Open Access Article
Open Access ArticleAn organic–inorganic hybrid K2TiF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Mn4+ red-emitting phosphor with remarkable improvement of emission and luminescent thermal stability†
Mn4+ red-emitting phosphor with remarkable improvement of emission and luminescent thermal stability†
Yan Yua,
Lin Wanga,
Daishu Denga,
Xue Zhonga,
Jiawei Qianga,
Tianman Wanga,
Chunxiang Wua,
Sen Liao *ab and
Yingheng Huang*ab
*ab and
Yingheng Huang*ab
aSchool of Chemistry and Chemical Engineering, Guangxi University, Nanning, Guangxi 530004, China. E-mail: liaosen@gxu.edu.cn; liaosen380@hotmail.com; huangyingheng@163.com; Fax: +86 771 3233718; Tel: +86 771 3233718
bGuangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, School of Resources, Environment and Materials, Guangxi University, Nanning, Guangxi 530004, China
First published on 28th January 2022
Abstract
A new type of monoethanolamine (MEA) and Mn4+ co-doped KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ (K2TiF6
MEAH+, Mn4+ (K2TiF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1MEAH+, 0.06Mn4+) red emitting phosphor was synthesized by an ion exchange method. The prepared Mn4+ co-doped organic–inorganic hybrid red phosphor exhibits sharp red emission at 632 nm and the emission intensity at room temperature is 1.43 times that of a non-hybrid control sample KTF
0.1MEAH+, 0.06Mn4+) red emitting phosphor was synthesized by an ion exchange method. The prepared Mn4+ co-doped organic–inorganic hybrid red phosphor exhibits sharp red emission at 632 nm and the emission intensity at room temperature is 1.43 times that of a non-hybrid control sample KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ (K2TiF6
Mn4+ (K2TiF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06Mn4+). It exhibits good luminescent thermal stability at high temperatures, and the maximum integrated PL intensity at 150 °C is 2.34 times that of the initial value at 30 °C. By coating a mixture of KTF
0.06Mn4+). It exhibits good luminescent thermal stability at high temperatures, and the maximum integrated PL intensity at 150 °C is 2.34 times that of the initial value at 30 °C. By coating a mixture of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, a yellow phosphor (YAG
MEAH+, Mn4+, a yellow phosphor (YAG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce3+) and epoxy resin on a blue InGaN chip, a prototype WLED (white light-emitting diode) with CCT = 3740 K and Ra = 90.7 is assembled. The good performance of the WLED shows that KTF
Ce3+) and epoxy resin on a blue InGaN chip, a prototype WLED (white light-emitting diode) with CCT = 3740 K and Ra = 90.7 is assembled. The good performance of the WLED shows that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ can provide a new choice for the synthesis of new Mn4+ doped fluoride phosphors.
MEAH+, Mn4+ can provide a new choice for the synthesis of new Mn4+ doped fluoride phosphors.
1 Introduction
In recent years, WLEDs (white light-emitting diodes) with stable performance, energy saving, and outstanding luminescent characteristics have increasingly become an important requirement in the display and lighting fields.1–5 At the moment, the commercially available WLEDs are composed of yellow emitting phosphors and blue emitting chips.6 Although the device can produce white light, due to the lack of a red light component, its corresponding disadvantages are low color rendering index (Ra < 80) and high color temperature (CCT > 4500 K). So, it cannot well meet the requirements of the lighting field.7 In order to obtain WLEDs with excellent performance, it is urgent to find a suitable red-emitting phosphor.Among the many types of red phosphors, Mn4+ doped fluoride red phosphors have been widely reported due to their advantages such as high quantum efficiency at room temperature, effective absorption of near-UV and blue light, and narrow-band red light emission.8–11 However, their inherent shortcoming is poor thermal stability of luminescence, and it is likely that the luminescent intensity will drop rapidly due to luminescent thermal quenching at high temperatures. Recently, a method to improve the luminous efficiency of inorganic phosphors is to graft with organic ligands, which can effectively promote the transfer of energy from the coordinated ligands to the activated ions, and results enhancement of the emission intensity.12,13 And because organic–inorganic hybrid metal halide perovskite materials have good color tunability and high PLQE,14,15 application of an organic–inorganic hybrid matrix for the synthesis of Mn4+ doped emitting phosphors has attracted the attention of some researchers. For example, T. Jüstel et al. reported an organic–inorganic hybrid [C(NH2)3]2GeF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ red phosphor that has narrow red emission at low temperatures (3 K) but exhibits severe luminescence quenching at room temperature.16 Cai et al. reported an organic–inorganic hybrid [(CH3)4]2TiF6
Mn4+ red phosphor that has narrow red emission at low temperatures (3 K) but exhibits severe luminescence quenching at room temperature.16 Cai et al. reported an organic–inorganic hybrid [(CH3)4]2TiF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ red phosphor can emission efficiently in the range of 50–300 K.17 Although the above attempts are not satisfactory, they provide us with an idea to solve the problem of low luminescent thermal stability of red phosphors and to find new Mn4+ doped red phosphors.
Mn4+ red phosphor can emission efficiently in the range of 50–300 K.17 Although the above attempts are not satisfactory, they provide us with an idea to solve the problem of low luminescent thermal stability of red phosphors and to find new Mn4+ doped red phosphors.
Therefore, in order to synthesize a new type of Mn4+ doped fluoride red phosphor with excellent performances, we doped part of MEAH+ in the K2TiF6 matrix, and synthesized Mn4+ doped KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ red phosphor on this basis. The prepared sample not only exhibits strong narrow red light emission at room temperature, but also exhibits strong negative luminescent thermal quenching, suggesting that the sample has great application prospects in WLEDs.
MEAH+, Mn4+ red phosphor on this basis. The prepared sample not only exhibits strong narrow red light emission at room temperature, but also exhibits strong negative luminescent thermal quenching, suggesting that the sample has great application prospects in WLEDs.
2 Experimental and methodology
The experiments and methodology are described in the ESI.†3 Results and discussion
3.1 Structure, morphology and composition properties
Fig. 1 depicts the XRD patterns and FTIR spectra of samples (i–ii) and matrix. In Fig. 1(a), all diffraction peaks of the two samples are indexed in agreement with the standard data of space group PDF#08-0488 (pure hexagonal phase K2TiF6 with space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1), and the indexed results are listed in Table 1. The order of the lattice volume size of the samples is PDF#08-0488 > (ii) > (i). The ionic radii of Ti4+ and Mn4+ are 0.61 Å and 0.53 Å, respectively,18 so the lattice volume will shrink when Mn4+ occupies the octahedral lattice sites of Ti4+. The absence of any impurity peaks in the XRD patterns of KTF
m1), and the indexed results are listed in Table 1. The order of the lattice volume size of the samples is PDF#08-0488 > (ii) > (i). The ionic radii of Ti4+ and Mn4+ are 0.61 Å and 0.53 Å, respectively,18 so the lattice volume will shrink when Mn4+ occupies the octahedral lattice sites of Ti4+. The absence of any impurity peaks in the XRD patterns of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ also show that MEAH+ is doped into the KTF matrix. Fig. 1(b) shows that the 2θ angles of KTF
MEAH+, Mn4+ also show that MEAH+ is doped into the KTF matrix. Fig. 1(b) shows that the 2θ angles of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are both slightly shifted to a large angle. It indicates that the lattice volumes of both samples are slightly contracted and there is lattice distortion.
MEAH+, Mn4+ are both slightly shifted to a large angle. It indicates that the lattice volumes of both samples are slightly contracted and there is lattice distortion.
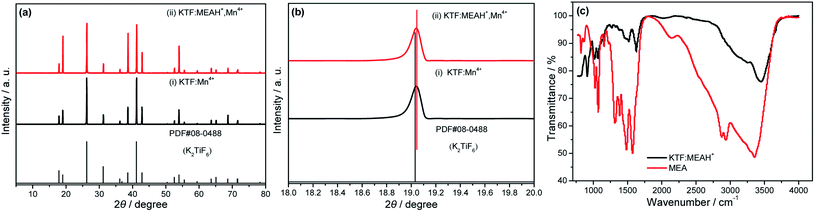 | ||
Fig. 1 XRD patterns and FTIR spectra of samples (i–ii) and matrix, (i) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and (ii) KTF Mn4+ and (ii) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+: (a) full XRD patterns, (b) expanded XRD patterns, (c) FTIR spectra of KTF MEAH+, Mn4+: (a) full XRD patterns, (b) expanded XRD patterns, (c) FTIR spectra of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+ and MEA. MEAH+ and MEA. | ||
| a = b/Å | c/Å | α = β/° | γ/° | V/Å3 | |
|---|---|---|---|---|---|
a (i) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and (ii) KTF Mn4+ and (ii) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. MEAH+, Mn4+. |
|||||
| PDF#08-0488 | 5.7271 | 4.6619 | 90 | 120 | 132.4 |
| (i) | 5.723 | 4.65712 | 90 | 120 | 132.1 |
| (ii) | 5.72339 | 4.66014 | 90 | 120 | 132.2 |
Fig. S1† shows the results obtained under natural light conditions using the ninhydrin method for KTF and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1MEAH+ (KTF
0.1MEAH+ (KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+), respectively. It can be seen from Fig. S1† that KTF does not change color, and KTF
MEAH+), respectively. It can be seen from Fig. S1† that KTF does not change color, and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+ turns blue. In order to further confirm that MEAH+ is doped into the lattice of the red phosphor, the infrared spectra of KTF
MEAH+ turns blue. In order to further confirm that MEAH+ is doped into the lattice of the red phosphor, the infrared spectra of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+ and MEA were tested, and the corresponding infrared spectra were provided in Fig. 1(c). It is not difficult to see from Fig. 1(c) that some peaks of KTF
MEAH+ and MEA were tested, and the corresponding infrared spectra were provided in Fig. 1(c). It is not difficult to see from Fig. 1(c) that some peaks of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+ show a slight red shift relative to MEA, but many peaks of the two can be matched. From the results, it can be concluded that MEAH+ is indeed doped into the lattice of the matrix.
MEAH+ show a slight red shift relative to MEA, but many peaks of the two can be matched. From the results, it can be concluded that MEAH+ is indeed doped into the lattice of the matrix.
Fig. 2 shows the SEM images and EDS patterns of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. Fig. 2(a) depicts that KTF
MEAH+, Mn4+. Fig. 2(a) depicts that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is composed of polyhedrons with a length of approximately 15 μm, and Fig. 2(b) depicts that KTF
Mn4+ is composed of polyhedrons with a length of approximately 15 μm, and Fig. 2(b) depicts that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are composed of regular hexahedrons with a length of approximately 16–18 μm. The addition of MEAH+ not only made the sample smooth, but also changed the morphology of KTF
MEAH+, Mn4+ are composed of regular hexahedrons with a length of approximately 16–18 μm. The addition of MEAH+ not only made the sample smooth, but also changed the morphology of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. Fig. 2(c) and (d) illustrate that KTF
MEAH+, Mn4+. Fig. 2(c) and (d) illustrate that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is composed of K, Ti, F, and Mn elements, and KTF
Mn4+ is composed of K, Ti, F, and Mn elements, and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ include K, Ti, F, Mn, C, O, and N elements. Since MEAH+ contains C, O, and N elements, the results in Fig. 2(d) can indicate that MEAH+ has successfully entered the K2TiF6 matrix.
MEAH+, Mn4+ include K, Ti, F, Mn, C, O, and N elements. Since MEAH+ contains C, O, and N elements, the results in Fig. 2(d) can indicate that MEAH+ has successfully entered the K2TiF6 matrix.
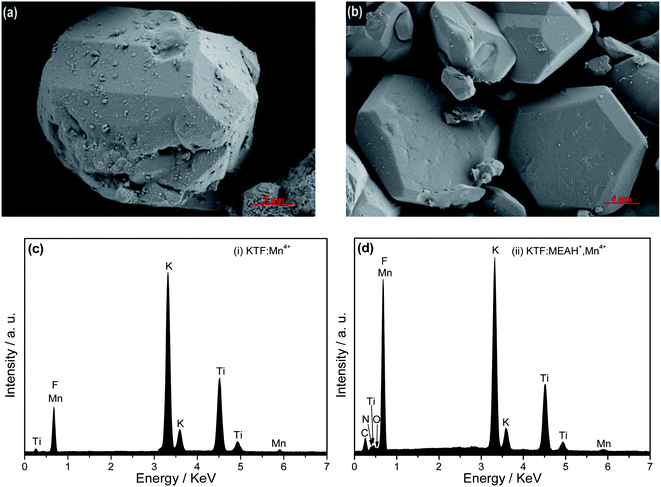 | ||
Fig. 2 SEM images and EDS spectra of two samples, (i) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and (ii) KTF Mn4+ and (ii) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+: (a and b) SEM images (c and d) EDS spectra. MEAH+, Mn4+: (a and b) SEM images (c and d) EDS spectra. | ||
3.2 Luminescent properties of room temperature
Fig. 3(a) and (b) show the luminescent characteristics of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. It can be seen from Fig. 3(a) that the luminous intensity ratio of KTF
MEAH+, Mn4+. It can be seen from Fig. 3(a) that the luminous intensity ratio of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+ and Mn4+KTF
MEAH+ and Mn4+KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is 1.43, indicating that the luminous intensity of the former is enhanced by doping of MEAH+. Fig. 3(a) and (b) indicate that the two samples have different emission and excitation bands, and the positions of the main peaks are almost the same. In the PLE spectra, there are two broad excitation bands centered at ∼360 nm and ∼460 nm, which originate from the 4A2g → 4T1g and 4A2g → 4T2g transitions of Mn4+ in octahedral symmetry, respectively. Under the excitation of 467 nm, the emission spectra have several sharp peaks in the range of 600–660 nm, of which the strongest peak is at 632 nm. They are attributed to the spin-forbidden d–d transition (2Eg → 4A2g) of Mn4+, and are activated by the [MnF6]2− vibration mode.19–22
Mn4+ is 1.43, indicating that the luminous intensity of the former is enhanced by doping of MEAH+. Fig. 3(a) and (b) indicate that the two samples have different emission and excitation bands, and the positions of the main peaks are almost the same. In the PLE spectra, there are two broad excitation bands centered at ∼360 nm and ∼460 nm, which originate from the 4A2g → 4T1g and 4A2g → 4T2g transitions of Mn4+ in octahedral symmetry, respectively. Under the excitation of 467 nm, the emission spectra have several sharp peaks in the range of 600–660 nm, of which the strongest peak is at 632 nm. They are attributed to the spin-forbidden d–d transition (2Eg → 4A2g) of Mn4+, and are activated by the [MnF6]2− vibration mode.19–22
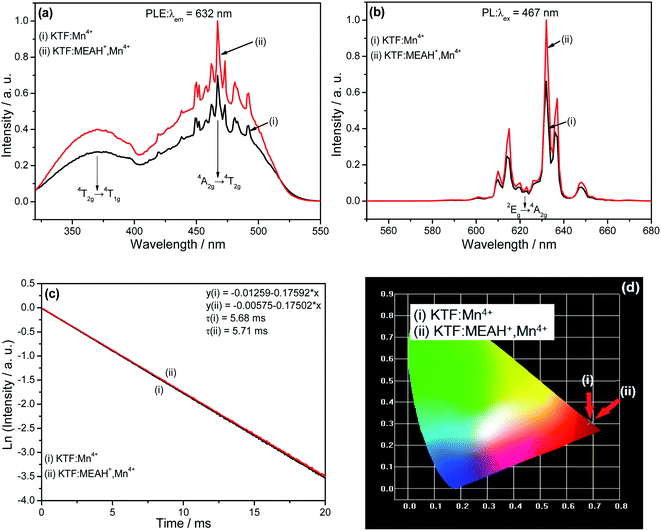 | ||
Fig. 3 Luminescent properties of two samples, (i) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and (ii) KTF Mn4+ and (ii) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+: (a) and (b) PLE and PL spectra of samples (i)–(ii), (c) decay curves, (d) CIE chromaticity diagram. MEAH+, Mn4+: (a) and (b) PLE and PL spectra of samples (i)–(ii), (c) decay curves, (d) CIE chromaticity diagram. | ||
Fig. 3(c) depicts the lifetime curves of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. They can be fitted by linear functions with constant terms. The lifetimes of the two samples are 5.68, 5.71 ms, respectively. It can be seen that the lifetime of KTF
MEAH+, Mn4+. They can be fitted by linear functions with constant terms. The lifetimes of the two samples are 5.68, 5.71 ms, respectively. It can be seen that the lifetime of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is improved after organic–inorganic hybridization. Theoretically, when energy transfer occurs between the donor and the acceptor, the lifetime of the donor will decrease, and the lifetime of the acceptor will increase.23 It is not difficult to see that the lifetimes of KTF
MEAH+, Mn4+ is improved after organic–inorganic hybridization. Theoretically, when energy transfer occurs between the donor and the acceptor, the lifetime of the donor will decrease, and the lifetime of the acceptor will increase.23 It is not difficult to see that the lifetimes of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are longer than the lifetime of KTF
MEAH+, Mn4+ are longer than the lifetime of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, indicating that there is energy transfer from MEAH+ (donor) to Mn4+ (acceptor).
Mn4+, indicating that there is energy transfer from MEAH+ (donor) to Mn4+ (acceptor).
The internal quantum yield (QYi) values of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are determined by eqn (S1).†24–26 The QYi of KTF
MEAH+, Mn4+ are determined by eqn (S1).†24–26 The QYi of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are determined to be 97.48 and 99.67%, respectively. The results of lifetime and QYi show that luminescent performances can be enhanced by doping of MEAH+.
MEAH+, Mn4+ are determined to be 97.48 and 99.67%, respectively. The results of lifetime and QYi show that luminescent performances can be enhanced by doping of MEAH+.
Based on the emission spectra data in Fig. 3(b), the chromaticity coordinates of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ were calculated. The calculation results are shown in (Fig. 3(d)). The details are as follows: (a) KTF
MEAH+, Mn4+ were calculated. The calculation results are shown in (Fig. 3(d)). The details are as follows: (a) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is (0.6918, 0.3081), (b) KTF
Mn4+ is (0.6918, 0.3081), (b) KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is (0.693, 0.3069).
MEAH+, Mn4+ is (0.693, 0.3069).
Eqn (S2)† is used to calculate the color purity of the sample.27–29 The calculated color purity values of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are 99.57 and 99.93%, respectively.
MEAH+, Mn4+ are 99.57 and 99.93%, respectively.
3.3 Luminescent properties of different samples
The effect of different molar ratios (atomic ratios) of Mn4+ on the fluorescence properties of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, xMn4+ (x = molar ratios of Mn/(Ti + Mn)) is shown in Fig. 4(a). The effect curve shown in Fig. 4(a) is a nonlinear curve with a maximum value. First of all, the curve increases with increasing x, reaching a maximum at x = 0.06, and decreases when x exceeds 0.06 due to quenching of the concentration.
MEAH+, xMn4+ (x = molar ratios of Mn/(Ti + Mn)) is shown in Fig. 4(a). The effect curve shown in Fig. 4(a) is a nonlinear curve with a maximum value. First of all, the curve increases with increasing x, reaching a maximum at x = 0.06, and decreases when x exceeds 0.06 due to quenching of the concentration.
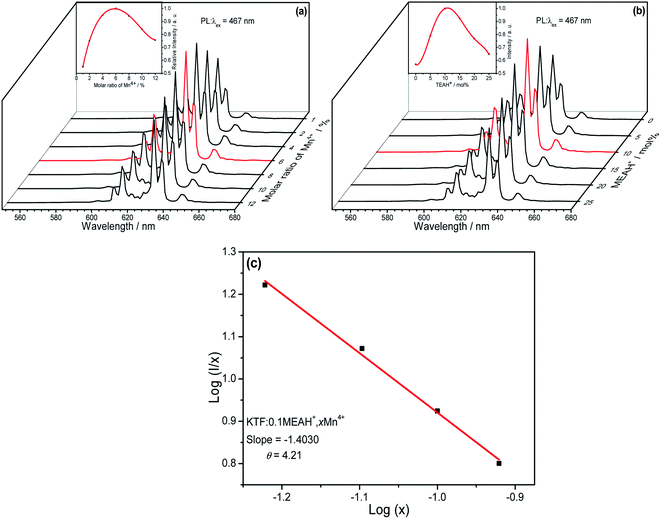 | ||
Fig. 4 The luminescent properties of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1MEAH+, xMn4+ and KTF 0.1MEAH+, xMn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yMEAH+, 0.06Mn4+ (a) and (b) PL spectra, (c) the related type line of multipolar interaction. yMEAH+, 0.06Mn4+ (a) and (b) PL spectra, (c) the related type line of multipolar interaction. | ||
Fig. 4(b) is the emission spectra of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yMEAH+, Mn4+ under different y (y = molar ratio of MEA/Ti, Mn4+ is fixed at 6%). The effect of y on the emission intensity is also a nonlinear curve with a maximum value. First, the emission intensity of this curve increases with the increase of y, and reaches the apex when y = 10%, and then when y > 10%, the emission intensity decreases due to the quenching of the concentration.
yMEAH+, Mn4+ under different y (y = molar ratio of MEA/Ti, Mn4+ is fixed at 6%). The effect of y on the emission intensity is also a nonlinear curve with a maximum value. First, the emission intensity of this curve increases with the increase of y, and reaches the apex when y = 10%, and then when y > 10%, the emission intensity decreases due to the quenching of the concentration.
The quenching mechanism of Mn4+ concentration in KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, xMn4+ can be explored by calculating the critical distance (Rc) between Mn4+ ions. The eqn (S3)† is used to calculate Rc.30
MEAH+, xMn4+ can be explored by calculating the critical distance (Rc) between Mn4+ ions. The eqn (S3)† is used to calculate Rc.30
For KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, V = 132.1 Å3, xc = 6%, and N = 4; Rc can be calculated as 10.17 Å. Since the Rc of the exchange interaction mechanism is less than 5 Å, and the current Rc is greater than 5 Å, suggesting that the quenching mechanism of Mn4+ concentration in KTF
MEAH+, Mn4+, V = 132.1 Å3, xc = 6%, and N = 4; Rc can be calculated as 10.17 Å. Since the Rc of the exchange interaction mechanism is less than 5 Å, and the current Rc is greater than 5 Å, suggesting that the quenching mechanism of Mn4+ concentration in KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ may not be an exchange interaction mechanism, but a multipolar interaction mechanism.
MEAH+, Mn4+ may not be an exchange interaction mechanism, but a multipolar interaction mechanism.
The related type of multipolar interaction can be determined according to the eqn (S4).†30,31 Fig. 4(c) depicts that the dependence curve of Log(I/x) on Log(x) for KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, xMn4+ is a straight line with a slope of −1.4030. The slope corresponds to θ = 4.21. Therefore, the quenching of Mn4+ concentration in the KTF
MEAH+, xMn4+ is a straight line with a slope of −1.4030. The slope corresponds to θ = 4.21. Therefore, the quenching of Mn4+ concentration in the KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, xMn4+ sample can be identified as a mechanism of dipole–dipole interaction.
MEAH+, xMn4+ sample can be identified as a mechanism of dipole–dipole interaction.
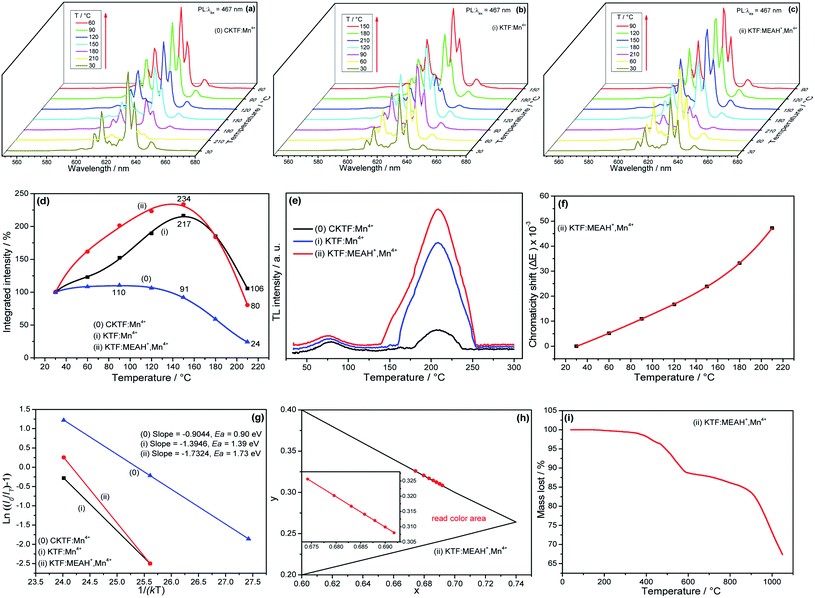
3.4 Analyses of thermal performances
Because the operating temperature of high-power LED devices may reach 150 °C, the luminescent thermal stability of phosphors is also an important evaluation index.32 To compare the luminescent thermal stability of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ with commercial red phosphors, sample (0) CKTF
MEAH+, Mn4+ with commercial red phosphors, sample (0) CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ (commercial – K2TiF6
Mn4+ (commercial – K2TiF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06Mn4+) was synthesized. The luminescent thermal stabilities of three samples were tested, and the results were depicted in Fig. 5(a–c). The PL spectrum intensities of the three samples are significantly affected by temperature. It is clear from Fig. 5(d) that the effect of temperature on the PL integrated intensities of all three samples are curves with a maximum value, indicating that the three samples all have negative thermal quenching (NTQ) effect. But, there is a huge difference between the three curves. Similar to results in many reports,33–35 when the temperature increases, the PL integrated intensity of CKTF
0.06Mn4+) was synthesized. The luminescent thermal stabilities of three samples were tested, and the results were depicted in Fig. 5(a–c). The PL spectrum intensities of the three samples are significantly affected by temperature. It is clear from Fig. 5(d) that the effect of temperature on the PL integrated intensities of all three samples are curves with a maximum value, indicating that the three samples all have negative thermal quenching (NTQ) effect. But, there is a huge difference between the three curves. Similar to results in many reports,33–35 when the temperature increases, the PL integrated intensity of CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ only slightly increases, then reaches the maximum value at 90 °C, and the PL intensity is only 110% of its initial value at 30 °C. Unlike CKTF
Mn4+ only slightly increases, then reaches the maximum value at 90 °C, and the PL intensity is only 110% of its initial value at 30 °C. Unlike CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, the integrated PL intensities of KTF
Mn4+, the integrated PL intensities of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ all reach the maximum at 150 °C. The maximum values of KTF
MEAH+, Mn4+ all reach the maximum at 150 °C. The maximum values of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are 217 and 234% of their initial values at 30 °C, respectively. So, the strength order of the effect for the three samples is as follows: KTF
MEAH+, Mn4+ are 217 and 234% of their initial values at 30 °C, respectively. So, the strength order of the effect for the three samples is as follows: KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ > KTF
MEAH+, Mn4+ > KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ > CKTF
Mn4+ > CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, indicating that KTF
Mn4+, indicating that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ have high luminescent thermal stability.
MEAH+, Mn4+ have high luminescent thermal stability.
Regarding the mechanism of the NTQ effect, it has been reported that as the temperature increases, the extra energy of the electron traps formed from the matrix defects is transferred to Mn4+, and leads to producing the effect.36–38 So, the improvement mechanism of luminescent thermal stability for KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ can be attributed to increasing of electron traps induced by co-doping of MEAH+.
MEAH+, Mn4+ can be attributed to increasing of electron traps induced by co-doping of MEAH+.
Since CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, KTF
Mn4+, KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are only different in the synthesis methods, but also in the NTQ effect, suggesting that it is our synthesized method that provide more suitable electron traps for KTF
MEAH+, Mn4+ are only different in the synthesis methods, but also in the NTQ effect, suggesting that it is our synthesized method that provide more suitable electron traps for KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+.
MEAH+, Mn4+.
In order to determine whether the doping of MEAH+ can lead to the increase of the electron traps, we conducted thermo-luminescence (TL) tests on CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, KTF
Mn4+, KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+. As shown in Fig. 5(e), there are mainly two traps located at 78 and 208 K in the TL curves. Compared with the trap depth of KTF
MEAH+, Mn4+. As shown in Fig. 5(e), there are mainly two traps located at 78 and 208 K in the TL curves. Compared with the trap depth of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, the trap depth of KTF
Mn4+, the trap depth of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is larger, which may indicate that the doping of MEAH+ can cause the increase of the electron traps.
MEAH+, Mn4+ is larger, which may indicate that the doping of MEAH+ can cause the increase of the electron traps.
In order to quantitatively describe the color stability, the eqn (S5)† is used to calculate the chromaticity shift (ΔE) of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ at different temperatures.39,40 As shown in Fig. 5(f), as the temperature increases, the chromaticity shift of KTF
MEAH+, Mn4+ at different temperatures.39,40 As shown in Fig. 5(f), as the temperature increases, the chromaticity shift of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ gradually increases. According to the curve, the ΔE of KTF
MEAH+, Mn4+ gradually increases. According to the curve, the ΔE of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ at 175 °C is 31.56 × 10−3, which is smaller than that of CSASNE (44 × 10−3, at 175 °C). Because the smaller ΔE is, the more stable chromaticity is, the result indicates that KTF
MEAH+, Mn4+ at 175 °C is 31.56 × 10−3, which is smaller than that of CSASNE (44 × 10−3, at 175 °C). Because the smaller ΔE is, the more stable chromaticity is, the result indicates that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ has high chromaticity stability and make it a potential phosphor for high-quality WLEDs.
MEAH+, Mn4+ has high chromaticity stability and make it a potential phosphor for high-quality WLEDs.
To further understand the characteristics of luminescent thermal quenching, the eqn (S6)† is used to calculate Ea values of CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+, KTF
Mn4+, KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+.41,42 Using the slopes of the three straight lines in Fig. 5(g), the Ea values of the three samples can be calculated to be 0.90, 1.39 and 1.73, respectively. Since the larger Ea, the harder the luminescent thermal quenching occurs, so the order of luminescent thermal stability is KTF
MEAH+, Mn4+.41,42 Using the slopes of the three straight lines in Fig. 5(g), the Ea values of the three samples can be calculated to be 0.90, 1.39 and 1.73, respectively. Since the larger Ea, the harder the luminescent thermal quenching occurs, so the order of luminescent thermal stability is KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ > KTF
MEAH+, Mn4+ > KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ > CKTF
Mn4+ > CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+.
Mn4+.
Fig. 5(h) depicts the color coordinates of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ at different temperatures. Fig. 5(h) shows that the color coordinates of KTF
MEAH+, Mn4+ at different temperatures. Fig. 5(h) shows that the color coordinates of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ are only slightly shifted, showing that the color stability of KTF
MEAH+, Mn4+ are only slightly shifted, showing that the color stability of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is high enough. The slight shift of the color coordinates may be resulted from the slight broadening of the PL peak.
MEAH+, Mn4+ is high enough. The slight shift of the color coordinates may be resulted from the slight broadening of the PL peak.
To confirm the chemical thermal stability of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, TG measurement was performed. Fig. 5(i) depicts the TG curve of KTF
MEAH+, Mn4+, TG measurement was performed. Fig. 5(i) depicts the TG curve of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ from 40 °C to 1050 °C. From Fig. 5(i), it can be seen that KTF
MEAH+, Mn4+ from 40 °C to 1050 °C. From Fig. 5(i), it can be seen that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ started to decompose at about 166 °C, indicating that KTF
MEAH+, Mn4+ started to decompose at about 166 °C, indicating that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ has good chemical thermal stability. The KTF
MEAH+, Mn4+ has good chemical thermal stability. The KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ has a relatively large mass loss from 360 °C, which is similar to our previous study.43 In general, KTF
MEAH+, Mn4+ has a relatively large mass loss from 360 °C, which is similar to our previous study.43 In general, KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is able to meet the requirements of WLEDs.
MEAH+, Mn4+ is able to meet the requirements of WLEDs.
3.5 Performances of WLEDs
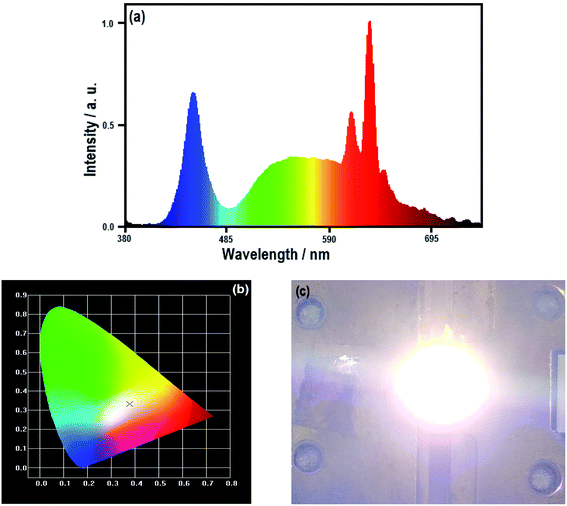
In order to evaluate the application potential of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, a mixture of KTF
MEAH+, Mn4+, a mixture of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, YAG
MEAH+, Mn4+, YAG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce3+ and epoxy resin is coated on blue InGaN chips and assembled into prototype WLED. The performance of the WLED is shown in Fig. 6.
Ce3+ and epoxy resin is coated on blue InGaN chips and assembled into prototype WLED. The performance of the WLED is shown in Fig. 6.
Fig. 6(a) is the electroluminescent spectrum of the WLEDs, which has blue, yellow and red emission bands. From the emission data of Fig. 6(a), the CIE chromaticity diagram (0.3773, 0.3332) in Fig. 6(b) can be acquired, and the color coordinate point is in the white light region. Fig. 6(c) is a luminous photo, which shows warm white light. The results in Fig. 6(b) and (c) show that WLEDs emits good quality warm white light (CCT = 3740 K, Ra = 90.7).
4 Conclusions
In general, a new type of Mn4+ doped organic–inorganic hybrid KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ red emitting phosphor was synthesized by ion exchange method. By comparing KTF
MEAH+, Mn4+ red emitting phosphor was synthesized by ion exchange method. By comparing KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ in luminous intensity and QYi, it can be found that KTF
MEAH+, Mn4+ in luminous intensity and QYi, it can be found that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ has high luminous intensity and nearly perfect quantum efficiency. The luminous intensity ratio of KTF
MEAH+, Mn4+ has high luminous intensity and nearly perfect quantum efficiency. The luminous intensity ratio of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ and KTF
MEAH+, Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is 1.43, and the QYi of KTF
Mn4+ is 1.43, and the QYi of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is 99.76%. When comparison of the thermal stability of CKTF
MEAH+, Mn4+ is 99.76%. When comparison of the thermal stability of CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ and KTF
Mn4+ and KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, where CKTF
MEAH+, Mn4+, where CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ was synthesized with a commercially available matrix, it can be found that at 150 °C, the integrated PL intensity of CKTF
Mn4+ was synthesized with a commercially available matrix, it can be found that at 150 °C, the integrated PL intensity of CKTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn4+ is only 91% of its initial value, while the integrated PL intensity of KTF
Mn4+ is only 91% of its initial value, while the integrated PL intensity of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is 234% of its initial value. The results suggest that KTF
MEAH+, Mn4+ is 234% of its initial value. The results suggest that KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+ is a red emitting phosphor with excellent luminescent thermal stability. By coating a mixture of KTF
MEAH+, Mn4+ is a red emitting phosphor with excellent luminescent thermal stability. By coating a mixture of KTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MEAH+, Mn4+, YAG
MEAH+, Mn4+, YAG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce3+ and epoxy resin on a blue InGaN chip, prototype WLEDs with good warm white light (CCT = 3740 K, Ra = 90.7) were assembled. The results show that a new type of organic–inorganic hybrid red emitting phosphor has been successfully synthesized, which provides a new choice for the synthesis of red emitting phosphor.
Ce3+ and epoxy resin on a blue InGaN chip, prototype WLEDs with good warm white light (CCT = 3740 K, Ra = 90.7) were assembled. The results show that a new type of organic–inorganic hybrid red emitting phosphor has been successfully synthesized, which provides a new choice for the synthesis of red emitting phosphor.
Author contributions
Yan Yu: methodology, formal analysis, investigation, writing-original draft. Lin Wang: investigation. Daishu Deng: investigation. Xue Zhong: investigation. Jiawei Qiang: investigation. Tianman Wang: investigation. Chunxiang Wu: investigation. Sen Liao: conceptualization, supervision. Yingheng Huang: review & editing, visualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the National Natural Science Foundation of China (Grant No. 21661006 and No. 21965004), the Natural Science Foundation of Guangxi Zhuang Autonomous Region, China (Grant No. 2019GXNSFDA245022, No. 2020GXNSFAA159036), the Scientific Research Foundation of Guangxi University (Grant No. XDZ140116), the Innovation Project of Guangxi Graduate Education (Grant No. YCSW2020015), the Students Experimental Skills and Innovation Ability Training Fund Project of Guangxi University (No. 202010593186).References
- Z. W. Jia, C. X. Yuan, Y. F. Liu, X. J. Wang, P. Sun, L. Wang, H. C. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed.
- Z. Abbas, S. Azam, A. I. Bashir, A. Marriam, M. Waqas, T. Alshahrani and B. U. Haq, Phys. Scr., 2020, 96, 015801 CrossRef.
- W. Xie, J. X. Li, C. X. Tian, Z. S. Wang, M. B. Xie, C. W. Zou, G. H. Sun and F. W. Kang, Solid State Sci., 2018, 76, 92–99 CrossRef CAS.
- J. Y. Wang, T. C. Lang, S. Q. Fang, T. Han, M. S. Cai, M. G. Wang, S. X. Cao, L. L. Peng, B. T. Liu, E. F. Polisadova and A. N. Yakovlev, ACS Sustainable Chem. Eng., 2021, 9, 2717–2726 CrossRef CAS.
- H. B. Nasrabadi, E. Madirov, R. Popescu, L. Štacková, P. Štacko, P. Klán, B. S. Richards, D. Hudry and A. Turshatov, J. Mater. Chem. C, 2021, 9, 16313–16323 RSC.
- Q. Yao, P. Hu, P. Sun, M. Liu, R. Dong, K. F. Chao, Y. F. Liu, J. Jiang and H. C. Jiang, Adv. Mater., 2020, 32, e1907888 CrossRef PubMed.
- W. M. Ming, H. L. Shi and M. H. Du, J. Mater. Chem. C, 2018, 6, 4171–4176 RSC.
- Y. Y. Zhou, H. Ming, S. Zhang, T. T. Deng, E. H. Song and Q. Y. Zhang, Chem. Eng. J., 2021, 415, 128974 CrossRef CAS.
- D. X. Shi, Z. B. Liang, X. Zhang, Q. Zhou, Z. L. Wang, M. M. Wu and Y. Q. Ye, J. Lumin., 2020, 226, 117491 CrossRef CAS.
- S. Tang, Y. Liu, H. Li, Q. Zhou, K. M. Wang, H. J. Tang and Z. L. Wang, J. Lumin., 2020, 224, 117291 CrossRef CAS.
- J. M. Ha, E. Novitskaya, N. Lam, M. Sanchez, Y. H. Kim, Z. Z Li, W. B. Im, O. A. Graeve and J. McKittrick, J. Lumin., 2020, 218, 116835 CrossRef CAS.
- K. N. Narasimhamurthy, G. P. Darshan, S. C. Sharma, H. B. Premkumar, H. Adarsha and H. Nagabhushana, J. Colloid Interface Sci., 2021, 600, 887–897 CrossRef CAS PubMed.
- Q. Zhu, J. J. Liu, X. D. Li, X. D. Sun and J.-G. Li, Appl. Surf. Sci., 2019, 489, 142–148 CrossRef CAS.
- C. K. Zhou, Y. Tian, Z. Yuan, H. R. Lin, B. H. Chen, R. Clark, T. Dilbeck, Y. Zhou, J. Hurley, J. Neu, T. Besara, T. Siegrist, P. Djurovich and B. W. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 44579–44583 CrossRef CAS PubMed.
- M. Worku, L. J. Xu, M. Chaaban, A. Ben-Akacha and B. W. Ma, APL Mater., 2020, 8, 10902 CrossRef CAS.
- F. Baur, D. Böhnisch and T. Jüstel, ECS J. Solid State Sci. Technol., 2020, 9, 046003 CrossRef CAS.
- P. Q. Cai, S. Wang, T. M. Xu, Y. Tang, X. L. Yuan, M. J. Wan, Q. Ai, J. J. Si, X. Yao, Y. G. Cao, M. K. Rabchinskii, P. N. Brunkov and Z. G. Liu, J. Lumin., 2020, 228, 117661 CrossRef CAS.
- H. M. Zhu, C. C. Lin, W. Q. Luo, S. T. Shu, Z. G. Liu, Y. S. Liu, J. T. Kong, E. Ma, Y. G. Cao, R. S. Liu and X. Y. Chen, Nat. Commun., 2014, 5, 4312 CrossRef CAS PubMed.
- H. H. Zhang, Y. Y. Chen, X. Y. Zhu, K. L. Liu, H. C. Zhou, X. K. Sun, N. N. Li and Y. L. Feng, J. Lumin., 2021, 236, 118131 CrossRef CAS.
- Y. J. Wang, C. K. Yu, Y. Y. Zhou, E. H. Song, H. Ming and Q. Y. Zhang, J. Alloys Compd., 2021, 855, 157347 CrossRef CAS.
- L. P. Dong, L. Zhang, Y. C. Jia, B. Q. Shao, W. Lü, S. Zhao and H. P. You, ACS Sustainable Chem. Eng., 2020, 8, 3357–3366 CrossRef CAS.
- T. C. Lang, J. Y. Wang, T. Han, M. S. Cai, S. Q. Fang, Y. Z. Zhong, L. L. Peng, S. X. Cao, B. T. Liu, E. Polisadova, V. Korepanov and A. Yakovlev, Inorg. Chem., 2021, 60, 1832–1838 CrossRef CAS PubMed.
- W. Li, H. R. Zhang, S. Chen, Y. L. Liu, J. L. Zhuang and B. F. Lei, Adv. Opt. Mater., 2016, 4, 427–434 CrossRef CAS.
- J. Wu, Z. Y. Li, L. Luo, Y. H. Xiong, L. Y. Jiang, R. Guo and L. L. Meng, J. Alloys Compd., 2021, 863, 158058 CrossRef CAS.
- L. Q. Xi, Y. X. Pan, M. M. Zhu, H. Z. Lian and J. Lin, Dalton Trans., 2017, 46, 13835–13844 RSC.
- L. Shi, Y. J. Han, Z. X. Ji and Z. W. Zhang, J. Mater. Sci.: Mater. Electron., 2019, 30, 3107–3113 CrossRef CAS.
- L. Y. Wang, E. H. Song, Y. Y. Zhou, T. T. Deng, S. Ye and Q. Y. Zhang, J. Mater. Chem. C, 2017, 5, 7253–7261 RSC.
- W. Li, L. L. Sun, B. Devakumar, N. Ma, Z. J. Zhang and X. Y. Huang, J. Photochem. Photobiol., A, 2021, 410, 113166 CrossRef CAS.
- M. M. Zhu, Y. X. Pan, L. Q. Xi, H. Z. Lian and J. Lin, J. Mater. Chem. C, 2017, 5, 10241–10250 RSC.
- L. F. Yuan, Y. H. Jin, G. T. Xiong, H. Y. Wu, J. C. Li, H. Liu, L. Chen and Y. H. Hu, J. Alloys Compd., 2021, 870, 159394 CrossRef CAS.
- N. Ma, W. Li, B. Devakumar, S. Y. Wang, L. L. Sun, Z. J. Zhang and X. Y. Huang, J. Lumin., 2021, 233, 117901 CrossRef CAS.
- L. Huang, Y. W. Zhu, X. J. Zhang, R. Zou, F. J Pan, J. Wang and M. M. Wu, Chem. Mater., 2016, 28, 1495–1502 CrossRef CAS.
- Y. W. Zhu, Y. Liu, L. Huang, T. T. Xuan and J. Wang, Sci. China: Technol. Sci., 2017, 60, 1458–1464 CrossRef CAS.
- L. Y. Wang, E. H. Song, T. T. Deng, Y. Y. Zhou, Z. F. Liao, W. R. Zhao, B. Zhou and Q. Y. Zhang, Dalton Trans., 2017, 46, 9925–9934 RSC.
- M. M. Zhu, Y. X. Pan, X. A. Chen, H. Z. Lian and J. Lin, J. Am. Ceram. Soc., 2018, 101, 4983–4993 CrossRef CAS.
- S. Q. Fang, T. C. Lang, T. Han, J. Y. Wang, J. Y. Yang, S. X. Cao, L. L. Peng, B. T. Liu, A. N. Yakovlev and V. I. Korepanov, Chem. Eng. J., 2020, 389, 124297 CrossRef CAS.
- X. T Fan, W. B. Chen, S. Y. Xin, Z. C. Liu, M. Zhou, X. Yu, D. C. Zhou, X. H. Xu and J. B. Qiu, J. Mater. Chem. C, 2018, 6, 2978–2982 RSC.
- W. Q. Xie, P. P. Li, Y. Wang, Q. J. Zhu, Y. J. Zhang, Y. J. Cai, S. Q. Xu and J. J. Zhang, J. Mater. Chem. C, 2019, 7, 8655–8659 RSC.
- F. Hong, G. Pang, L. J. Diao, Z. D. Fu, G. X. Liu, X. T. Dong, W. S. Yu and J. X. Wang, Dalton Trans., 2020, 49, 13805–13817 RSC.
- Y. Y. Liu, J. Gao, W. Shi, X. Y. Feng, Z. J. Zhou, J. X. Wang, J. L. Guo, R. Y. Kang, B. Deng and R. J. Yu, Ceram. Int., 2021, 47, 18814–18823 CrossRef CAS.
- Y. W. Zhu, L. Huang, R. Zou, J. H. Zhang, J. B. Yu, M. M. Wu, J. Wang and Q. Su, J. Mater. Chem. C, 2016, 4, 5690–5695 RSC.
- P. Xue and L. H. Tian, Opt. Mater., 2021, 115, 111063 CrossRef CAS.
- Y. M. Liu, T. M. Wang, Z. P. Chen, K. Y. Chen, M. M. Guan, Y. H. Huang, S. Liao and H. X. Zhang, J. Mater. Sci.: Mater. Electron., 2018, 29, 12536–12542 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08734g |
| This journal is © The Royal Society of Chemistry 2022 |