Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
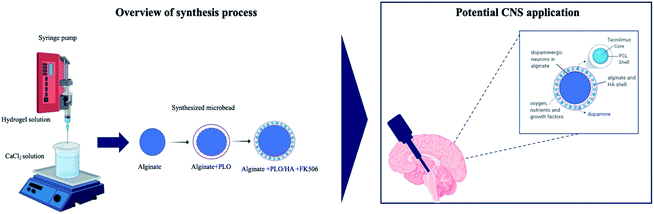
An alginate-based encapsulation system for delivery of therapeutic cells to the CNS
Despoina Eleftheriadou ab,
Rachael E. Evansab,
Emily Atkinsonab,
Ahmed Abdallaab,
Francesca K. H. Gavinsab,
Ashleigh S. Boydd,
Gareth R. Williams
ab,
Rachael E. Evansab,
Emily Atkinsonab,
Ahmed Abdallaab,
Francesca K. H. Gavinsab,
Ashleigh S. Boydd,
Gareth R. Williams b,
Jonathan C. Knowles
b,
Jonathan C. Knowles c,
Victoria H. Roberton
c,
Victoria H. Roberton ab and
James B. Phillips
ab and
James B. Phillips *ab
*ab
aUCL Centre for Nerve Engineering, University College London, London, UK
bUCL School of Pharmacy, University College London, London, WC1N 1AX, UK. E-mail: jb.phillips@ucl.ac.uk
cBiomaterials & Tissue Engineering, UCL Eastman Dental Institute, London, UK
dUCL Institute of Immunity and Transplantation, Royal Free Hospital, London, UK
First published on 1st February 2022
Abstract
Treatment options for neurodegenerative conditions such as Parkinson's disease have included the delivery of cells which release dopamine or neurotrophic factors to the brain. Here, we report the development of a novel approach for protecting cells after implantation into the central nervous system (CNS), by developing dual-layer alginate beads that encapsulate therapeutic cells and release an immunomodulatory compound in a sustained manner. An optimal alginate formulation was selected with a view to providing a sustained physical barrier between engrafted cells and host tissue, enabling exchange of small molecules while blocking components of the host immune response. In addition, a potent immunosuppressant, FK506, was incorporated into the outer layer of alginate beads using electrosprayed poly-ε-caprolactone core–shell nanoparticles with prolonged release profiles. The stiffness, porosity, stability and ability of the alginate beads to support and protect encapsulated SH-SY5Y cells was demonstrated, and the release profile of FK506 and its effect on T-cell proliferation in vitro was characterized. Collectively, our results indicate this multi-layer encapsulation technology has the potential to be suitable for use in CNS cell delivery, to protect implanted cells from host immune responses whilst providing permeability to nutrients and released therapeutic molecules.
1. Introduction
As life expectancy has increased dramatically over the past century, conditions associated with ageing have become more prevalent.1 Neurodegenerative diseases, such as Alzheimer's disease, and Parkinson's disease (PD), represent a daunting worldwide challenge for society and healthcare providers. These disorders are associated with extensive loss of neuronal cells, reflecting cellular demise, and are clinically characterized by progressive cognitive, motor, and behavioural impairments. Final stage patients are left bed-ridden and dependent on specialist care.2–4Recent advances in the area of regenerative medicine have yielded new opportunities to develop targeted therapies. Two of those, cell- and neurotrophic factor delivery, have been trialed in humans to-date. The first aims at replacing lost neurons and functional reinnervation.5 In the case of PD, this approach involves transplanting new cells capable of forming network connections and producing dopamine.6,7 However, both pre-clinical and clinical evidence shows high rates of cell death after implantation, which could be partially attributed to the hostile mechanical and chemical host tissue environment that cells encounter and activation of the host immune system.8–10 For instance, only 1% to 20% of grafted neurons survive in animal models of PD.11–13
Deficiency of neurotrophic factors, such as cerebral dopamine neurotrophic factor (CDNF), neurturin and glial cell line-derived neurotrophic factor (GDNF) has also been related to neurodegenerative diseases. Therefore, delivery of these factors has been the basis of the second neuro-regenerative strategy which, instead of replacing lost neurons, focuses on employing neurotrophins to enhance the growth and function of viable neurons in the affected areas.14,15 Despite early optimism, this approach has also proved challenging, mainly due to the short half-life of proteins in vivo and challenges with targeting delivery, with clinical outcomes not meeting the expectations of the preclinical data.16,17
An alternative strategy for targeted dopamine replacement and neuroprotection could be achieved by the delivery of therapeutic cells which can release dopamine and secrete neurotrophic factors without needing to integrate synaptically and form cell–cell connections with host cells.18,19 Using this approach would enable therapeutic cells to remain permanently encapsulated following transplantation, separating them physically from direct interaction with host cells whilst enabling the exchange of soluble factors required to sustain the engrafted cells and elicit their therapeutic effects. Established protocols exist for the reliable generation of neural progenitors which can either release dopamine or be used as delivery vehicles for trophic factors such as GDNF following stable transfection.20,21 A commonly applied technology of immunoisolation is microencapsulation of cells in spherical beads made of hydrogels that allow passage of oxygen and nutrients while protecting transplanted cells. This inhibits immune recognition by restricting cellular interactions and leads to a significant increase in the survival of transplanted cells.22 While encapsulated cell therapy has been extensively studied for the treatment of conditions like diabetes,23,24 the use of this approach in therapy for CNS disorders remains limited.25 Alginate based 3D platforms have been shown to not interfere with the dopaminergic potential of encapsulated cells. Encapsulation in alginate beads facilitated the early onset of neuronal dopamine generation compared with conventional 2D systems, with 3D differentiated cells showing higher dopamine secretion.26 A recent clinical trial that studied the efficacy of immunoprotected (alginate-encapsulated) porcine choroid plexus cells for xenotransplantation in patients with PD showed that this technology can be relatively safe and well-tolerated.27
Still, an outstanding issue with microencapsulation that requires resolution is the inability of this passive protective barrier to efficiently protect cells from exposure to cytokines and other small diffusible cytotoxic molecules produced by stimulated immune cells.28 Thus, surface modification,29,30 or immobilization and release of immunomodulatory molecules might also be considered an effective strategy to suppress the host immune response upon implantation.
Here, we have developed a composite cell encapsulation system, consisting of dual-layer micro-scale beads that can maintain cell survival while concurrently being able to release an immunomodulatory compound in a sustained manner. The use of multi-layer biotechnology allows the design of cell encapsulation vehicles with optimal degradation rates and desirable physicochemical properties. The proposed system consists of (a) an inherently non-degradable core for cell encapsulation and (b) an outer hybrid polymer layer that will ultimately degrade for FK506 encapsulation and controlled delivery. This approach can allow the transplantation of cells that release therapeutic soluble factors while protected from the host immune response.
For the core of the beads, we chose alginate; an inert, readily available, nontoxic biomaterial with tunable properties. Alginate is one of the most frequently used biomaterials for cell encapsulation,22 mainly due to its ability to form gels under conditions suitable for cell survival.31 For the outer layer, a composite of alginate and hyaluronic acid (HA) was selected. Both alginate and HA can be manipulated to present mechanical properties that comply with those of native brain tissue.32 Alginate has been shown to not interfere with the survival, differentiation, maturation or growth factor secretion of encapsulated neuronal cells.26,33,34 Moreover, it has a low capacity to support cell–matrix interaction owing to the lack of suitable mammalian cell adhesion molecules, and low protein adsorption capacity.26,35,36 HA is a biocompatible and bioresorbable material that plays a widespread role in cellular signaling, differentiation, proliferation, and cell migration in the CNS.37,38 Survival of encapsulated cells in alginate beads was demonstrated here using human neuroblastoma SH-SY5Y cells, a catecholaminergic neuronal cell line commonly used in vitro in models of neurodegenerative disease.39
To further reduce the host tissue immune response following transplantation, we incorporated FK506, a widely used potent immunosuppressant, into the outer layer of the encapsulation system. FK506 is a macrolide drug that exerts its immunosuppressive effects by binding to FK506-binding proteins and ultimately preventing T-cell activation.40,41 Although FK506 is commonly used in allograft transplant rejection prophylaxis,42 systemic administration has been associated with nephrotoxicity, neurotoxicity, and gastric disturbances.43 These side-effects could potentially be reduced using a local/implantable delivery system. Compared to traditional dosing regimens, implantable formulations require a considerably lower pharmaceutical dosage and allow the local drug concentration to remain within the therapeutic window over an extended period, thus minimizing side effects and ensuring efficacy. Furthermore, the use of hydrogels can help to achieve continuous drug release at isolated sites, where it is difficult to maintain nanoparticles for long time periods.44,45 Therefore, FK506 was incorporated into the outer layer of alginate beads either in soluble form or in nanoparticles. To provide appropriate stability and release kinetics, the nanoparticles were formed using a core–shell approach through coaxial electrospraying of poly(ε-caprolactone) (PCL) with FK506. This demonstrates a novel method for protecting implanted cells through localized FK506 release as part of a cellular encapsulation system suitable for delivery of therapeutic cells to the CNS.
2. Materials and methods
2.1. Reagents
Unless otherwise stated all cell culture materials and chemical reagents were purchased from Sigma Aldrich (Gillingham, UK) or Thermo Fisher Scientific (Loughborough, UK). Alginic acid sodium salt powder was purchased from Sigma Aldrich (Gillingham, UK). FK506 was obtained from Abcam (Cambridge, MA, USA). MagCellect Rat CD4 + T cell isolation kits were sourced from R&D systems (Minneapolis, MN, USA). Cell activation cocktail was provided by Biolegend (San Diego, CA, USA). A FK506 enzyme linked immunosorbent assay (ELISA) kit was purchased from Abnova (Taipei City, Taiwan). Running buffer and calibration beads for flow cytometry were obtained from Miltenyi Biotec (Gladbach, Germany).Collection and use of tissue from animals were conducted in accordance with the UK Animals (Scientific Procedures) Act (1986) and the European Communities Council Directives (86/609/EEC) and approved by the UCL Animal Welfare and Ethical Review Body.
2.2. Material preparation and characterisation
For imaging purposes, DAPI (4′,6-diamidino-2-phenylindole) and FITC positive PCL nanoparticles were incorporated in the core and shell of alginate + PLO/HA beads respectively. Multichannel image acquisition was performed using an inverted fluorescence microscope (EVOS Fl) with 4× objective. Confocal microscopy (LSM710, Zeiss, Oberkochen, Germany) was used to capture z-scans of a bead section using a 40× lens.
Absorption properties were evaluated by estimating the weight swelling ratio (SW) based on eqn (1),
| SW = (Wswollen − Wdry)/Wdry, | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 crosslinker to polymer solution was maintained constant for all measurements. To determine the linear viscoelastic region of hydrogels, separate strain sweep tests were made. Based on these results, a common strain value was chosen and later used to record viscoelastic properties during oscillatory experiments at a fixed strain of 0.01%, which was within the linear region, under constant frequency of 1 Hz.
1 crosslinker to polymer solution was maintained constant for all measurements. To determine the linear viscoelastic region of hydrogels, separate strain sweep tests were made. Based on these results, a common strain value was chosen and later used to record viscoelastic properties during oscillatory experiments at a fixed strain of 0.01%, which was within the linear region, under constant frequency of 1 Hz.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v to obtain a 5% w/v solution. An FK506 core solution was dissolved in ethanol to a final concentration of 0.1% w/v. Both solutions were loaded into disposable plastic syringes, mounted to feed a stainless-steel coaxial spinneret (inner/outer needle internal diameters: 0.5/1.0 mm). The solutions were ejected through the spinneret using two separate syringe pumps (KDS100, Cole-Parmer, London, UK) at constant ratio flow rates of 1
1 v/v to obtain a 5% w/v solution. An FK506 core solution was dissolved in ethanol to a final concentration of 0.1% w/v. Both solutions were loaded into disposable plastic syringes, mounted to feed a stainless-steel coaxial spinneret (inner/outer needle internal diameters: 0.5/1.0 mm). The solutions were ejected through the spinneret using two separate syringe pumps (KDS100, Cole-Parmer, London, UK) at constant ratio flow rates of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 core to shell (core; 0.01 ml h−1, shell; 0.1 ml h−1). The spinneret was connected to the positive electrode of a high-voltage DC power supply (18–20 kV, HCP35-35000, FuG Elektronik, Schechen, Germany), and nanoparticles were collected on a grounded stainless-steel plate 18 cm from the tip. To improve the recoverability of the particles, the collector plate was pre-coated with a film of 5% w/v polyvinylpyrrolidone (PVP) dissolved in ethanol. The film was allowed to dry in air before spinning commenced. For fluorescent NPs, instead of FK506 the core consisted of fluorescein sodium salt dissolved in ethanol (5 mg ml−1).
10 core to shell (core; 0.01 ml h−1, shell; 0.1 ml h−1). The spinneret was connected to the positive electrode of a high-voltage DC power supply (18–20 kV, HCP35-35000, FuG Elektronik, Schechen, Germany), and nanoparticles were collected on a grounded stainless-steel plate 18 cm from the tip. To improve the recoverability of the particles, the collector plate was pre-coated with a film of 5% w/v polyvinylpyrrolidone (PVP) dissolved in ethanol. The film was allowed to dry in air before spinning commenced. For fluorescent NPs, instead of FK506 the core consisted of fluorescein sodium salt dissolved in ethanol (5 mg ml−1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 1 min), and the remaining solution was analysed using UV-Visible spectroscopy (Lambda 25, PerkinElmer, Beaconsfield, UK) at 205 nm. Standard purified FK506 concentrations (0–200 ng ml−1 in DPBS buffer pH 7.4) were used to generate a calibration curve. For NPs embedded in the outer layer of ALG + PLO/HA beads, sampling was performed as previously described, while FK506 release was quantified using an ELISA kit according to the manufacturer's protocol. The encapsulation efficiency of the PCL nanoparticles was assessed following the in vitro release study. The nanoparticles were isolated by centrifugation as mentioned earlier and burst with pure acetonitrile. A calibration curve was plotted using acetonitrile and the remaining tacrolimus was determined using the UV spectrometer. Then, the encapsulation efficiency of the particles was determined using the following equation
000 rpm, 1 min), and the remaining solution was analysed using UV-Visible spectroscopy (Lambda 25, PerkinElmer, Beaconsfield, UK) at 205 nm. Standard purified FK506 concentrations (0–200 ng ml−1 in DPBS buffer pH 7.4) were used to generate a calibration curve. For NPs embedded in the outer layer of ALG + PLO/HA beads, sampling was performed as previously described, while FK506 release was quantified using an ELISA kit according to the manufacturer's protocol. The encapsulation efficiency of the PCL nanoparticles was assessed following the in vitro release study. The nanoparticles were isolated by centrifugation as mentioned earlier and burst with pure acetonitrile. A calibration curve was plotted using acetonitrile and the remaining tacrolimus was determined using the UV spectrometer. Then, the encapsulation efficiency of the particles was determined using the following equation| Encapsulation efficiency (%) = (encapsulated drug (mg)/amount of drug in feedstock (mg)) × 100%. |
For transmission electron microscopy (TEM), samples of liquid NP suspensions were dropped with a Pasteur pipette onto a carbon/formvar coated copper grid. After 15 s excess sample was blotted off with filter paper. Then a drop of stain (1% uranyl acetate) was added if required and blotted after 15 seconds. The grid was placed into a specimen holder and inserted into a Philips/FEI CM 120 BioTwin TEM (FEI Company, Hillsboro, OR, USA) for imaging at 120 kV.
2.3. Cell culture
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v Hams F12
1 v/v Hams F12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eagle's Minimum Essential medium (EMEM) media supplemented with 1% non-essential amino acid solution, 2 mM L-glutamine, 15% v/v fetal bovine serum (FBS) and 1% v/v penicillin/streptomycin (P/S). Cells were passaged when 70–80% confluency was reached by trypsinization, centrifugation at 100 × g for 5 minutes and re-suspension in fresh media, then seeded at the desired density. Flasks were kept in a humidified incubator at 37 °C with 5% CO2 in air. To test viability, cells were encapsulated in 2% alginate beads produced using a 21 G needle and syringe pump for reproducibility. Cellular beads were then transferred to a 6-well plate and cultured for further analysis.
Eagle's Minimum Essential medium (EMEM) media supplemented with 1% non-essential amino acid solution, 2 mM L-glutamine, 15% v/v fetal bovine serum (FBS) and 1% v/v penicillin/streptomycin (P/S). Cells were passaged when 70–80% confluency was reached by trypsinization, centrifugation at 100 × g for 5 minutes and re-suspension in fresh media, then seeded at the desired density. Flasks were kept in a humidified incubator at 37 °C with 5% CO2 in air. To test viability, cells were encapsulated in 2% alginate beads produced using a 21 G needle and syringe pump for reproducibility. Cellular beads were then transferred to a 6-well plate and cultured for further analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 v/v dilution) was added and plates were incubated for 15 min at 37 °C then washed briefly with 1.0 ml of culture media. Finally, an additional 1.0 ml of culture media was added to each well prior to image acquisition. For cell viability, multichannel image acquisition was initially captured using rhodamine (for propidium iodide – PI) and fluorescein isothiocyanate (FITC; for Syto21) filters in a fluorescence microscope (Zeiss-Axio Lab. A1, Zeiss, Oberkochen, Germany) with 20× objectives. Manual cell counting of FITC-stained cells in five pre-selected areas based on a sampling protocol led to the determination of the total number of cells and calculation of the percentage of cell viability.
1000 v/v dilution) was added and plates were incubated for 15 min at 37 °C then washed briefly with 1.0 ml of culture media. Finally, an additional 1.0 ml of culture media was added to each well prior to image acquisition. For cell viability, multichannel image acquisition was initially captured using rhodamine (for propidium iodide – PI) and fluorescein isothiocyanate (FITC; for Syto21) filters in a fluorescence microscope (Zeiss-Axio Lab. A1, Zeiss, Oberkochen, Germany) with 20× objectives. Manual cell counting of FITC-stained cells in five pre-selected areas based on a sampling protocol led to the determination of the total number of cells and calculation of the percentage of cell viability.3. Results and discussion
3.1. Developing materials for encapsulating therapeutic cells
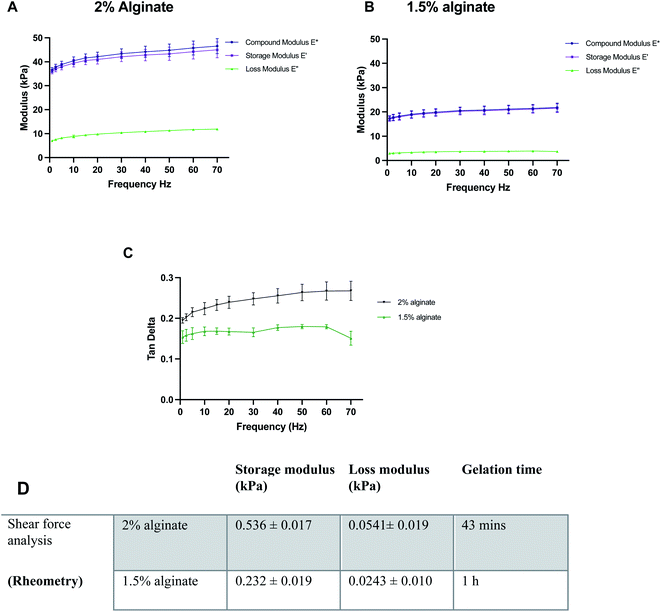
Before the synthesis of the beads, mechanical properties of alginate were optimised using 100 μl alginate gels cast in ThinCerts™ in 24-well plates to allow for easy testing. DMA analysis revealed that alginate formulations can be benchmarked and tuned to mimic the stiffness properties of the brain, by adjusting the concentration of polymer. Moreover, the results verify the viscoelastic behaviour of the hydrogels as can be seen by the high storage modulus and low loss modulus (Fig. 2A–C).DMA was performed at 23 °C. However, under physiological conditions, the alginate gels could be mildly stiffer than measured; a 10% increase in the stiffness of alginate gels from 23 °C to 37 °C has been previously reported.48 Thus, the temperature for rheological measurements was set to 37.5 °C. For rheological assessment, single frequency oscillatory tests were conducted to evaluate the time-dependent viscoelastic shear behaviour of selected alginate hydrogels. The variation in viscosity was determined as a function of time. Hydrogels were tested at a constant oscillation frequency of 1 Hz, which is within their linear viscoelastic region. The end of the crosslinking process was defined as the time point where six consecutive measurements did not differ by more than 0.5%. Both 1.5% w/v and 2% w/v alginate hydrogels exhibited a much higher storage modulus than their respective loss modulus, indicating that the materials behave like a viscoelastic gel. Moreover, in all formulations, the concentration of crosslinker was found to be sufficient and led to the formation of fully gelled alginate gels. Higher alginate concentration resulted in shorter gelation time. Gelation was completed at 43 min and 1 h for 2 and 1.5% w/v alginate hydrogels respectively (Fig. 2D).
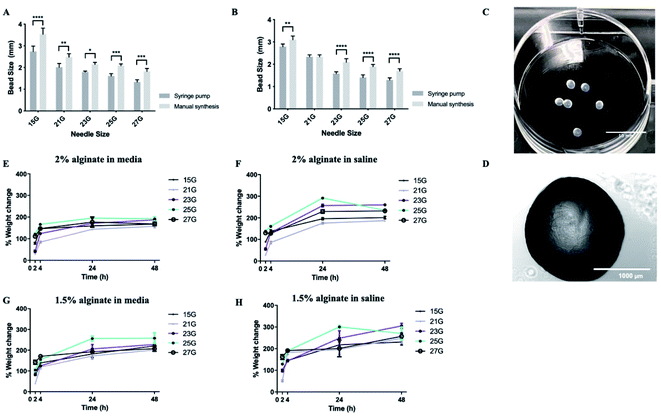
Subsequently, synthesis methods were optimized to control the morphological properties of the alginate beads which is another major influencer of the functional survival of encapsulated cells.49,50 Beads displayed varying sizes and swelling rates, depending on the synthesis methods and experimental conditions. Of the methods tested, manual synthesis was the least technically challenging.51 However, compared to dropwise dispersion from the syringe by hand, beads generated using a syringe pump exhibited less variability in their size. Additionally, in terms of translational potential, the robustness of the automated bead-generation system allows for further scaling up of the process. While the size of the beads makes their delivery with narrow cannulae problematic, it could be preferable for CNS therapy where large-sized retrievable microbeads may be more suitable for safety reasons in case of complications.52,53 Beads synthesized from 2% w/v alginate solution were characterized as having smaller diameters than those from 1.5% w/v when all the other parameters of the synthesis protocol remained the same (Fig. 3A and B). It should be noted that, in all experimental conditions, alginate beads displayed a rather smooth surface without visible defects, as identified by light microscopy (Fig. 3C and D). This is important as broken beads or beads with a rough morphology have been previously linked to the protrusion of cells35 and inflammatory responses.54
With regards to the swelling ratio, this was significantly higher for beads immersed in saline solution than those in media, with the effect being more pronounced in the case of beads composed of 1.5% w/v alginate (Fig. 3E–H). The swelling behaviour of the beads was also influenced by the gauge of the needle used to produce them, with small-diameter beads exhibiting higher swelling rates, due to their greater surface area. Previous research suggests hydrogels that promote neural survival are more likely to exhibit swelling (up to 366%), which could be a reflection on the extracellular ion environment that is favorable to neurons.55,56 Therefore, some degree of swelling is acceptable. Another important consideration is the effect of pH on the stability of the alginate beads. Local acidosis is observed in several neurological conditions and is often related to neuroinflammation. However, the potential reduction of pH is unlikely to cause the degradation of alginate microcapsules. Previous research demonstrated that alginate beads remain intact between pH = 1 to pH = 7 but exhibit some swelling at pH > 3 due to loss of negative charges (pKa ≈ 3.5).57
Upon optimization of the synthesis protocol, beads produced from both 1.5% and 2% w/v alginate solutions were stable (did not collapse or rupture) up to 28 days. 2% alginate beads synthesized with 21 gauge needles were most favourable as they exhibited smaller diameters and less swelling.
3.2. Survival and protection of SH-SY5Y cells in alginate hydrogels
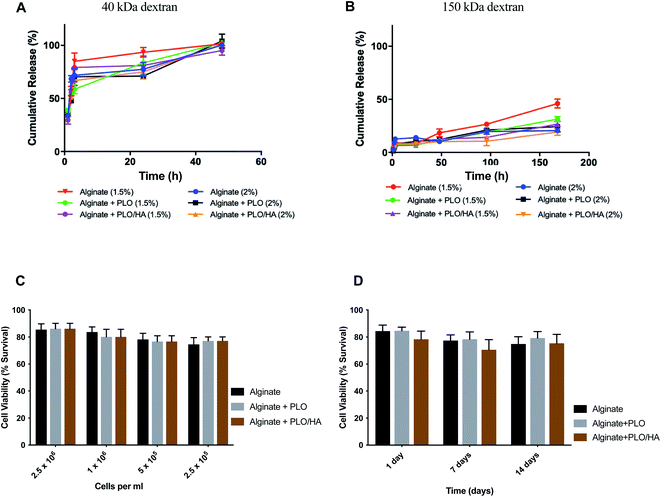
Based on previous work, the addition of a polycation coating, an extra polymeric layer or a combination of both can lower the surface roughness compared to conventional cell-loaded alginate beads.35 By using a polycation coating, followed by the addition of an external layer, beads allowing diffusion within a specific molecular cut-off can be produced, the hypothesis being that the material will exclude key components of the immune system from reaching the encapsulated cells without restricting the diffusion of nutrients and release of bioactive agents.Solute and antibody permeability was modelled by measuring the release of 40 kDa and 150 kDa molecular weight dextran from beads made from 1.5% and 2% w/v alginate solutions with and without additional surface coatings (PLO or PLO/HA). These studies demonstrated that the rate of molecular diffusion in the beads is a function of both the molecular weight of diffusing molecules as well as the initial alginate concentration and the presence of additional coating. In agreement with previous reports, the rates of diffusion increase as the initial concentration of the polymer decreases.58,59 In the case of 40 kDa dextran, the molecule diffused through the matrix easily, with the whole amount released after 48 h of incubation for all conditions (Fig. 4A). In contrast, although there was a certain level of transport through the polymeric network for the high molecular weight 150 kDa dextran, its diffusion was much more restricted. Coating of the beads was found to reduce the release of 150 kDa dextran by providing an additional barrier. The extent of the effect varied between time points, with the highest difference observed between at 96 h and 168 h for 1.5% alginate (p < 0.0001 for alginate vs. alginate + PLO/HA) and at 96 h 2% alginate (p < 0.001 for alginate vs. alginate + PLO/HA) (Fig. 4B).
This suggests that beads are suitable for encapsulation of cells as they allow the transport of essential nutrients whilst protecting against the infiltration of immune cells. This is confirmed by the findings that both alginate compositions tested here supported SH-SY5Y cell survival (Fig. 4C and D). To test viability, cells were encapsulated in 2% alginate beads produced using a 21G needle and syringe pump for reproducibility. The increase of cell concentration from 2.5 × 105 to 2.5 × 106 cells per ml of alginate did not have a noticeable influence on cell survival (Fig. 4C). Moreover, encapsulation of SH-SY5Y cells sustained their viability at over 77% for up to 14 days (Fig. 4D). Our results are in accordance with the conclusions of previous work on the proliferation of neuronal cells embedded in soft alginate substrates.60–62 While the results are promising, future studies should investigate the long-term viability of cells in these materials in vivo.
3.3. Refining the microbead design to reduce the local immune response
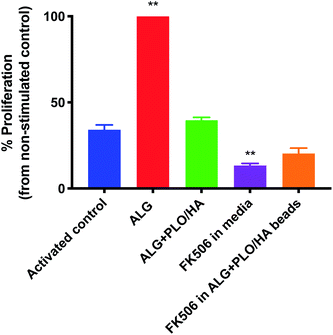
In addition to oxygen and nutrient supply and physical protection during injection, a key consideration for biomaterial encapsulation of implanted cells is positive modulation of the host tissue immune response. The extent to which alginate beads, modified or unmodified, were able to elicit an immune response was examined in vitro using a T-cell proliferation assay (Fig. 5). To our knowledge, the effect of alginate on T-cell proliferation has not been extensively studied. Unmodified alginate beads elicited a strong response compared to the control (65.9% increase). PLO/HA coating reduced the proliferation to 41.2 ± 1.7%. It should be noted that this proliferation index was comparable to the stimulated control, which exhibited 34.6 ± 2.9% cellular division, implying that alginate + PLO/HA beads are not strongly immunogenic.To further reduce the immune response, we explored the co-administration of the immunomodulatory agent FK506, an approved immunosuppressive agent used for solid organ transplantation in humans. Neuroprotective and neurotrophic effects of FK506 have been previously demonstrated on dopaminergic neurons. For example, Castilho et al. have illustrated that treatment of rat embryonic dopaminergic neurons with immunophilin ligands FK506 and cyclosporin A is protective both in vitro and following grafting to the rat brain.63 Given its widespread use and proven efficacy in rejection prophylaxis for transplantation, FK506 is an appropriate drug candidate for incorporation into the beads. Furthermore, while FK506 has been used in the clinic for years, it has a narrow therapeutic index, variable bioavailability, and is associated with adverse effects and interactions.64,65 Therefore, local administration may be beneficial in terms of patient safety and increased efficiency. Fig. 5 shows that T-cell proliferation was further reduced by the addition of FK506 in free form and encapsulated in the bead coating (to 13.6 ± 1.2% and 21.2 ± 3.1% respectively), which implies that encapsulation of FK506 did not alter the ability of the drug to suppress the T-cell response.
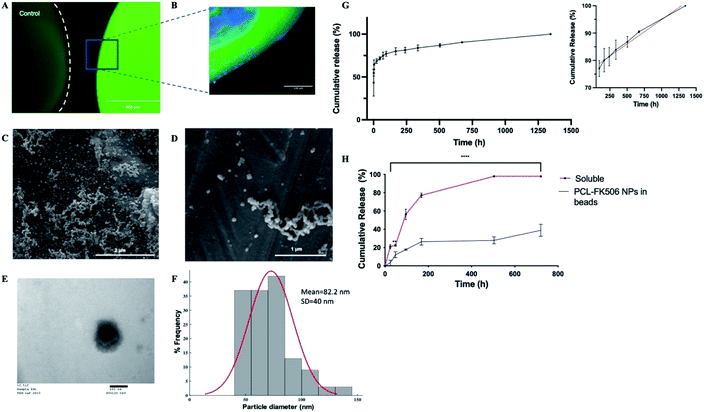
To provide local drug release, FK506 was incorporated in the outer layer of ALG + PLO/HA beads in soluble form or in nanoparticles (Fig. 6A and B). To prolong FK506 release from the beads, a novel drug delivery system was developed by loading the drug into core–shell nanoparticles (PCL-FK506 NPs) synthesized by coaxial electrospraying (Fig. 6C–F). Nanoparticles were successfully generated within a clinically acceptable size range (20–100 nm); not big enough to be destructive or small enough to be cleared.66–69 SEM and TEM images of the resulting nanoparticles can be seen in Fig. 6C–E. Mean size of the PCL-FK506 NPs was found to be 82 ± 40 nm (Fig. 6F). The entrapment efficiency of FK506 in PCL NPs was found to be 61.8%.
The drug release profile for the nanoparticles followed biphasic kinetics with approximately 60% of the drug being released during the initial burst (Fig. 6G). This could be attributed to the high surface area of the NPs. After the initial burst, a linear release profile was observed from 96 hours to 1344 hours (Fig. 6G inset). Electrosprayed PCL-FK506 nanoparticles were incorporated into the outer layer of the alginate beads, leading to a more sustained release compared with incorporation of FK506 in solution, with approximately 39% of the drug being released within 30 days (Fig. 6H). For FK506 incorporated in the outer layer of the beads in solution the release occurred in two phases, an initial slow-rate over the first 72 hours followed by a burst of release on the fourth day, and 97.5% of the drug was released by 336 hours (14 days). The longer-term prolonged release profile achieved by incorporation of FK506-loaded nanoparticles in the outer layer of the beads is particularly important as clinical trials of transplantation to the brain have shown that immunosuppression is required to prevent rejection and suggest that there is an optimum duration of immunosuppression which is required throughout the transplantation and observational period (typically at least 6 months); withdrawal of treatment too early affects functional outcomes.70–72
4. Conclusion
In summary, we have developed a novel alginate-based cell-encapsulation and drug delivery system that can maintain cell viability for up to 14 days in vitro as demonstrated with the SH-SY5Y cell line, with the potential to allow release of bioactive molecules while protecting implanted cells. Synthesis methods and material properties have been optimized for delivery to the brain, and a polymer coating incorporating nanoparticles for slow release of the immunosuppressant FK506 allows for reduction of the host immune response to implanted material and cells.Author contributions
DE and REE were responsible for the conceptualization, methodology and investigation and for drafting the manuscript. EA, AA and FG were responsible for data/evidence collection and formal analysis. ASB contributed to conceptualization and formal analysis. GRW and JCK contributed to methodology, writing – review and editing. VHR contributed to writing – review and editing and methodology. JBP provided supervision and guidance throughout.Conflicts of interest
None of the authors have any relevant conflicts of interest to declare.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) (EP/R004463/1) and the UCL Rosetrees Stoneygate Prize 2018. REE and FKHG received funding from EPSRC through the award of PhD studentships in the Centre for Doctoral Training in Advanced Therapeutics & Nanomedicines (EP/L01646X/1). EA received funding from EPSRC through the award of PhD studentship in the Centre for Doctoral Training in Transformative Pharmaceutical Technologies (EP/S023054/1).References
- V. Canudas-Romo, E. DuGoff, A. W. Wu, S. Ahmed and G. Anderson, North Am. Actuar. J., 2016, 20(3), 276–285 CrossRef.
- M. D. Welsh, Curr. Neurol. Neurosci. Rep., 2001, 1, 346–349 CrossRef CAS PubMed.
- A. M. Gorman, J. Cell. Mol. Med., 2008, 12, 2263–2280 CrossRef CAS PubMed.
- Y. E. Choonara, V. Pillay, L. C. du Toit, G. Modi, D. Naidoo, V. M. Ndesendo and S. R. Sibambo, Int. J. Mol. Sci., 2009, 10, 2510–2557 CrossRef CAS PubMed.
- R. A. Barker, K. Farrell, N. V. Guzman, X. He, S. E. Lazic, S. Moore, R. Morris, P. Tyers, R. Wijeyekoon, D. Daft, S. Hewitt, V. Dayal, T. Foltynie, Z. Kefalopoulou, P. Mahlknecht, N. P. Lao-Kaim, P. Piccini, H. Bjartmarz, A. Björklund, O. Lindvall, J. Nelander-Wahlestedt, M. Parmar, G. Paul, H. Widner, A. Church, S. Dunnett, K. Peall, A. Rosser, J. M. Gurruchaga, S. Palfi, T. Piroth, C. Winkler and T. consortium, Nat. Med., 2019, 25, 1045–1053 CrossRef CAS PubMed.
- T. Ramos-Moreno, J. G. Lendínez, M. J. Pino-Barrio, A. del Arco and A. Martínez-Serrano, PLoS One, 2012, 7, e52714 CrossRef CAS PubMed.
- Z. Fuxing, X. Feng, W. Xia, L. Xueyuan, W. Renzhi, G. Wei and B. Xinjie, Stem Cells, 2017, 35, 1519–1531 CrossRef PubMed.
- G. Q. Daley, Cell Stem Cell, 2012, 10, 740–749 CrossRef CAS PubMed.
- A. Trounson and C. McDonald, Cell Stem Cell, 2015, 17, 11–22 CrossRef CAS PubMed.
- F. Colombo, G. Sampogna, G. Cocozza, S. Y. Guraya and A. Forgione, J. Microsc. Ultrastruct., 2017, 5, 1–8 CrossRef PubMed.
- P. Brundin, J. Karlsson, M. Emgård, G. S. Kaminski Schierle, O. Hansson, Å. Petersén and R. F. Castilho, Cell Transplant., 2000, 9, 179–195 CrossRef CAS PubMed.
- A. Boronat-García, M. Guerra-Crespo and R. Drucker-Colín, World J. Transplant., 2017, 7, 179–192 CrossRef PubMed.
- R. A. Barker, M. Parmar, A. Kirkeby, A. Björklund, L. Thompson and P. Brundin, J. Parkinson's Dis., 2016, 6, 57–63 Search PubMed.
- T. B. Sampaio, A. S. Savall, M. E. Z. Gutierrez and S. Pinton, Neural Regener. Res., 2017, 12, 549–557 CrossRef CAS PubMed.
- H. J. Huttunen and M. Saarma, Cell Transplant., 2019, 28, 349–366 Search PubMed.
- A. Kirkeby and R. A. Barker, Nat. Rev. Neurol., 2019, 15, 312–314 CrossRef PubMed.
- G. Paul and A. M. Sullivan, Eur. J. Neurosci., 2019, 49, 440–452 CrossRef PubMed.
- P. Garcia, I. Youssef, J. K. Utvik, S. Florent-Béchard, V. Barthélémy, C. Malaplate-Armand, B. Kriem, C. Stenger, V. Koziel, J.-L. Olivier, M.-C. Escanye, M. Hanse, A. Allouche, C. Desbène, F. T. Yen, R. Bjerkvig, T. Oster, S. P. Niclou and T. Pillot, J. Neurosci., 2010, 30, 7516–7527 CrossRef CAS PubMed.
- D. F. Emerich, J. H. Kordower, Y. Chu, C. Thanos, B. Bintz, G. Paolone and L. U. Wahlberg, Neural Plast., 2019, 2019, 6286197 Search PubMed.
- S. Nolbrant, A. Heuer, M. Parmar and A. Kirkeby, Nat. Protoc., 2017, 12, 1962–1979 CrossRef CAS PubMed.
- A. A. Akhtar, G. Gowing, N. Kobritz, S. E. Savinoff, L. Garcia, D. Saxon, N. Cho, G. Kim, C. M. Tom, H. Park, G. Lawless, B. C. Shelley, V. B. Mattis, J. J. Breunig and C. N. Svendsen, Stem Cell Rep., 2018, 10, 1696–1704 CrossRef CAS PubMed.
- G. Orive, E. Santos, D. Poncelet, R. M. Hernández, J. L. Pedraz, L. U. Wahlberg, P. De Vos and D. Emerich, Trends Pharmacol. Sci., 2015, 36, 537–546 CrossRef CAS PubMed.
- A. Omer, V. Duvivier-Kali, J. Fernandes, V. Tchipashvili, C. K. Colton and G. C. Weir, Transplantation, 2005, 79(1), 52–58 CrossRef CAS PubMed.
- C. Y. Cheung and K. S. Anseth, Bioconjugate Chem., 2006, 17, 1036–1042 CrossRef CAS PubMed.
- M. P. Zanin, L. N. Pettingill, A. R. Harvey, D. F. Emerich, C. G. Thanos and R. K. Shepherd, J. Controlled Release, 2012, 160, 3–13 CrossRef CAS PubMed.
- J. Kim, P. Sachdev and K. Sidhu, Stem Cell Res., 2013, 11, 978–989 CrossRef CAS PubMed.
- B. Snow, E. Mulroy, A. Bok, M. Simpson, A. Smith, K. Taylor, M. Lockhart, B. B. J. Lam, C. Frampton, P. Schweder, B. Chen, G. Finucane, A. McMahon and L. Macdonald, Parkinsonism Relat. Disord., 2019, 61, 88–93 CrossRef PubMed.
- Y. Koyama, Neurochem. Int., 2014, 78, 35–42 CrossRef CAS PubMed.
- G. Orive, M. De Castro, H.-J. Kong, R. M. Hernández, S. Ponce, D. J. Mooney and J. L. Pedraz, J. Controlled Release, 2009, 135, 203–210 CrossRef CAS PubMed.
- E. Bazigou, J. T. Wilson and J. E. Moore, et al., Microvasc. Res., 2014, 96, 38–45 CrossRef PubMed.
- A. M. A. Rokstad, I. Lacík, P. de Vos and B. L. Strand, Adv. Drug Delivery Rev., 2014, 67–68, 111–130 CrossRef CAS PubMed.
- R. D. Bartlett, D. Eleftheriadou, R. Evans, D. Choi and J. B. Phillips, Biomaterials, 2020, 120303 CrossRef CAS PubMed.
- M. Chayosumrit, B. Tuch and K. Sidhu, Biomaterials, 2010, 31, 505–514 CrossRef CAS PubMed.
- C. Addae, X. Yi, R. Gernapudi, H. Cheng, A. Musto and E. Martinez-Ceballos, Differentiation, 2012, 83, 233–241 CrossRef CAS PubMed.
- S. V. Bhujbal, B. De Haan, S. P. Niclou and P. De Vos, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- L. Li, A. E. Davidovich, J. M. Schloss, U. Chippada, R. R. Schloss, N. A. Langrana and M. L. Yarmush, Biomaterials, 2011, 32, 4489–4497 CrossRef CAS PubMed.
- T. M. Fowke, R. N. Karunasinghe, J.-Z. Bai, S. Jordan, A. J. Gunn and J. M. Dean, Sci. Rep., 2017, 7, 44135 CrossRef CAS PubMed.
- A. Cañibano-Hernández, L. Saenz del Burgo, A. Espona-Noguera, G. Orive, R. M. Hernández, J. Ciriza and J. L. Pedraz, Mol. Pharm., 2017, 14, 2390–2399 CrossRef PubMed.
- H. Xicoy, B. Wieringa and G. J. M. Martens, Mol. Neurodegener., 2017, 12, 10 CrossRef PubMed.
- K. Tamura, T. Fujimura, K. Iwasaki, S. Sakuma, T. Fujitsu, K. Nakamura, K. Shimomura, T. Kuno, C. Tanaka and M. Kobayashi, Biochem. Biophys. Res. Commun., 1994, 202, 437–443 CrossRef CAS PubMed.
- F. J. Dumont, Curr. Med. Chem., 2000, 7, 731–748 CrossRef CAS PubMed.
- I. J. Saldanha, O. Akinyede and K. A. Robinson, Cochrane Database Syst. Rev., 2015,(11), CD009421 Search PubMed.
- J. Kruh and C. S. Foster, in New Treatments in Noninfectious Uveitis, Karger Publishers, 2012, vol. 51, pp. 29–46 Search PubMed.
- L. K. Widjaja, M. Bora, P. N. Chan, V. Lipik, T. T. Wong and S. S. Venkatraman, J. Biomed. Mater. Res., Part A, 2014, 102(9), 3056–3065 CrossRef PubMed.
- K. Tajdaran, M. S. Shoichet, T. Gordon and G. H. Borschel, Biotechnol. Bioeng., 2015, 112, 1948–1953 CrossRef CAS PubMed.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- P. Stagnaro, I. Schizzi, R. Utzeri, E. Marsano and M. Castellano, Carbohydr. Polym., 2018, 185, 56–62 CrossRef CAS PubMed.
- C. Ganachaud, D. Bernin, D. Isaksson and K. Holmberg, Appl. Microbiol. Biotechnol., 2013, 97, 4403–4414 CrossRef CAS PubMed.
- A. King, A. Andersson, B. L. Strand, J. Lau, G. Skjak-Bræk and S. Sandler, Transplantation, 2003, 76, 275–279 CrossRef PubMed.
- K. Yu, Y. Mei, N. Hadjesfandiari and J. N. Kizhakkedathu, Colloids Surf., B, 2014, 124, 69–79 CrossRef CAS PubMed.
- S. Swioklo, P. Ding, A. W. Pacek and C. J. Connon, Process Biochem., 2017, 59, 289–296 CrossRef CAS.
- S. V. Bhujbal, P. de Vos and S. P. Niclou, Adv. Drug Delivery Rev., 2014, 67–68, 142–153 CrossRef CAS PubMed.
- P. de Vos, H. A. Lazarjani, D. Poncelet and M. M. Faas, Adv. Drug Delivery Rev., 2014, 67–68, 15–34 CrossRef CAS PubMed.
- P. De Vos, B. De Haan and R. Van Schilfgaarde, Biomaterials, 1997, 18, 273–278 CrossRef CAS PubMed.
- M. Matyash, F. Despang, R. Mandal, D. Fiore, M. Gelinsky and C. Ikonomidou, Tissue Eng., Part A, 2012, 18, 55–66 CrossRef CAS PubMed.
- C. K. Kuo and P. X. Ma, J. Biomed. Mater. Res., Part A, 2008, 84(4), 899–907 CrossRef PubMed.
- Y. Li, M. Hu, Y. Du, H. Xiao and D. J. McClements, Food Hydrocolloids, 2011, 25, 122–130 CrossRef CAS.
- J. S. Moon, H. M. Jeon, W. Meng, T. Akaike and I. K. Kang, J. Biomater. Sci., Polym. Ed., 2005, 16, 1245–1259 CrossRef CAS PubMed.
- N. Wang, G. Adams, L. Buttery, F. H. Falcone and S. Stolnik, J. Biotechnol., 2009, 144, 304–312 CrossRef CAS PubMed.
- N. O. Dhoot, C. A. Tobias, I. Fischer and M. A. Wheatley, J. Biomed. Mater. Res., Part A, 2004, 71, 191–200 CrossRef PubMed.
- L. N. Novikova, A. Mosahebi, M. Wiberg, G. Terenghi, J. O. Kellerth and L. N. Novikov, J. Biomed. Mater. Res., Part A, 2006, 77, 242–252 CrossRef PubMed.
- E. K. Purcell, D. Ph, A. Singh, B. Tech and D. R. Kipke, Tissue Eng., Part C, 2009, 15, 541–550 CrossRef CAS PubMed.
- R. F. Castilho, O. Hansson and P. Brundin, Exp. Neurol., 2000, 164, 94–101 CrossRef CAS PubMed.
- R. Venkataramanan, A. Swaminathan, T. Prasad, A. Jain, S. Zuckerman, V. Warty, J. McMichael, J. Lever, G. Burckart and T. Starzl, Clin. Pharmacokinet., 1995, 29, 404–430 CrossRef CAS PubMed.
- G. L. Plosker and R. H. Foster, Drugs, 2000, 59, 323–389 CrossRef CAS PubMed.
- J. H. Kim, J. H. Kim, K. W. Kim, M. H. Kim and Y. S. Yu, Nanotechnology, 2009, 20(50), 505101 CrossRef PubMed.
- X. Duan and Y. Li, Small, 2013, 9(9–10), 1521–1532 CrossRef CAS PubMed.
- D. H. Jo, J. H. Kim, T. G. Lee and J. H. Kim, Nanomedicine, 2015, 11(7), 1603–1611 CrossRef CAS PubMed.
- S. Ohta, E. Kikuchi, A. Ishijima, T. Azuma, I. Sakuma and T. Ito, Sci. Rep., 2020, 10, 18220 CrossRef CAS PubMed.
- A. E. Rosser and A.-C. Bachoud-Lévi, Clinical trials of neural transplantation in Huntington's disease, Progress in Brain Research, ed. S. B. Dunnett and A. Björklund, Elsevier, 2012, vol. 200, Ch. 17, pp. 345–371, ISSN 0079-6123, ISBN 9780444595751 Search PubMed.
- G. E. Tullis, K. Spears and M. D. Kirk, Stem Cells Int., 2014, 2014, 507905 Search PubMed.
- R. Aron Badin, M. Vadori, B. Vanhove, V. Nerriere-Daguin, P. Naveilhan, I. Neveu, C. Jan, X. Lévèque, E. Venturi, P. Mermillod, N. Van Camp, F. Dollé, M. Guillermier, L. Denaro, R. Manara, V. Citton, P. Simioni, P. Zampieri, D. D’avella, D. Rubello, F. Fante, M. Boldrin, G. M. De Benedictis, L. Cavicchioli, D. Sgarabotto, M. Plebani, A. L. Stefani, P. Brachet, G. Blancho, J. P. Soulillou, P. Hantraye and E. Cozzi, Am. J. Transplant., 2016, 16, 2016–2029 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |