samples (Fig. 6d). In neutral medium, the ZLDH material released almost 91% of intercalated NO3− in 30 days, in acidic medium, and it released almost 100% of the intercalated NO3− ions. It retained 8.227% (3.466 mg) in a neutral medium and 0% of the intercalated nitrate in an acidic medium. The C-3 composite retained greater net contents of NO3− ions for the identical time period in both neutral and acidic media. It retained 14.545% (2.744 mg) in a neutral medium and 9.53% (1.798 mg) of the total intercalated nitrate in an acidic medium for 30 days.
3.7 Study of growth of A. brasilense after inoculation in LDH-composite samples
Observations from the catalase test mentioned in Section 2.5 showed the formation of bubbles after applying 3% H2O2 onto the Azospirillum-streaked agar plates confirming the bacterial species to be A. brasilense.37 The presence and growth of A. brasilense in the C-3 composite sample were established by streaking sample solutions loaded with A. brasilense into malate–agar plates on the day of loading and the plates were incubated for 24 hours at 30 °C. The similar streaking process was repeated after 15 and 30 days of loading Azospirillum broth to the sample solution. The growth of A. brasilense in the agar plates confirmed the presence of the bacteria in the sample on the first day of streaking, after 15 days, and after 30 days (Fig. 7).3.8 Nitrate assimilation studies of A. brasilense-loaded samples
The nitrate assimilation by the bacteria A. brasilense was established by confirming the presence of NO2− ions in Azospirillum-loaded samples of ZLDH, composite C-3, and KNO3 (Table 1) with the test kit mentioned in Section 2.8.2.40 Control solutions without the presence of the bacterial broth showed no traces of NO2− ions, and only NO3− ion was detected. It was observed that in the bacterial presence, all the NO3− ions present in the KNO3 solution and released from ZLDH and C-3 samples were not converted into NO2− ions. Spectrophotometric tests and the kit tests showed the presence of both NO2− and NO3− ions in the test solutions.| Interval | Samples | ||||||
|---|---|---|---|---|---|---|---|
| ZLDH (+) | C-3 (+) | KNO3 (+) | CMCH (+) | (+) | ZLDH (−) | C-3 (−) | |
| a (+) loaded with Azospirillum; (−) no Azospirillum; 0 ppm = *, 0–0.05 ppm = **, 0.05–0.1 ppm = ***, 0.1–0.2 ppm = ****, 0.2–0.5 ppm = *****, 0.5–1.0 ppm = ******, 1.0 ppm = *******. | |||||||
| Day-1 | * | * | * | * | * | * | * |
| Day-2 | * | * | * | * | * | * | * |
| Day-3 | ** | * | ** | * | * | * | * |
| Day-4 | ** | ** | *** | * | * | * | * |
| Day-5 | ** | ** | **** | * | * | * | * |
| Day-7 | *** | ** | **** | * | * | * | * |
| Day-10 | **** | *** | **** | * | * | * | * |
| Day-13 | **** | *** | **** | * | * | * | * |
| Day-15 | ***** | **** | **** | * | * | * | * |
| Day-16 | ***** | **** | ***** | * | * | * | * |
| Day-17 | ****** | ****** | ****** | * | * | * | * |
| Day-30 | ******* | ****** | ******* | * | * | * | * |
A. brasilense is reported to have various useful plant functions other than nitrate assimilation, including phytohormone production and atmospheric nitrogen fixation. In culture media reach with a carbon source, fixation of atmospheric nitrogen and subsequent conversion into NH3/NH4+ by nitrogen-fixing bacteria were established by Kanimozhi et al.44 The presence of NH3/NH4+ in the test solutions was confirmed by testing with Nessler's reagent, and quantitative detection was performed using the test kit mentioned in Section 2.8.3.41,42 The pH of all the samples post-loaded with A. brasilense was found to be in the range of 5–6 during the observation period. The amount of NH3/NH4+ present in all the samples on the day of bacterial inoculation was observed to be in the range of 0–0.25 mg L−1. After three days, the amount raised to 0.25–0.50 mg L−1 range, which remain unchanged in the following period for non-nitrogenous samples. The value increased to 0.5–1.0 mg L−1 range in solutions containing ZLDH, C-3 and KNO3 (Fig. 8). This could be attributed to the fact that a particular volume of bacterial broth containing particular colony-forming units of the bacteria can only convert a certain amount of atmospheric nitrogen.37 The additional quantity of NH3/NH4+ present in ZLDH, C-3 and KNO3 samples contributes to the nitrate–nitrite–ammonium conversion occurring in the systems. Control solutions without Azospirillum incorporation showed no traces of NH3/NH4+ in the solution.
 | ||
| Fig. 8 Amount of NH3/NH4+ present in A. brasilense-loaded (+) and non-loaded (−) samples; AZS signifies A. brasilense alone. | ||
3.9 Study of release of Zn from bacteria-loaded C-3 samples
C-3 composite and ZLDH sample solutions without loading with A. brasilense exhibited 0 mg L−1 Zn content from day-1 to day-30. Azospirillum broth itself showed no traces of Zn2+. Only ZLDH and C-3 samples loaded with A. brasilense showed the presence of Zn2+ from day-5 onwards after inoculation (Fig. 9). The amount of Zn2+ was observed to increase from day-5 to day-30 for LDH. The C-3 sample first exhibited an increase in Zn content from day-5 to day-15, and then again decreased on day-20, and the value was raised on day-30.4. Discussions
The FTIR spectra (Fig. 1) of the ZLDH, CMCH and C-3 samples present the composite sample C-3 exhibiting characteristic peaks for both LDH and hydrogel part. A band at 564 cm−1 is due to the superimposition of characteristic bands of hydrotalcite due to Zn–Al–OH translational modes in LDH layers.13,14,37,45 The PXRD (Fig. 2a and b) patterns exhibit peaks appearing in the ZLDH spectra also appearing in the C-3 spectra, having a lower intensity, predicting the loss of crystallinity from original LDH while forming a composite with the hydrogel material, having amorphous nature. The peak at 2θ value of 12.3° demonstrates the intercalation of NO3− in the LDH layers, with a basal spacing distance of 7.196 Å for the 003 plane.14 Thermal analysis (Fig. 2c) shows the thermal stability of the C-3 sample in the intermediate range of the parent LDH and hydrogel material, confirming the formation of the composite between the two materials. The second break corresponds to desorption of strongly held water molecules in the gallery region and dehydroxylation of LDH octahedral layers. However, the third break is observed in the range of 570–640 °C due to major mass loss in connection with the elimination of intercalated anions from the gallery regions.11,13 The first weight loss for C-3 samples (Fig. 2e) is due to the elimination of adsorbed water molecules from the system. The second break depicting major mass loss due to the decomposition of the hydrogel sample and dehydroxylation of LDH interlayers. The third break is due to the elimination of intercalated anions from LDH gallery regions, as suggested by Allou et al.11 and Sharif et al.13The composite formulation of these two materials led to some morphological modifications to their respective surfaces observed in the SEM micrograph (Fig. 3). The composite formulation caused the LDH material to compress and become more compact, unlike its previous flake-like structures.13 The water retention studies (Fig. 4) show the C-3 sample exhibiting decent water swelling and retaining property in both acidic and neutral media. This can be governed by the formation of composites between CMCH and ZLDH. LDH without possessing water-retaining property itself on composite formulation with hydrogel inherits this ability. The faster water release in an acidic medium could be attributed to the possibility of the formation of hydrogen bonding in the samples. The hydrogen bonding acts as a physical cross-linker, constricting the hydrogel structure, and thus, preventing water absorption and retention in the sample matrices.18
At a neutral pH, nitrate release from the ZLDH sample was observed to be fastest in carbonated water (Fig. 6). This can be correlated with the high affinity of CO32− ions for the LDH layers, displacing the intercalated nitrates faster when present in abundance.14 Due to dissolved CO2 in distilled water and the presence of atmospheric CO2, NO3− ions were also displaced from LDH layers in distilled water and Milli-Q water, yet the displacement is slower, leading to slower nitrate release. The comparative nitrate release from ZLDH, C-3 and KNO3 makes ZLDH and C-3 to be slower nitrate release sources than the potential commercial nitrate fertilizer KNO3, which releases almost 100% nitrate content merely during 5 minutes. C-3 performed slower nitrate release than the ZLDH sample, establishing the composite formulation inducing the slower nutrient ion release nature in the LDH material. Nitrate release profiles of ZLDH and C-3 at neutral and acidic pH exhibits greater retaining and slower nitrate release from the C-3 sample, demonstrating the protection offered by the polymer matrix to the LDH counterpart in the composite sample, retaining greater NO3− content in its matrix and performing slower nitrate release. For improved nitrate assimilation, A. brasilense bacterial broth was cultured and added to the C-3 composite sample and the differences were observed by testing the presence of NO2− ions. The growth of A. brasilense streaked from the loaded samples onto the agar plates shows the active presence of the bacteria up to 30 days post loading (Fig. 7). The presence of NO2− ions only in bacteria-loaded samples releasing NO3− ions shows reduction of released NO3− ions facilitated by the bacterial activity. The conversion of NO3− ions released from the ZLDH and C-3 composite to NO2− ions by the bacteria (nitrate reductase enzyme present in the bacteria is responsible for the function) establishes its ability in promoting nitrate assimilation and enhancing nutrient uptake in plants30,31,46 (Table 1). The presence of NH3/NH4+ in bacteria-loaded samples indicates the conversion of atmospheric nitrogen into ammonia by the bacteria, containing the nitrogenase enzyme responsible for this function. The additional quantity of NH3/NH4+ present in bacteria-loaded ZLDH, C-3 and KNO3 samples demonstrates the complete conversion of NO3− into NH4+. In the presence of the bacteria, a fraction of NO3− ions were reduced to NO2− and NO2− ions were reduced to NH4+ (Fig. 8).
The presence of Zn2+ in bacteria-loaded ZLDH and C-3 samples (Fig. 9) attributes to the partial dissolution of Zn2+ from ZLDH and composite structures in an Azospirillum broth environment with pH ranging from 5 to 6.43 The enzymes present in A. brasilense along with acidic pH may also be a governing factor in these findings. The released Zn2+ could be utilized by the plant as an added nutrient apart from the targeted N fertilization. This further improves the multi-functionality of the composite system.
5. Conclusions
In this work, a multifunctional nitrogen fertilizer has been formulated for the dual delivery of NO3− and NH4+. Nitrate-intercalated Zn–Al-layered double hydroxide was utilized as a slow-release NO3− source, which was incorporated onto citric acid cross-linked carboxymethyl cellulose and hydroxyethyl cellulose-based hydrogel for the preparation of the composite system. The water retention studies of the composite were carried out in neutral and acidic media taking the parent hydrogel and layered double hydroxide material as positive and negative controls. The composite exhibited a decent water retention property in both the media. Nitrate release studies demonstrated the composite sample to be capable of holding greater net nitrate content in both neutral and acidic media, establishing slower nitrate release from the composite compared to the layered double hydroxide material alone. Additional incorporation of A. brasilense into the composite system aided enhanced nitrate assimilation by converting a portion of NO3− ions released from the gallery regions to NO2− supported by the nitrite test and increased NH3/NH4+ availability via atmospheric N2 fixation and complete reduction of a fraction of nitrate released, established by the ammonium test. The degradation study of the composite further showed the availability of Zn2+ in the solution that plants can utilize as an essential nutrient. Thus, the formulated multifunctional fertilizer system possesses the ability to supply plant-accessible N in dual forms (NO3− and NH4+), improve nitrate assimilation, and provide Zn upon degradation. This biodegradable multifunctional fertilizer composite with the residual bacteria could also uplift the overall soil fertility regime.Author contributions
Rimjim Gogoi: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft; Arup Borgohain: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft; Madhusmita Baruah: Data curation, Formal analysis, Investigation, Methodology, Writing-original draft; Tanmoy Karak: Methodology, Project administration, Resources, Validation, Visualization, Writing - review & editing. Jiban Saikia: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Validation, Visualization, Writing - review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. S. thanks UGC, India (Grant no. F.30-467/2019-BSR) for financial support. R. G. and M. B. thanks Mr Abhijit Saikia of Green Agro Enterprise, for providing the bacterial strain for the work. We thank Dibrugarh University for providing all the infrastructural facility. The authors are grateful to the Department of Science and Technology for financial support under DST-FIST programme and UGC, New Delhi for Special Assistance Programme (UGC-SAP) to the Department of Chemistry, Dibrugarh University.References
- M. R. Maghsoodi, N. Najafi, A. Reyhanitabar and S. Oustan, Geoderma, 2020, 379, 114644 CrossRef CAS.
- L. M. Llive, M. Perullini, P. R. Santagapita, A. SchneiderTeixeira and L. Deladino, Eur. Polym. J., 2020, 138, 109955 CrossRef CAS.
- D. T. Gungula, F. P. Andrew, J. Joseph, S. A. Kareem, J. T. Barminas, E. F. Adebayo, A. M. Saddiq, V. T. Tame, I. Dere, W. J. Ahinda and R. Ator, Results Mater., 2021, 12, 100223 CrossRef CAS.
- P. Vejan, T. Khadiran, R. Abdullah and N. Ahmad, J. Control. Release, 2021, 339, 321–334 CrossRef CAS.
- F. Mao, P. Hao, Y. Zhu, X. Kong and X. Duan, Chinese J. Chem. Eng., 2022, 41, 42–48 CrossRef.
- P. Lyu, G. Wang, Y. Cao, B. Wang and N. Deng, Chemosphere, 2021, 276, 130116 CrossRef.
- M. P. Bernardo, G. G. F. Guimaraes, V. F. Majaron and C. Ribeiro, ACS Sustainable Chem. Eng., 2018, 6, 5152–5161 CrossRef CAS.
- X. Jiang, B. Yan, J. Chen, W. Li, J. Hu and Y. Guan, Chem. Eng. J., 2019, 378, 122154 CrossRef CAS.
- L. P. F. Benício, V. R. L. Constantino, F. G. Pinto, L. Vergütz, J. Tronto and L. M. da Costa, ACS Sustainable Chem. Eng., 2017, 5, 399–409 CrossRef.
- C. Jaubertie, M. J. Holgado, M. S. San Romen and V. Rives, Chem. Mater., 2006, 18, 3114–3121 CrossRef CAS.
- N. B. Allou, A. Yadav, M. Pal and R. L. Goswamee, Carbohydr. Polym., 2018, 186, 282–289 CrossRef CAS.
- M. Z. bin Hussein, Z. Zainal, A. H. Yahaya and D. W. V. Foo, J. Control. Release, 2002, 82, 417–427 CrossRef CAS.
- S. N. M. Sharif, N. Hashim, I. M. Isa, S. A. Bakar, M. I. Saidin, M. S. Ahmad, M. Mamat and M. Z. Hussein, Mater. Chem. Phys., 2020, 251, 123076 CrossRef CAS.
- V. L. N. Nunes, R. L. Mulvaney, R. B. Cantarutti, F. G. Pinto and J. Tronto, Nitrogen, 2020, 1, 125–136 CrossRef.
- S. Barkhordari, M. Yadollahi and H. Namazi, J. Polym. Res., 2014, 21, 1–9 CAS.
- G. F. de Castro, E. M. Mattiello, J. A. Ferreira, L. Zotarelli and J. Tronto, New J. Chem., 2020, 44, 10066–10075 RSC.
- G. F. de Castro, L. Zotarelli, E. M. Mattiello and J. Tronto, New J. Chem., 2020, 44, 16965–16976 RSC.
- S. B. Nia, M. Pooresmaeil and H. Namazi, Int. J. Biol. Macromol., 2020, 155, 1401–1409 CrossRef PubMed.
- C. Buchmann, Z. Steinmetz, M. Brax, S. Peth and G. E. Schaumann, Geoderma, 2020, 362, 114142 CrossRef CAS.
- C. Demetri, R. D. Sole, F. Scalera, A. Sannino, G. Vasapollo, A. Maffezzoli, L. Ambrosio and L. Nicolais, J. Appl. Polym. Sci., 2008, 110, 2453–2460 CrossRef.
- I. Kassem, Z. Kassab, M. Khouloud, H. Sehaqui, R. Bouhfid, J. Jacquemin, A. E. K. Qaiss and M. el Achaby, Int. J. Biol. Macromol., 2020, 162, 136–149 CrossRef CAS PubMed.
- B. Tomadoni, M. F. Salcedo, A. Y. Mansilla, C. A. Casalongué and V. A. Alvarez, Eur. Polym. J., 2020, 137, 109953 CrossRef CAS.
- V. L. N. Nunes, R. L. Mulvaney, R. B. Cantarutti, F. G. Pinto and J. Tronto, Nitrogen, 2020, 1, 125–136 CrossRef.
- L. P. F. Benício, F. G. Pinto and J. Tronto, Layered Double Hydroxide Polymer Nanocomposites, Elsevier, 2020, pp. 715–741 Search PubMed.
- M. R. Khodabakshi and M. H. Baghersad, RSC Adv., 2021, 11, 38961–38976 RSC.
- N. Sharma, A. Singh and R. K. Dutta, Polym. Bull., 2021, 78, 2933–2950 CrossRef CAS.
- L. Lisuzzo, G. Cavallaro, S. Milioto and G. Lazzara, Appl. Clay Sci., 2021, 213, 106231 CrossRef CAS.
- L. Lisuzzo, G. Cavallaro, S. Milioto and G. Lazzara, J. Nanostructure Chem., 2021, 11, 663–673 CrossRef CAS.
- M. I. Piash, K. Iwabuchi, T. Itoh and K. Uemura, Geoderma, 2021, 397, 115100 CrossRef CAS.
- T. Hachiya and H. Sakakibara, J. Exp. Bot., 2017, 68, 2501–2512 CAS.
- C. Masclaux-Daubresse, F. Daniel-Vedele, J. Dechorgnat, F. Chardon, L. Gaufichon and A. Suzuki, Ann. Bot., 2010, 105, 1141–1157 CrossRef PubMed.
- M. L. Han, Q. Y. Lv, J. Zhang, T. Wang, C. X. Zhang, R. J. Tan, Y. L. Wang, L. Y. Zhong, Y. Q. Gao, Z. F. Chao, Q. Q. Li, G. Y. Chen, Z. Shi, H. X. Lin and D. Y. Chao, Mol. Plant, 2022, 15, 167–178 CrossRef CAS.
- H. Hatami, A. Fotovat and A. Halajnia, Eurasian Soil Sci., 2021, 54, 431–440 CrossRef CAS.
- S. Lopez-Rayo, A. Imran, H. C. B. Hansen, J. Schjo-erring and J. Magid, J. Agric. Food Chem., 2017, 65, 8779–8789 CrossRef CAS PubMed.
- M. R. Berber and I. H. Hafez, Bull. Environ. Contam. Toxicol., 2018, 101, 751–757 CrossRef CAS PubMed.
- M. Raffi and P. B. B. N. Charyulu, Asian J. Biol. Life Sci., 2012, 1, 213–218 CAS.
- G. Pandiarajan, N. T. Balaiah and B. M. Kumar, J. Biofertil. Biopestic., 2012, 3, 1–4 Search PubMed.
- M. T. Downes, Water Res., 1978, 12, 673–675 CrossRef CAS.
- L. J. Kamphake, S. A. Hannah and J. M. Cohen, Water Res., 1967, 1, 205–216 CrossRef CAS.
- I. Franklin, A. Ormaza-Gonzalez and P. Villalba-Flor, Water Res., 1994, 28, 2223–2228 CrossRef.
- J. Sun, X. Peng, J. V. Impe and J. Vanderleyden, Appl. Environ. Microbiol., 2000, 66, 113–117 CrossRef CAS PubMed.
- S. Yu, E. Kyaw, T. Lynn, Z. Latt, W. W. Mon, M. Nwe, A. Aung and T. Sev, J. Sci. Innov. Res., 2017, 6, 63–67 Search PubMed.
- P. Borah, N. Gujre, E. R. Rene, L. Rangan, R. K. Paul, T. Karak and S. Mitra, Chemosphere, 2020, 254, 126852 CrossRef CAS PubMed.
- K. Kanimozhi and A. Panneerselvam, Chem. Sin., 2010, 1, 138–145 CAS.
- M. Z. bin Hussein, Z. Zainal, A. H. Yahaya and D. W. V. Foo, J. Control. Release, 2002, 82, 417–427 CrossRef CAS.
- J. T. Lin and V. Stewart, Nitrate Assimilation by Bacteria, Academic Press Ltd., 1998, vol. 39 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08759b |
| This journal is © The Royal Society of Chemistry 2022 |