DOI:
10.1039/D1RA08744D
(Paper)
RSC Adv., 2022,
12, 4417-4427
Chitin/egg shell membrane@Fe3O4 nanocomposite hydrogel for efficient removal of Pb2+ from aqueous solution†
Received
30th November 2021
, Accepted 28th January 2022
First published on 3rd February 2022
Abstract
The development of adsorbents by using the byproducts or waste from large-scale industrial and agricultural production is of great significance, and is considered to be an economic and efficient strategy to remove the heavy metals from polluted water. In this work, a novel chitin/EM@Fe3O4 nanocomposite hydrogel was obtained from a NaOH/urea aqueous system, where the proteins of egg shell membrane and Fe3O4 nanoparticles were chemically bonded to chitin polymer chains with the help of epichlorohydrin. Due to the existence of a large number of –NH2, –OH, –CONH–, –COOH and hemiacetal groups, the adsorption efficiency for Pb2+ into the absorbent was dramatically enhanced. The experimental results revealed that the adsorption behavior strongly depends on various factors, such as initial pH, initial Pb2+ concentration, incubation temperature and contact time. The kinetic experiments indicated that the adsorption process for Pb2+ in water solution agreed with the pseudo-second-order kinetic equation. The film diffusion or chemical reaction is the rate limiting process in the initial adsorption stage, and the adsorption of Pb2+ into the nanocomposite hydrogel can well fit the Langmuir isotherm. Thermodynamic analysis demonstrated that such adsorption behaviors were dominated by an endothermic (ΔH° > 0) and spontaneous (ΔG° < 0) process.
1. Introduction
In response to the national policy on the energy saving and carbon emission reduction, the electric car/bicycle industries have developed rapidly in recent years especially in China. As one of the most important parts, the battery has been paid great attention. Among those various batteries, the lead acid battery is still considered to be an essential species and widely used, due to its high stability, reliability and low cost. However, the extensive use and potential leakage of Pb2+ ions in the related industries, such as smelting, battery recycling, painting and mining, can result in high levels of contamination in water and thus accumulation via the food chain. Even very low levels of Pb2+ intake can give rise to serious irreversible injuries on human beings' nervous system, reproductive system, immune system, and organs.1,2 Hence, effective removal of Pb2+ from industrial wastewater will contribute to environmental sustainability.
Up to now, diverse remediation technologies have been introduced to remove Pb2+ from wastewater, such as chemical reduction, electrocoagulation, ion exchange, membrane separation as well as adsorption.3–6 Of the current strategies, adsorption has emerged as an effective way for water treatment due to its many advantages, including cost-efficiency, easy handling, availability of plentiful adsorbents and environment-friendly manner. Numerous absorbents, such as activated carbon, polymeric and lignocellulosic materials, inorganic nanoparticles and organic frameworks have been well developed to absorb the heavy metal ions from the aquatic environments.7–13 Recently, concerns have been devoted into the utilization of biomass and agricultural wastes to remove heavy metal ions, where these adsorbents are usually derived from the byproducts or wastes from large-scale industrial and agricultural production.13–15 Such behavior is of high significance for the developing countries, since huge amounts of agricultural wastes have been effectively recycled for the sake of saving the natural resources. For example, the orange and cucumber peels have been employed to treat the contaminated water.14,16 The presence of carboxyl, hydroxyl, and carbonyl groups is likely responsible for the efficient adsorption of heavy metal ions. Egg shell membrane (EM) is a naturally-occurring biomaterial generated by poultry and regarded as a biowaste. This semipermeable membrane is composed of the highly cross-linked protein fibers, and identified as a highly-value material in biomedical engineering benefitting from its nontoxic, collagen-rich and biocompatible characteristics.16 Moreover, the unique 3D fibrous net-work and a large number of functional groups (–COOH, –NH2 and –OH) would render the material with a strong ability to bind and capture metal ions.17,18
Chitin is another abundant and renewable biomass resource. It is composed of β-(1-4)-linked 2-acetamido-2-deoxy-D-glucose, and widely exists in different crustaceans, mollusks, algae, insects, fungi, and yeasts on earth.19,20 The annual production of chitin is estimated to be about 1010 to 1011 tons from living organisms, and more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons could be available every year from shellfish wastes.21 This polysaccharide possesses a number of amide (–NH–) and hydroxyl (–OH) groups in the polymer chains, which will facilitate the chelation to Ar3+, Cd2+, Co2+, Cu2+, Mn2+ and Pb2+ ions in aqueous system.22 Thus, the chitin-derived materials as the low-cost absorbents have been largely applicated in the water treatment. This occurrence is further enhanced with the development of solvent systems of the chitin. The as-fabricated materials in terms of chitin fibers, crystals whisker, films, hydrogels, aerogels, microspheres and hybrid nanocomposites displayed high performance for the removal of heavy metal ions, organic dyes or pesticide.21,23–25
000 tons could be available every year from shellfish wastes.21 This polysaccharide possesses a number of amide (–NH–) and hydroxyl (–OH) groups in the polymer chains, which will facilitate the chelation to Ar3+, Cd2+, Co2+, Cu2+, Mn2+ and Pb2+ ions in aqueous system.22 Thus, the chitin-derived materials as the low-cost absorbents have been largely applicated in the water treatment. This occurrence is further enhanced with the development of solvent systems of the chitin. The as-fabricated materials in terms of chitin fibers, crystals whisker, films, hydrogels, aerogels, microspheres and hybrid nanocomposites displayed high performance for the removal of heavy metal ions, organic dyes or pesticide.21,23–25
With respect to the advantages of these biomass resources, in this work, we aim to combine the chitin and egg shell membrane to create a novel kind of nanocomposite hydrogel to efficiently remove Pb2+ in an economical way. In the design, the magnetic Fe3O4 nanoparticles were also incorporated into the hydrogel matrix. On one hand, the Fe3O4 nanoparticles have a suitable framework for interaction with Pb2+, leading to enhanced adsorption efficiency;26 on the other hand, the introduction of magnetic particles could provide the convenience for the ultimate separation. Thus, the impacts of the environmental pH condition, initial Pb2+ concentration and incubation temperature on Pb2+ adsorption into the chitin/EM@Fe3O4 nanocomposite hydrogel were investigated in detail, respectively. The corresponding adsorption kinetics and isotherm behaviors were also explored. We believe that this work provides a promising strategy for the construction of novel biomass-based absorbents for rapid and high-capacity removal of heavy metal ions.
2. Experimental
2.1 Materials
Chitin powder with a degree of acetylation about 98% was purchased from Jinke Chitin Co. Ltd (Zhejiang, China). The weight-average molecular weight (Mw) of chitin was evaluated to be 5.0 × 105 by dynamic light scattering (DLS, ALV/CGS-8F, ALV, Germany) in 5% LiCl/DMAc (w/w). Raw egg shell membranes were collected from local school restaurants. All of the chemical reagents including NaOH, urea, HCl, Pb(NO3)2, epichlorohydrin, EDTA·2Na, H2O2 and alcohol were analytical grade from commercial sources in China. Trisodium citrate dihydrate, FeCl3·6H2O, NaAc, ethylene glycol were obtained from Aladdin Chemical Co. Ltd (Shanghai, China).
2.2 Preparation of highly water-dispersible Fe3O4 nanoparticles
The magnetite Fe3O4 nanoparticles were prepared through a modified solvothermal reaction.27 Briefly, 2.025 g of FeCl3·6H2O, 5.781 g of NaAc, and 0.684 g of trisodium citrate dihydrate were dissolved in 150 mL of ethylene glycol. After vigorous stir for 1 h at room temperature, the as-formed homogeneous black mixture was transferred into a Teflon-lined stainless-steel autoclave (200 mL) with the further incubation at 200 °C for 10 h. During the process Fe3+ was partly reduced into Fe2+. Finally, the obtained Fe3O4 product was washed with ethanol for 6–10 times with the help of a magnet field, and then dried in oven at 60 °C for further use.
2.3 Pre-treatment of raw egg shell membranes
Raw egg shell membranes were obtained by peering manually from discarded eggshells and composed of both inner and outer membranes. Subsequently, the membranes were treated with HCl (5.0 wt%) for 4 h to remove the CaCO3 residues, and thoroughly washed in deionized water. After incubation in 0.1 mmol L−1 EDTA aqueous solution for 24 h and fully rinse with deionized water, the fresh egg shell membranes (denoted as EM) were collected. These products were finally dried at 60 °C and ground into powders for the preparation of nanocomposite hydrogels.
2.4 Fabrication of the chitin/EM@Fe3O4 nanocomposite hydrogel
The fabrication of chitin/EM@Fe3O4 nanocomposite hydrogel was mainly involved two steps, the dissolution of egg shell membranes and chitin in NaOH/urea solvent system. Firstly, 1.0 g EM powders were totally dissolved into 11.0 wt% NaOH aqueous solution upon heating and sonification. After cooling down to room temperature, certain amount of urea was added to form the 1.0 wt% EM/11.0 wt% NaOH/4.0 wt% urea/80.0 wt% H2O mixture system. Subsequently, 4.0 g chitin powders were dispersed into the above mixture solution with stirring for 5 min, and then was stored under refrigeration (−30 °C) for 4 h.28 The frozen solid was thawed and stirred extensively at room temperature. After the freeze/thawing manipulation were repeated 3 cycles, 2.0 mL epichlorohydrin as cross-linker and 1.0 mL Fe3O4 aqueous dispersion were added into 100 g of the mixture solution and stirred at 0 °C for 0.5 h to obtain a homogeneous solution, which was then subjected to centrifugation for 10 min at 4 °C. In the following, the obtained uniform solution was cast into a Petri dish and kept at ambient temperature for 12 h allowing the gelation. Finally, the as-prepared hydrogel was immersed in distilled water for 3 days to remove any residues. The nanocomposite hydrogel sample was labeled as chitin/EM@Fe3O4. For the preparation of chitin and chitin/EM hydrogels, similar experimental procedures with a fixed concentration of epichlorohydrin (2.0 mL/100 g) were displayed. For the preparation of nanocomposite hydrogel beads, the obtained pre-gel solution was allowed to drip into hot water by an injector to get the raw beads. After thorough washing with ultrapure water, the hydrogel beads were collected.
2.5 Adsorption behaviors studies
The adsorption behaviors of Pb2+ into chitin/EM@Fe3O4 hydrogel including the effects of environmental pH condition and incubation temperature, kinetic mechanisms, adsorption isotherms and thermodynamic parameters were explored intensively. All the experiments were performed in 50 mL plastic centrifuge tubes containing 30 mL of Pb2+ stock solution by utilizing the batch equilibrium method. During the whole process, all the tubes were sealed with the caps and placed in a thermostatic water bath shaker at a speed of 150 rpm. In the adsorption isotherm, the initial concentrations of Pb2+ varied from 0.5 to 20.0 mmol L−1 at the incubation temperature of 293 K. To each container, approximate 1.0 g hydrogel samples were added. After incubation for 24 h, the equilibrium concentration of Pb2+ in each tube was determined with ICP-OES spectrometer. Thus, the adsorption capacity, qe (mmol g−1), of Pb2+ into chitin/EM@Fe3O4 hydrogel can be calculated as:29| |
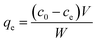 | (1) |
where c0 and ce are the initial and equilibrium concentrations of Pb2+ (mmol L−1), respectively, V is the volume of Pb2+ aqueous solution (L) in each tube and W is the mass of chitin/EM@Fe3O4 hydrogel samples (g).
For the kinetic studies, 1.0 g chitin/EM@Fe3O4 hydrogel samples were added to 30 mL of Pb2+ aqueous solution. The concentration was kept at 5.0 mmol L−1 and the pH value was set to be 5.0. At different time intervals, the final concentration of Pb2+ in each container was analyzed by ICP-OES. To investigate the effect of pH on the Pb2+ adsorption capacity, the pH values were changed from 1.0 to 5.0 by adding diluted HCl solution. At higher pH condition, the Pb2+ ions are easy to precipitate. In order to investigate the impact of incubation temperature on the adsorption efficiency, the batch adsorption studies were performed at the temperatures of 277, 293 and 310 K, respectively.
2.6 Desorption and regeneration studies
In order to evaluate the desorption and regeneration abilities of chitin/EM@Fe3O4 hydrogel, 1.0 g samples were fistly placed in 30 mL of 5.0 mmol L−1 Pb2+ aqueous solution. After incubation for 24 h at 293 K, the adsorbents and aqueous solution were collected, respectively. The aqueous solution was used to evaluate the adsorption capacity and desorption efficiency. The adsorbents were regenerated by using 10 mL of 0.05 mol L−1 HCl as the static eluting solution for 3 times. Each static elution time was set to be 4 h. Then, the adsorbent was fully washed with deionized water for the subsequent adsorption studies. For comparison, the hydrogel samples were also regenerated by using 10 mL of 0.1 mol L−1 EDTA as the static eluting solution for 3 times. Each static elution time was set to be 4 h. After that, the adsorbent was fully washed by 1.0 mol L−1 NaCl solution and deionized water for the succeeding adsorption performance. This adsorption–desorption cycle in both cases was repeated 3 times under the same condition.
2.7 Characterization
The wide-angle X-ray diffraction (XRD) patterns for each sample were recorded in reflection mode on a Rigaku SmartLab diffractometer equipped with a CuKα radiation source (λ = 1.542 Å) operated at 40 kV and 30 mA. The samples were scanned at 4° min−1 and at a step size of 2.5° in 2θ. The FT-IR spectra were recorded in the wavenumber range from 4000 to 700 cm−1 using the attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer, USA) at room temperature. The samples were freeze-dried in a conventional freezer dryer and then dried at 45 °C in a vacuum chamber to eliminate water from the samples. Thermogravimetric analysis (TGA) tests were performed on a TG instrument (PerkinElmer Co., USA) in an atmosphere of nitrogen at a heating rate of 10 °C min−1 from 50 to 650 °C. High resolution transmission electron microscopy (HR-TEM) and SAED images were acquired using a JEM-2011 transmission electron microscope (JEOL Ltd, Japan) operated at 200 kV. Magnetic characterization was carried out on a vibrating sample magnetometer (PPMS-9, Quantum Design, USA). Particle size and zeta potential were measured using a Nano-ZS ZEN3600 (Malvern Instruments, UK) at 25 °C. The exact concentration of Pb2+ in solution was determined by the ICP-OES instrument with an RF generator power 1150 W (Thermo, USA). The auxiliary, carrier and plasma gas (Ar) flow rates were set to be 0.5, 0.5 and 12.0 L min−1, respectively. The wavelength of emission line for Pb is 220.353 nm. The leaching concentration of Fe2+/Fe3+ in solution was determined by the ICP-MS instrument (Agilent 7700, USA), and the total weight content of Fe in chitin/EM@Fe3O4 hydrogel sample was measured by the ICP-OES instrument (Agilent 725ES, USA). The wavelength of emission line for Fe is 239.56 nm.
3. Results and discussion
3.1 Structure characterization of chitin/EM@Fe3O4 hydrogel
The prerequisite for preparation of chitin/EM@Fe3O4 nanocomposite hydrogel is the dissolution of both chitin and egg shell membrane in an appropriate solvent system. In the design, we attempted to fabricate this novel adsorbing material by dissolving them in the 11.0 wt% NaOH/4.0 wt% urea solvent system. One possible reason for the choice can be attributed to the fact that as-prepared hydrogel from the alkali/urea solvent system is expected to display excellent mechanical properties,20 which will guarantee the stability and integrity of the absorbents in the recycle processing. During the whole preparation, egg shell membranes were firstly allowed to be dissolved in 11.0 wt% NaOH aqueous solution upon heating, and then the chitin powders were dispersed and finally dissolved in the 11.0 wt% NaOH/4.0 wt% urea solution via freeze–thawing cycle treatment. By addition of water-dispersible Fe3O4 nanoparticles and chemical-crosslink agent of epichlorohydrin into the mixture, the chitin/EM@Fe3O4 nanocomposite hydrogel was obtained after thorough washing in water. As shown in Fig. 1a, the hydrogel samples showed different morphologies. The pure chitin hydrogel is transparent and colorless, while the chitin/EM hydrogel displays the yellowish color and higher swelling behavior, indicating the successful chemical crosslinking between EM protein and chitin polymer chains. By comparison, the chitin/EM@Fe3O4 nanocomposite hydrogel shows the dark-brown color, as a result of the existence of Fe3O4 nanoparticles in the matrix. Meanwhile, by immersing the nanocomposite hydrogel into water for 1 week, the Fe3O4 nanoparticles were hardly observed and separated from the hydrogel matrix, illustrating the strong affinity to the matrix and high stability of the bio-absorbents. It should be mentioned that we didn't adopt the fabrication strategy by in situ synthesis of Fe3O4 in the hydrogel matrix, because Fe3+ ions are not easy to penetrate into the deep interior of the hydrogel, finally leading to the gradient distribution of Fe3O4 in the matrix. This heterogeneous structure will display an adverse effect on the adsorption capacity.
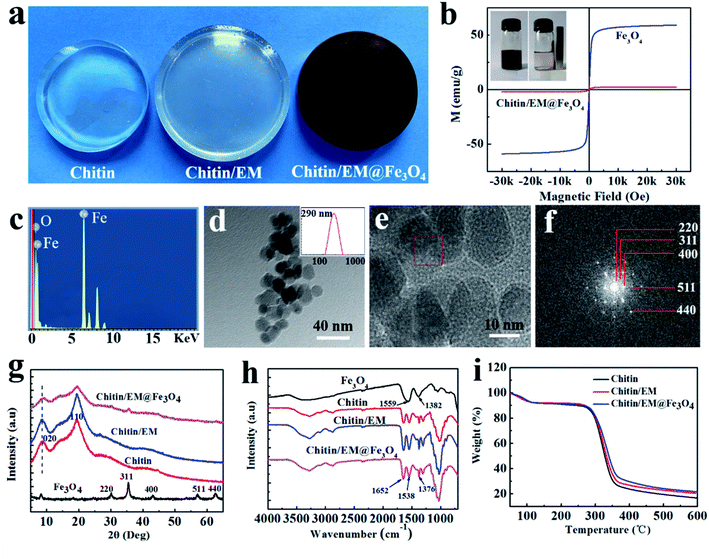 |
| | Fig. 1 (a) Photographs of the as-prepared hydrogel samples. (b) Magnetic hysteresis loops of Fe3O4 particles and chitin/EM@Fe3O4 hydrogel sample. (Inset) Pictures of Fe3O4 nanoparticles in aqueous solution before and after suffering from a magnetic field. (c) EDS spectra for the chitin/EM@Fe3O4 hydrogel sample. (d) TEM image and (e) high resolution TEM image of the Fe3O4 nanoparticles dispersed in chitin/EM@Fe3O4 hydrogel. (Inset) The corresponding size distribution of the Fe3O4 particles. (f) SAED pattern for the corresponding location. XRD patterns (g), TGA curves (h) and (i) FT-IR spectra for the specimens. | |
The existence of Fe3O4 nanoparticles in the nanocomposite hydrogel was firstly studied using a vibrating sample magnetometer (shown in Fig. 1b). The results demonstrate that the nanocomposite hydrogel possesses a superparamagnetic character. Both the chitin/EM@Fe3O4 hydrogel samples and Fe3O4 nanoparticles had no remanence or coercivity at 300 K, and the magnetization saturation values (Ms) were evaluated to be 2.8 and 58.2 emu g−1, respectively. The smaller Ms value can be ascribed to the lower content of Fe3O4 nanoclusters in the nanocomposite hydrogel. Nevertheless, the as-prepared hydrogel beads (shown in Fig. S1†) can be still easily separated from aqueous solution under an external magnetic field.
Further evidence was collected from the EDS pattern (Fig. 1c). The observation for the elements of Fe and O in the spectrum confirmed the successful synthesis of the magnetite particles. The particle size analysis and TEM images (Fig. 1d and e) reveal that the Fe3O4 particles have a uniform size of about 290 nm, and dispersed in the hydrogel matrix. The particles are composed of nanocrystals with the size of about 5–20 nm, where the nanocrystals seem to be connected with each other similar like the appearance of pomegranate. Selected-area electron diffraction (SAED) (Fig. 1f) recorded on the edge of a magnetite particle presents the polycrystalline-like phase pattern. This structure may provide them with a large surface area for adsorption of guest molecules, such as the heavy metal ions or organic dyes.30
Fig. 1g presents the XRD patterns for Fe3O4, chitin, chitin/EM and chitin/EM@Fe3O4, respectively. The typical pattern of Fe3O4 particles exhibits the strong diffraction peaks at 2θ =30.1°, 35.4°, 43.1°, 57.0° and 62.7°, corresponding to the reflection planes of (220), (311), (400), (511) and (440), respectively. This result indicates that the magnetite particles possess the polycrystalline structure, which is in good agreement with the SAED. In the case of chitin pure hydrogel, two main semi-crystalline peaks at 2θ =8.6° and 19.4° occurred, which are assigned to the (020) and (110) reflections, respectively. For the chitin/EM nanocomposite hydrogel, it shows the similar diffraction behavior to the chitin, implying that the EM was greatly degraded into small peptide sections during the fabrication process. The occurrence of the diffraction peaks at 2θ =30.1° and 35.4° in the chitin/EM@Fe3O4 nanocomposite hydrogel samples proves that Fe3O4 particles have been well incorporated into the hydrogel matrix. In addition, the diffraction peak at 2θ =8.6° in the chitin/EM sample shifted to 9.3°. The strong interaction between the Fe3O4 particles and hydrogel polymer network and certain chemical-crosslink reaction may commonly contribute to this phenomenon. More information on the mutual interactions was identified from ATR-FTIR spectra (Fig. 1h). As shown, all the tested samples show the prominent absorption bands around 3287–3432 cm−1, which are ascribed to the stretching mode of O–H and N–H groups. These features ensure that the EM proteins and Fe3O4 particles could be chemically bonded to chitin in presence of epichlorohydrin. The respective peaks in the nanocomposite hydrogel located at 1652 cm−1, 1538 cm−1 and 1376 cm−1 mainly originate from the amide I, amide II and amide-III bands of chitin. The characteristic peaks at 1559 and 1382 cm−1 associated with carboxylate groups in the Fe3O4 particles shifted to the lower wavenumber region, and seem to be overlapped by the amide (II and III) bands, suggesting the strong hydrogen bonding interactions between Fe3O4 particles and the polymer chains. Thermogravimetric analysis (Fig. 1i) illustrate that the thermal stability of chitin/EM@Fe3O4 nanocomposite hydrogel was slightly enhanced by incorporation of EM and Fe3O4 particles. According to the percentage of weight loss in N2 atmosphere, the contents for EM and Fe3O4 particles in the given nanocomposite system were estimated to be 23.1 wt% and 3.1 wt%, respectively.
3.2 Adsorption kinetics of Pb2+ into chitin/EM@Fe3O4 hydrogel
Fig. 2 presents the equilibrium adsorption capacity for Pb2+ into different adsorbents. As indicated, three hydrogel species displayed the differentiated efficiency. A relatively higher qe value was observed in the chitin/EM hydrogel samples than chitin pure hydrogel, implying that the abundant groups such as the –NH2 and –COOH in the EM protein will provide more feasibility to anchor the heavy metal ions. Among these adsorbents, the chitin/EM@Fe3O4 nanocomposite hydrogel showed the highest qe value. The addition of Fe3O4 nanoparticles in the hydrogel matrix is favorable for the chelation to Pb2+ ions. The citrate stabilized Fe3O4 nanoparticles own a large number of –COOH groups on the surface.30 Besides, the magnetite nanoparticles show the characteristics of high surface area and unique cubic inverse spinel structure. There features are conductive to the interactions with Pb2+ ions.26 With respect to the improvement in adsorption capacity, the chitin/EM@Fe3O4 nanocomposite hydrogel can be taken as the promise candidate for water treatment.
 |
| | Fig. 2 The equilibrium adsorption capacity of Pb2+ in chitin, chitin/EM and chitin/EM@Fe3O4 hydrogels at 20 °C in pH = 5 aqueous solution. | |
The adsorption behaviors of Pb2+ into chitin/EM@Fe3O4 hydrogel were extensively investigated. Fig. 3a depicts the dependence of the qe value for Pb2+ adsorption into nanocomposite adsorbent versus the incubation time in aqueous solution. The qe increased almost linearly in the initial period, implying a quick and efficient adsorption process on the surface of chitin/EM@Fe3O4 hydrogel. Upon 24 h incubation, the biosorption equilibrium was established. The time profile for the Pb2+ uptake in water was smooth and continuous until the equilibrium, suggesting that the monolayer adsorption possibly occurred.31 To analyze the adsorption kinetics of Pb2+ into the as-prepared nanocomposite hydrogel, several basic models including the pseudo-first-order, kinetics pseudo-second-order kinetics, and intraparticle diffusion models were applied. The related parameters obtained from these equations will cast great significance on how to design and model the adsorption process. The pseudo-first-order kinetic equation can be expressed as follows:32
| |
 | (2) |
 |
| | Fig. 3 (a) The effect of contact time on the Pb2+ adsorption in chitin/EM@Fe3O4 hydrogel at 20 °C in pH = 5 aqueous solution. The corresponding pseudo-first-order model (b), pseudo-second-order model (c), intra-particle diffusion plots (d) and (e) Boyd plots for Pb2+ adsorption in chitin/EM@Fe3O4 hydrogel at 20 °C in pH = 5 aqueous solution. | |
The pseudo-second-order kinetic model can be depicted as:29
| |
 | (3) |
where
qt is the adsorption amount of Pb
2+ (mmol g
−1) in chitin/EM@Fe
3O
4 hydrogel at different time
t.
q1 (mmol g
−1) and
k1 (h
−1) are the maximum adsorption capacity and rate constants for the pseudo-first-order model, while
q2 (mmol g
−1) and
k2 (g mmol
−1 h
−1) represent the maximum adsorption capacity and rate constants for the pseudo-second-order model. The values of
k1,
k2,
q1,
q2 and correlation coefficients of
R12 and
R22 for Pb
2+ adsorption can be easily drawn from the linear fitting plots of ln(
qe −
qt)
versus t (
Fig. 3b) and
t/
qt versus t (
Fig. 3c), respectively. As listed in
Table 1, the maximum adsorption capacity of
q2 for Pb
2+ in aqueous solution is almost same to the actual experimental data (
Fig. 3a). Meanwhile, the correlation coefficients (
R22) for pseudo-second-order kinetic model is 0.9966, which is much closer to 1.0. Hence, the adsorption in water solution follows the pseudo-second-order kinetic model, implying that Pb
2+ adsorption in chitin/EM@Fe
3O
4 hydrogel is controlled by the inner surface adsorption. In other words, the adsorption behavior for Pb
2+ is dominated by chemical adsorption.
33
Table 1 Kinetic parameters for adsorption of Pb2+ into chitin/EM@Fe3O4 hydrogel at 20 °C in aqueous solution
| Pseudo-first-order kinetics |
Pseudo-second-order kinetics |
| k1 (h−1) |
qe (mmol g−1) |
R12 |
k2 (g mmol−1 h−1) |
qe (mmol g−1) |
R22 |
| 0.0034 |
0.0214 |
0.9199 |
0.3789 |
0.0467 |
0.9966 |
The adsorption process was further investigated by employing the intra-particle diffusion model. Generally, in many cases the intra-particle diffusion into the interior of the adsorbent through the pores is considered to be the rate-controlling step, where the uptake amount of solutes increases almost linearly with t0.5 rather than with the contact time t.34 Therefore, the adsorption equation can be referred to:
where
kpi is the intra-particle diffusion rate constant at stage
i (mmol g
−1 h
−0.5). The intercept of
Ci represents the thickness of boundary layer. According to the theory,
qt versus t0.5 should be linear when the intra-particle diffusion occurred in the adsorption process. Otherwise, additional mechanisms may be involved. As shown in
Fig. 3d, the intra-particle diffusion fitting plot for Pb
2+ uptake into chitin/EM@Fe
3O
4 hydrogel is consist of three stages. All the linear lines for each stage did not pass through the origin, demonstrating that the intra-particle diffusion could not solely determine the overall rate of mass transfer at the initial stage of adsorption process. Both the film diffusion (chemical reaction) at the first linear region and the following pore diffusion at the second linear segments contribute to the adsorption rate. More evidence could be clarified from the Boyd's model,
35 which is given as:
| |
 | (5) |
where
F is the fractional attainment of equilibrium at different contact times
t, and
Bt is a function of
F| |
 | (6) |
where
qt and
qe are the amount of Pb
2+ loaded (mmol g
−1) into chitin/EM@Fe
3O
4 hydrogel at time
t and at equilibrium state, respectively. Then the
eqn (5) could be rewritten as:
| | |
Bt = −0.4977 − ln(1 − F)
| (7) |
The rate-controlling step in the adsorption process of Pb2+ into chitin/EM@Fe3O4 hydrogel will be easily distinguished from the Boyd plots. If the values of Bt change linearly along with the incubation time t and pass through the origin, the rate of mass transfer is controlled by the pore diffusion. If the plot profile is nonlinear or linear without passing through the origin, the film diffusion or chemical reaction will mainly dominate the adsorption rate. As illustrated in Fig. 3e, the fitting plot for the adsorption process of Pb2+ exhibit the nonlinear behavior, clearly indicating that the film diffusion or chemical reaction is the rate-limiting step in the initial period and then follows the intra-particle diffusion.
3.3 Adsorption isotherms of Pb2+ into chitin/EM@Fe3O4 hydrogel
The adsorption efficiency strongly depends on the initial concentration of heavy metal ions in solution. Usually, the adsorption capacity is enhanced along with the increase of the concentration of metal ions in the solution.31 As shown in Fig. 4a, the amount of Pb2+ (qe) adsorbed into chitin/EM@Fe3O4 hydrogel increased from 0.013 to 0.070 mmol g−1, when the initial concentration of Pb2+ increased from 0.7 to 18.0 mmol L−1 at 293 K. This relationship between the adsorption capacity at the fixed temperature and the ions concentration at equilibrium can be easily described by the equilibrium adsorption isotherms, including the Langmuir and Freundlich equations. It is of great importance to learn more about the mutual interactions and optimize the dosage of adsorbents. The Langmuir isotherm model is established in the following form:31| |
 | (8) |
where qmax (mmol g−1) is the maximum adsorption capacity associated with the monolayer coverage on the surface, and b is the Langmuir constant (L mmol−1), reflecting the uptake efficiency. Unlike Langmuir model, the Freundlich isotherm is supposed to be an empirical equation to study the multilayer adsorption process on heterogeneous surface:36| |
 | (9) |
where KF is the Freundlich constant, and the exponent 1/n is the heterogeneity factor, indicating the adsorption capacity and intensity, respectively.
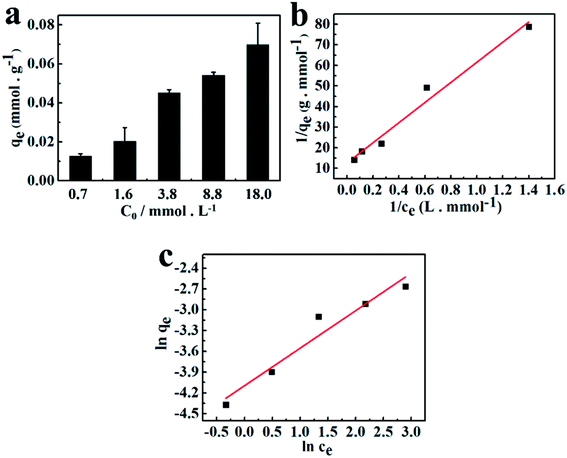 |
| | Fig. 4 (a) Adsorption isotherms for Pb2+ adsorption on chitin/EM@Fe3O4 hydrogel at 20 °C in pH = 5 aqueous solution. The related Langmuir isotherms (b) and (c) Freundlich isotherms for Pb2+ adsorption in chitin/EM@Fe3O4 hydrogel. | |
Fig. 4b and c present the Langmuir and Freundlich profiles for Pb2+ adsorption in absorbents at 293 K, respectively, and the related parameters are summarized in Table 2. In Langmuir model, the value of qmax was estimated to be 0.079 mmol g−1, implying that the as-fabricated chitin/EM@Fe3O4 nanocomposite hydrogel could display high adsorption capacity for Pb2+ in aqueous solution. As for the Freundlich plot fitting, the value of 1/n was determined to be 0.542 at 293 K, revealing that Pb2+ could be easily anchored into the absorbents. In Freundlich equation, the constant (1/n) is closely related to the adsorption intensity of the adsorbent. When the value of 1/n ranges 0.5 < 1/n ≤ 1, adsorbates are easy to be anchored on the matrix.37 Based on the fact that the regression coefficient of R2 obtained from the Langmuir model (R2 = 0.9727) is higher than that for the Freundlich model (R2 = 0.9301), it can be concluded that the uptake process of Pb2+ into the chitin/EM@Fe3O4 hydrogel prefers to the Langmuir adsorption. Namely, the adsorption process in water solution is primarily dominated by monolayer adsorption.
Table 2 Langmuir and Freundlich parameters for Pb2+ adsorption into chitin/EM@Fe3O4 hydrogel at 20 °C in aqueous solution
| Langmuir parameters |
Freundlich parameters |
| qmax (mmol g−1) |
B (L mmol−1) |
R2 |
1/n |
KF (mmol g−1) |
R2 |
| 0.079 |
0.259 |
0.9727 |
0.542 |
0.017 |
0.9301 |
3.4 Thermodynamic parameters of Pb2+ adsorption
Fig. 5a depicts the effects of incubation temperature on the uptake capacity for Pb2+ adsorption into chitin/EM@Fe3O4 hydrogel in water solution. The equilibrium adsorption capacity slightly increased from 0.043 to 0.050 mmol g−1, as the temperature was elevated from 4 to 37 °C, revealing the endothermic nature of this adsorption process. With the aim to intensively explore the endothermic nature in the adsorption process, the thermodynamic parameters, such as standard free energy (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) are determined by utilizing the following equations38.: | |
ΔG°= −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (10) |
| |
 | (11) |
 |
| | Fig. 5 (a) Effects of temperature on uptake capacity of Pb2+ adsorption into chitin/EM@Fe3O4 hydrogel at 20 °C in pH = 5 aqueous solution. (b) The corresponding Van't Hoff plots for Pb2+ adsorption in chitin/EM@Fe3O4 hydrogel. | |
The values of ΔH° and ΔS° can be calculated by van't Hoff equation:
| |
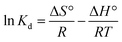 | (12) |
where
Kd is the standard thermodynamic equilibrium constant at the absolute temperature of
T (K),
R is the gas constant (8.314 J mol
−1 K
−1).
As shown in Table 3, the value of ΔG° for Pb2+ adsorption into chitin/EM@Fe3O4 hydrogel was negative, indicating that the adsorption of Pb2+ into absorbents underwent a favorable and spontaneous process. The decreased value of ΔG° with an increase in temperature suggests that the higher temperature is beneficial to the Pb2+ adsorption in solution. Furthermore, according to the linear fitting profile of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus 1/T (Fig. 5b), the values of ΔH° and ΔS° in water solution were determined to be 48.6 kJ mol−1 and 267.3 J mol−1 K−1. The positive value of ΔH° demonstrates the endothermic nature of the adsorption process and the existence of an energy barrier. The large positive value of ΔS° proves the higher binding ability of Pb2+ to the nanocomposite matrix and the increased randomness at the solid–solution interface.39
Kd versus 1/T (Fig. 5b), the values of ΔH° and ΔS° in water solution were determined to be 48.6 kJ mol−1 and 267.3 J mol−1 K−1. The positive value of ΔH° demonstrates the endothermic nature of the adsorption process and the existence of an energy barrier. The large positive value of ΔS° proves the higher binding ability of Pb2+ to the nanocomposite matrix and the increased randomness at the solid–solution interface.39
Table 3 Thermodynamic parameters for Pb2+ adsorption into chitin/EM@Fe3O4 hydrogel at 20 °C in aqueous solution
| Temperature (K) |
ΔH° (kJ mol−1) |
ΔS° (J mol−1 K−1) |
ΔG° (kJ mol−1) |
| 277 |
48.6 |
267.3 |
−5.58 |
| 293 |
|
|
−6.05 |
| 310 |
−6.74 |
3.5 Effects of pH on Pb2+ adsorption behavior
Plenty of function groups such as the hydroxy, amino, carboxyl, acetyl amino and hemiacetal groups exist in the chitin/EM@Fe3O4 nanocomposite hydrogel. The protonation/deprotonation of these groups is susceptible to the environmental pH conditions. Therefore, pH plays an important role in regulating the adsorption behavior for metal ions. To avoid the precipitation of Pb2+ in higher condition, the pH values of the solutions were adjusted within the range of 1.0–5.0 for investigation. As illustrated in Fig. 6, the as-prepared adsorbent performs different behaviors for Pb2+ adsorption during the pH ranges. The adsorption capacity increased sharply as the pH value increased from 1.0 to 5.0. The zeta potentials for the hydrogel samples are positive (Fig. S2†), revealing that the amino groups are protonated in the whole pH range. This occurrence would result in the electrostatic repulsive force with Pb2+ and prevent the metal complex formation. Hence, the increase in adsorption capacity is mainly relevant to the de-protonation of the carboxyl groups and the electronegative oxygen atoms in hydroxy, acetyl amino and hemiacetal groups. In condition of low pH, the protonation of active sites such as carboxylates takes place, which is unfavorable for the Pb2+ binding to the biosorbents. Meanwhile, abundant hydrogen ions (H+) will restrain the activity of electronegative oxygen, thus leading to the decrease in adsorption capacity.40 As the pH increases, the ionization of –COOH groups from the citric stabilized Fe3O4 nanoparticle and EM protein occurred, and in turn the increased negative charges of –COO− groups will give rise to the stronger binding ability to heavy metal ions.41 Moreover, the lone pair of electrons in the neutral oxygen atoms (the hemiacetal oxygen atom of the anhydro-glucose at the non-reducing end and the hydroxyl groups of an anhydro-glucose unit at the reducing end) under high pH condition is beneficial to the formation of coordination bonds between O atoms and the lead atoms.42
 |
| | Fig. 6 Effects of pH on the uptake capacity of Pb2+ adsorption in chitin/EM@Fe3O4 hydrogel at 20 °C in aqueous solution. | |
3.6 Regeneration
As an ideal adsorbent, how to recover and maintain the original adsorption capacity is vital for the practical application. In this study, Pb2+-loaded nanocomposite hydrogels were regenerated by using 0.05 mol L−1 HCl aqueous solution. For comparison, 0.1 mol L−1 EDTA aqueous solution was also introduced as another static eluting agent. Since the component of Fe3O4 nanoparticles in chitin/EM@Fe3O4 nanocomposite hydrogel may be dissolved or chelated in the eluting solution, the weight loss of Fe was firstly explored. According to the original and final weight contents of Fe in hydrogel samples, the weight loss can be calculated as:| |
 | (13) |
where m0(Fe) (g) is the original weight content of Fe in hydrogel sample, and mt(Fe) (g) is the weight content of Fe after incubating the hydrogel sample in the eluting solution for the given time of t. As shown in Fig. 7, after immersing the hydrogel samples in the eluting agents of diluted HCl and EDTA aqueous solutions for 48 h, the corresponding loss amounts of Fe from the matrix were evaluated to be 10.6% and 5.7%, respectively. The values of weight loss for the hydrogel samples in HCl aqueous solution are relatively higher than those in EDTA aqueous solution, implying that the nanocomposite bio-sorbents displayed higher stability in EDTA solution during the regenerated process. Although the phenomena of weight loss would occur in both cases, the values are acceptable and can still be utilized as the candidate eluting agents. Alternatively, in order to maximally reduce the weight loss of Fe during the regeneration, the elution time can be limited to be 0.5–1 h in each cycle, which has been demonstrated to be sufficient to remove the adsorbed heavy metal ions from the matrix. Similar processing methods have been readily applicated in the regeneration of various adsorbents, such as Fe3O4/sawdust carbon,43 Fe3O4-modified sugarcane bagasse44 or CMC/alginate/graphene oxide@Fe3O4.45
 |
| | Fig. 7 The weight loss of Fe as a function of immersing time for chitin/EM@Fe3O4 hydrogel samples in different eluting media at 20 °C. | |
Further, the regeneration behaviors were investigated. As listed in Table 4, the desorption ratios of Pb2+ from the matrix by using EDTA aqueous solution were slightly higher than diluted HCl solution in each cycle, confirming the better elution ability. Irrespective of the experimental deviation, these results reveal that most of the Pb2+ adsorbed in chitin/EM@Fe3O4 hydrogel could be removed in both cases, testifying the potential re-usability of the adsorbents. When the regenerated materials were placed in Pb2+ aqueous solution upon the same incubation condition, they displayed a slight decrease tendency in the loading efficiency after each cycle. The existence of the small and reasonable decrease in adsorption capacity may be due to the static elution method, resulting in the incomplete desorption of ions from the matrix. In spite of this, the developed chitin/EM@Fe3O4 hydrogel could still be a good candidate for future application.
Table 4 Repeated adsorption capacity and desorption efficiency of Pb2+ into chitin/EM@Fe3O4 hydrogel in the desorption–regeneration cycle
| Cycle number |
Adsorption capacity (mmol g−1) |
Desorption efficiency (%) |
| HCl-1 |
0.0453 |
88.96 |
| HCl-2 |
0.0403 |
92.01 |
| HCl-3 |
0.0387 |
81.27 |
| EDTA-1 |
0.0467 |
94.43 |
| EDTA-2 |
0.0436 |
94.21 |
| EDTA-3 |
0.0407 |
92.17 |
4. Conclusion
A novel kind of chitin/EM@Fe3O4 nanocomposite hydrogel derived from the biowastes of egg shell membrane and chitin was successfully prepared from the NaOH/urea aqueous system. The obtained hydrogel adsorbent could display the enhanced adsorption efficiency for Pb2+ in aqueous solution with the addition of egg shell membrane and citric stabilized Fe3O4 nanoparticles in the matrix. The good correlation coefficient (0.9966) demonstrates that the adsorption process in water solution obeys the pseudo-second-order kinetic model, implying that the Pb2+ uptake into the hydrogel is mainly controlled by the inner surface adsorption. Moreover, the adsorption behaviors strongly depend on the initial concentration of the Pb2+, pH value and the incubation temperature. The adsorption process of Pb2+ into this absorbent can be suitably described by the Langmuir isotherm, and the thermodynamic analysis indicates that the adsorption behavior was spontaneous and endothermic. In addition, the nanocomposite bio-sorbents could still display high uptake capacity after three cycles. This work will pave a way for the utilization of novel biomass-based absorbents for rapid and high-capacity removal of heavy metal ions.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
Dr Qy Liu gratefully acknowledges the financial support from Natural Science Foundation of China (51603195). Jie Ren thanks Postgraduate Education Innovation Project of Shanxi Province, China (2020SY400).
References
- Y.-M. Liu, X.-J. Ju, Y. Xin, W.-C. Zheng, W. Wang, J. Wei, R. Xie, Z. Liu and L.-Y. Chu, ACS Appl. Mater. Interfaces, 2014, 6, 9530–9542 CrossRef CAS PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- X.-J. Ju, S.-B. Zhang, M.-Y. Zhou, R. Xie, L. Yang and L.-Y. Chu, J. Hazard Mater., 2009, 167, 114–118 CrossRef CAS PubMed.
- I. Hajdu, M. Bodnár, Z. Csikós, S. Wei, L. Daróczi, B. Kovács, Z. Győri, J. Tamás and J. Borbély, J. Membr. Sci., 2012, 409, 44–53 CrossRef.
- S. Liang, X. Guo, N. Feng and Q. Tian, J. Hazard. Mater., 2009, 170, 425–429 CrossRef CAS PubMed.
- X. He, D.-P. Yang, X. Zhang, M. Liu, Z. Kang, C. Lin, N. Jia and R. Luque, Chem. Eng. J., 2019, 369, 621–633 CrossRef CAS.
- X. Li, J. Xing, C. Zhang, B. Han, Y. Zhang, T. Wen, R. Leng, Z. Jiang, Y. Ai and X. Wang, ACS Sustainable Chem. Eng., 2018, 6, 10606–10615 CrossRef CAS.
- S. Abbaszadeh, S. R. W. Alwi, C. Webb, N. Ghasemi and I. I. Muhamad, J. Clean. Prod., 2016, 118, 210–222 CrossRef CAS.
- N. Nematidil, M. Sadeghi, S. Nezami and H. Sadeghi, Carbohydr. Polym., 2019, 222, 114971 CrossRef CAS PubMed.
- J. Jiang, Y. Long, X. Hu, J. Hu, M. Zhu and S. Zhou, J. Solid State Chem., 2020, 289, 121491 CrossRef CAS.
- R.-S. Wang, Y. Li, X.-X. Shuai, R.-H. Liang, J. Chen and C.-M. Liu, Polymers, 2021, 13, 2453 CrossRef CAS PubMed.
- Q. Sun, B. Aguila, J. Perman, L. D. Earl, C. W. Abney, Y. Cheng, H. Wei, N. Nguyen, L. Wojtas and S. Ma, J. Am. Chem. Soc., 2017, 139, 2786–2793 CrossRef CAS PubMed.
- M. Basu, A. K. Guha and L. Ray, J. Clean. Prod., 2017, 151, 603–615 CrossRef CAS.
- N. Feng, X. Guo, S. Liang, Y. Zhu and J. Liu, J. Hazard Mater., 2011, 185, 49–54 CrossRef CAS PubMed.
- D. Sud, G. Mahajan and M. P. Kaur, Bioresour. Technol., 2008, 99, 6017–6027 CrossRef CAS PubMed.
- S. Park, K. S. Choi, D. Lee, D. Kim, K. T. Lim, K.-H. Lee, H. Seonwoo and J. Kim, Biosyst. Eng., 2016, 151, 446–463 CrossRef.
- B. Liu and Y. Huang, J. Mater. Chem., 2011, 21, 17413–17418 RSC.
- A. Mittal, M. Teotia, R. Soni and J. Mittal, J. Mol. Liq., 2016, 223, 376–387 CrossRef CAS.
- M. N. R. Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef CAS.
- Y. Zhong, J. Cai and L.-N. Zhang, Chin. J. Polym. Sci., 2020, 1–14 CAS.
- L. Qiao, L. Zhao and K. Du, Chem. Eng. J., 2020, 393, 124818 CrossRef CAS.
- D. Liu, Y. Zhu, Z. Li, D. Tian, L. Chen and P. Chen, Carbohydr. Polym., 2013, 98, 483–489 CrossRef CAS PubMed.
- Y. Duan, A. Freyburger, W. Kunz and C. Zollfrank, ACS Sustainable Chem. Eng., 2018, 6, 6965–6973 CrossRef CAS.
- G. I. Tovar Jimenez, A. Valverde, C. Mendes-Felipe, S. Wuttke, A. Fidalgo-Marijuan, E. S. Larrea, L. Lezama, F. Zheng, J. Reguera and S. Lanceros-Méndez, ChemSusChem, 2021, 14, 2892–2901 CrossRef CAS PubMed.
- B. Duan, Y. Huang, A. Lu and L. Zhang, Prog. Polym. Sci., 2018, 82, 1–33 CrossRef CAS.
- R. Singh and R. Bhateria, ACS Omega, 2020, 5, 28305–28318 CrossRef CAS PubMed.
- J. Duan, X. He and L. Zhang, Chem. Commun., 2015, 51, 338–341 RSC.
- M. He, Z. Wang, Y. Cao, Y. Zhao, B. Duan, Y. Chen, M. Xu and L. Zhang, Biomacromolecules, 2014, 15, 3358–3365 CrossRef CAS PubMed.
- T. Hou, H. Zhang, D. He, Q. Liu, Z. Zhang, L. Xiao, W. Li and M. Barnes, RSC Adv., 2018, 8, 36858–36868 RSC.
- J. Liu, Z. Sun, Y. Deng, Y. Zou, C. Li, X. Guo, L. Xiong, Y. Gao, F. Li and D. Zhao, Angew. Chem., Int. Ed., 2009, 48, 5875–5879 CrossRef CAS PubMed.
- H. Tang, W. Zhou and L. Zhang, J. Hazard Mater., 2012, 209, 218–225 CrossRef PubMed.
- H. Tu, M. Huang, Y. Yi, Z. Li, Y. Zhan, J. Chen, Y. Wu, X. Shi, H. Deng and Y. Du, Appl. Surf. Sci., 2017, 426, 545–553 CrossRef CAS.
- A. S. Özcan and A. Özcan, J. Colloid Interface Sci., 2004, 276, 39–46 CrossRef PubMed.
- B. Hameed, I. Tan and A. Ahmad, J. Colloid Interface Sci., 2008, 144, 235–244 CAS.
- A. Özcan, E. M. Öncü and A. S. Özcan, J. Hazard Mater., 2006, 129, 244–252 CrossRef PubMed.
- G. Bayramoglu, A. Denizli, S. Bektas and M. Y. Arica, Microchem. J., 2002, 72, 63–76 CrossRef CAS.
- B. Samiey and M. R. Dargahi, Cent. Eur. J. Chem., 2010, 8, 906–912 CAS.
- D. Ding, Y. Zhao, S. Yang, W. Shi, Z. Zhang, Z. Lei and Y. Yang, Water Res., 2013, 47, 2563–2571 CrossRef CAS PubMed.
- X.-F. Sun, S.-G. Wang, X.-W. Liu, W.-X. Gong, N. Bao, B.-Y. Gao and H.-Y. Zhang, Bioresour. Technol., 2008, 99, 3475–3483 CrossRef CAS PubMed.
- K. Zhang, Z. Li, N. Deng, J. Ju, Y. Li, B. Cheng, W. Kang and J. Yan, Cellulose, 2019, 26, 945–958 CrossRef CAS.
- Z. P. Li, X. R. Duan, C. H. Liu and B. A. Du, Anal. Biochem., 2006, 351, 18–25 CrossRef CAS PubMed.
- F. Zhao, E. Repo, Y. Song, D. Yin, S. B. Hammouda, L. Chen, S. Kalliola, J. Tang, K. C. Tam and M. Sillanpää, Green Chem., 2017, 19, 4816–4828 RSC.
- N. Kataria and V. Garg, Chemosphere, 2018, 208, 818–828 CrossRef CAS PubMed.
- G. Liu, L. Liao, Z. Dai, Q. Qi, J. Wu, L. Q. Ma, C. Tang and J. Xu, Chem. Eng. J., 2020, 395, 125108 CrossRef CAS.
- Z. Wu, W. Deng, W. Zhou and J. Luo, Carbohydr. Polym., 2019, 216, 119–128 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08744d |
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *b and
Haiqiang Wang
*b and
Haiqiang Wang a
a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons could be available every year from shellfish wastes.21 This polysaccharide possesses a number of amide (–NH–) and hydroxyl (–OH) groups in the polymer chains, which will facilitate the chelation to Ar3+, Cd2+, Co2+, Cu2+, Mn2+ and Pb2+ ions in aqueous system.22 Thus, the chitin-derived materials as the low-cost absorbents have been largely applicated in the water treatment. This occurrence is further enhanced with the development of solvent systems of the chitin. The as-fabricated materials in terms of chitin fibers, crystals whisker, films, hydrogels, aerogels, microspheres and hybrid nanocomposites displayed high performance for the removal of heavy metal ions, organic dyes or pesticide.21,23–25
000 tons could be available every year from shellfish wastes.21 This polysaccharide possesses a number of amide (–NH–) and hydroxyl (–OH) groups in the polymer chains, which will facilitate the chelation to Ar3+, Cd2+, Co2+, Cu2+, Mn2+ and Pb2+ ions in aqueous system.22 Thus, the chitin-derived materials as the low-cost absorbents have been largely applicated in the water treatment. This occurrence is further enhanced with the development of solvent systems of the chitin. The as-fabricated materials in terms of chitin fibers, crystals whisker, films, hydrogels, aerogels, microspheres and hybrid nanocomposites displayed high performance for the removal of heavy metal ions, organic dyes or pesticide.21,23–25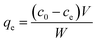







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd
Kd

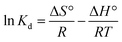
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus 1/T (Fig. 5b), the values of ΔH° and ΔS° in water solution were determined to be 48.6 kJ mol−1 and 267.3 J mol−1 K−1. The positive value of ΔH° demonstrates the endothermic nature of the adsorption process and the existence of an energy barrier. The large positive value of ΔS° proves the higher binding ability of Pb2+ to the nanocomposite matrix and the increased randomness at the solid–solution interface.39
Kd versus 1/T (Fig. 5b), the values of ΔH° and ΔS° in water solution were determined to be 48.6 kJ mol−1 and 267.3 J mol−1 K−1. The positive value of ΔH° demonstrates the endothermic nature of the adsorption process and the existence of an energy barrier. The large positive value of ΔS° proves the higher binding ability of Pb2+ to the nanocomposite matrix and the increased randomness at the solid–solution interface.39






