Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
First-row transition metal doped germanium clusters Ge16M: some remarkable superhalogens†
Huu Tho Nguyen a,
Ngo Tuan Cuong
a,
Ngo Tuan Cuong b,
Ngo Thi Lancd,
Nguyen Thanh Tung
b,
Ngo Thi Lancd,
Nguyen Thanh Tung c,
Minh Tho Nguyen
c,
Minh Tho Nguyen e and
Nguyen Minh Tam
e and
Nguyen Minh Tam *fg
*fg
aFaculty of Natural Sciences Education, Sai Gon University, 273 An Duong Vuong Street, Ho Chi Minh City, Vietnam
bCenter for Computational Science, Faculty of Chemistry, Hanoi National University of Education, Hanoi, Vietnam
cInstitute of Materials Science and Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Vietnam
dInstitute of Science and Technology, TNU-University of Sciences, Tan Thinh Ward, Thai Nguyen City, Vietnam
eInstitute for Computational Science and Technology (ICST), Quang Trung Software City, Ho Chi Minh City, Vietnam
fLaboratory of Theoretical and Computational Biophysics, Advanced Institute of Materials Science, Ton Duc Thang University, Ho Chi Minh City, Vietnam. E-mail: nguyenminhtam@tdtu.edu.vn
gFaculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Vietnam
First published on 4th May 2022
Abstract
A theoretical study of geometric and electronic structures, stability and magnetic properties of both neutral and anionic Ge16M0/− clusters with M being a first-row 3d transition metal atom, is performed using quantum chemical approaches. Both the isoelectronic Ge16Sc− anion and neutral Ge16Ti that have a perfect Frank–Kasper tetrahedral Td shape and an electron shell filled with 68 valence electrons, emerge as magic clusters with an enhanced thermodynamic stability. The latter can be rationalized by the simple Jellium model. Geometric distortions from the Frank–Kasper tetrahedron of Ge16M having more or less than 68 valence electrons can be understood by a Jahn–Teller effect. Remarkably, DFT calculations reveal that both neutral Ge16Sc and Ge16Cu can be considered as superhalogens as their electron affinities (≥3.6 eV) exceed the value of the halogen atoms and even that of icosahedral Al13. A detailed view of the magnetic behavior of Ge16M0/− clusters shows that the magnetic moments of the atomic metals remain large even when they are quenched upon doping. When M goes from Sc to Zn, the total spin magnetic moment of Ge16M0/− increases steadily and reaches the maximum value of 3 μB with M = Mn before decreasing towards the end of the first-row 3d block metals. Furthermore, the IR spectra of some tetrahedral Ge16M are also predicted.
1. Introduction
Along with silicon, germanium is one of the most important microelectronic materials. The last several decades have witnessed a continuing interest in the clusters of this semiconductor element since their bulk materials can no longer satisfy the current needs of the miniaturization of electronic devices.1–11 Both silicon and germanium do not favor sp2-hybridization such as carbon whose small clusters tend to form linear or planar cyclic structures, but rather prefer 3D species arising from a tetrahedral sp3 hybridization. Consequently, pure germanium clusters with high symmetry are often unstable in the form of empty caged structures. Since the first observation of the far more abundant Si15M and Si16M that was reported in 1987 from the laser photoionization time of flight mass spectra,12 subsequent investigations using ab initio calculations on the geometrical and electronic structures of the Si15 and Si16 clusters doped with several transition metals such as Cr, Mo, and W have been performed.13 These studies showed that the transition metal dopant is often located inside a polyhedral cage forming the Si15M and Si16M clusters that have high thermodynamic stability and low magnetic moments as compared the M metal dopants. In a recent review article, Kumar et al.14 analyzed in detail the electronic and geometrical structures of the Si15 and Si16 clusters doped with several transition metals and showed that the transition metal atom is always endohedrally doped within the silicon cage. This thus demonstrates that introduction of hetero-atoms as dopants into hollow cages can supply us with a valuable pathway to stabilize endohedral cage-like clusters as well as to adjust their many novel physico-chemical properties. Motivated by such a fundamental feature, along with several studies performed on doped silicon clusters, a large number of both experimental and theoretical investigations on germanium clusters doped by various chemical elements have been carried out.15–39Transition metal atoms that have unpaired valence electrons in their nd electronic configurations, are inherently magnetic elements. They have been considered as interesting dopants in clusters since interactions between these impurities and the host are expected to alter both electronic and geometrical structures and thereby to generate the doped clusters possessing some novel physico-chemical properties. Moreover, as stated above, due to their high coordination number, transition metals can endohedrally be doped and stabilize the caged structures and simultaneously tailor magnetic properties of host clusters. Indeed, previous studies of singly transition metal doped germanium clusters showed that starting from the size n = 9, the Ge unit absorbs the Ni and Ru dopant endohedrally in giving rise to the most stable isomers of GenNi and GenRu.22,24 The metal atom is encapsulated inside a germanium cage at n = 10 when the dopant is Ti, V, and Cu,20,25,29 and the critical size for the heavy metal W atom being completely enclosed into a caged germanium framework in the GenW clusters turns out to be at n = 12.33 A theoretical investigation34 on divalent-metal atom doped silicon, germanium and tin clusters XnM (X = Si, Ge, Sn; n = 8–12 and 14) demonstrated that the 12- and 14-atom clusters can be transformed into magic clusters upon doping. Particularly, the manganese-doped X12Mn was found to be an icosahedral superatom with a high magnetic moment of 5 μB.34 In an examination of doubly iron-doped germanium clusters, Liang and co-workers also indicated that both neutral and cationic states of GenFe20/− adopt polyhedral cage-like shapes with one Fe atom located inside the cage with 9 ≤ n ≤ 12.26 Soon after the theoretical prediction of metal-encapsulated silicon cages,40 Kumar and Kawazoe performed a series of calculations to explore the possible germanium cages stabilized by metal doping. Analogous to M@Sin clusters, they explored M@Gen (n = 14–16 and M = Ti, Zr, Hf, Fe, Ru, Os) clusters with various possible cage configurations such as the Frank–Kasper (FK) polyhedron, capped decahedron, fullerene-like cage and cubic cage.41–44 Remarkably, the Ge16M sizes have been one of the most attractive germanium clusters that have been reported so far. A quantum chemical study of Kumar et al.41 revealed that the very stable ground states of Ge16M clusters, with M being elements of Group IVb including Ti, Zr, and Hf, are FK tetrahedra characterized by large HOMO–LUMO energy gaps. Surprisingly, the energy gap for Ge16Zr is even larger than the value for the lowest-energy isomer of FK Si16Zr discovered before.40 Most recently, Du and co-workers carried out an investigation on the interaction in dimers of well-known endohedrally doped clusters, including several X16M clusters with X being the tetravalent elements, and found that Ge16Ti cage clusters emerge as suitable building blocks to assemble generating solids and nanostructures with enhanced stabilities and diverse physical properties.45
To date, many transition metal-silicon and transition metal-germanium clusters have been examined, and the understanding on the cage-like silicon and germanium structures stabilized by doping of some transition metals has well been established on the basis of the concept of filling the electron shells for superatoms within a spherical potential model, and also of various electron counting rules including the Wade–Mingos rules, systems with 18 and 32 electrons.14 However, to the best of our knowledge, until recently only one study on the trimeric Ge2M including all 3d transition metals M was reported.46 Therefore, systematic theoretical studies on a certain series of transition metal-germanium clusters are still necessary in order to understand more deeply the relationship between structures and electronic properties of the transition metal doped Ge cluster, especially the Ge16 ones bearing the characteristic Frank–Kasper geometry. In this context, we set out to perform a systematic theoretical investigation on the geometries, stability, and magnetic properties of the germanium clusters doped by one atom belonging to the 3d row transition metals in both neutral and anionic states Ge16M0/−, with M going from Sc to Zn. Using density functional theory (DFT) calculations, we thoroughly determine the geometries of the lowest-lying equilibrium structures and thereby explore their structural evolution, as well as assign their electronic configurations, energetic parameters and magnetic properties. In particular, some systems behaving as strong superhalogens are discovered.
2. Computational methods
On the basis of a reliability test that has been obtained from a previous study on germanium-based clusters,21 we select the hybrid B3PW91 functional in conjunction with the 6-311+G(d) basis sets as implemented in Gaussian 09 package47 for all electronic structure calculations carried out in this work. The unrestricted formalism is used for species with an open electronic shell. The search for local energy minima is conducted using the two approaches. First, plausible structures of Ge16M clusters are generated using a stochastic algorithm,48 which was improved based on the random kick procedure reported by Saunders.49 By another way, initial structures of each Ge16M are manually constructed by adding the M atom at all possible positions on the surfaces of the reported low-lying isomers of Ge16.10,11 The initial guess structures are then geometrically optimized using the hybrid B3PW91 functional in conjugation with the small LANL2DZ basis set. Several local minima obtained by different approaches turn out to be identical. The local energy minima having relative energies of <5 eV with respect to the lowest-lying isomer are then reoptimized at different spin states using the same B3PW91 functional but with the larger 6-311+G(d) basis set. Harmonic vibrational frequencies and zero-point energy (ZPE) corrections of the Ge16M clusters are subsequently calculated at the same level. Unless otherwise stated, relative energies quoted hereafter are determined from B3PW91/6-311+G(d) + ZPE computations.Furthermore, the natural bond orbital (NBO) analyses are performed by using the NBO 3.0 program implemented in the Gaussian package to examine the electronic configuration and thereby rationalize the magnetic and chemical bonding properties of the clusters considered. Based on the NBO analyses, the magnetic moments including the total (TMMs) and local (LMMs) values are defined as the difference between the numbers of spin-up and spin-down electrons occupying the molecular/atomic orbitals of the cluster/atom.
3. Results and discussion
3.1. Geometrical structures
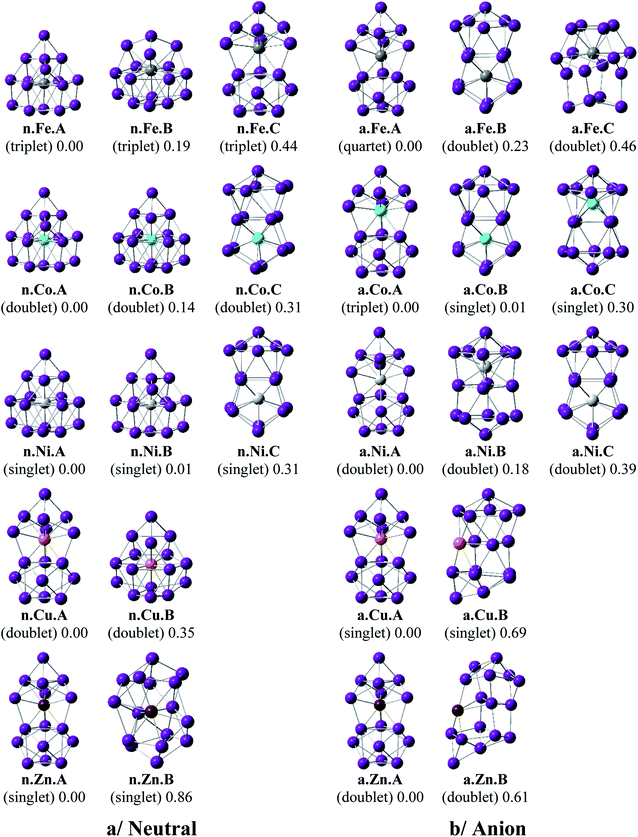
Shapes of the optimized equilibrium structures of both neutral and anionic series of Ge16M clusters, their spin states, and DFT relative energies are shown in Fig. 1 and 2. Because of the large number of isomers located on the potential energy surface of each cluster, only some low lying isomers whose relative energies are close to the corresponding ground state structure are presented for each dopant M. | ||
| Fig. 1 Geometry, relative energy, and spin state (in the bracket) of the most stable isomers Ge16M0/−, with M = Sc, Ti, V, Cr, and Mn using (U)B3PW91/6-311+G(d) optimizations. | ||
 | ||
| Fig. 2 Geometry, relative energy, and spin state (in the bracket) of the most stable isomers Ge16M0/−, with M = Fe, Co, Ni, Cu, and Zn using (U)B3PW91/6-311+G(d) optimizations. | ||
As for a convention, a X.M.Y label is used to denote the isomers considered, in which X = n and a stand for a neutral and anionic state, respectively, M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn, and Y = A, B, C… refers to the different isomers with increasing relative energy. Thus, X.M.A invariably refers to the lowest-energy isomer of the X.M series.
The main characteristics of the geometrical features can briefly be summarized as follows:
For M = Sc, Ti and V, our calculated results are in good agreement with the previous studies.36,41 The most stable Ge16M in both neutral and anionic states consistently prefer a FK structure in which the dopant atom is endohedrally located at the central position of the FK Ge16 cage. Remarkably, the lowest-lying isomers of both isoelectronic Ge16Sc− anion and Ge16Ti neutral are much more stable than the next isomers with large relative energy gaps of 0.84 and 0.95 eV, respectively. The most stable Ge16V in both neutral and anionic states still retain the FK form, but their relative energy gaps decrease to <0.5 eV. Besides, the low-lying isomers of Ge16V− anion are found to exist in the triplet state.
For Ge16Cr, the FK is no longer the most stable form, as they are 0.24 and 0.14 eV higher in energy than the neutral and anion, respectively, of another endohedral structure in which the Cr atom is encapsulated in a C3v cage. Ge16Cr also favors high spin multiplicity, corresponding to the triplet and quartet states for the neutral and anion, respectively.
The three lowest lying isomers of the neutral Ge16Mn prefer endohedrally doped structures and are stable in a quartet state. n.Mn.A becomes 0.25 eV more stable than the other cage n.Mn.B. In the anionic state, however, a competition in energy among three most stable isomers emerges with relative energy gaps of <0.1 eV. Particularly, the a.Mn.A, constructed from fusion of two Ge10 in which the endohedral Ge atom, or the Ge atom at the vertex of the lower Ge10 block, is substituted by the Mn atom,48 and the FK a.Mn.B, are energetically degenerate within a small energy difference of only 0.02 eV, and both of them have a magnetic moment of 4 μB, arising from a quintet spin state. Remarkably, the geometry of a.Mn.A is also retained as the most stable one for all remaining Ge16M− anions, with M being Fe, Co, Ni, Cu and Zn, despite a competition in energy in Ge16Co− where the triplet state a.Co.A is only 0.01 eV lower in energy than the singlet a.Co.B. Accordingly, both Co derivatives a.Co.A and a.Co.B are energetically degenerate.
The shapes of the low lying isomers of the neutral Ge16Fe, Ge16Co, and Ge16Ni are similar to that of the Ge16Mn. However, optimization calculations indicate that the two endohedral isomers n.Ni.A and n.Ni.B of Ge16Ni are again practically degenerate with a negligible energy gap of 0.01 eV. Finally, the lowest-lying isomers of Ge16Cu and Ge16Zn exhibit the same shape in both neutral and anionic states.
3.2. Stabilities
In order to probe the inherent thermodynamic stability of the Ge16M clusters considered, their average binding energies (Eb) are examined and compared to those of the relevant pure germanium clusters Ge17 in both neutral and anionic states. The Eb values of the Ge16M clusters can conventionally be defined in eqn (1) and (2):| Eb(Ge16M) = [16E(Ge) + E(M) − E(Ge16M)]/17 | (1) |
| Eb(Ge16M−) = [15E(Ge) + E(Ge−) + E(M) − E(Ge16M−)]/17 | (2) |
Similarly, for the neutral Ge17 and anionic Ge17−, the Eb can be defined by eqn (3) and (4), respectively, as follows:
| Eb(Ge17) = [17E(Ge) − E(Ge17)]/17 | (3) |
| Eb(Ge17−) = [16E(Ge) + E(Ge−) − E(Ge17−)]/17 | (4) |
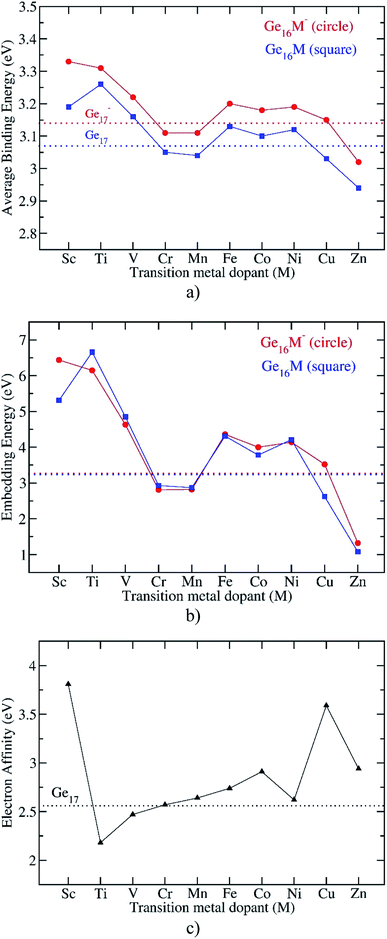 | ||
| Fig. 3 Evolution of the average binding energy, embedding energy, and electron affinity of the Ge16M clusters considered. Values are obtained from (U)B3PW91/6-311+G(d) + ZPE computations. | ||
To reinforce the above findings, we further examine the embedding energy (EE) of the clusters considered. Embedding energy is defined as the energy gained in incorporating a M-dopant into the Ge16 hosts and defined by eqn (5):
| EE(Ge16M0/−) = E(Ge160/−) + E(M) − E(Ge16M0/−) | (5) |
The Eb values of all anionic Ge16M− and pure Ge17− clusters are obviously higher than those of the neutral counterparts, as shown in Fig. 3a. An examination of the computed adiabatic electron affinities (EA) of neutral Ge16M, in comparison to that of Ge17, can thus give us a better insight into this feature. As shown in Fig. 3c, except for Ti and V, the first-row transition metal doped germanium clusters Ge16M have the larger EA values than that of Ge17. When the M-dopant varies from Sc to Zn, the EA of Ge16M takes the largest value of 3.8 eV at Ge16Sc, then decrease sharply and reaches the lowest value of 2.2 eV at the next member Ge16Ti. Then, the EA value gradually increases as M-dopant goes from Ti to Co, then slightly decreases at Ge16Ni before strongly increases at the coinage metal Ge16Cu and finally decreases again at Ge16Zn.
The large EA of Ge16Sc can be interpreted in the same way as that applied to the neutral Al13, which is well-known for its very large electron affinity exceeding that of halogen atoms and has thus been named as a superhalogen.50 Similarly, the neutral Ge16Sc, as formed from the detachment of one electron from the closed-shell structure of the anion Ge16Sc− possessing an enhanced thermochemical stability, also has a very large electron affinity. As stated above, calculations reveal that the EA of Ge16Sc amounts to 3.8 eV, which is even larger than that of 3.6 eV of Al13.51 In contrast to Ge16Sc, the following neutral member Ge16Ti, which is stabilized by a closed shell filled by 68 valence electrons in a singlet state, exhibits the smallest EA value due to the low stability of the corresponding anion. In this context, Ge16Sc can be considered as a superhalogen.
The enhanced stability of both isoelectronic Ge16Ti and Ge16Sc− FK structures can be rationalized by examining their MO pictures under the viewpoint of the electronic shells in a Jellium model (JM),52 which is successfully applied to clarify the stability of various structural motifs of atomic clusters in previous studies, particularly those based on Group IVa atoms.48,53,54 According to this simple model, the valence electrons are freely movable in a simple mean-field potential constructed by the nuclei of atoms; the valence electrons fill the orbitals following the pattern of atomic orbitals (AO) as [1S2 1P6 1D10 2S2 1F14 2P6 1G18 2D10…] corresponding to the numbers of electrons of 2, 8, 18, 20, 34, 40, 58 and 68, etc., that emerge as the magic numbers consistent with a complete filling of the successive electronic shells. As a consequence, a cluster that possesses a valence electron number belonging to this magic number series is able to attain an enhanced thermodynamic stability.
Both Ge16Ti and Ge16Sc− FK's are characterized by a closed electronic shell configuration with the magic number of 68 valence electrons. The shapes of the relevant MOs and their energy levels, as illustrated in Fig. 4, reveal the great similarity between their densities of states (DOS) and thus prove that they have similar electronic structure as well as the thermochemical stability. The 68 valence electrons of each cluster are distributed in the following orbital configuration:
| [(1A1)2 (1T2)6 (1E)4 (2T2)6 (2A1)2 (3T2)6 (1T1)6 (3A1)2 (4A1)2 (4T2)6 (2T1)6 (5T2)6 (2E)4 (3E)4 (6T2)6]. |
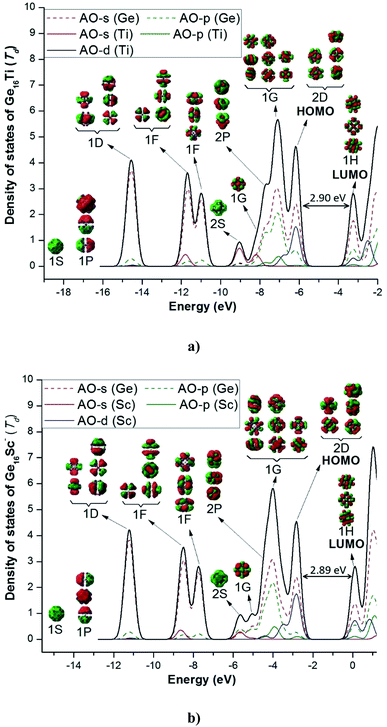 | ||
| Fig. 4 Total (DOS) and partial (pDOS) densities of state of (a) Ge16Ti and (b) Ge16Sc−. Shapes of orbitals of clusters are obtained from B3PW91/6-311+G(d) calculations. | ||
This corresponds to a sequence of electronic shell model as:
| [1S2 1P6 1D10 1F14 2S2 1G2 2P6 1G16 2D10] |
For both clusters, the lowest-lying MOs include an s-type valence orbital of the 1S and three p-type orbitals of the 1P subshells. The MOs of both 1D and 1F subshells are mainly composed of s-AO of Ge and the remaining AOs of both germanium and the transition metal dopant with much smaller contributions. For the neutral Ge16Ti, the MO of 2S subshell are principally formed by the s-AO(Ti), whereas the MO of 2S of anion Ge16Sc− are constructed by interaction between s-AO(Sc) and p-AOs(Ge). The MOs of 2P and 1G subshells are constructed by a combination of both s- and p-AOs of Ge-atoms. Finally, the 2D subshell is composed of both s- and p-AO of Ge and d-AO of the metal impurity. In general, the electronic configuration of the both neutral Ge16Ti and anion Ge16Sc− basically satisfies the electronic shell model of [1S2 1P6 1D10 1F14 2S2 1G2 2P6 1G16 2D10] and makes them the enhanced stability species with a magic number of 68 valence electrons.
Remarkably, in addition to the finding of Ge16Sc superhalogen, calculations reveal that the ground state of the anion Ge16Cu− also has a closed electronic shell and high stability as compared to its neutral form, and being much more stable than the second isomer with a large relative energy gap of 0.69 eV. This is caused by the fact that the neutral Ge16Cu has a large electron affinity of 3.6 eV, again approximate to those of the chlorine atom and Al13 mentioned above. Accordingly, Ge16Cu can also be considered as a superhalogen. The large EA of Ge16Cu can be interpreted based on MO approaches. Of the Ge16M clusters in both neutral and anionic forms, the electronic structure of the anion Ge16Cu− can be considered as a closed-shell by 68 electrons in the pool of valence electrons of the whole cluster such as in the case of Ge16Sc− and Ge16Ti even though it possesses a non-spherical like geometry. This could be rationalized by considering the following fact. Each Ge delocalizes four electrons whereas the Cu dopant delocalizes three of its eleven 4s13d10 valence electrons including one 4s and two 3d into the pool, and while the added electrons are also delocalized in the shell of the entire cluster, the eight remaining 3d electrons are localized in four 3d orbitals of the central Cu. The reason for such a behaviour of the Cu atom in the Ge16Cu− anion is that it is located at the trigonal-prismatic hole formed by six nearest Ge atoms. Following the ligand field effects induced by the trigonal-prismatic coordination within a C3v point group of the whole Ge16Cu− cluster, the five degenerate 3d AOs of the Cu atoms split into three groups of  irreducible representations. While the two former groups have lower energies, the latter possessing higher energy matches the energies of the valence AOs of the Ge neighboring atoms, and thereby combine well with them to form shell MOs of the resulting cluster. Based on shapes and relative energies of the MOs, we can now assign the electronic energy levels of the anion Ge16Cu− as [1S2 1P6 1D6 1F6 1D4 1F8 3dCu8 2S2 2P2 1G6 2P4 1G10 2D4 1G2 2D6]. The images of the 3dCu orbitals are represented in the insert of Fig. 5.
irreducible representations. While the two former groups have lower energies, the latter possessing higher energy matches the energies of the valence AOs of the Ge neighboring atoms, and thereby combine well with them to form shell MOs of the resulting cluster. Based on shapes and relative energies of the MOs, we can now assign the electronic energy levels of the anion Ge16Cu− as [1S2 1P6 1D6 1F6 1D4 1F8 3dCu8 2S2 2P2 1G6 2P4 1G10 2D4 1G2 2D6]. The images of the 3dCu orbitals are represented in the insert of Fig. 5.
3.3. Electron shell of 68 valence electrons and Jahn–Teller distortion
Let us now describe in some detail the electronic configurations of the neutral Ge16Ti as well as the anionic Ge16Sc− that possess FK shape in Td symmetry. Each Ge atom in Ge16Ti contributes four valence electrons, whereas the Sc and Ti atom contribute three and four, respectively, to its cluster shell. The number of valence electrons contributed by the constitution atoms to the cluster shell amounts to 68 that occupy thus 34 shell MOs. As described in Section 3.2 above, the electronic configuration of this cluster can be written as [1S2 1P6 1D10 2S2 1F14 2P6 1G18 2D10] in which the HOMO is the 2D shell and the LUMO is the 1H. In a Td point group, the D shell orbitals of either Ge16Ti or Ge16Sc− split into 2-fold and 3-fold degenerate orbitals, corresponding to an E irreducible representation and a T irreducible representation, respectively, or it could be written as D = E + T. The F shell orbital of both Ge16Ti and Ge16Sc− split to 1-fold and 3-fold and 3-fold degenerate orbitals, namely F = T + T + A. In the same vein, the G shell orbital splits to 1-fold and 3-fold, 3-fold and 2-fold degeneracy orbitals, namely G = A + 2T + E. Therefore, the electron shell configuration of each cluster could be written as follows: [1S2 1P6 1D2-folds4 1D3-folds6 2S2 1F3-folds6 1F3-folds6 1F1-fold2 2P6 1G1-fold2 1G3-folds6 1G3-folds6 1G2-folds4 2D10].A closer look at the shapes of shell MOs shows that the ordering of some shell MOs alters, and the shell electron configuration of the cluster becomes: [1S2 1P6 1D2-folds4 1D3-folds6 1F1-fold2 1F3-folds6 1F3-folds6 2S2 1G1-fold2 2P6 1G3-folds6 1G3-folds6 1G2-folds4 2D2-folds4 2D3-fold6] as this can be seen in Fig. 6a and c.
Nevertheless, geometry optimizations reveal that only FK structures with a closed shell filled by 68 valence electrons, including neutral Ge16Ti and anionic Ge16Sc− clusters, possess a Td high symmetry whereas the remaining FK structures, which enclose more or less than 68 valence electrons, exist at lower symmetry. Such a geometrical distortion can be understood by the Jahn–Teller effect. Let us first examine the open-shell neutral Ge16Sc having 67 valence electrons due to the fact that the HOMO of the anion Ge16Sc− is a 2D shell orbital; the latter has the value of azimuthal quantum number l equal to 2 and a 5-fold degeneracy. In the T point group, the 2D shell orbitals are reduced to the E + T irreducible representations in which the T orbitals, or the 2Dthree-fold levels are higher energetically. As an electron is removed from such a HOMO, namely the 2Dthree-fold of the anionic Ge16Sc−, to form the neutral Ge16Sc, the corresponding SOMO is triply degenerate and the Td structure of the Ge16Sc neutral is subjected to a distortion to a C3v group, accompanying a splitting of the T MOs, or the 2Dthree-fold one, into E + A2 orbitals, and the A2 orbital is now singly occupied (cf. Fig. 6b).
In the case of 69 electrons of the Ge16Ti− anion, it is worth to note that the LUMO of the Ge16Ti neutral cluster corresponds to a 1H shell orbital which has the value of azimuthal quantum number l of 5 and a 11-fold degeneracy. In the T point group, the 1H shell is reduced into E + 3T irreducible representations. In going from the neutral Ge16Ti to the anionic Ge16Ti−, the incoming electron fills in one of the degenerate LUMO of T representation, which thus causes a distortion of the Td structure of the Ge16Ti neutral, again to a C3V structure of the resulting Ge16Ti− anion, accompanying with a splitting of the T MOs into E + A2 orbitals, of which the A2 orbital accommodates the unpaired electron (cf. Fig. 6d).
It is also worth mentioning that the vertical electron detachment energy between the neutral Ge16Sc and its anion at the geometrical structure of the anion which has 68 electrons, amounts to 3.95 eV, whereas the adiabatic detachment energy is 3.81 eV. Thus, the energy gain of the Ge16Sc neutral upon distortion is 0.14 eV. Similarly, the energy gain of Ge16Ti− due to a distortion from Td to C3v symmetry is calculated at a value of 0.23 eV. Such a small but significant amount of energy originates in an intrinsic instability of the T1 or the 2Dthree-fold1 electron configuration of the HOMOs of Ge16Sc, as well as the T1 or 1Hthree-fold1 configuration of the LUMOs of Ge16Ti−, all in Td symmetry. The latter are therefore distorted to C3v point group yielding such an energy gain of the system.
The SOMO–LUMO gap of 1.1 eV in the 67 electrons neutral Ge16Sc is very close to the SOMO–LUMO gap in the 69 electrons of anionic Ge16Ti−. This reflects the fact that both systems undergo a comparable symmetry reduction when going from the Ge16Sc− to Ge16Sc as well as from Ge16Ti to Ge16Ti−, as this is illustrated in Fig. 6b and d. In summary, two forces are combined in the formation of the C3v structures of 67-electron Ge16Sc neutral and 69 electron Ge16Ti− anion: the major intrinsic stability of the 68 electron Ge16Sc− and Ge16Ti counterparts which favor a Td symmetry, and the reorganization energy gained during the symmetry lowering from Td to C3v point group. This also makes the average binding energy of Ge16Sc− much higher than that of its neutral, while the average binding energy of Ge16Ti− is only slightly higher.
3.4. Spin magnetic moments
It is typical that when a metallic cluster is doped by a transition metal atom, the outer-most orbitals of the impurity including d and s shell can combine with the valence orbitals of the host to form shell orbitals of the resulting doped clusters. For the investigated Ge16M, as each Ge atom delocalizes its 4 valence electrons, the corresponding Ge16M− anion can approach 68 electrons if the metal dopant could delocalize its 3 valence electrons. The remaining valence electrons of the metal will combine to build up its magnetic moment. Fig. 7 shows the change in the total spin magnetic moment (TMM) value of Ge16M in both neutral and anionic states as M goes from Sc to Zn. The perception of how the total and local spin magnetic moments of the clusters arise can be confirmed in considering the calculated total and local spin magnetic moments on each constituent atoms of the clusters that are listed in Table 1. The total spin magnetic moment of the Ge16M− anion increases steadily from 1 μB for the Ti dopant to 4 μB for the Mn dopant, then decreases to 1 μB for the Ni dopant. For the Ge16M− anions, their magnetic moments are mostly held on the transition metal atoms, being 0.4, 1.5, 2.9, 3.1, 2.2, and 1.4 for the Ti, V, Cr, Mn, Fe, and Co dopants, respectively. Exceptions include the Ge16Ni− and Ge16Zn− in which the total magnetic moments are delocalized all over the entire skeleton. | ||
| Fig. 7 Total spin magnetic moment (μB) of Ge16M in comparison with Ge17 at both neutral and anionic states. | ||
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Atom | Ge16Sc | Ge16V | Ge16Cr | Ge16Mn | Ge16Fe | Ge16Co− | Ge16Cu |
| Ge (1) | 0.0 | 0.1 | −0.3 | 0.0 | 0.1 | 0.0 | 0.0 |
| Ge (2) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ge (3) | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ge (4) | 0.1 | −0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ge (5) | 0.1 | 0.0 | −0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| Ge (6) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ge (7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ge (8) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ge (9) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ge (10) | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ge (11) | 0.0 | −0.1 | −0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| Ge (12) | 0.0 | 0.0 | −0.1 | 0.1 | 0.1 | 0.0 | 0.4 |
| Ge (13) | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| Ge (14) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ge (15) | 0.0 | 0.0 | 0.0 | 0.1 | −0.1 | 0.0 | 0.0 |
| Ge (16) | 0.1 | −0.1 | 0.0 | 0.0 | −0.1 | 0.0 | 0.0 |
| M (17) | 0.2 | 1.0 | 2.6 | 2.7 | 1.7 | 0.9 | 0.0 |
| TMM | 1.0 | 1.0 | 2.0 | 3.0 | 2.0 | 1.0 | 1.0 |
| (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atom | Ge16Ti− | Ge16V− | Ge16Cr− | Ge16Mn− | Ge16Fe− | Ge16Co− | Ge16Ni− | Ge16Zn− | Ge17− |
| Ge (1) | 0.1 | −0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Ge (2) | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 |
| Ge (3) | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 |
| Ge (4) | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| Ge (5) | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 |
| Ge (6) | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 |
| Ge (7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| Ge (8) | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 |
| Ge (9) | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 |
| Ge (10) | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 |
| Ge (11) | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 |
| Ge (12) | 0.1 | 0.0 | 0.0 | 0.2 | 0.2 | 0.2 | 0.0 | 0.1 | 0.1 |
| Ge (13) | 0.1 | 0.1 | −0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 |
| Ge (14) | 0.0 | 0.1 | −0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.5 |
| Ge (15) | 0.1 | 0.0 | −0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 |
| Ge (16) | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| M (17) | 0.4 | 1.5 | 2.9 | 3.1 | 2.2 | 1.4 | 0.2 | 0.0 | 0.0 |
| TMM | 1.0 | 2.0 | 3.0 | 4.0 | 3.0 | 2.0 | 1.0 | 1.0 | 1.0 |
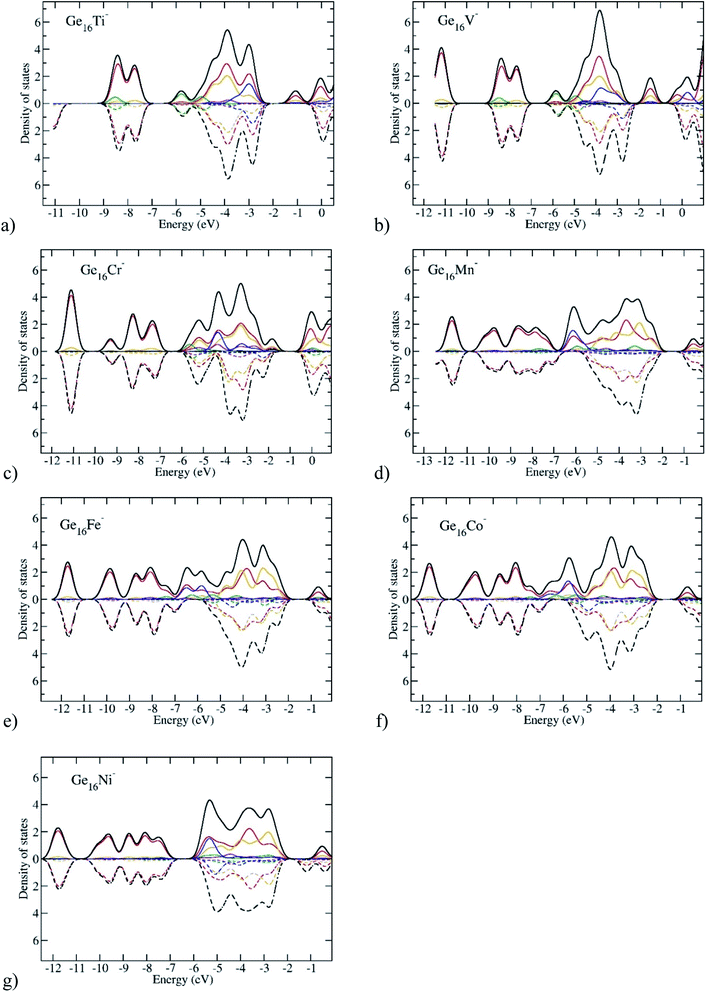
In order to gain more insight into the spin magnetic behavior of Ge16M clusters, along with Table 1 listing total and local spin magnetic moments, the total density of states (TDOS) and partial density of states (PDOS) of the anionic Ge16M− clusters from the Ti to Ni dopants are plotted in Fig. 8. Spin-up and spin-down densities of states are plotted separately on the same graph for each of the clusters. It has been stated that the relative shift between the spin-up and spin-down bands indicates the degree of spin-exchange splitting; the larger the shift of DOS bands, the larger the magnetic moment of the cluster.55 As we glance at Fig. 8, we can realize that the shift is large for the Ge16M− (M = Cr, Mn, Fe, Co) clusters suggesting that they possess high spin magnetic moments, while it is slight in the cases of Ge16Ti− and Ge16Ni− clusters.
The spin magnetic moment of the whole cluster is mainly created by the unpaired electrons, and the spin magnetic moments localized on the metal dopant arise from the density of unpaired electron contained in its orbitals or the difference in partial density of α- and β-electrons of the M atom. For the Ti and V clusters (Fig. 8a and b), the α-HOMO states are located at distinctive energy level as compared to the α-, β-inner states, while for the other Ge16M, with M being Cr, Mn, Fe, Co, and Ni, the α-HOMO states are situated in almost the same energy region as with the α, and β-inner states. For the DOS of Ge16Cr− anion, in the domain of states ranging from −4.3 to −2.0 eV, there is an obvious presence of the density of α-state of d orbitals of Cr (the blue curve) without the β-state counterpart. This information implies that the total magnetic moment of the cluster is mainly dominated by Cr-d states, while Ge-s and Ge-p states make rather a small contribution. A similar argument can be made for the successive clusters. For Ge16Mn− in the domain from ca. −6.5 to −2.0 eV there are densities of α-states of s- and d-orbitals of Mn (the green and blue curves) without their β-state counterparts; for Ge16Fe− in the domain from ca. −7.0 to −2.0 eV there are densities of α-states of s- and d-orbitals of Fe (the green and blue curves) without their β-state counterparts; for Ge16Co− in the domain from ca. −7.0 to −2.0 eV there are densities of α-states of s- and d-orbitals of Co (the green and blue curves) without their β-state counterparts. This observation is in line with the calculated local magnetic moments on the Cr, Mn, Fe, Co atoms which amount to 2.9, 3.1, 2.2 and 1.4 μB, respectively. Thus, although the Ge16 cage somehow quenches the usually large magnetic moments of free transition metal atoms, the latter property remain substantial in the doped derivatives.
3.5. IR spectra
As discussed above, several lower-energy structural and spin isomers for each Ge16M cluster are considered, and we report herein only the lowest-energy isomers for certain species. In some cases, the energy difference of the most stable isomers is really small that cannot allow us to distinguish the ground state structure. Moreover, no infrared (IR) spectrum of any GenM has been reported both experimentally and theoretically so far. The IR spectra are expected to provide us with a fingerprint for assignment of the cluster in terms of their metallic dopant, especially for the geometrical structure. For the purpose to help for distinguishing low-lying energy isomers, the calculated vibrational spectra of two lowest-lying isomers for some selected Ge16M, namely the Ge16Sc and Ge16Ti in both neutral and anionic forms, are plotted in Fig. 9. The vibrational frequency range goes from 0 to 350 cm−1, as no signals are found at higher photon energy. As it could be seen in Fig. 9a and b, the vibrational spectra for the ground state of Ge16Sc− anion and Ge16Ti neutral, each has 68 valence electrons and is in Td symmetry, are relatively simple featuring a highly intense peak, centered at ∼304 and 292 cm−1 with T degenerate modes, respectively.Unlike the IR spectra of the ground states, those of the next isomers turn out to be more complicated and characterized by several highly intense peaks in the range of 260 to 300 cm−1 (see Fig. 9c and d). The following frequencies can be noted. While for the ground state of Ge16Sc neutral, isomer n.Sc.A has νstretching Sc–Ge being of ∼298 cm−1, the νstretching Sc–Ge of the next isomer n.Sc.B is of ∼280 and 290 cm−1. For the Ge16Sc− anion, FK structure a.Sc.A has a νstretching Sc–Ge of ∼304 cm−1, while νstretching Sc–Ge of the isomer a.Sc.B is of ∼264, 279 and 289 cm−1. Although for Ge16Ti, FK structure n.Ti.A only has one peak at 292 cm−1, being three-fold degenerate Sc–Ge stretching mode, for the next isomer n.Ti.B, the Sc–Ge stretching modes appear at three frequencies ∼263, 275 and 298 cm−1. For the anion Ge16Ti−, a.Ti.A in distorted FK shape (C3v) leads its νstretching Sc–Ge being of ∼246 and 281 cm−1, which is a two-fold degenerate mode E, while a.Ti.B isomer has a νstretching Sc–Ge being of ∼252, 270 and 279 cm−1. The large difference in vibrational spectra for both nearly degenerate isomers and their high intense peaks can be used to assign the ground state structure when they can be generated experimentally and characterized spectroscopically.
By analysis of their IR spectra, a symmetry lowering of the cluster in going down from 68 to 67 as well as going up to 69 valence electrons can be recognized. The IR spectrum of Ge16Ti is characterized by a single peak centered at 292 cm−1, corresponding to the vibrational modes of Ti atom inside the Ge16 cage. Although the cluster has 45 vibrational modes in total, only the vibrational modes of the Ti atom inside the cage are IR active with notably high intensity. Other modes that correspond to deformation of the Ge16 cage do not result in significant IR intensity. The stretching modes of the Ti atom inside the Ge16 cage belong to the T2 irreducible representation. As the cluster receives one electron to form the Ge16Ti−, the anion is distorted to C3v point group and the ν(Ti–Ge16) T2 mode is reduced to the E + A2 modes. As this could be seen in Fig. 9, the difference of 22 cm−1 between the E and A2 modes is relatively significant in view of the low frequency. It is worth noting that for Ge16Ti−, its A2 ν(Ti–Ge16) stretching mode has a lower frequency than the E ν(Ti–Ge16) stretching. The added electron in the Ge16Ti− anion causes the cluster to distort from Td to C3v point group, giving rise to a lowering of electron density along one of the three ν(Ti–Ge16) stretching modes.
4. Concluding remarks
In the present theoretical study, the geometric and electronic structures, thermodynamic stability, and magnetic properties of the 16-atom germanium clusters doped with the first-row 3d transition metal atoms, Ge16M with M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn, in both neutral and anionic states, were investigated using quantum chemical (DFT) methods.The most stable isomers of Ge16M, as M goes from Sc to V, prefer a Frank–Kasper (FK) structure in which the metal dopant is endohedrally encapsulated at the central position of a Ge16 FK cage. In particular, both the anionic Ge16Sc− and neutral Ge16Ti whose electronic shells are filled by a magic number of 68 valence electrons, are characterized by a perfect FK tetrahedral geometry and enjoy an enhanced thermochemical stability with high average binding energies and embedding energies. Their higher stability can be interpreted in terms of the electronic shells of the Jellium model. Analyses of electronic configuration also indicate that the geometric distortions from an FK tetrahedron Ge16M having more or less than 68 valence electrons are caused by a Jahn–Teller effect arising from the degenerate frontier orbitals.
Perhaps most interestingly is the result obtained from energy calculations that revealed that both neutrals Ge16Sc and Ge16Cu emerge as superhalogens, due to a characteristic that each possesses a large electron affinity of 3.8 and 3.6 eV, respectively. These electron affinities exceed the values of halogen atoms and even that of the well-known superhalogen Al13 (∼3.6 eV). Moreover, a comprehensive picture of the magnetic behavior is displayed for Ge16M clusters at both neutral and anionic states, in which the observed dopant-dependent magnetic moment can be understood by a charge distribution analysis. As M goes from the left to the right side on the first-row transition metal atoms in the Periodic Table, corresponding to Sc to Zn, the total magnetic moment of Ge16M first takes a low value at M = Sc and Ti, then increases steadily and reaches the maximum value of 3 μB at M = Mn, before decreasing towards the end of the row due to the fact that these magnetic moments are mostly held on the metal dopants. This result opens up an avenue that a magnetically inert germanium cluster can be induced to a relatively high magnetic moment following doping by a suitable transition metal impurity. Finally, the IR spectra of FK Ge16M are simulated as a helpful guide for future experimental assignment of these degenerate ground state clusters.
Funding information
This work is funded by VinGroup (Vietnam) and supported by VinGroup Innovation Foundation (VinIF) under project code VinIF.2020.DA21.Conflicts of interest
There are no conflicts of interest to declare.References
- E. F. Archibong and A. St-Amant, J. Chem. Phys., 1998, 109, 962–972 CrossRef CAS.
- K. A. Gingerich, R. W. Schmude, M. Sai Baba and G. Meloni, J. Chem. Phys., 2000, 112, 7443–7448 CrossRef CAS.
- B.-x. Li and P.-l. Cao, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 15788–15796 CrossRef CAS.
- Z.-Y. Lu, C.-Z. Wang and K.-M. Ho, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 2329–2334 CrossRef CAS.
- J. Zhao, J. Wang and G. Wang, Phys. Lett. A, 2000, 275, 281–286 CrossRef CAS.
- C. Zhao and K. Balasubramanian, J. Chem. Phys., 2001, 115, 3121–3133 CrossRef CAS.
- S. Ma and G. Wang, J. Mol. Struct.: THEOCHEM, 2006, 767, 75–79 CrossRef CAS.
- G. Pacchioni and J. Koutecký, J. Chem. Phys., 1986, 84, 3301–3310 CrossRef CAS.
- J. Wang, G. Wang and J. Zhao, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 205411 CrossRef.
- S. Bulusu, S. Yoo and X. C. Zeng, J. Chem. Phys., 2005, 122, 164305 CrossRef CAS PubMed.
- W. An, Phys. Chem. Chem. Phys., 2018, 20, 25746–25751 RSC.
- S. M. Beck, J. Chem. Phys., 1987, 87, 4233–4234 CrossRef CAS.
- V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 073404 CrossRef.
- J. Zhao, Q. Du, S. Zhou and V. Kumar, Chem. Rev., 2020, 120, 9021–9163 CrossRef CAS PubMed.
- J. De Haeck, T. B. Tai, S. Bhattacharyya, H. T. Le, E. Janssens, M. T. Nguyen and P. Lievens, Phys. Chem. Chem. Phys., 2013, 15, 5151–5162 RSC.
- G. Gopakumar, P. Lievens and M. T. Nguyen, J. Phys. Chem. A, 2007, 111, 4353–4361 CrossRef CAS PubMed.
- V. T. Ngan, J. De Haeck, H. T. Le, G. Gopakumar, P. Lievens and M. T. Nguyen, J. Phys. Chem. A, 2009, 113, 9080–9091 CrossRef CAS PubMed.
- N. M. Tam, V. T. Ngan and M. T. Nguyen, Chem. Phys. Lett., 2014, 595–596, 272–276 CrossRef.
- X.-J. Li and K.-H. Su, Theor. Chem. Acc., 2009, 124, 345 Search PubMed.
- S. Mahtout, C. Siouani and F. Rabilloud, J. Phys. Chem. A, 2018, 122, 662–677 CrossRef CAS PubMed.
- S. Shi, Y. Liu, C. Zhang, B. Deng and G. Jiang, Comput. Theor. Chem., 2015, 1054, 8–15 CrossRef CAS.
- K. Dhaka, R. Trivedi and D. Bandyopadhyay, J. Mol. Model., 2013, 19, 1473–1488 CrossRef CAS PubMed.
- X.-J. Hou, G. Gopakumar, P. Lievens and M. T. Nguyen, J. Phys. Chem. A, 2007, 111, 13544–13553 CrossRef CAS PubMed.
- Y. Jin, S. Lu, A. Hermann, X. Kuang, C. Zhang, C. Lu, H. Xu and W. Zheng, Sci. Rep., 2016, 6, 30116 CrossRef CAS PubMed.
- M. Kumar, N. Bhattacharyya and D. Bandyopadhyay, J. Mol. Model., 2012, 18, 405–418 CrossRef CAS PubMed.
- X.-Q. Liang, X.-J. Deng, S.-J. Lu, X.-M. Huang, J.-J. Zhao, H.-G. Xu, W.-J. Zheng and X. C. Zeng, J. Phys. Chem. C, 2017, 121, 7037–7046 CrossRef CAS.
- C. Liu, I. A. Popov, L.-J. Li, N. Li, A. I. Boldyrev and Z.-M. Sun, Chem.–Eur. J., 2018, 24, 699–705 CrossRef CAS PubMed.
- W. Qin, W.-C. Lu, L.-H. Xia, L.-Z. Zhao, Q.-J. Zang, C. Z. Wang and K. M. Ho, AIP Adv., 2015, 5, 067159 CrossRef.
- C. Siouani, S. Mahtout, S. Safer and F. Rabilloud, J. Phys. Chem. A, 2017, 121, 3540–3554 CrossRef CAS PubMed.
- V. T. Tran, M. T. Nguyen and Q. T. Tran, J. Phys. Chem. A, 2017, 121, 7787–7796 CrossRef CAS PubMed.
- V. T. Tran and Q. T. Tran, J. Comput. Chem., 2018, 39, 2103–2109 CrossRef CAS PubMed.
- R. Trivedi, K. Dhaka and D. Bandyopadhyay, RSC Adv., 2014, 4, 64825–64834 RSC.
- J. Wang and J.-G. Han, J. Phys. Chem. A, 2006, 110, 12670–12677 CrossRef CAS PubMed.
- V. Kumar and Y. Kawazoe, Appl. Phys. Lett., 2003, 83, 2677–2679 CrossRef CAS.
- D. Bandyopadhyay, P. Kaur and P. Sen, J. Phys. Chem. A, 2010, 114, 12986–12991 CrossRef CAS PubMed.
- J. Atobe, K. Koyasu, S. Furuse and A. Nakajima, Phys. Chem. Chem. Phys., 2012, 14, 9403–9410 RSC.
- S. Jaiswal and V. Kumar, Comput. Theor. Chem., 2016, 1075, 87–97 CrossRef CAS.
- H. T. Pham, C.-T. D. Phan, M. T. Nguyen and N. M. Tam, RSC Adv., 2020, 10, 19781–19789 RSC.
- N. A. Borshch, N. S. Pereslavtseva and S. I. Kurganskii, Russ. J. Phys. Chem. B, 2015, 9, 9–18 CrossRef CAS.
- V. Kumar and Y. Kawazoe, Phys. Rev. Lett., 2001, 87, 045503 CrossRef CAS PubMed.
- V. Kumar and Y. Kawazoe, Phys. Rev. Lett., 2002, 88, 235504 CrossRef PubMed.
- V. Kumar and Y. Kawazoe, Appl. Phys. Lett., 2002, 80, 859–861 CrossRef CAS.
- V. Kumar, A. K. Singh and Y. Kawazoe, Nano Lett., 2004, 4, 677–681 CrossRef CAS.
- A. K. Singh, V. Kumar and Y. Kawazoe, J. Phys. Chem. B, 2005, 109, 15187–15189 CrossRef CAS PubMed.
- Q. Du, Z. Wang, S. Zhou, J. Zhao and V. Kumar, Phys. Rev. Mater., 2021, 5, 066001 CrossRef CAS.
- N. D. Minh, C. H. Cuong, N. T. Trung and V. T. Ngan, Theor. Chem. Acc., 2018, 137, 131 Search PubMed.
- M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, et al., Gaussian 09 Revision: D.01, 2009 Search PubMed.
- T. B. Tai and M. T. Nguyen, J. Chem. Theory Comput., 2011, 7, 1119–1130 CrossRef CAS PubMed.
- M. Saunders, J. Comput. Chem., 2004, 25, 621–626 CrossRef CAS PubMed.
- E. Bergeron Denis, A. W. Castleman, T. Morisato and N. Khanna Shiv, Science, 2004, 304, 84–87 CrossRef CAS PubMed.
- Q. A. Smith and M. S. Gordon, J. Phys. Chem. A, 2011, 115, 899–903 CrossRef CAS PubMed.
- M. Brack, Rev. Mod. Phys., 1993, 65, 677–732 CrossRef CAS.
- N. M. Tam, T. B. Tai and M. T. Nguyen, J. Phys. Chem. C, 2012, 116, 20086–20098 CrossRef CAS.
- N. M. Tam, T. B. Tai, V. T. Ngan and M. T. Nguyen, J. Phys. Chem. A, 2013, 117, 6867–6882 CrossRef PubMed.
- C.-G. Li, J. Zhang, W.-Q. Zhang, Y.-N. Tang, B.-Z. Ren and Y.-F. Hu, Sci. Rep., 2017, 7, 17516 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d1ra08527a |
| This journal is © The Royal Society of Chemistry 2022 |